Abstract
Immunoglobulin G (IgG), IgA, and IgM antibodies were measured in serum samples from 71 organ donors, 81 kidney transplant recipients at transplantation, and 67 patients during the posttransplant period by using a virus-like particle-based enzyme-linked immunosorbent assay (ELISA). BK virus (BKV) and JC virus DNA were detected in urine and plasma by real-time PCR. IgG antibodies to BKV were demonstrated in the majority (80.3 to 100%) of patients irrespective of clinical category, but titers were highest in patients with active viral replication. IgA antibodies were present with greater frequency (72.7 to 81.3% versus 0 to 23.6%; P < 0.001) and higher titer (mean optical density, 0.11 to 0.15 versus 0.05 to 0.08; P < 0.001) in patients who were BKV DNA positive than those who were BKV DNA negative. IgM antibodies showed a similar pattern of reactivity but lower frequency in the setting of active viral replication (9.1 to 43.7% versus 0 to 1.4%; P < 0.001). A rise in IgG level of >0.577 optical density (OD) units or a rise in IgA or IgM level of >0.041 OD units was strongly associated with active viral replication. Urine viral load showed a positive correlation with IgM titer (r = 0.22) but a negative correlation with IgG titer (r = −0.28) and IgA titer (r = −0.1). Chronic dialysis patients typically did not have serologic or virologic evidence of active BKV infection. Anti-BKV titers did not rise in patients with JC viruria. In conclusion, measurement of anti-BKV antibody titer and class response can be used to detect the onset of viral replication. ELISAs can be quite specific despite considerable sequence homology between BK virus and JC virus.
BK virus (BKV) and JC virus (JCV) are the two polyomavirus species most commonly implicated in human disease (9). BKV infection is believed to occur during childhood via the respiratory route. This is followed by viral latency in the urogenital tract. BKV reactivation with urinary excretion of virus occurs in 10 to 60% of renal transplant patients and BKV nephropathy in 1 to 10% of renal transplant patients in different studies. Viral nephropathy can also occur in the setting of congenital immunodeficiency and AIDS (12-14, 24, 25, 35, 37, 41, 44-46, 48, 55). The diagnosis of BKV nephropathy is primarily based on histologic examination. High levels of circulating virus in the plasma develop in patients with tissue-destructive disease and can be regarded as a surrogate marker of viral nephropathy (38).
JCV is also a ubiquitous virus acquired early in life. JCV excretion has been noted in the urine of up to 70% of healthy individuals, particularly in the Far East and among Pacific Islanders (1, 5, 6, 29, 30). In patients with AIDS, JCV causes progressive multifocal leukoencephalopathy. Using PCR, JCV DNA can be amplified in up to 75% of blood samples and 92% of cerebrospinal fluid samples obtained from patients with progressive multifocal leukoencephalopathy and systemic lupus erythematosus (15, 18, 39, 54). Viral DNA has also been documented in human neoplasms, including brain tumors and carcinoma of the colon, and nephrectomy specimens with renal cell carcinoma (33). Rarely, JCV can result in interstitial nephritis within the transplanted kidney (28, 57).
The humoral immune response to polyomavirus infection in humans is not well characterized. Most publications date back to the 1970s and 1980s and are based primarily on the hemagglutination inhibition assay (8, 10, 11, 19, 20, 26, 31, 36, 50, 51). Recent studies have used enzyme-linked immunosorbent assay (ELISA) technology (21, 47), and two have studied clinically well-characterized subpopulations of kidney transplant patients. Bohl et al. showed that the titer of anti-BKV antibodies in kidney donors predicted the frequency, magnitude, and duration of posttransplant BKV viruria (4). Hariharan et al. focused on patients with biopsy-proven nephropathy and showed a temporal correlation between elimination of BKV and development of immunoglobulin G (IgG) antibodies to BKV VP-1 (22). We measured IgG, IgM, and IgA levels in defined categories of patients and correlated antibody level with quantitative BKV and JCV viral load.
MATERIALS AND METHODS
Study population.
The study subjects included 71 organ donors, 81 kidney transplant recipients at the time of transplantation, and 67 transplant patients in the posttransplantation period. All transplant patients were recruited from the Thomas E. Starzl Institute Kidney Transplant Program at the University of Pittsburgh Medical Center (Table 1). Archival recipient baseline and deceased donor serum samples were obtained from a sample bank maintained by The Center for Organ Recovery and Education, Pittsburgh, PA. Posttransplant urine and plasma samples were obtained from a subset of patients initially sampled at the time of transplantation. Patients studied in the posttransplantation period contributed from 1 to 11 blood specimens (median, 2 specimens). The samples were collected at a median time interval of 9 months after transplant (range, 0.5 to 80 months). The sample collection protocols were approved by the University of Pittsburgh Institutional Review Board (IRB protocol no. 000586). Immunosuppression consisted primarily of pretransplant induction with thymoglobulin or alemtuzumab (Campath) followed by tacrolimus monotherapy. Random midstream urine samples were collected and assayed for viral DNA without centrifugation. Blood samples from transplant patients were collected in EDTA tubes, and the plasma fraction was separated out by low-speed centrifugation. All samples were frozen at −80°C within 6 h and assayed up to 2 years posttransplant.
TABLE 1.
Frequencies of anti-BKV VP-1 antibodies in different patient categories
| Sample category | No. (percentage) of patients positive for:
|
||
|---|---|---|---|
| IgG | IgA | IgM | |
| Donors (n = 71) | 57 (80.3) | 16 (22.5) | 1 (1.4) |
| Recipient baseline (n = 81) | 66 (81.5) | 19 (23.4) | 0 (0) |
| Posttransplant BKV PCR negative (n = 21) | 20 (95.2) | 5 (23.6) | 0 (0) |
| JCV viruria (n = 19) | 18 (94.7) | 0 (0) | 0 (0) |
| Posttransplant viruria (n = 11) | 11 (100) | 8 (72.7) | 1 (9.1) |
| Posttransplant viremia (n = 16) | 16 (100) | 13 (81.3)a | 7 (43.7)b |
P < 0.001 versus all other sample groups tested for IgA BKV antibodies.
P < 0.009 versus all other sample groups tested for IgM BKV antibodies.
BKV and JCV PCR assays.
DNA was extracted from plasma or urine as previously described (42, 43). Briefly, 5 ml of unspun urine was extracted using a QIAamp Blood Maxi kit (catalog no. 51192; QIAGEN), and 200 μl of plasma was extracted using a QIAamp Blood Mini kit (catalog no. 51104; QIAGEN). The final extraction volumes were 200 μl and 40 μl for urine and plasma, respectively. Quantitative real-time PCR assays for BK and JC viruses were performed using a Roche LightCycler using virus-specific primers and probes targeted against the VP-1 gene (42, 43). Amplification reactions were run in a reaction volume of 20 μl containing 2 μl DNA sample, Roche 10× FasTaq Hyb mastermix (Roche Applied Science), 2.5 mM magnesium chloride, a 500 nM concentration of each forward and reverse primer, and a 200 nM concentration of each probe. No-template control and negative control samples (human genomic DNA extracted from tonsil tissue) were included in each run. Thermal cycling was initiated with a first FasTaq activation step of 10 min at 95°C, followed by 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 5 s, with F3 channel fluorescence data collection at a single point during the annealing phase. Real-time PCR amplification data were analyzed with software provided by the manufacturer. Standard curves for the quantification of JCV and BKV were constructed using serial dilutions of a plasmid containing the entire linearized genome of the JCV Mad1 strain or BKV Dun strain ranging from 1 to 109 genomic copies of JCV DNA per PCR. The sensitivity of both the BKV and JCV quantitative PCR assays was 10 copies of viral genomic DNA.
VLP-based ELISA.
BKV virus-like particles (VLPs) were generated in insect cells from a recombinant baculovirus expressing BKV VP-1 protein as previously described (17). The ELISA was performed as previously described with minor modifications. Briefly, enzyme immunoassay (EIA) plates (PolySorp; Nunc, Naperville, IL) were incubated overnight with VLP protein (30 ng/well). Serum specimens (1:400 dilution for IgG and 1:100 dilution for IgA and IgM) were then tested in duplicate according to a standard EIA protocol. After development of the EIA reaction with 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrogen peroxide solution, absorbance was measured at 405 nm. For IgA and IgM measurements, serum was preincubated for 30 min at room temperature with 20% (vol/vol) goat anti-human IgG (catalog no. I-1011; Sigma). Goat anti-human IgG (5%) was also included in the serum dilution buffer during incubation in antigen-coated wells. Positive and negative control sera, sensitivity controls, and reproducibility controls were included in each run. Runs where reference serum values fell outside the expected standard deviation were repeated. Results are recorded both as an optical density value and as a categorical variable (seropositive or seronegative) based on an assay cutoff. The IgG assay cutoff was determined by comparison with the distribution of values obtained for serum samples from 74 children 2 to 5 years old. An iterative statistical approach was used to exclude outliers in the distribution of these control sera until no remaining values were greater than 3 standard deviations (SD) above the mean optical density (OD) value or until five rounds of the iterative procedure were executed. Seropositivity was defined as 3 SD above the mean OD obtained for the negative control sera (minus outliers). The cut off point for IgG seropositivity was an OD value greater than 0.100 unit. The IgA and IgM cutoffs were determined in a similar manner using serum samples from a subset of 25 children. The IgA and IgM cutoff points for seropositivity were OD values greater than 0.070 unit and 0.115 unit, respectively.
Statistical analysis.
Optical densities were treated as a continuous variable and compared between groups using the Mann-Whitney test. Proportions of different patient categories testing positive for specific antibodies were compared by a chi-square test or Fisher's exact test. Differences in viral loads were evaluated by the Mann-Whitney test. Correlations between viral load and serologic parameters were sought by generating scatter plots based on log viral copy number and optical density readings. All statistical analyses were performed using SigmaStat (San Rafael, CA) software, version 2.03. Regression lines and 95% prediction intervals were generated by SigmaPlot version 8.02 (Systat Software Inc., Point Richmond, CA).
RESULTS
The patients studied ranged in age from 17 to 74 years, with a male-to-female ratio of 2.1:1. Subjects were included in the group that was positive for BKV DNA by PCR posttransplant (the posttransplant BKV PCR-positive group) if BK viral DNA could be amplified from any urine or blood sample. Based on this criterion, 27 posttransplant patients were scored as BKV PCR positive and 40 as BKV PCR negative. The latter group included 19 patients with JCV viruria (Table 1). A seropositive status was assigned whenever the optical density in the ELISA was above the cutoff for seropositivity. Data on BKV serology in patients with JCV viruria were analyzed separately as well as after inclusion in a group designated the posttransplant BKV PCR-negative group.
IgG antibodies to BKV VLPs were demonstrated in 57/71 (80.3%) samples obtained from kidney donors, 66/81 (81.5%) baseline samples from transplant recipients, 38/40 (95.0%) posttransplant BKV PCR-negative samples (21 BKV PCR negative and 19 JCV PCR positive), and 27/27 (100%) posttransplant BKV PCR-positive samples (11 with viruria and 16 with viremia). Within the posttransplant PCR-negative group, 18/19 (94.7%) patients with JCV viruria were BKV seropositive (Table 1). IgA antibodies to BKV VP-1 were detected in 22.5% of donors, 23.4% of recipient baseline samples, and 23.6% of posttransplant patients negative by BKV PCR (Table 1). In patients with BKV viremia or viruria, the frequency of IgA antibodies was 81.3% or 72.7%, respectively. None of the patients with JCV viruria were BKV IgA seropositive. IgM antibodies to BKV VLPs showed the same pattern of increased reactivity in samples derived from patients with BKV viremia or BKV viruria (43.7% of viremic patients versus 9.1% of viruric patients versus 0% of recipient baseline samples), but a significant number of patients who demonstrated an IgA antibody response did not mount a detectable IgM response. No IgM anti-BKV antibodies were detected in patients with JCV viruria, again indicating a lack of cross-reactivity between BKV and JCV epitopes detected by this assay.
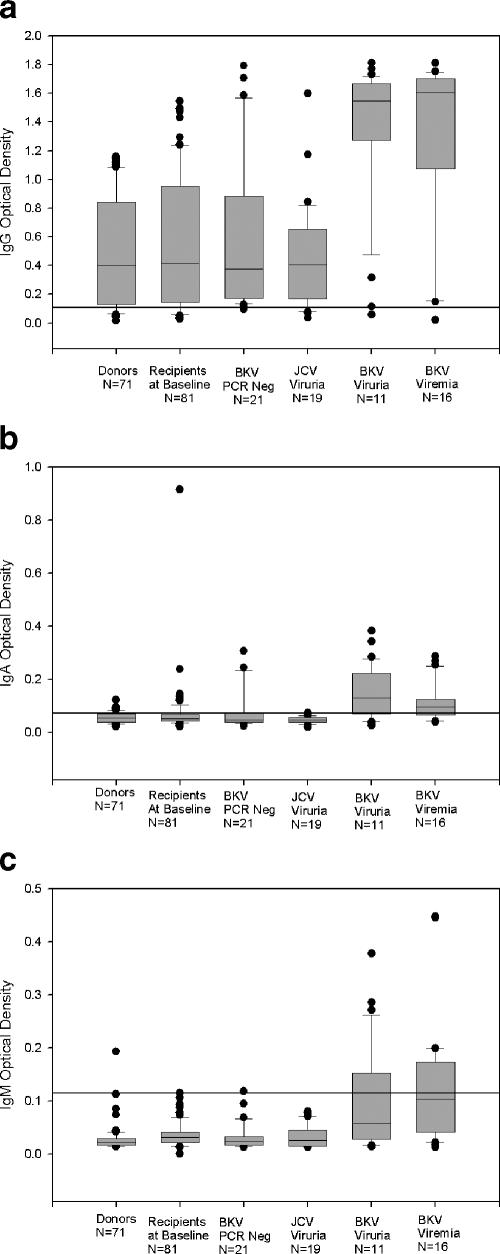
In addition to characterizing the serologic status of each patient as a categorical variable (seropositive or seronegative), we also analyzed optical density readings as a measure of antibody titers. Posttransplant patients without BKV viremia or viruria had a lower IgG optical density reading than those with active BKV viruria or viremia (Fig. 1). Optical density measurements in patients with BKV viremia did not differ significantly from patients with BKV viruria. Active BKV infection (viruria or viremia) also resulted in increased titers of IgA and IgM anti-BKV VP-1 antibodies (P < 0.001 versus all other categories studied).
FIG. 1.
Box plots showing optical density readings in the ELISA for IgG (a), IgA (b), or IgM (c) anti-BKV antibodies in different study categories. Each box plot depicts measurements from the 25th to 75th percentile (SigmaPlot 8.0). The error bars correspond to the 10th and 90th percentiles. The horizontal bar in each box represents the median value. Outliers are indicated by the solid circles. The thick horizontal line indicates the optical density cutoff used to define patients as seropositive or seronegative. Subject categories, with the number of subjects in parentheses, are as follows: donors (71), recipient baseline samples (81), posttransplant BKV PCR negative (21), JCV viruria (19), BKV viruria (11), and BKV viremia (16). Optical densities in lanes 5 and 6 are higher than all other categories (P < 0.05).
A change in optical density (ΔOD), defined as the peak posttransplant value minus the baseline or first available sample, could be calculated for 31 patients who had more than one sample studied. Of these, 12 patients had viral replication, defined as viruria or viremia, and 10/12 had an IgG ΔOD of >0.577. Both of the patients with a ΔOD lower than this cutoff were patients with persistent replication in which serology was done after replication had already started. Ten of 11 patients with viral replication (there are no available data on the 12th patient) had an IgA ΔOD greater than an arbitrarily selected cutoff of 0.041, but two samples with a ΔOD value of more than this cutoff were PCR negative. Only 7/11 patients were IgM seropositive, and 7/7 had an IgM ΔOD that was greater than an arbitrarily selected cutoff of 0.041. One patient with an IgM optical density of 0.117 was negative for BKV DNA by PCR.
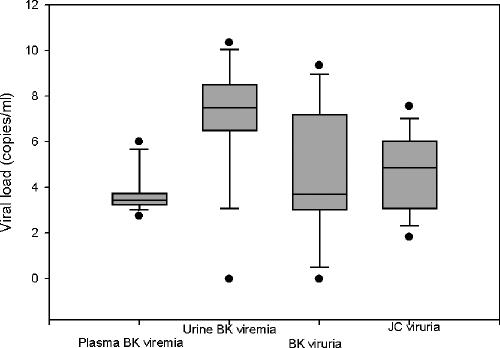
Viral genomic loads in samples obtained from different clinical settings are presented in Table 2. Donor and recipient baseline samples generally had no detectable viral DNA, except for low viral loads (250 to 1,980 copies/ml) in four donor and two recipient urine samples. Sixteen patients showed BKV viremia, and these patients also had BKV viruria. The urinary BKV load in the viremic patients (range, 0 to 2.29E10 copies/ml; median, 6.39E7 copies/ml) was higher than that in all other categories (P < 0.001). The median urine viral load in this group of patients is approximately 4 logs higher than the plasma load (range, 5.6E2 to 3.55E5 copies/ml; median, 2.62E3 copies/ml). Box plots show overlap in the range of urinary viral loads observed in patients with and without BKV viremia (Fig. 2). BKV viral load in the viruric patients without viremia varied from 2.0E2 to 2.28E9 copies/ml (median, 5.38E3 copies/ml). Four BKV viremia patients had biopsy-proven viral nephropathy, evidenced by the presence of viral inclusions, interstitial nephritis, and a positive in situ hybridization test for BKV DNA. Testing for JCV DNA was performed in a limited subset of 19 patients to address the issue of cross-reactivity between BKV and JCV antibodies. In these patients, a substantial JCV burden was found despite the fact that JCV is usually not considered a clinically significant pathogen in the kidney. JCV viral loads ranged from 6.92E1 to 3.72E7 copies/ml. The median JCV load (4.57E4 copies/ml) was lower than the urinary BKV load only in patients who developed BKV viremia (P < 0.001). None of the patients with JCV viruria had histologic evidence of viral nephropathy on biopsy.
TABLE 2.
BKV and JCV viral loads in different clinical settingsa
| Sample category | No. of samples | No. of copies/ml
|
||
|---|---|---|---|---|
| Minimum | Maximum | Median | ||
| Donors | 39 | 0 | 1,930 | 0 |
| Recipient baseline | 31 | 0 | 1,980 | 0 |
| Posttransplant BKV PCR negative | 21 | 0 | 0 | 0 |
| BKV viremia (plasma) | 16 | 5.6E2 | 3.55E5 | 2.62E3 |
| BKV viremia (urine) | 16 | 0 | 2.29E10 | 6.39E7b |
| BKV viruria | 11 | 200 | 2.28E9 | 5,380 |
| JCV viruria | 19 | 6.92E1 | 3.72E7 | 4.57E4 |
For patients with BKV viremia, urinary and plasma viral loads are presented separately. For all other patient categories, only urine measurements are presented, since plasma samples tested negative. For patients with multiple samples, the peak viral load was used for analysis.
P < 0.001 versus BKV viremia (plasma), donors, recipient baseline, and PCR-negative groups (Mann-Whitney test).
FIG. 2.
Box plot showing BKV and JCV viral loads (log transformed) in posttransplant PCR-positive subjects. Subject categories, with the number of subjects in parentheses, are as follows: plasma levels in patients with BKV viremia (16), urinary BKV load in patients with viremia (16), urinary BKV load in patients with asymptomatic viruria but no viremia (11), and JCV DNA load in patients with JC viruria (19). Urinary viral loads were higher in patients with BKV viremia than in patients with BKV viruria or JCV viruria (P < 0.001). The box plot depicts measurements from the 25th to the 75th percentile (SigmaPlot 8.0). The error bars correspond to the 10th and 90th percentiles. The horizontal bar in each box represents the median value. Outliers are indicated by the solid circles.
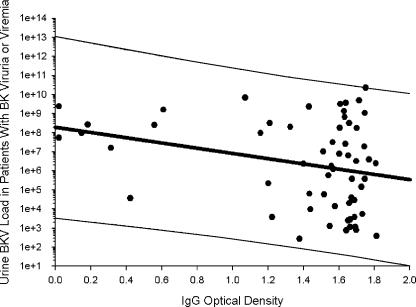
When all viruric samples studied were analyzed, urinary BKV load showed a weak negative correlation with IgG (r = −0.28, P = 0.03) (Fig. 3). A similar relationship was seen with IgA optical density (r = −0.1), indicating a possible protective role for IgG and IgA antibodies. However, urinary viral load showed a positive correlation with the IgM optical density (r = 0.22). On repeating the analyses and including only patients with viremia, the inverse relationship between viral load and IgA antibody titer became accentuated (r = −0.61). Correlations between plasma viral load and antibody titer were also observed but with weaker correlation coefficients (IgG, −0.05; IgA, −0.14; and IgM, −0.17).
FIG. 3.
Semilog plot of urinary viral load versus anti-BKV IgG using only samples from patients with active BKV viremia or viruria. The optical density shows a negative correlation with increasing viral load. The linear regression line in the middle is bracketed by two hairlines, which indicate the 95% prediction intervals calculated by SigmaPlot 8.0.
This is a cross-sectional study and did not have a uniform sampling protocol for all donor-recipient pairs. Within the framework of this limitation, we found that viruria developed in 9/56 (16.1%) patients receiving kidneys from BKV IgG-positive donors compared to 5/15 (33.3%) patients who received organs from BKV IgG-negative donors. This apparent difference is not meaningful considering the small number of data points and the overall prevalence of viruria in our kidney transplant program, which is approximately 30% without regard to donor serologic status. Considering kidney transplant donors and recipients from which both donor and recipient sera were available prior to transplantation, 4 of 22 (18.2%) were judged to be in the high-risk category for viral transmission (i.e., recipient seronegative and donor seropositive). Two of these patients developed viruria in the posttransplant period, and one progressed to nephropathy. A larger study with more systematic and frequent sampling is needed to comprehensively address the risk of BKV transmission as a function of the serologic status of the donor and recipient.
DISCUSSION
Classical studies on polyomavirus serology have relied on hemagglutination inhibition or immunofluorescence antibody techniques (8, 19, 26, 36, 51). More recently, ELISA technology has been used to define polyomavirus immunity. Rollison et al. found anti-BKV IgG antibodies in 85% of prediagnostic serum samples from a case-control study of brain tumors using a whole-virus ELISA (47). Hamilton et al. found that antibody titers to JCV or BKV determined by hemagglutination inhibition were lower than those determined by whole-virus ELISA, although results obtained by both techniques showed good overall correlation (21). We previously reported a BKV seroprevalence of approximately 65% in non-Hodgkin's lymphoma case-control studies (16). Stolt et al. analyzed serum samples from Swedish children and found that seroprevalence peaked at 98% at 7 to 9 years of age, followed by a minor decrease (52). Bohl et al. and Hariharan et al. have used ELISA technology to measure anti-BKV antibodies in kidney transplant patients (4, 22). Our data confirm that organ transplant recipients can successfully mount a response to BKV, despite being on immunosuppressive medications, including lymphocyte depletion regimens that require administration of alemtuzumab. Normal antibody responses to cytomegalovirus and Epstein-Barr virus have been recorded for kidney transplant patients treated with anti-thymocyte globulin (27). On the other hand, some investigators have documented derangements in immune response to Epstein-Barr virus, hepatitis C virus, and human immunodeficiency virus agents in immunosuppressed individuals (2, 23, 34, 40, 49, 53).
The data presented show that antibodies to BKV VP-1 protein can be detected in the serum of most transplant recipients. Furthermore, the presence of serum IgM or IgA or a high titer of IgG to BKV was shown to be an acceptable marker for BKV replication. Among patients with paired serum samples, we found that most of those with BKV viruria or viremia showed a substantial increase in IgG, IgA, and IgM antibody levels, indicating that transplant recipients are capable of mounting anamnestic antibody responses to BKV. Given the convenience of serologic testing, measurement of BKV antibodies could potentially be used as a primary screening test. Detection of IgA or IgM class antibodies or an increase in IgG antibodies above a defined threshold could be an indication for performing quantitative PCR to determine the exact viral load.
IgG and IgA antibodies to BKV VP-1 showed a negative correlation with urinary viral load. The correlation coefficients are low, and the extent to which these antibodies are effective in viral neutralization remains to be determined. Viral DNA remained detectable concurrently with high antibody titers, but what proportion of it remained infectious cannot be determined by our data. In a small series of six patients with BKV nephropathy, clinical recovery and clearance of viral load seemed to coincide temporally with the development of BKV-specific antibodies (22). However, these data are difficult to interpret, since patients with persistent viruria and progressive tissue damage were not included for comparison. Recently, Bohl et al. published data showing that antibody titers in kidney transplant donors reflect primarily the activity and transmissibility of BKV infection (3, 4). Preexisting recipient antibodies seemed to provide no protection from donor-derived virus, suggesting that anti-VP-1 antibodies to BKV are nonneutralizing in nature. Additional studies are needed to resolve this issue.
Even though BKV and JCV are closely related viruses, patients with JCV viruria did not show any increase in the frequency or titer of IgA or IgM antibodies. Adsorption experiments published by Viscidi and Clayman confirm that cross-reactivity between JCV and BKV is not a significant problem for the ELISA used in our studies (56). Hamilton et al. observed that only 25.4% of sera tested had antibody titers to JCV and BKV which were identical or closely related (21). A lack of cross-reactivity between BKV and JCV in antibody assays may, at first glance, appear surprising given the fact that these BKV and JCV VP-1 proteins share 87% amino acid homology. However, we believe that it demonstrates how the immune system can target different antigen epitopes and permit one virus to replicate in the presence of active immunity to another closely related virus. These considerations do not exclude the possibility that some viral antigenic epitopes may be shared between BKV and JCV. Indeed, two recent studies on cell-mediated immunity against BKV have demonstrated such cross-reactive epitopes (7, 32).
It is of interest that patients with chronic renal failure on dialysis did not show serologic evidence of BKV reactivation. On the other hand, it is to be noted that several donor and baseline recipient sera were classified as seropositive for IgA. Further work is needed to determine the clinical significance of these IgA antibodies, which are not accompanied by viral DNA in the urine or plasma. Study of a larger numbers of patients, with a systematic posttransplant sampling protocol, is needed to provide a numerical assessment of the risk of viruria and viremia in kidney transplant patients stratified by donor and recipient serologic status.
Acknowledgments
This work was supported by NIH grants RO-1 AI 51227 and AI 63360.
We declare that we have no commercial or other association that might pose a conflict of interest with regard to this work.
REFERENCES
- 1.Agostini, H. T., C. F. Ryschkewitsch, and G. L. Stoner. 1996. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J. Clin. Microbiol. 34:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodeus, M., F. Smets, R. Reding, E. Sokal, J. B. Otte, P. Goubau, and L. van Renterghem. 1999. Epstein-Barr virus infection in sixty pediatric liver graft recipients: diagnosis of primary infection and virologic follow-up. Pediatr. Infect. Dis. J. 18:698-702. [DOI] [PubMed] [Google Scholar]
- 3.Bohl, D. L., G. Storch, C. Ryschkewitsch, M. Gaudreault-Keener, M. Schnitzler, E. O. Major, and D. C. Brennan. 2005. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am. J. Transplant. 5:2213-2221. [DOI] [PubMed] [Google Scholar]
- 4.Bohl, D. L., C. Ryschkewitsch, E. O. Major, G. A. Storch, and D. C. Brennan. 2005. BK virus antibody titers markedly increase with viremia. Am. J. Transplant. 5(Suppl. 11):273A. [Google Scholar]
- 5.Chang, D., M. Wang, W. C. Ou, M. S. Lee, H. N. Ho, and R. T. Tsai. 1996. Genotypes of human polyomaviruses in urine samples of pregnant women in Taiwan. J. Med. Virol. 48:95-101. [DOI] [PubMed] [Google Scholar]
- 6.Chang, H., M. Wang, R. T. Tsai, H. S. Lin, J. S. Huan, W. C. Wang, and D. Chang. 2002. High incidence of JC viruria in JC-seropositive older individuals. J. Neurovirol. 8:447-451. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y. P., J. Trofe, J. Gordon, R. A. Du Pasquier, P. Roy-Chaudhury, M. J. Kuroda, E. S. Woodle, K. Khalili, and I. J. Koralnik. 2006. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J. Virol. 80:3495-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman, D. V., S. D. Gardner, and A. M. Field. 1973. Human polyomavirus infection in renal allograft recipients. Br. Med. J. 3:371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demeter, L. M. 1995. JC, BK, and other polyomaviruses: progressive multifocal leukoencephalopathy, p. 1400-1406. In G. L. Mandel, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, N.Y.
- 10.De Stasio, A., P. Mastromarino, A. Pana, L. Seganti, S. Sinibaldi, P. Valenti, and N. Orsi. 1980. Characterization of a glycoprotein inhibitor present in human serum and active towards BK virus hemagglutination. Microbiologica 3:293-305. [Google Scholar]
- 11.De Stasio, A., N. Orsi, A. Pana, L. Seganti, S. Sinibaldi, and P. Valenti. 1979. The importance of pre-treatment of human sera for the titration of hemagglutination-inhibiting antibodies towards BK virus. Ann. Sclavo 21:249-257. [PubMed] [Google Scholar]
- 12.Drachenberg, C. B., C. O. Beskow, C. B. Cangro, P. M. Bourquin, A. Simsir, J. Fink, M. R. Weir, D. K. Klassen, S. T. Bartlett, and J. C. Papadimitriou. 1999. Human polyoma virus in renal allograft biopsies: morphological findings and correlation with urine cytology. Hum. Pathol. 30:970-977. [DOI] [PubMed] [Google Scholar]
- 13.Drachenberg, C. B., J. C. Papadimitriou, R. Wali, C. L. Cubitt, and E. Ramos. 2003. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am. J. Transplant. 3:1383-1392. [DOI] [PubMed] [Google Scholar]
- 14.Drachenberg, R. C., C. B. Drachenberg, J. C. Papadimitriou, E. Ramos, J. C. Fink, R. Wali, M. R. Weir, C. B. Cangro, D. K. Klassen, A. Khaled, R. Cunningham, and S. T. Bartlett. 2001. Morphological spectrum of polyoma virus disease in renal allografts: diagnostic accuracy of urine cytology. Am. J. Transplant. 1:373-381. [PubMed] [Google Scholar]
- 15.Dubois, V., H. Dutronc, M. E. Lafon, V. Poinsot, J. L. Pellegrin, J. M. Ragnaud, A. M. Ferrer, and H. J. Fleury. 1997. Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type 1-infected patients. J. Clin. Microbiol. 35:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engels, E. A., D. E. Rollison, P. Hartge, D. Baris, J. R. Cerhan, R. K. Severson, W. Cozen, S. Davis, R. J. Biggar, J. J. Goedert, and R. P. Viscidi. 2005. Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int. J. Cancer 117:1013-1019. [DOI] [PubMed] [Google Scholar]
- 17.Engels, E. A., R. P. Viscidi, D. A. Galloway, J. J. Carter, J. R. Cerhan, S. Davis, W. Cozen, R. K. Severson, S. de Sanjose, J. S. Colt, and P. Hartge. 2004. Case-control study of simian virus 40 and non-Hodgkin lymphoma in the United States. J. Natl. Cancer Inst. 96:1368-1374. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante, P., R. Caldarelli-Stefano, E. Omodeo-Zorini, A. E. Cagni, L. Cocchi, F. Suter, and R. Maserati. 1997. Comprehensive investigation of the presence of JC virus in AIDS patients with and without progressive multifocal leukoencephalopathy. J. Med. Virol. 52:235-242. [DOI] [PubMed] [Google Scholar]
- 19.Gardner, S. D., A. M. Field, D. V. Coleman, and B. Hulme. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1:1253-1257. [DOI] [PubMed] [Google Scholar]
- 20.Gardner, S. D., E. F. MacKenzie, C. Smith, and A. A. Porter. 1984. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J. Clin. Pathol. 37:578-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton, R. S., M. Gravell, and E. O. Major. 2000. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J. Clin. Microbiol. 38:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariharan, S., E. P. Cohen, B. Vasudev, R. Orentas, R. P. Viscidi, J. Kakela, and B. DuChateau. 2005. BK virus specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am. J. Transplant. 5:2719-2724. [DOI] [PubMed] [Google Scholar]
- 23.Henle, W., and G. Henle. 1981. Epstein-Barr virus-specific serology in immunologically compromised individuals. Cancer Res. 41:4222-4225. [PubMed] [Google Scholar]
- 24.Hirsch, H. H., M. Mohaupt, and T. Klimkait. 2001. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J. Infect. Dis. 184:1494-1496. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch, H. H., W. Knowles, M. Dickenmann, J. Passweg, T. Klimkait, M. J. Mihatsch, and J. Steiger. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal transplant recipients. N. Engl. J. Med. 347:488-496. [DOI] [PubMed] [Google Scholar]
- 26.Hogan, T. F., E. C. Borden, J. A. McBain, B. L. Padgett, and D. L. Walker. 1980. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann. Intern. Med. 92:373-378. [DOI] [PubMed] [Google Scholar]
- 27.Hornef, M. W., G. Bein, L. Fricke, J. Steinhoff, H. J. Wagner, W. Hinderer, H. H. Sonneborn, and H. Kirchner. 1995. Coincidence of Epstein-Barr virus reactivation, cytomegalovirus infection, and rejection episodes in renal transplant recipients. Transplantation 60:474-480. [DOI] [PubMed] [Google Scholar]
- 28.Kazory, A., D. Ducloux, J. M. Chalopin, R. Angonin, B. Fontaniere, and H. Moret. 2003. The first case of JC virus allograft nephropathy. Transplantation 76:1653-1655. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura, T., Y. Aso, N. Kuniyoshi, K. Hara, and Y. Yogo. 1990. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J. Infect. Dis. 161:1128-1133. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura, T., C. Sugimoto, A. Kato, H. Ebihara, M. Suzuki, F. Taguchi, K. Kawabe, and Y. Yogo. 1997. Persistent JC virus (JCV) infection is demonstrated by continuous shedding of the same JCV strains. J. Clin. Microbiol. 35:1255-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knowles, W. A., P. Pipkin, N. Andrews, A. Vyse, P. Minor, D. W. Brown, and E. Miller. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71:115-123. [DOI] [PubMed] [Google Scholar]
- 32.Krymskaya, L., M. C. Sharma, J. Martinez, W. Haq, E. C. Huang, A. P. Limaye, D. J. Diamond, and S. F. Lacey. 2005. Cross-reactivity of T lymphocytes recognizing a human cytotoxic T-lymphocyte epitope within BK and JC virus VP1 polypeptides. J. Virol. 79:11170-11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laghi, L., A. E. Randolph, D. P. Chauhan, G. Marra, E. O. Major, J. V. Neel, and C. R. Boland. 1999. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc. Natl. Acad. Sci. USA 96:7484-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lok, A. S. F., D. Chien, Q. L. Choo, T. M. Chan, E. K. W. Chiu, I. K. P. Cheng, M. Houghton, and G. Kuo. 1993. Antibody response to core, envelope and nonstructural hepatitis C virus antigens—comparison of immunocompetent and immunosuppressed patients. Hepatology 18:497-502. [PubMed] [Google Scholar]
- 35.Mylonakis, E., N. Goes, R. H. Rubin, A. B. Cosimi, R. B. Colvin, and J. A. Fishman. 2001. BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation 72:1587-1592. [DOI] [PubMed] [Google Scholar]
- 36.Nastac, E., M. Stoian, M. Hozoc, M. Iosipenco, and M. Melencu. 1982. Prevalence of hemagglutination-inhibiting antibodies to BK virus in patients with endemic Balkan nephropathy. Virologie 33:169-170. [PubMed] [Google Scholar]
- 37.Nickeleit, V., H. H. Hirsch, I. F. Binet, F. Gudat, O. Prince, P. Dalquen, G. Thiel, and M. J. Mihatsch. 1999. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J. Am. Soc. Nephrol. 10:1080-1089. [DOI] [PubMed] [Google Scholar]
- 38.Nickeleit, V., T. Klimkait, I. F. Binet, P. Dalquen, V. Del Zenero, G. Thiel, M. J. Mihatsch, and H. H. Hirsch. 2000. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N. Engl. J. Med. 342:1309-1315. [DOI] [PubMed] [Google Scholar]
- 39.Perrons, C. J., J. D. Fox, S. B. Lucas, N. S. Brink, R. S. Tedder, and R. F. Miller. 1996. Detection of polyomaviral DNA in clinical samples from immunocompromised patients: correlation with clinical disease. J. Infect. 32:205-209. [DOI] [PubMed] [Google Scholar]
- 40.Ragni, M. V., O. K. Ndimbie, E. O. Rice, F. A. Bontempo, and S. Nedjar. 1993. The presence of hepatitis C virus (HCV) antibody in human immunodeficiency virus-positive hemophilic men undergoing HCV seroreversion. Blood 82:1010-1015. [PubMed] [Google Scholar]
- 41.Ramos, E., C. B. Drachenberg, J. C. Papadimitriou, O. Hamze, J. C. Fink, D. K. Klassen, R. C. Drachenberg, A. Wiland, R. Wali, C. B. Cangro, E. Schweitzer, S. T. Bartlett, and M. R. Weir. 2002. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J. Am. Soc. Nephrol. 13:2145-2151. [DOI] [PubMed] [Google Scholar]
- 42.Randhawa, P., A. Ho, R. Shapiro, A. Vats, P. Swalsky, S. Finkelstein, J. Uhrmacher, and K. Weck. 2004. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J. Clin. Microbiol. 42:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randhawa, P., J. Uhrmacher, W. Pasculle, A. Vats, R. Shapiro, B. Eghtsead, and K. Weck. 2005. A comparative study of BK and JC virus infections in organ transplant recipients. J. Med. Virol. 77:238-243. [DOI] [PubMed] [Google Scholar]
- 44.Randhawa, P. S., and A. J. Demetris. 2000. Nephropathy due to polyomavirus type BK. N. Engl. J. Med. 342:1361-1363. [DOI] [PubMed] [Google Scholar]
- 45.Randhawa, P. S., S. Finkelstein, V. Scantlebury, R. Shapiro, C. Vivas, M. Jordan, M. M. Pickin, and A. J. Demetris. 1999. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 67:103-109. [DOI] [PubMed] [Google Scholar]
- 46.Randhawa, P. S., A. Vats, R. Shapiro, K. Weck, and V. Scantlebury. 2002. BK virus: discovery, epidemiology, and biology. Graft 5(Suppl.):S19-S27. [Google Scholar]
- 47.Rollison, D. E., K. J. Helzlsouer, A. J. Alberg, S. Hoffman, J. Hou, R. Daniel, K. V. Shah, and E. O. Major. 2003. Serum antibodies to JC virus, BK virus, simian virus 40, and the risk of incident adult astrocytic brain tumors. Cancer Epidemiol. Biomarkers Prev. 12:460-463. [PubMed] [Google Scholar]
- 48.Rosen, S., W. Harmon, A. M. Krensky, P. J. Edelson, B. L. Padgett, B. W. Grinnell, M. J. Rubino, and D. L. Walker. 1983. Tubulo-interstitial nephritis associated with polyomavirus (BK type) infection. N. Engl. J. Med. 308:1192-1196. [DOI] [PubMed] [Google Scholar]
- 49.Shah, G. J., A. J. Demetris, J. S. Gavaler, J. H. Lewis, S. Todo, T. E. Starzl, and D. H. Vanthiel. 1992. Incidence, prevalence, and clinical course of hepatitis C following liver transplantation. Gastroenterology 103:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah, K., R. Daniel, D. Madden, and S. Stagno. 1980. Serological investigation of BK papovavirus infection in pregnant women and their offspring. Infect. Immun. 30:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoian, M., M. Hozoc, M. Iosipenco, E. Nastac, and M. Melencu. 1983. Serum antibodies to papova viruses (BK and SV 40) in subjects from the area with Balkan endemic nephropathy. Virologie 34:113-117. [PubMed] [Google Scholar]
- 52.Stolt, A., K. Sasnauskas, P. Koskela, M. Lehtinen, and J. Dillner. 2003. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 84:1499-1504. [DOI] [PubMed] [Google Scholar]
- 53.Sumaya, C. V., R. N. Boswell, Y. Ench, D. L. Kisner, E. M. Hersh, J. M. Reuben, and P. W. A. Mansell. 1986. Enhanced serological and virological findings of Epstein-Barr virus in patients with AIDS and AIDS-related complex. J. Infect. Dis. 154:864-869. [DOI] [PubMed] [Google Scholar]
- 54.Sundsfjord, A., A. Osei, H. Rosenqvist, M. Van Ghelue, Y. Silsand, H. J. Haga, O. P. Rekvig, and U. Moens. 1999. BK and JC viruses in patients with systemic lupus erythematosus: prevalent and persistent BK viruria, sequence stability of the viral regulatory regions, and nondetectable viremia. J. Infect. Dis. 180:1-9. [DOI] [PubMed] [Google Scholar]
- 55.Vallbracht, A., J. Lohler, J. Gossmann, T. Gluck, D. Petersen, H. J. Gerth, M. Gencic, and K. Dorries. 1993. Disseminated BK type polyomavirus infection in an AIDS patient associated with central nervous system disease. Am. J. Pathol. 143:29-39. [PMC free article] [PubMed] [Google Scholar]
- 56.Viscidi, R., and B. Clayman. 2006. Serological cross reactivity between polyomavirus capsids. Adv. Exp. Med. Biol. 577:73-84. [DOI] [PubMed] [Google Scholar]
- 57.Wen, M. C., C. L. Wang, M. Wang, C. H. Cheng, M. J. Wu, C. H. Chen, K. H. Shu, and D. Chang. 2004. Association of JC virus with tubulointerstitial nephritis in a renal allograft recipient. J. Med. Virol. 72:675-678. [DOI] [PubMed] [Google Scholar]