Abstract
GPR7 and GPR8 are orphan G protein-coupled receptors that are highly similar to each other. These receptors are expressed predominantly in brain, suggesting roles in central nervous system function. We have purified an endogenous peptide ligand for GPR7 from bovine hypothalamus extracts. This peptide, termed neuropeptide B (NPB), has a C-6-brominated tryptophan residue at the N terminus. It binds and activates human GPR7 or GPR8 with median effective concentrations (EC50) of 0.23 nM and 15.8 nM, respectively. In situ hybridization shows distinct localizations of the prepro-NPB mRNA in mouse brain, i.e., in paraventricular hypothalamic nucleus, hippocampus, and several nuclei in midbrain and brainstem. Intracerebroventricular (i.c.v.) injection of NPB in mice induces hyperphagia during the first 2 h, followed by hypophagia. Intracerebroventricular injection of NPB produces analgesia to s.c. formalin injection in rats. Through EST database searches, we identified a putative paralogous peptide. This peptide, termed neuropeptide W (NPW), also has an N-terminal tryptophan residue. Synthetic human NPW binds and activates human GPR7 or GPR8 with EC50 values of 0.56 nM and 0.51 nM, respectively. The expression of NPW mRNA in mouse brain is confined to specific nuclei in midbrain and brainstem. These findings suggest diverse physiological functions of NPB and NPW in the central nervous system, acting as endogenous ligands on GPR7 and/or GPR8.
There are a large number of orphan G protein-coupled receptors (GPCR) whose cognate ligands have yet to be identified (1, 2). The search for endogenous ligands of orphan GPCRs is important because GPCRs are in general excellent drug targets (3). GPR7 and GPR8 are two orphan GPCRs (4). They were originally cloned from human genomic DNA by degenerative PCR using primers based on the sequences of the δ-opioid receptor and somatostatin receptor. Indeed, GPR7 and GPR8 each share ≈40% overall amino acid identities with opioid and somatostatin receptors. Human GPR7 and GPR8 are 70% identical to each other at the nucleotide level and 64% identical at the amino acid level. Interestingly, no orthologue exists for the GPR8 gene in rodents, indicating that GPR8 may have originated as a replicate of GPR7 after divergence of the rodent from other species in mammalian evolution (5).
The observation that GPR7 and GPR8 mRNAs are expressed in distinct areas in the central nervous system (CNS) (4, 5), together with the similarity of GPR7 and GPR8 with opioid and somatostatin receptors, strongly argue for the existence of endogenous peptide ligand(s) in CNS. In this study, we have purified a neuropeptide ligand for GPR7 from the bovine hypothalamus. This peptide, termed neuropeptide B (NPB), consists of 29 aa with a unique brominated N-terminal tryptophan. Through EST database searches, we also identified another isopeptide of the same family, termed neuropeptide W (NPW). While this manuscript was being prepared, three papers were published reporting two endogenous ligands for GPR7 and GPR8 (6–8). Sequence comparisons revealed that the two peptides described in this study are identical to the peptides identified by the authors of these papers. The biochemical and physiological studies by us and others together support the idea that NPB and NPW function as endogenous ligands for GPR7 and GPR8, with pleiotropic effects in brain.
Methods
All of the detailed information about the experimental procedures is provided in Supporting Methods, which is published as supporting information on the PNAS web site, www.pnas.org. Culture of Xenopus laevis melanophores and DNA transfections by electroporation were done as described (9). For purifying NPB, the acid/acetone extraction of frozen bovine hypothalami was performed as described (10, 11). The details of HPLC procedures and the following cDNA cloning are described in supporting information. All synthetic peptides were synthesized by a standard Fmoc solid-phase synthesis method (12). Molecular weights of the purified and synthetic peptides were measured by matrix-assisted laser desorption/ionization–time of flight mass spectrometry. Ltk− cells, stably cotransfected with the human GPR7 cDNA and the Gqi chimeric G protein α subunit cDNA, were used for the cytoplasmic Ca2+ transient assay (13). Cytoplasmic calcium levels were monitored by using Fura-2/AM as described (14). In situ hybridization on mouse brain slices was performed as described (15). Mice and rats were housed under controlled lighting and temperature. All experimental procedures involving animals were approved by the institutional committee, and were in accordance with National Institutes of Health guidelines. For intracerebroventricular (i.c.v.) cannulation, a guide cannula was implanted stereotaxically into the lateral ventricle under sterile conditions. Food intake was measured as described (10). The open field apparatus with 15 × 15 infrared beam arrays at 2.4-cm intervals was used for locomotion analysis. The paw flick test and formalin test were performed as described (16) for the analysis of analgesic effects.
Results
Purification of NPB as GPR7 Agonist.
We fractionated acid-acetone extracts of bovine hypothalami with a C18 reverse-phase HPLC column, and screened these fractions by the melanosome aggregation assay in X. laevis melanophores transfected with the human GPR7 cDNA. We detected a robust activity in fractions eluted at ≈24% CH3CN in 0.1% TFA (see Fig. 5, which is published as supporting information on the PNAS web site). We purified the active substance in these fractions through five additional steps of reverse-phase HPLC. Matrix-assisted laser desorption/ionization–time of flight mass spectrometry of the purified substance gave a monoisotopic MH+ mass of 3,238.7. Automated Edman degradation revealed the sequence XYKPTAGQGYYSVGRAAGLLSGFHRSPYA, where X indicates an undefined hydrophobic amino acid. We determined that this residue is C-6-l-brominated tryptophan (for details, see Supporting Results and Fig. 6, which are published as supporting information on the PNAS web site).
cDNA and Amino Acid Sequences of NPB and NPW.
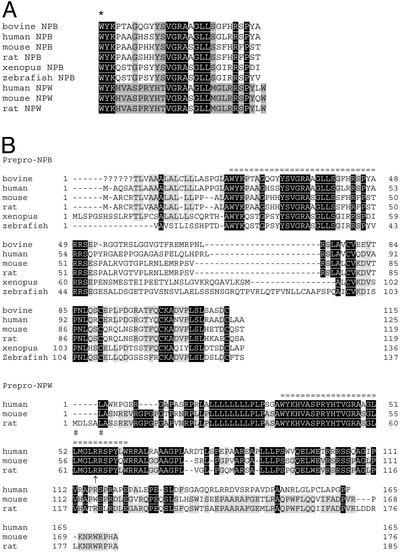
Based on the amino acid sequences of the purified bovine NPB peptide, we searched the National Center for Biotechnology Information EST database by using the TBLASTN algorithm with the default set of stringency parameters. This resulted in identification of EST sequences encoding the human (AW058203), mouse (AI430753), rat (BG376816), Xenopus (AL640188), and zebrafish (BM571370) orthologues of the prepro-NPB precursor. A full-length cDNA encoding bovine prepro-NPB was obtained from bovine spinal cord mRNA. All prepro-NPB orthologues deduced from these cDNAs contained the putative mature NPB peptides consisting of 29 aa (Fig. 1). The first predicted amino acid of all of these mature NPB orthologues is tryptophan.
Figure 1.
Amino acid sequences of NPB and NPW. (A) Amino acid sequences of mature NPB and NPW peptides. An asterisk indicates the posttranslational bromination site of the native bovine NPB. Peptide sequences of other species are deduced from cDNA sequences. Amino acid identities between NPB and NPW are shown in black. Shaded residues are conserved only within the NPB or NPW. (B) Deduced amino acid sequences of prepro-NPB and NPW precursor polypeptides. Mature peptides are marked by equal signs. Question marks indicate undetermined sequence of bovine prepro-NPB. Human and mouse prepro-NPW cDNA do not have a translation initiator ATG codon; putative translation initiation sites are indicated by a pound sign. An arrow indicates a possible additional processing site for NPW. Identical amino acids within the orthologues are shown in black. Lightly shaded residues are conserved in more than four of six (NPB) or two of three (NPW) species.
In addition to these NPB orthologues, we obtained the human (AI500303) and rat (BQ134951) cDNAs for a paralogous prepro-peptide, termed NPW. The mouse prepro-NPW cDNA was obtained by RACE using mouse lung mRNA. Prepro-peptides of NPW deduced from these cDNAs contained the putative 30-amino acid mature NPW sequences (Fig. 1). All of these mature NPWs had an N-terminal tryptophan. All mature NPWs also had a pair of arginine residues in their 24th and 25th positions (Fig. 1B). Therefore, a shorter form of NPW (23 aa) may also be produced as a result of proteolytic processing at this site. The amino acid sequences of both NPB and NPW exhibited no meaningful similarity to any other known peptides, including somatostatins and opioid peptides.
In Vitro Pharmacology of NPB and NPW.
Melanophore assay.
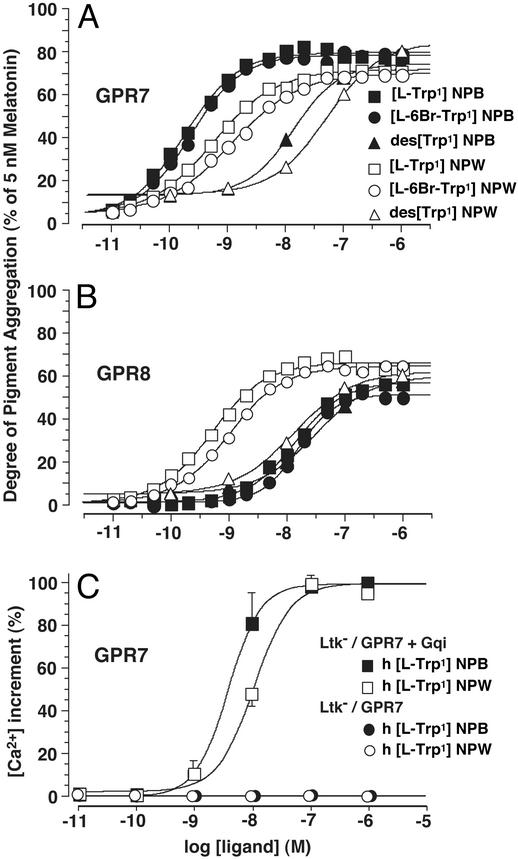
X. laevis melanophores were transiently transfected with the human GPR7 or GPR8 cDNA, and challenged with various forms of synthetic human NPB and NPW. Both brominated and nonbrominated NPB potently stimulated the aggregation of melanosomes in melanophores transfected with GPR7 cDNA. EC50 values were 0.18 and 0.23 nM, respectively, with approximately the same efficacies (Fig. 2A). When the first tryptophan residue was deleted (des[l-Trp-1]NPB), the potency on GPR7 dramatically decreased (EC50 = 13.4 nM). GPR8 showed a lower apparent affinity for NPB as compared with NPW (Fig. 2B). EC50 values of the brominated and nonbrominated NPB on GPR8 were 14.1 and 15.8 nM, respectively.
Figure 2.
In vitro pharmacology of NPB and NPW assessed by melanophore pigment aggregation assay (A and B) and [Ca2+]i transient assay (C) in mammalian cells. (A and B) X. laevis melanophores were transiently transfected with human GPR7 (A) or GPR8 (B) cDNA, and challenged with various forms of synthetic human NPB and NPW peptides. Brominated NPB and nonbrominated NPB have approximately equal potencies on GPR7 (A), whereas both of them have much weaker potencies on GPR8 (B). Brominated NPW and nonbrominated NPW both have similar potencies on GPR7 and GPR8. des[l-Trp-1]NPB and des[l-Trp-1]NPW are both much less potent on GPR7 and GPR8. Data are the mean of eight separate experiments. (C) Ltk− cells were stably cotransfected with the human GPR7 cDNA and Gqi chimera cDNA, and challenged with nonbrominated synthetic human NPB and NPW. NPB has a modestly higher potency than NPW. When Gqi cDNA is not cotransfected, neither NPB nor NPW could induce the calcium mobilization (open and filled circles are all overlapping). Peak [Ca2+]i values are represented as percentages of the maximum response. Data are presented as the mean ± SEM of three experiments performed in duplicate.
Nonbrominated NPW showed similar activities on GPR7 and GPR8 with EC50 values of 0.56 and 0.51 nM, respectively. Synthetic brominated NPW had a slightly lower potency for both receptors than the nonbrominated peptide (EC50 = 1.15 and 1.0 nM). When the first tryptophan residue of NPW was deleted (des[l-Trp-1]NPW), its activity on GPR7 and GPR8 also declined dramatically, with EC50 values of 49.9 and 13.5 nM, respectively.
Cytoplasmic Ca2+ transient assay.
We analyzed the intracellular calcium mobilization induced by synthetic NPB and NPW in mouse Ltk− cells. The human GPR7 cDNA was stably transfected into Ltk− cells with or without the Gqi chimeric G protein α subunit cDNA, which can redirect Giα-driven signal to the Gq signaling pathway (13). Both NPB and NPW induced the rapid mobilization of intracellular calcium at doses from 10−7 to 10−9 M, with EC50 values of 3.9 and 10.9 nM, respectively (Fig. 2C). Neither NPB nor NPW induced a detectable calcium mobilization when the Gqi cDNA was not cotransfected, indicating that GPR7 transduces its signal through G proteins with a Gi-class, but not Gq-class, α subunit. This is consistent with the findings above that NPB and NPW induce melanosome aggregation in Xenopus melanophores, which is well documented to occur through the Gi-coupled signaling pathway.
Unusual Start Codon in Mouse and Human NPW cDNA.
In the rat prepro-NPW cDNA, a typical ATG translation initiation codon was found at the 5′ end of the encoded signal peptide. In human and mouse prepro-NPW cDNAs, however, no ATG codon was found at or near the corresponding position; instead, there was a CTG codon (Fig. 7A, which is published as supporting information on the PNAS web site). We predicted that this CTG codon functions as the translation initiator, because it could potentially start an ORF that encodes appropriate prepro-NPW. We mutated the CTG codon of the mouse prepro-NPW cDNA into ATG or CGG, and made a cDNA construct with each mutation with a FLAG epitope fused to the C terminus of the coding sequence. These constructs were transiently transfected into Chinese hamster ovary (CHO) cells and cell lysates were then processed for Western blotting with the anti-FLAG antibody. Equal amounts of the mouse NPW prepropeptide were translated from mRNAs containing either the CTG or ATG initiator codon in CHO cells (Fig. 7B). This demonstrates that the CTG codon in this context was used as a translation initiator with efficiency virtually equal to the canonical ATG codon.
Tissue Distributions of NPB and NPW mRNAs.
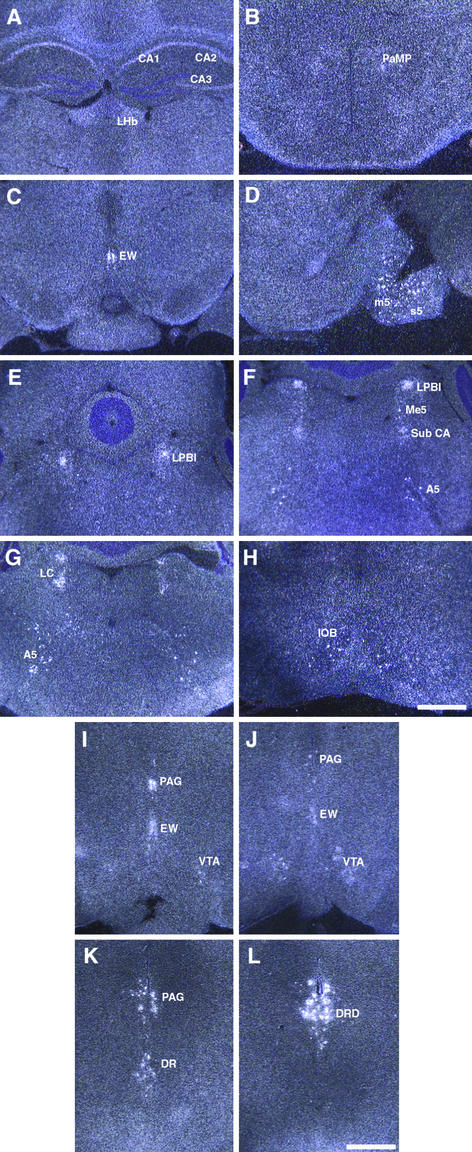
In in situ hybridization to mouse brain sections, expression of NPB mRNA was detected in hippocampus (CA1, CA2, and CA3 in Fig. 3A), lateral habenular nucleus (LHb in Fig. 3A), paraventricular hypothalamic nucleus, medial parvicellular part (PaMP in Fig. 3B), Edinger–Westphal (EW in Fig. 3C) nucleus, motor root of the trigeminal nerve (m5 in Fig. 3D), sensory root of the trigeminal nerve (s5 in Fig. 3D), lateral parabrachial nucleus internal part (LPBI in Fig. 3 E and F), mesencephalic trigeminal nucleus (Me5 in Fig. 3F), subcoeruleus nucleus alpha part (Sub CA in Fig. 3F), locus coeruleus (LC in Fig. 3G), noradrenergic cell group A5 (A5 in Fig. 3 F and G) and inferior olive subnucleus B (IOB in Fig. 3H). The strongest expression of NPB was observed in the EW nucleus.
Figure 3.
In situ hybridization of NPB (A–H) and NPW (I–L) mRNA on mouse brain sections. (Scale bars are 300 μm.) (A–H) NPB mRNA in mouse brain. CA1–3, CA1–3 field of hippocampus; LHb, lateral habenular nucleus; PaMP, paraventricular hypothalamic nucleus medial parvicellular part; EW, EW nucleus; m5, motor root of the trigeminal nerve; s5, sensory root of the trigeminal nerve; LPBI, lateral parabrachial nucleus internal part; Me5, mesencephalic trigeminal nucleus; Sub CA, subcoeruleus nucleus α part; A5, noradrenergic cell group A5; LC, locus coeruleus; IOB, inferior olive subnucleus B. (I–L) NPW mRNA in mouse brain. PAG, periaqueductal gray matter; EW, EW nucleus; VTA, ventral tegmental area; DR, dorsal raphe nucleus; DRD, dorsal raphe nucleus dorsal part.
NPW mRNA was detected only in a few confined areas: periaqueductal gray matter (PAG in Fig. 3 I–K), ventral tegmental area (VTA in Fig. 3 I and J), EW nucleus (Fig. 3 I and J), and dorsal raphe nucleus (DR in Fig. 3K), especially the dorsal part of dorsal raphe nucleus (DRD in Fig. 3L).
In Vivo Pharmacological Effects of NPB.
Effects on food intake.
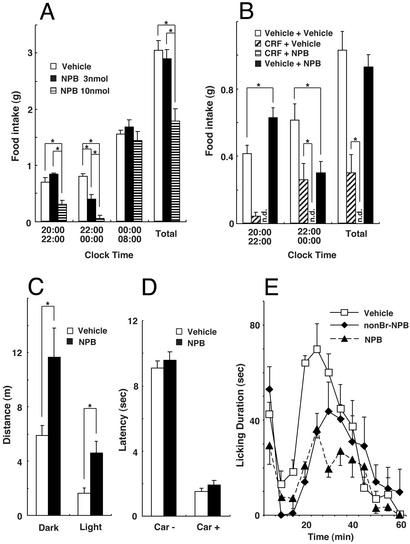
Hypothalamic nuclei such as the dorsomedial, paraventricular, and arcuate nuclei are well known to be involved in the regulation of feeding behavior (17). Expression of GPR7 mRNA in these nuclei prompted us to examine the role of NPB in feeding behavior in mice. When NPB was i.c.v. injected during the light phase, we observed no significant effects of NPB on feeding behavior (data not shown). In contrast, in the dark phase, i.c.v. administration of 3 nmol of NPB increased feeding, but only within the first 2 h (Fig. 4 A and B). A higher dose of NPB suppressed food intake in this interval. After 2 h, both doses of NPB exerted a significant anorexic effect. The colocalization in EW nucleus of NPB/NPW with urocortin, a stress-related neuropeptide of the corticotropin-releasing factor (CRF) family, leads us to test the effects of coadministration of NPB and CRF. The anorexic effect of NPB was markedly enhanced when CRF, a known anorexic peptide, was preadministered. The i.c.v. administration of these two peptides almost completely suppressed the food intake over 4 h (Fig. 4B).
Figure 4.
In vivo pharmacological effects of i.c.v.-injected NPB on food intake (A and B), locomotor activity (C), and nociception (D and E). (A) Vehicle or 3 or 10 nmol of synthetic rat NPB was i.c.v. injected in bolus into freely fed mice, and food consumption was measured. Injections were performed at 20:00 (beginning of dark phase) and food intake was measured at 22:00, 00:00, and 08:00 the next morning (end of dark phase). The data represent food intake between the indicated times (mean ± SEM, n = 4–5 per group). Asterisks indicate significant difference (P < 0.05, one-way ANOVA, Fisher's post hoc analysis). (B) Anorexic effect of NPB is enhanced by pretreatment with CRF. CRF (0.3 nmol) was i.c.v. injected 15 min before the injection of 3 nmol of synthetic rat NPB in mice. NPB was i.c.v. injected at 20:00 (beginning of dark phase), and food intake was measured at 22:00 and 00:00. n.d., no food intake detected (bars invisible). The data represent food intake between designated times in dark phase (mean ± SEM, n = 7 per group). Asterisks indicate the significant difference (P < 0.05, one-way ANOVA, Fisher's post hoc analysis). (C) NPB-induced hyperlocomotion in both dark and light phases. Three nanomols of synthetic rat NPB or vehicle was i.c.v. injected into rats placed in an open field apparatus. The data represent the distance traveled (meters) per 2 h (mean ± SEM, n = 6–8 per group). Asterisks indicate significant difference (P < 0.05, one-way ANOVA, Fisher's post hoc analysis). (D) Paw flick tests were performed 20 min after i.c.v. injection of vehicle or 3 nmol of synthetic rat NPB in rats. Tests were performed without (Car−) and with (Car+) the chemical inflammation induced by carrageenan. Two milligrams of carrageenan was injected into a hindpaw 3 h before the paw flick test. The data represent the latency (seconds) to flick the paw out of the path of the heat-producing light beam (mean ± SEM, n = 6–8 per Car− group and n = 4–5 per Car+ group). (E) Formalin tests were performed 10 min after injection of vehicle, 3 nmol of nonbrominated synthetic rat NPB, or 3 nmol of brominated NPB in rats. The data represent the licking duration (seconds per 5-min bin) at indicated minutes after injection of 50 μl of 5% formalin into a hindpaw (mean ± SEM, n = 4–5 per group). Asterisks indicate significant difference from vehicle treatment (P < 0.05, one-way ANOVA, Fisher's post hoc analysis).
Our data showing a biphasic (early orexigenic followed by delayed anorexic) effect of NPB are different from the simple orexigenic action of NPW reported by Shimomura et al. (7). Because rodents only have GPR7 that accepts both peptides with relatively high affinities, these differences cannot be ascribed to the different potency rank orders of the two peptides on GPR7 and GPR8. Although the details of experimental conditions differ between the two studies (7, 10), this finding indicates that further evaluation is required to establish the potential role of NPB and NPW in the regulation of food intake.
Effects on locomotor activity.
Locomotor activity was recorded in an open-field activity monitor over 2 h after i.c.v. injection of NPB in rats. In both dark and bright phase, 3 nmol of NPB induced a significant hyperlocomotion (Fig. 4C).
Analgesic effects.
Several distinct areas in CNS where the expression of NPB, NPW, or GPR7 mRNA was observed, particularly periaqueductal gray matter and amygdala, are known to express opioid receptors (18). This colocalization suggests the possibility that NPB, NPW, and GPR7 may have a role in the afferent pain pathway and/or regulation of pain-related affective behaviors. Possible analgesic effects of NPB were evaluated by the paw flick test and formalin test in rats. A heat-producing light beam aimed at a hindpaw was used to induce thermal pain, and the latency for paw flicking was examined with or without 3-nmol i.c.v. injection of NPB. NPB did not affect the behavioral response against the acute thermal stimulus (Fig. 4D). The chemical inflammation induced by carrageenan was further imposed on the same rats, and the response to the thermal stimulus was reexamined. Two milligrams of carrageenan significantly shortened the latency in paw flicking, and this was not affected by NPB. In contrast to these negative results, i.c.v. injection of NPB significantly reduced the licking duration in the formalin test (Fig. 4E). The time spent in licking the hindpaw after formalin injection is believed to correlate with perceived pain and the intensity of behavioral response to pain (16). Both brominated and nonbrominated NPB, administered i.c.v., were effective between 10 and 30 min after formalin injection, demonstrating a central analgesic effect of the peptide against chemically induced pain.
Discussion
The peptide ligand for GPR7 that we have isolated from bovine hypothalamus, termed NPB according to a previous paper (6), has a brominated N-terminal tryptophan. Additionally, at the mRNA level we have identified in silico a paralogous peptide, termed NPW (7). In this study, we did not attempt to purify NPW at the peptide level. NPW was shown by another group to have a short form (23 aa) as well as a long form (30 aa) (7). Both synthetic NPB and NPW activate GPR7 and GPR8 with EC50 values of subnanomolar to nanomolar. NPB had a modestly higher potency than NPW on the activation of GPR7, whereas GPR8 was activated much more potently by NPW.
We and the Takeda group have independently identified the bromination of the first tryptophan residue of NPB (6). Although bromine is the fifth most abundant inorganic anion in human plasma and tissues, its physiological roles in terrestrial animals have not yet been established. Nonspecific bromination on tyrosine residues of proteins found in bronchoalveolar lavage fluid was reported in patients with allergen-induced asthma (19). This bromination is catalyzed by eosinophil peroxidase, which is released from allergen-activated eosinophils. Bromine, as well as the pseudohalide thiocyanate, is a physiological substrate for this enzyme thereby generating a halogenating oxidant (20, 21). This is in contrast to neutrophil peroxidase, which uses chlorine under physiological concentrations of halides. Other than this example, to our knowledge, no posttranslationally brominated polypeptides have been reported in terrestrial animals. Some peptides from the venom of carnivorous marine cone snails, such as σ-conotoxin, are shown to have brominated tryptophan(s) (22–25). Like NPB, brominations on these peptides of conus venom were found at the C-6-position of the indole moiety. Although NPW has an N-terminal tryptophan, bromination of NPW has not yet been detected (7).
The role of bromination on the N-terminal tryptophan residue of NPB remains unknown. In the melanophore assay, nonbrominated NPB had essentially the same potency and efficacy as brominated NPB both on GPR7 and GPR8. Fujii et al. (6) have shown the similar results in cAMP inhibition and binding assays. Also in the formalin analgesia test, centrally administered brominated and nonbrominated NPB exerted essentially the same degree of analgesic effects. However, the physiological roles of bromotryptophan should be further investigated. For example, bromination might stabilize the peptide in local environments in vivo. Indeed, it is known that peptides with an N-terminal tryptophan tend to be more susceptible to amino-peptidases (“N-end rule”) (26), and bromination may block this degradation.
In the mouse and human prepro-NPW cDNAs, we found a CTG codon at the putative translation initiation site. By in vitro mutational analysis, we demonstrated that this CTG codon in the mouse prepro-NPW cDNA is in fact used as an efficient translation initiation site (see Fig. 7). The nucleotide sequence surrounding this initiator CTG matches Kozak's rule (27). Several genes have non-ATG translation initiators in addition to a downstream and in-frame ATG initiation codon (28–32). The non-ATG-initiated translation generates N-terminally extended proteins. In contrast, genes having a single non-ATG translation initiator without a downstream ATG are extremely rare in eukaryocytes (33–35). The biological significance of non-ATG-initiated translation of prepro-NPW remains to be investigated.
The distinct expression patterns of NPB and NPW mRNAs in brain suggest separate roles in CNS functions. In mouse brain, NPB mRNA was most abundantly expressed in the EW nucleus of the midbrain. A less prominent expression of NPW mRNA was also observed in the EW nucleus. This nucleus has been classically known for its function in pupillary light reflex. However, many neurons in the EW nucleus actually project to the spinal cord, especially to the superficial dorsal horn (36, 37). In light of the analgesic effect of NPB shown in the present study, this finding suggests the possibility that NPB/NPW originated in the EW nucleus may be involved in nociceptive processing in the dorsal horn (see below). In addition, recent studies show that the expression of urocortin in CNS is confined to the EW nucleus (38). Urocortin is a neuropeptide closely related to CRF, and is thought to mediate stress-induced behaviors (39). Further studies may provide a new insight into the physiological role of this nucleus.
GPR7 mRNA is expressed abundantly in amygdala in rat brain (5). In mouse brain, GPR7 mRNA is expressed also in the bed nucleus of stria terminalis as well as in amygdala (see Fig. 8, which is published as supporting information on the PNAS web site). A number of brainstem monoaminergic nuclei, which are known to send afferents to amygdala, express NPB and/or NPW mRNA abundantly. NPB mRNA is expressed in locus coeruleus, A5 noradrenergic cell group, nucleus parabrachialis (Fig. 3 E–G), and nucleus of the solitary tract (data not shown). NPW mRNA is expressed in dorsal raphe nucleus, ventral tegmental area, and periaqueductal gray matter (Fig. 3 I–L). The limbic system, including amygdala and bed nucleus of stria terminalis, might use NPB and NPW for relaying signals from the brainstem to govern affective behaviors. Licking response after pain stimulus by formalin is thought to be a complex supraspinal behavior, which reflects the affective and motivational aspects of nociceptive processing (40). In our study, NPB significantly reduced the licking response during the late phase of formalin test. It would be interesting to see whether NPB and NPW is involved in the nociceptive processing in the limbic system.
NPB did not exert appreciable analgesic activity in our paw flick tests, with or without preexisting inflammation induced by carrageenan. Although this may be in part due to the qualitative differences of heat-induced (paw flick test) versus chemically induced pain (formalin test), a plausible explanation is that NPB may be mainly involved in the supraspinal regulation of pain processing, which is known to be closely manifested in formalin-induced licking behavior. Indeed, the internal lateral parabrachial nucleus (LPBI) contains abundant NPB mRNA (Fig. 3 E and F). Neurons in the LPBI project almost exclusively to the intralaminar thalamic nuclei, and receive almost exclusively spinal afferents that are thought to be involved in nociception (41, 42).
We observed a short-lasting orexigenic effect of NPB in mice. Shimomura et al. (7) have also reported an orexigenic effect of the short-form NPW in the first 2 h of injection into rat brain. These orexigenic effects might reflect direct actions of NPB on GPR7-expressing “orexigenic neurons” in hypothalamic nuclei such as the dorsomedial, paraventricular, and arcuate nuclei. In this study, however, in addition to this early orexigenic effect, we observed a delayed but more marked anorexic effect of NPB after 2 h of i.c.v. injection. Furthermore, NPB markedly enhanced the well-established anorexic effect of CRF when coadministered, suggesting an interaction between the NPB/NPW and CRF/urocortin systems. It is tempting to hypothesize that the late-phase anorexic effect of NPB might be due to its involvement in the limbic system, i.e., it might be mediated through motivational components, which have influences on the hedonic aspect of eating.
In conclusion, our observations suggest the diverse functions of NPB and NPW in CNS acting as endogenous ligands for GPR7 and/or GPR8. Considering the distributions of NPB, NPW, and GPR7 mRNAs outside CNS (see Fig. 9, which is published as supporting information on the PNAS web site), it is obvious that these peptides may also function in peripheral organs as paracrine or endocrine factors.
Supplementary Material
Acknowledgments
We thank Michele Kelly, Mike Brown, and Joe Goldstein for critically reading the manuscript; Takashi Owa, Yoshiya Oda, Jon Willie, Mark Valasek, and Ichiyo Matsuzaki for helpful discussions; and Dr. Yuichiro Yamada at Kyoto University for providing plasmids. This work was supported in part by research grants from the Perot Family Foundation and Exploratory Research for Advanced Technology (Japan)/Japan Science and Technology Corporation. M.Y. is an Investigator and H.K. is an Associate of the Howard Hughes Medical Institute.
Abbreviations
- GPCR
G protein-coupled receptor
- NPB
neuropeptide B
- NPW
neuropeptide W
- TFA
trifluoroacetic acid
- CRF
corticotropin-releasing factor
- i.c.v.
intracerebroventricular
- EW
Edinger–Westphal
References
- 1.Civelli O, Nothacker H P, Saito Y, Wang Z, Lin S H, Reinscheid R K. Trends Neurosci. 2001;24:230–237. doi: 10.1016/s0166-2236(00)01763-x. [DOI] [PubMed] [Google Scholar]
- 2.Lee D K, George S R, Evans J F, Lynch K R, O'Dowd B F. Curr Opin Pharmacol. 2001;1:31–39. doi: 10.1016/s1471-4892(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Wise A, Gearing K, Rees S. Drug Discovery Today. 2002;7:235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- 4.O'Dowd B F, Scheideler M A, Nguyen T, Cheng R, Rasmussen J S, Marchese A, Zastawny R, Heng H H, Tsui L C, Shi X, et al. Genomics. 1995;28:84–91. doi: 10.1006/geno.1995.1109. [DOI] [PubMed] [Google Scholar]
- 5.Lee D K, Nguyen T, Porter C A, Cheng R, George S R, O'Dowd B F, Scheideler M A, Rasmussen J S, Marchese A, Zastawny R, et al. Brain Res Mol Brain Res. 1999;71:96–103. doi: 10.1016/s0169-328x(99)00171-0. [DOI] [PubMed] [Google Scholar]
- 6.Fujii R, Yoshida H, Fukusumi S, Habata Y, Hosoya M, Kawamata Y, Yano T, Hinuma S, Kitada C, Asami T, et al. J Biol Chem. 2002;277:34010–34016. doi: 10.1074/jbc.M205883200. [DOI] [PubMed] [Google Scholar]
- 7.Shimomura Y, Harada M, Goto M, Sugo T, Matsumoto Y, Abe M, Watanabe T, Asami T, Kitada C, Mori M, et al. J Biol Chem. 2002;277:35826–35832. doi: 10.1074/jbc.M205337200. [DOI] [PubMed] [Google Scholar]
- 8.Brezillon S, Lannoy V, Franssen J D, Le Poul E, Dupriez V, Lucchetti J, Detheux M, Parmentier M. J Biol Chem. 2003;278:776–783. doi: 10.1074/jbc.M206396200. [DOI] [PubMed] [Google Scholar]
- 9.DeCamp D L, Thompson T M, de Sauvage F J, Lerner M R. J Biol Chem. 2000;275:26322–26327. doi: 10.1074/jbc.M004055200. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli R M, Tanaka H, Williams S C, Richardson J A, Kozlowski G P, Wilson S, et al. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, Tanaka H, Richardson J A, Williams S C, Slaughter C A, Nakamura M, Chen J L, Yanagisawa M. J Biol Chem. 2001;276:28471–28477. doi: 10.1074/jbc.M103478200. [DOI] [PubMed] [Google Scholar]
- 12.Fields G B, Noble R L. Int J Pept Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 13.Conklin B R, Farfel Z, Lustig K D, Julius D, Bourne H R. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Moroi K, Iwai J, Takahashi H, Ohnuma N, Hori S, Takimoto M, Nishiyama M, Masaki T, Yanagisawa M, et al. J Biol Chem. 1998;273:11378–11383. doi: 10.1074/jbc.273.18.11378. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin I J, Shelton J, Garry D J, Richardson J A. Dev Dyn. 1997;208:75–84. doi: 10.1002/(SICI)1097-0177(199701)208:1<75::AID-AJA7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Tjolsen A, Berge O G, Hunskaar S, Rosland J H, Hole K. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M W, Woods S C, Porte D, Jr, Seeley R J, Baskin D G. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 18.Satoh M, Minami M. Pharmacol Ther. 1995;68:343–364. doi: 10.1016/0163-7258(95)02011-x. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Samoszuk M K, Comhair S A, Thomassen M J, Farver C F, Dweik R A, Kavuru M S, Erzurum S C, Hazen S L. J Clin Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss S J, Test S T, Eckmann C M, Roos D, Regiani S. Science. 1986;234:200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]
- 21.Slungaard A, Mahoney J R., Jr J Biol Chem. 1991;266:4903–4910. [PubMed] [Google Scholar]
- 22.Craig A G, Jimenez E C, Dykert J, Nielsen D B, Gulyas J, Abogadie F C, Porter J, Rivier J E, Cruz L J, Olivera B M, McIntosh J M. J Biol Chem. 1997;272:4689–4698. doi: 10.1074/jbc.272.8.4689. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez E C, Craig A G, Watkins M, Hillyard D R, Gray W R, Gulyas J, Rivier J E, Cruz L J, Olivera B M. Biochemistry. 1997;36:989–994. doi: 10.1021/bi962840p. [DOI] [PubMed] [Google Scholar]
- 24.Rigby A C, Lucas-Meunier E, Kalume D E, Czerwiec E, Hambe B, Dahlqvist I, Fossier P, Baux G, Roepstorff P, Baleja J D, et al. Proc Natl Acad Sci USA. 1999;96:5758–5763. doi: 10.1073/pnas.96.10.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalume D E, Stenflo J, Czerwiec E, Hambe B, Furie B C, Furie B, Roepstorff P. J Mass Spectrom. 2000;35:145–156. doi: 10.1002/(SICI)1096-9888(200002)35:2<145::AID-JMS922>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Varshavsky A. Proc Natl Acad Sci USA. 1996;93:12142–12149. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak M. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acland P, Dixon M, Peters G, Dickson C. Nature. 1990;343:662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- 29.Bruening W, Pelletier J. J Biol Chem. 1996;271:8646–8654. doi: 10.1074/jbc.271.15.8646. [DOI] [PubMed] [Google Scholar]
- 30.Florkiewicz R Z, Sommer A. Proc Natl Acad Sci USA. 1989;86:3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hann S R, King M W, Bentley D L, Anderson C W, Eisenman R N. Cell. 1988;52:185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- 32.Saris C J, Domen J, Berns A. EMBO J. 1991;10:655–664. doi: 10.1002/j.1460-2075.1991.tb07994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falvey E, Fleury-Olela F, Schibler U. EMBO J. 1995;14:4307–4317. doi: 10.1002/j.1460-2075.1995.tb00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imataka H, Gradi A, Sonenberg N. EMBO J. 1998;17:7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao J H, Davidson I, Matthes H, Garnier J M, Chambon P. Cell. 1991;65:551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]
- 36.Loewy A D, Saper C B. Brain Res. 1978;150:1–27. doi: 10.1016/0006-8993(78)90650-9. [DOI] [PubMed] [Google Scholar]
- 37.Loewy A D, Saper C B, Yamodis N D. Brain Res. 1978;141:153–159. doi: 10.1016/0006-8993(78)90624-8. [DOI] [PubMed] [Google Scholar]
- 38.Weninger S C, Dunn A J, Muglia L J, Dikkes P, Miczek K A, Swiergiel A H, Berridge C W, Majzoub J A. Proc Natl Acad Sci USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weninger S C, Peters L L, Majzoub J A. Endocrinology. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- 40.Donahue R R, LaGraize S C, Fuchs P N. Brain Res. 2001;897:131–138. doi: 10.1016/s0006-8993(01)02103-5. [DOI] [PubMed] [Google Scholar]
- 41.Fulwiler C E, Saper C B. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 42.Bourgeais L, Monconduit L, Villanueva L, Bernard J F. J Neurosci. 2001;21:2159–2165. doi: 10.1523/JNEUROSCI.21-06-02159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.