Abstract
Microbodies usually house catalase to decompose hydrogen peroxide generated within the organelle by the action of various oxidases. Here we have analyzed whether peroxisomes (i.e., catalase-containing microbodies) exist in Neurospora crassa. Three distinct catalase isoforms were identified by native catalase activity gels under various peroxisome-inducing conditions. Subcellular fractionation by density gradient centrifugation revealed that most of the spectrophotometrically measured activity was present in the light upper fractions, with an additional small peak coinciding with the peak fractions of HEX-1, the marker protein for Woronin bodies, a compartment related to the microbody family. However, neither in-gel assays nor monospecific antibodies generated against the three purified catalases detected the enzymes in any dense organellar fraction. Furthermore, staining of an N. crassa wild-type strain with 3,3′-diaminobenzidine and H2O2 did not lead to catalase-dependent reaction products within microbodies. Nonetheless, N. crassa does possess a gene (cat-4) whose product is most similar to the peroxisomal type of monofunctional catalases. This novel protein indeed exhibited catalase activity, but was not localized to microbodies either. We conclude that N. crassa lacks catalase-containing peroxisomes, a characteristic that is probably restricted to a few filamentous fungi that produce little hydrogen peroxide within microbodies.
Microbodies are nearly ubiquitous organelles of the eukaryotic cell. They usually house a number of hydrogen peroxide-producing oxidases as well as catalase, which quickly removes the hydrogen peroxide generated within microbodies (10). Proteins are targeted to the lumen of microbodies by either of two peroxisomal targeting signals (PTS) (19, 48). The predominant signal is the PTS1, a tripeptide located at the C terminus and composed of the amino acids SKL or conservative variants thereof (16, 30). Depending on species, cell type, or developmental state, distinct types of microbodies can be prevalent, which emerge upon differential protein import. The various types are termed according to their marker enzyme content, such as peroxisomes, glyoxysomes, glycosomes, or Woronin bodies (4, 24). Remarkably, filamentous ascomycetes harbor at least two distinct types of microbodies within a single cell: (i) microbodies with a metabolic function (peroxisomes or glyoxysomes), which house the key enzymes of the glyoxylate cycle and a complete fatty acid β-oxidation system; and (ii) the Woronin body, which is required to seal septal pores after hyphal wounding. The Woronin body was identified as a microbody-like organelle because an anti-SKL antibody specifically recognized the dominant protein of this organelle (24). This protein was recently identified as HEX-1 (21, 49). HEX-1 indeed harbors the PTS1 sequence SRL, aggregates within the Woronin body, and gives rise to the typical hexagonal shape of this specialized organelle.
Interestingly, glyoxysomes of the filamentous fungus Neurospora crassa were reported to lack catalase activity. Instead, catalase activity was detected in organelles with higher density than glyoxysomes (25, 53). Further support for the existence of such an additional microbody-like compartment was provided by Wanner and Theimer (53), who subjected the N. crassa slime mutant, which lacks a rigid cell wall, to 3,3′-diaminobenzidine (DAB) staining. The DAB reaction product that is generated upon catalase-dependent hydrogen peroxide decomposition was absent from glyoxysomes but was found in crescent-shaped structures in close proximity to vacuoles. However, in the reports mentioned, the identity of this catalase-containing organelle remained elusive. Notably, in a more recent report, catalase activity was detected in Woronin body-enriched fractions (49). Since in sucrose density gradients the Woronin body sediments at a significantly higher density than glyoxysomes, the Woronin body might in fact represent the catalase-containing organelle described above. On the other hand, Woronin bodies are not associated with vacuoles and their hexagonal shape does not resemble the prolate structures seen by Wanner and Theimer (53).
Three catalases have been described in N. crassa: catalase 1 (CAT-1) and catalase 3 represent the typical large monofunctional catalases, whereas catalase 2 is a member of the catalase-peroxidase family and is possibly derived from a bacterial enzyme. All three isozymes are present throughout the N. crassa asexual life cycle, albeit to varying levels: CAT-1 is highly abundant in conidia, CAT-2 is mainly found in aerial hyphae and conidia (37), and CAT-3 activity increases during exponential growth and is induced under various stress conditions (6, 33). Subcellular localization of the N. crassa catalases has not been thoroughly studied. Evidence exists that CAT-3 is processed and secreted; however, since only a little extracellular CAT-3 activity has been found, it has been suggested that most of the enzyme is either bound to the cell wall or remains within the cell (34). Completion of the N. crassa genome (14) revealed a fourth putative catalase that belongs to the family of small-subunit monofunctional catalases and is most similar to peroxisomal catalases of animals and yeasts (22). Thus, current knowledge is commensurate with the existence of aperoxisomal compartment in N. crassa that is distinct from glyoxysomes. To clarify whether or not peroxisomes exist in N. crassa, we have thoroughly analyzed catalase activities under peroxisome-inducing conditions. Neither cytochemistry nor catalase activity gels supported the existence of a microbody-associated catalase. Likewise, the application of antibodies against the three characterized catalase isozymes failed to detect a lumenal catalase. Finally, characterization of the novel CAT-4 revealed that this protein is a bona fide catalase; however, this protein is not targeted to organelles. The impact of our finding of a eukaryote devoid of peroxisomal catalase is discussed.
MATERIALS AND METHODS
Strains and culture conditions.
The Neurospora crassa wild-type strains St. Lawrence 74-OR8-1a (FGSC#988) and 74-OR23-1A (FGSC#987) were used for all biochemical experiments of this work. Strains Nc15 and Nc21 were generated by integrating the expression constructs MF272 (green fluorescent protein [GFP] expression) (13) and CW20 (GFP-CAT-4), respectively, into the his-3 locus of strain N623 (FGSC#6103) by homologous recombination, followed by a screening of prototrophic His+ transformants for expression of GFP by immunoblotting. Strain Nc23 was similarly generated by integrating plasmid pCW22 (CAT-4) into strain N623 and screening for expression of CAT-4. Wild-type Aspergillus tamarii (DSM 825; ATCC 10836) was obtained from DSMZ,Braunschweig, Germany. Strains were maintained on Vogel's medium N supplemented with 2% sucrose or, for the induction of microbodies, 1 mM oleic acid plus 1% (wt/vol) Tergitol, 40 mM acetate, or 1% (vol/vol) ethanol. All manipulations were carried out according to standard Neurospora techniques (9).
Yeast strains used were wild-type Saccharomyces cerevisiae UTL-7A; its derivative, yHPR251, which harbors an integrated copy of a PTS2-DsRed construct (47); and the catalase-less strain GA1-7D cta1Δ ctt1Δ (kindly provided by C. Schüller, Max Perutz Laboratories, Vienna, Austria). Escherichia coli strain DH5α was used for all plasmid amplifications and isolations. Standard media for the cultivation of yeast and bacterial strains were prepared as described previously (43).
Plasmids and cloning procedures.
To clone the reading frame of cat-4 (NCU05169.2), primer pair RE1490/RE1491 was used (RE1490, 5′-AATTGGATCCATGTCTTCAAACGACGCAC-3′; RE1491 5′-AATTGAATTCTCACTCATCATCCTTCGAATC-3′). Due to our failure to amplify cat-4 from cDNA library M-1 (FGSC, Kansas City, MO), genomic wild-type DNA was used as template in the PCR. The single 60-bp intron of the gene that follows codon 3 could be gapped by primer RE1490, which contained the complementary sequence of the first three codons, yet annealed to the sequence 3′ of the intron. The PCR product was subcloned into pBluescript SK+ (Stratagene), and its identity was verified by sequencing (MWG-BIOTECH AG, Ebersberg, Germany). The insert was lifted as a BamHI-EcoRI fragment and cloned into appropriately cut pYPGE15 (5), designed for constitutive expression of CAT-4 in S. cerevisiae (pCW21). To express an N-terminal GFP fusion of CAT-4 in yeast, pUG36 (42), kindly provided by J. H. Hegemann, was appropriately cut (pHPR349).
For the expression of an N-terminal GFP fusion of CAT-4 in N. crassa, GFP was amplified from pMF272 (13) with primer pair RE1372/RE1373 (RE1372, 5′-AATCTAGAATGGTGAGCAAGGGCGAG-3′; RE1373 5′-AAGGATCCCTTGTACAGCTCGTCCAT-3′), cut with XbaI and BamHI, and cloned together with the BamHI-EcoRI CAT-4 fragment into XbaI-EcoRI-cut pMF272 (pCW20). For the expression of untagged CAT-4 in N. crassa, the GFP open reading frame of pMF272 was replaced by the BamHI-EcoRI CAT-4 fragment (pCW22).
Purification of N. crassa catalases.
All procedures were carried out at 4°C. For the separation and purification of three different catalases from N. crassa, all purification steps were analyzed by native polyacrylamide gel electrophoresis (PAGE) followed by in-gel activity staining of catalases. Mechanical lysis of up to 80 g of frozen mycelia grown for 48 h on acetate-containing medium was performed by rapid agitation with glass beads (0.1 to 0.2 mm) in 200 ml buffer A at pH 6.7 (50 mM Tris, 1 mM EDTA, 0.5 mM EGTA, 0.5 mM benzamidine, 0.5 mM dithioerythrol [DTE], 0.1 mM phenylmethylsulfonyl fluoride) using a Beadbeater (Biospec Products, Inc.). To prevent overheating of the sample during the homogenization, the Beadbeater, with the ice water jacket installed, was operated 15 times for 15 s each with intervals of 30 s. The homogenate was decanted from the settled glass beads, passed through four layers of gauze, and subjected to centrifugation at 17,000 × g for 20 min. The supernatant was loaded directly onto a coupled column system consisting of a cation-exchange column (phosphocellulose P-11, 5 by 15.5 cm; Whatman) and a “dye-ligand” column (Blue-Sepharose Cl-6B, 1.5 by 13.5 cm). The flowthrough was subjected to anion-exchange chromatography using a Whatman DEAE cellulose DE52 column (2.5 by 11 cm) equilibrated with buffer A (pH 6.7).
Bound protein including CAT-3 was eluted with a 200-ml linear gradient of potassium chloride (0 to 0.2 M in buffer A, pH 6.7). Peak catalase activity fractions were pooled and loaded onto a hydroxylapatite (HA) column (2.5 by 5 cm; Bio-Rad) equilibrated in buffer B at pH 6.7 (50 mM Tris, 0.5 mM benzamidine, 0.5 mM DTE, 0.1 mM PMSF). CAT-3 was eluted with a 100-ml linear gradient of 0 to 0.3 M potassium phosphate. Catalase-containing fractions of the flowthrough from the DE52 column equilibrated with buffer A (pH 6.7) were pooled and adjusted to a pH value of 7.5 by titration with a 0.1 N sodium hydroxide solution. CAT-2 and CAT-1 were separated by anion-exchange chromatography using a Whatman DEAE cellulose DE52 column (2.5 by 10 cm) equilibrated with buffer A (pH 7.5). Only CAT-2 bound to the column and was eluted with a 200-ml linear gradient of potassium chloride (0 to 0.2 M in buffer A, pH 6.7).
CAT-1 was purified from cells grown on sucrose for 48 h by mechanical homogenization as described above and following the purification protocol described by Jacob and Orme-Johnson (20) with some modifications: e.g., using the Beadbeater procedure as described above for mechanical homogenization. The final purification step for all three catalases, CAT-1, CAT-2, and CAT-3, was size exclusion chromatography performed on a Sephacryl S-300 HR column (3 by 126 cm; GE Healthcare, Freiburg, Germany) equilibrated with 50 mM Tris, pH 7.5, 0.5 mM benzamidine, 0.5 mM DTE, and 150 mM sodium chloride.
Generation and usage of antisera and immunoprecipitation.
Antibodies against N. crassa MFP (50) and TIM-23 (35), GFP (41), and yeast Cta1p (17) and Pcs60p (3) were described previously. The antibody against yeast Pgk1p was commercially obtained (Molecular Probes, Eugene, OR). Antibodies against CAT-1, CAT-2, CAT-3, HEX-1, thiolase, and mitochondrial enoyl-coenzyme A (CoA) hydratase were generated in rabbits with solutions of native, highly purified proteins. A detailed description of the purifications of the latter three proteins will be reported elsewhere. Immunoblotting was performed according to standard protocols. Immunoreactive complexes were detected with the ECL enhanced chemiluminescence system from GE Healthcare.
To test for the specificity of the anti-CAT-1 antibody, increasing amounts of antiserum or preimmune serum were added to crude wild-type extract prepared from sucrose-grown hyphae. The soluble fractions were separated from the precipitates by centrifugation and were subjected to an in-gel catalase assay. For the isolation of CAT-1 by immunoprecipitation, 30 mg of a postorganellar supernatant prepared from sucrose-grown mycelia was incubated with 50 μl Dynabeads M-280 sheep anti-rabbit immunoglobulin G (Invitrogen, Karlsruhe, Germany) coated with anti-CAT-1 antibodies. Following triple washing with phosphate-buffered saline, beads were boiled in 50 μl of 1× sodium dodecyl sulfate (SDS) sample buffer. The sample was separated by SDS-PAGE, and the precipitated 80-kDa protein was analyzed by electrospray ionization-mass spectrometry (ESI-MS).
Preparation of crude protein extracts, differential centrifugation, and sucrose density gradient centrifugation.
For subcellular fractionation, cultures were inoculated with conidia (105/ml) in Vogel's minimal medium and were shaken (100 rpm) at 30°C for 24 h before shifting hyphae to the various peroxisome-inducing conditions. Hyphae were harvested by filtration, washed with water, mixed with 1 g/g wet weight quartz sand and 4 volumes of isolation buffer (150 mM Tricine, pH 7.4, 0.44 M sucrose, 10 mM KCl, 5 mM MgCl2, 1 mM EDTA), and ground with a pestle in a mortar at 4°C. The homogenate was squeezed through four layers of cheesecloth and subjected to centrifugation at 2,500 × g for 5 min. The resulting supernatant was taken as crude extract.
For differential centrifugation, 10 ml of crude extract was separated in a pellet and supernatant fraction through centrifugation at 25,000 × g for 20 min. For density gradient centrifugation, 10 ml of crude extract was layered on top of a linear gradient of 30 to 60% (wt/wt) sucrose (in 10 mM Tricine, pH 7.4, 1 mM EDTA). The gradient was subjected to centrifugation for 90 min at 38,000 × g at 4°C in a Sorvall SV288 vertical rotor. Fractions of 1 ml were collected from the bottom to the top, and their protein concentration was determined spectrophotometrically at 280 nm. Sucrose density was determined refractometrically.
Yeast lysates were prepared and fractionated by differential centrifugation as described previously (11).
Enzyme assays.
Catalase (EC 1.11.1.6), urate oxidase (EC 1.7.3.3), isocitrate lyase (EC 4.1.3.1), enoyl-CoA hydratase (EC 4.2.1.17), and 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) activities of MFP, fumarase (EC 4.2.1.2), and cytochrome c oxidase (1.9.3.1) were assayed by established procedures (25). The catalase activity of CAT-4 was similarly assayed, with the exception that various amounts of NADPH were added to the assay mixture. In-gel catalase activity assays were carried out as described by Woodbury et al. (55). In brief, samples were separated on native polyacrylamide gels, which were then incubated with 0.1% H2O2 for 10 min, washed twice with water, and treated with a solution containing 1% FeCl3 and 1% K3(Fe(CN)6). Upon emergence of the unstained catalase bands, reactions were stopped by washing the gels with water.
Fluorescence microscopy.
Live yeast cells were analyzed for GFP and DsRed fluorescence as described previously (41). N. crassa mycelia were similarly prepared. After overnight growth in Vogel's minimal medium, hyphae were harvested and grown overnight in induction medium (1× Vogel's salt, 0.05% [wt/vol] Tween 40, 0.1% [wt/vol] oleic acid) at 30°C. For inspection, a suspension of mycelia was placed on a slide, mixed with an equal volume of 1% (wt/vol) low-melting-point agarose in H2O, and sealed with a coverslip. All micrographs were recorded on a Zeiss Axioplan 2 microscope with a Zeiss Plan-Apochromat ×100/1.4 oil objective and an Axiocam MR digital camera and were processed with AxioVision 4.2 software (Zeiss, Jena, Germany).
Electron microscopy.
For overall cell morphology, cells were fixed in 1.5% KMnO4 for 20 min, poststained in 0.5% uranyl acetate, subsequently dehydrated via an ethanol series, and embedded in Epon 812. For detection of catalase activity, cells were prefixed in 3% glutaraldehyde in 0.1 M cacodylate buffer. Catalase activity was detected with DAB and 0.06% hydrogen peroxide as described before (52). For immunocytochemistry, the glutaraldehyde-fixed cells were embedded in Unicryl; ultrathin sections were incubated with specific anti-CAT-1 antibodies and gold-conjugated goat anti-rabbit antiserum (54).
RESULTS
Expression of catalases under peroxisome-inducing conditions.
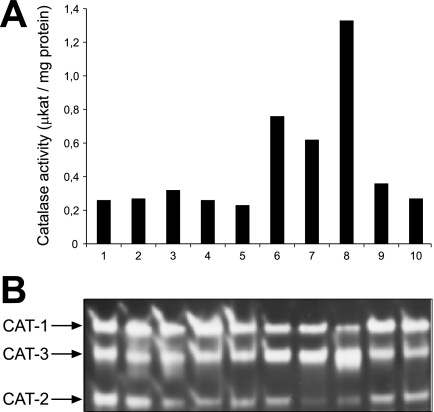
To analyze whether catalase activity is induced under conditions that require microbody-borne metabolism, wild-type cells were grown for 12 h in media containing ethanol (Fig. 1, lane 1), acetate (lane 2), or oleic acid (lane 3) as the sole carbon source. Under these conditions, the glyoxylate cycle enzymes and, in the last case, the β-oxidation enzymes are induced (25). In addition, media with d-methionine (lane 10), which induces the expression of d-amino acid oxidase (46), as well as media with uric acid (lane 9), which increases the levels of urate oxidase (36), were tested for coinduction of catalase. Total catalase activity did not increase significantly under any of these conditions relative to that in control cells that were grown for 24 h in medium with sucrose (lane 5) as the sole carbon source (Fig. 1A). In contrast, stationary-phase cells that had been grown for 96 h in sucrose medium (lane 8) exhibited a sevenfold increase in activity, as observed previously (6, 33). Since three differentially regulated catalase isozymes are described in N. crassa, the contribution of each enzyme to the total catalase activity was visualized on native acrylamide gels that had been loaded with equal amounts of enzyme activities. All three enzymes were clearly discernible under all conditions (Fig. 1B). More CAT-2 was observed in ethanol- and acetate-grown cells, and as expected, CAT-3 was predominant in cells grown to the stationary phase (33). Beyond that we did not obtain evidence for an additional catalase isozyme under all conditions tested.
FIG. 1.
N. crassa catalase activities under conditions that promote peroxisome proliferation. N. crassa mycelia were grown in liquid medium containing 2% (wt/vol) sucrose for 24 h, filtered, washed, and transferred to fresh minimal media containing the following carbon sources: 1% (vol/vol) ethanol (lane 1), 40 mM acetate (lane 2), 40 mM acetate plus 1 mM oleic acid (lane 3), 1 mM oleic acid (lane 4), and 2% sucrose (lane 5). In addition, sucrose-grown cells were supplemented with uric acid as the sole nitrogen source (lane 9) or d-methionine as the sole sulfur source (lane 10). After growth for 12 h, mycelia were harvested by filtration and cell extracts were examined for total catalase activity (A). To test the effect of mycelial age on catalase activity, hyphae were grown in standard sucrose medium for 24 h (lane 5), 48 h (lane 6), 72 h (lane 7), or 96 h (lane 8). (B) Samples with equal catalase activity (40 μkat) were separated by nondenaturing PAGE and analyzed for catalase activity by determining the appearance of H2O2-cleared zones after ferric cyanide/ferric chloride staining. The three catalase isoenzymes were designated according to Michán et al. (33).
Intracellular distribution of catalase activity.
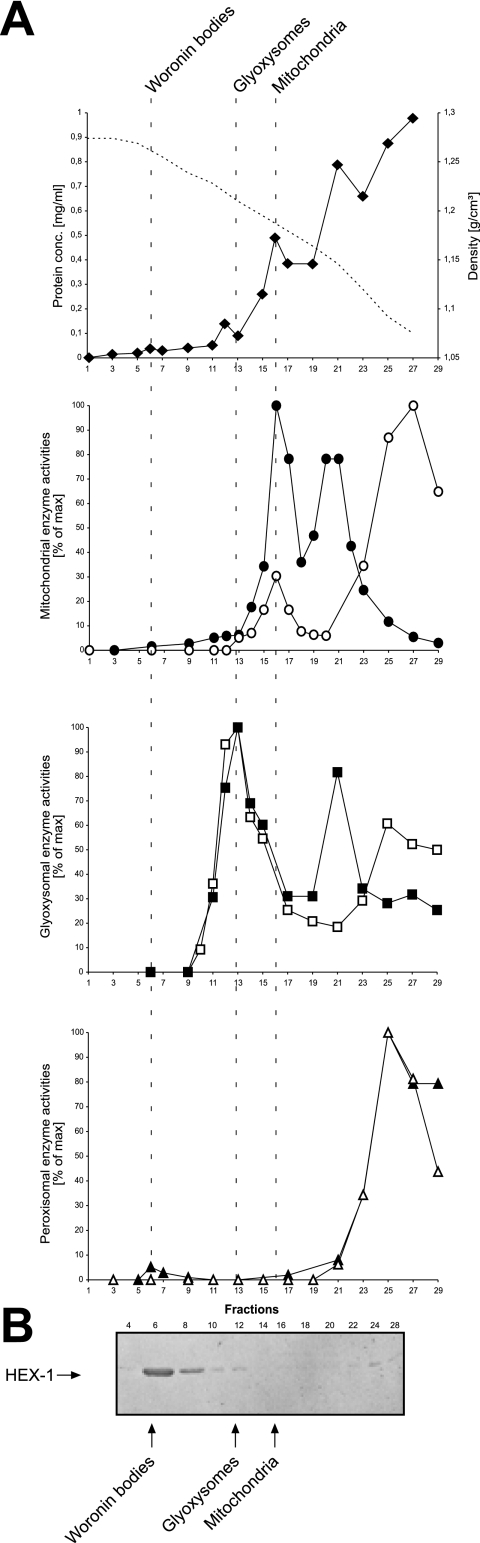
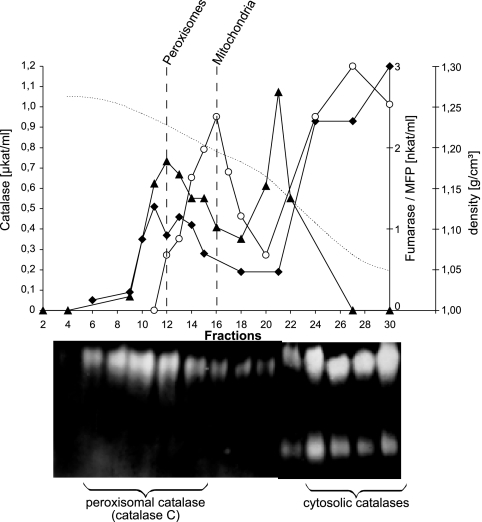
To determine the intracellular distribution of catalase activities in an oleic acid-induced N. crassa wild-type strain, a postnuclear supernatant devoid of cell debris and nuclei was separated on a continuous sucrose density gradient (30 to 60%). The resulting fractions were assayed for the mitochondrial marker enzymes fumarase and cytochrome c oxidase as well as for the glyoxysomal marker enzymes isocitrate lyase and the multifunctional β-oxidation enzyme (Fig. 2A). Peak Woronin body fractions were identified immunologically by using an anti-HEX-1 antibody (Fig. 2B). The organelles were separated well, with mitochondria being identified at a density of 1.19 g/cm3, glyoxysomes at 1.21 g/cm3, and Woronin bodies at 1.26 g/cm3. During cell breakage, a fraction of organelles always gets ruptured, particularly the mechanically and osmotically fragile microbodies (25). Portions of the glyoxysomal and mitochondrial matrix enzymes were therefore also found in the lighter fractions of the gradient where soluble proteins and possibly organellar remnants are recovered. Most of the measurable catalase activity was found in the soluble fractions and was entirely absent from mitochondria and glyoxysomes. Nonetheless, a small but significant fraction of catalase, estimated to account for 5% of total activity, cosedimented with the Woronin body fractions. A similar catalase distribution was obtained when cells were grown on acetate or sucrose as the sole carbon source (not shown). At the same time, no isocitrate lyase or fumarase activity could be measured in these fractions. We also tested the distribution of urate oxidase activity as this enzyme was reported to cosediment with catalase in the slime mutant (53). However, urate oxidase was exclusively found in the upper soluble fractions, indicating that urate oxidase is a soluble enzyme in N. crassa.
FIG. 2.
Subcellular localization of catalase activity by spectrophotometry. Cell lysate of N. crassa wild-type mycelia was loaded on top of a linear sucrose density gradient (30 to 60% [wt/wt]) and subjected to centrifugation at 48,000 × g for 90 min. Fractions were collected from the bottom (fraction 1) to the top (fraction 29) and were assayed for mitochondrial (cytochrome c oxidase • and fumarase ○), glyoxysomal (multifunctional β-oxidation enzyme ▪ and isocitrate lyase □), and peroxisomal (catalase ▴ and urate oxidase ▵) marker enzymes (A). Localization of Woronin bodies was determined by immunoblotting using anti-HEX-1 antibodies (B). Dashed vertical lines indicate the organellar peak fractions as denoted. Densities (dotted lines) and protein concentrations (⧫) of each fraction are also indicated.
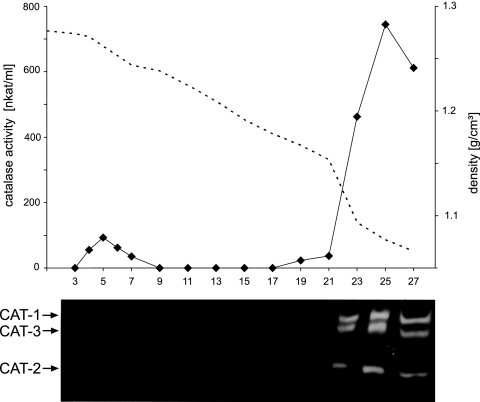
To analyze which of the catalase isozymes caused the observed catalase activity in Woronin bodies, fractions of a similar sucrose gradient were subjected to an in-gel catalase activity assay. Surprisingly, all three isozymes were only detected in the soluble fractions, although activity was clearly measurable also in the dense fractions (Fig. 3). Loading samples from the dense and soluble fractions that contained equal catalase activities (as measured with the liquid assay) also failed to reveal any catalase activity with the in-gel assay. These data were interpreted to mean that the measured consumption of hydrogen peroxide in the Woronin body fractions was not due to a catalase activity, albeit we could not entirely rule out that the sensitivity of the in-gel assay was too low to detect organellar catalase.
FIG. 3.
Subcellular localization of catalase activity by an in-gel activity assay. Cell lysate of N. crassa mycelia that had been grown for 12 h in oleic acid-containing medium was subjected to isopycnic 30 to 60% (wt/wt) sucrose density gradient centrifugation. One-milliliter fractions were collected from the bottom (fraction 1) to the top (fraction 29) and measured for catalase activity by a spectrophotometric assay. The dotted line indicates the densities of each fraction (upper panel). From the particulate fraction 5 and the supernatant fractions 23, 25, and 27, aliquots with 20 nkat catalase activity each were loaded on a native 7.5% polyacrylamide gel while from the remaining fractions aliquots of 100 μl were loaded. After separation under nondenaturing conditions, catalase activity staining was performed (lower panel).
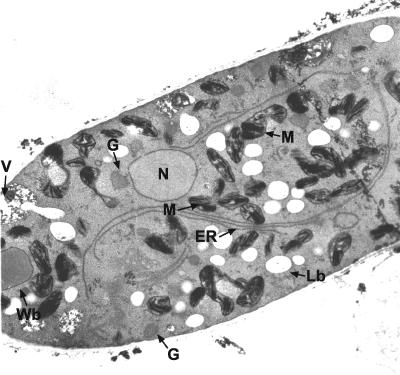
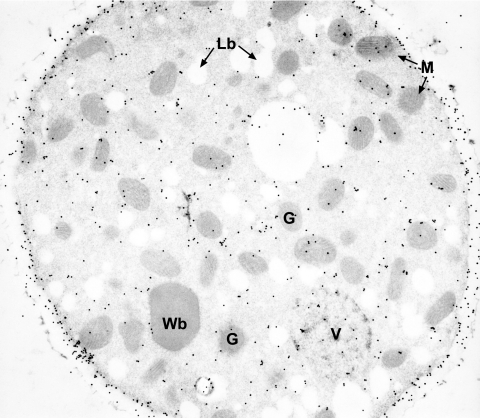
In an alternative approach, eventual organellar catalase activity was assayed cytochemically. Prefixed cells that had been induced by oleic acid were treated with H2O2 and DAB, and ultrathin sections thereof were subjected to electron microscopy. Neither glyoxysomes nor Woronin bodies contained a clear DAB reaction product that is formed in the presence of catalase activity (Fig. 4). Significant reaction products were only obtained for mitochondrial cristae, most likely caused by an intramitochondrial peroxidase reaction (39). Notably, the DAB-positive organelles adjoined to vacuoles as described for the N. crassa slime mutant were never observed, even when serial ultrathin sections of a cell were inspected (see Fig. S1 in the supplemental material). Thus, cytochemistry did not provide conclusive evidence for a compartmentalized catalase.
FIG. 4.
In situ catalase staining. Ultrathin sections of fixed oleic acid-grown N. crassa hyphae were stained with DAB and H2O2 for catalase activity. Visualization by electron microscopy revealed that only mitochondrial cristae showed an electron-dense reaction product. ER, endoplasmic reticulum; G, glyoxysomes; Lb, lipid bodies; M, mitochondria; N, nucleus; V, vacuole; Wb, Woronin body.
Purification and immunological detection of the catalase isozymes.
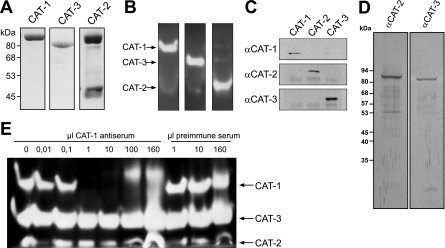
To detect the catalase proteins in situ, we set out to produce antibodies against the three isozymes. To that end, the three catalase activities were purified, starting with extracts from acetate (CAT-2)- or sucrose (CAT-1, CAT-3)-grown wild-type cells. For CAT-1, a previously established protocol was used (20). The various purification steps for CAT-2 and CAT-3 are summarized in Table 1. The purity of the enzymes was analyzed by denaturing SDS-PAGE (Fig. 5A) as well as by an in-gel catalase assay (Fig. 5B). One major band was seen for catalases 1 and 3 in the Coomassie-stained gel; the purified catalase 2 sample contained one additional protein of approximately 46 kDa. Nonetheless, since the activity assay revealed that each catalase gave rise to only one H2O2-reactive band, the samples were considered sufficiently pure to immunize rabbits. Tests for the specificity of the resulting antisera showed that all antibodies were monospecific. They did not significantly cross-react with the other purified catalases (Fig. 5C), and the antisera against CAT-2 and CAT-3 each gave rise to a single band at the expected sizes of 83 kDa and 79 kDa, respectively, in a Western blot performed with crude extract from oleic acid-induced wild-type mycelium (Fig. 5D). The specificity of the CAT-1 antiserum was further proved by an immunoprecipitation experiment. Incubation of crude wild-type extract with increasing amounts of antiserum led to depletion of isoform 1, but not of CAT-2 and CAT-3, in the unbound fraction (Fig. 5E).
TABLE 1.
Purification of three N. crassa catalases
| Fraction | Amt of protein (mg) | Total activity (mkat) | Sp act (mkat/mg) | Yield (%) | Fold purified | In-gel catalase activitya |
|---|---|---|---|---|---|---|
| Purification of CAT-3 and CAT-2 | ||||||
| Crude extract | 780 | 1.32 | 1.7 | 100 | 1 | CAT-1, CAT-2, CAT-3 |
| P11/Blue-Sepharose flowthrough | 191 | 1.14 | 6 | 86.5 | 3.5 | CAT-1, CAT-2, CAT- |
| DE52 pH 6.7 eluate | 24.3 | 0.35 | 14.4 | 26.5 | 8.5 | CAT-3 |
| HA eluate | 1.03 | 0.26 | 252 | 19.7 | 148 | CAT-3 |
| S300 peak fraction pool | 0.3 | 0.17 | 567 | 12.9 | 334 | CAT-3 |
| DE52 pH 6.7 flowthrough | 80.6 | 0.62 | 7.7 | 47 | 4.5 | CAT-1, CAT-2 (CAT-3) |
| DE52 pH 7.5 eluate | 5 | 0.03 | 5.6 | 2.1 | 3.3 | CAT-1, CAT-2 |
| S300 peak fraction pool | 1 | 0.02 | 21 | 1.6 | 12.4 | CAT-2 |
| Purification of CAT-1 | ||||||
| Crude extract | 2,037 | 0.84 | 0.4 | 100 | 1 | CAT-1, CAT-2, CAT-3 |
| Ammonium sulfate fractionation | 180 | 0.30 | 1.7 | 35 | 4.2 | CAT-1 (CAT-3) |
| DE52 eluate | 6.8 | 0.27 | 39.7 | 32 | 101.2 | CAT-1 |
| S300 peak fraction pool | 2.0 | 0.18 | 91.7 | 22 | 229.2 | CAT-1 |
Parentheses indicate residual activity (presence in low amounts).
FIG. 5.
Immunologic analysis of three purified catalase isoenzymes from N. crassa. (A and B) Determination of purity. For each isoenzyme, the gel filtration fraction with the highest specific catalase activity was analyzed by denaturing (A) and nondenaturing (B) PAGE. Catalases were visualized by Coomassie (A) and in-gel activity (B) staining, respectively. The sizes as predicted by the genome sequence are as follows: CAT-1, 85.5 kDa; CAT-2, 83.4 kDa; CAT-3, 79.2 kDa. (C to E) Specificity of antisera directed against the individual purified catalases. (C) Equal amounts (5 μg) of purified catalase isoenzymes were subjected to Western blot analysis. Primary rabbit antisera were used at dilutions of 1:1,000 and in combination with alkaline phosphatase-conjugated goat anti-rabbit antibodies (α-CAT-1, α-CAT-2, and α-CAT-3). (D) Crude extract (10 μg) from oleic acid-induced wild-type mycelium was analyzed by Western blotting using anti-CAT-2 and anti-CAT-3 antibodies (dilution of 1:20,000). (E) Immunoprecipitation of CAT-1. Increasing amounts of CAT-1 antiserum or preimmune serum were added to crude extract (200 μkat catalase activity), incubated for 1 h, and subjected to centrifugation. The soluble fraction was analyzed for the presence of catalase isoenzymes by an in-gel activity assay.
There is some ambiguity in the literature regarding the nomenclature of the three catalase isozymes. Originally described by Chary and Natvig (6), the catalase running most slowly in the native polyacrylamide gel was designated as CAT-3, the slightly faster running catalase as CAT-1, and the one with the highest mobility as CAT-2, the latter of which clearly represents the catalase-peroxidase family member (NCU05770.2). More recently, a cat-3 knockout strain (NCU00355.2) was described by the Hansberg laboratory that surprisingly lacked the catalase band of intermediate mobility (34), which would be referred to as CAT-1 according to the Chary and Natvig nomenclature (6). To therefore find out at the molecular level which protein is represented by the most slowly migrating catalase, the dominant immunoprecipitated protein of approximately 85 kDa that remained bound to the antibody after stringent washing was digested with trypsin and analyzed by ESI-MS. Gene product NCU08791.2 (N. crassa genome release 7 at the Broad Institute) could be clearly assigned to this sample (data not shown). Since this reading frame is identical to catalase 1 (GenBank accession no. AY027545), it became obvious that the catalase that was formerly known as isozyme 3 (6) is now termed CAT-1 and vice versa. We therefore also chose the nomenclature that was in line with the GenBank entries for CAT-1 and CAT-3.
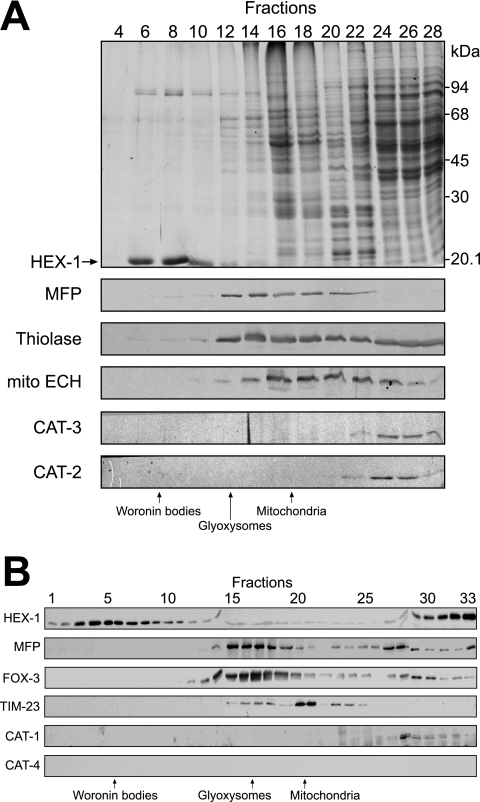
Application of anti-CAT-1 antibodies to thin sections of oleic acid-induced N. crassa cells revealed that subcellular compartments were not significantly labeled. CAT-1 was rather concentrated at the cell wall (Fig. 6), suggesting that a portion of CAT-1 is secreted. This observation is reminiscent of CAT-3, which is also secreted, yet only small percentage of CAT-3 is lost to the medium (34). The antibodies were also used to determine the distribution of the catalases within a sucrose density gradient. CAT-2 and CAT-3 did not enter the gradient and were localized to the soluble fractions (Fig. 7A). The same was true for CAT-1 (Fig. 7B). Taken together, none of the three measurable catalase activities was present within microbodies.
FIG. 6.
Immunocytochemical localization of CAT-1. Ultrathin sections of glutaraldehyde-fixed mycelia of N. crassa that had been grown on sucrose-containing medium were decorated with anti-CAT-1 antibodies and gold-conjugated goat anti-rabbit antiserum. G, glyoxysomes; Lb, lipid bodies; M, mitochondria; V, vacuole; Wb, Woronin bodies.
FIG. 7.
Subcellular localization of catalases by immunoblot analysis of sucrose density gradient fractions. Cell lysates of oleic acid-induced wild-type mycelia were layered on top of a 30 to 60% (wt/wt) sucrose gradient and subjected to isopycnic centrifugation. Fractions were collected from the bottom (fraction 1) to the top (fraction 28) of the gradient, and equal amounts of protein from the indicated fractions were analyzed by SDS-PAGE and immunoblotting. The following marker enzymes were analyzed: enoyl-CoA hydratase (mito ECH) for mitochondria; multifunctional β-oxidation enzyme (MFP) and 3-ketoacyl-CoA thiolase (thiolase) for glyoxysomes; and HEX-1 for Woronin bodies, identified as the dominant 21-kDa protein in the Coomassie-stained gel (arrow). Antibodies directed against CAT-2 and CAT-3 revealed that both catalases are localized to the cytosol (A). A similar gradient with 33 fractions was used to determine the localization of CAT-1 (B), except that HEX-1 was detected immunologically and TIM-23 served as a mitochondrial marker protein. The additional appearance of HEX-1 in the soluble fractions is due to the fraction of Woronin bodies that were ruptured during cell breakage. CAT-4 was not detected in any of the fractions with the cross-reacting anti-yeast Cta1p antibody.
Characterization of a novel N. crassa catalase.
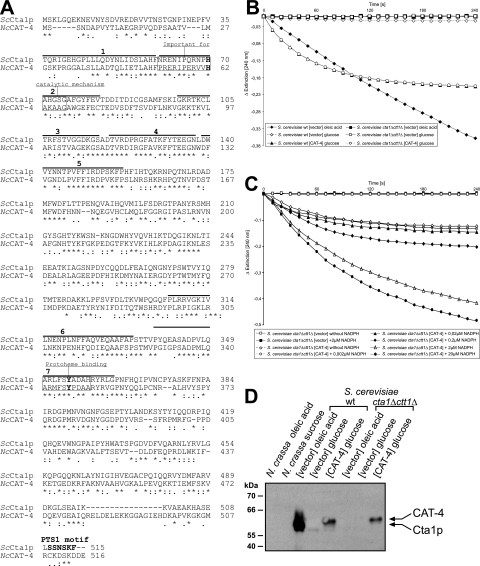
We and others (22) have searched the N. crassa genome sequence for the presence of catalase isozymes. Interestingly, there is indeed a fourth open reading frame with strong similarity to that for the catalases, NCU05169.2, hereafter referred to as cat-4 (Fig. 8A). Since under various growth conditions and developmental states only three distinct activities were detectable in native gels (Fig. 1), there was no evidence for a fourth catalase being produced in N. crassa. To determine experimentally whether this reading frame encodes a true catalase, we set out to amplify cat-4 from a cDNA library (FGSC, Kansas City, MO). However, this approach failed, probably due to a (very) low expression rate of this reading frame. We therefore amplified CAT-4 from genomic DNA by using a primer pair that allowed synthesis of the intron-less gene (see Materials and Methods for details). cat-4 was then cloned into an expression vector designed to heterologously express N. crassa CAT-4 in S. cerevisiae. Since the endogenous yeast catalases are completely repressed in the presence of 2% glucose (8, 40), catalase activity eventually measured under such conditions in the transformants must stem from the expressed cat-4 gene.
FIG. 8.
CAT-4 is a novel catalase from N. crassa. (A) Sequence alignment of NcCAT-4 (NCU05169.2) with S. cerevisiae Cta1p (ScCta1p). The amino acid sequences were aligned using CLUSTALW with its default parameters (http://www.ebi.ac.uk/clustalw/). Asterisks denote identical residues, double dots (:) and single dots denote positions with conserved and semiconserved substitutions, respectively (7). The consensus signatures for protoheme binding and the proximal active site (PROSITE; http://ca.expasy.org/prosite/) as well as the seven-element fingerprint of the catalase protein family (http://umber.sbs.man.ac.uk/dbbrowser/sprint/) are highlighted. The targeting signal of Cta1p according to Kragler et al. (28) is also denoted (PTS1). (B) Catalase enzyme activity assays. S. cerevisiae wild-type strain UTL7-A and the catalase-less mutant strain GA1-7D ctt1Δ cta1Δ were transformed with a plasmid designed to heterologously express CAT-4 from the constitutive PGK1 promoter (CAT-4) or as control with the empty vector (vector). Strains were grown in the presence of 2% glucose or 0.1% oleic acid as indicated in the legend and assayed for catalase activity. Endogenous yeast catalase activity was only measurable in oleic acid-induced cells (⧫), whereas CAT-4 was active in glucose-grown wild-type (▴) and cta1Δ ctt1Δ mutant (○) cells. (C) Protection of CAT-4 by NADPH. Catalase activity was determined as described for panel B, but increasing amounts of NADPH were added to the assay mixture. (D) Immunological detection and expression of CAT-4. The same yeast strains as well as N. crassa wild-type mycelia grown on sucrose or oleic acid were processed for SDS-PAGE and Western blot analysis. Anti-Cta1p antibodies were used at a dilution of 1:10,000 in combination with the ECL detection system.
A high catalase activity (461 ± 41 U/mg protein) was indeed measured in the wild-type S. cerevisiae strain expressing CAT-4, whereas the wild-type strain transformed with the empty vector control exhibited only very low catalase activity (8 ± 0.7 U/mg protein). Endogenous catalase activity (218 ± 11 U/mg protein) was measured in the latter strain upon induction by oleic acid (Fig. 8B). Expression of the cat-4 gene in a yeast strain devoid of Cta1p and Ctt1p, its endogenous two catalases, gave rise to similar catalase activities (578 ± 85 U/mg protein), thereby demonstrating that CAT-4 is a bona fide catalase (Fig. 8B). The observed rapid decrease in CAT-4 activity was prevented by the addition of NADPH (Fig. 8C), indicating that NADPH effectively protected CAT-4 against inactivation by its substrate, H2O2, as has been reported for other catalases (26). To see whether our highly specific antibody against S. cerevisiae peroxisomal catalase A (Cta1p) recognizes CAT-4, the same protein extracts were analyzed by immunoblotting. A band of the expected size for CAT-4 was clearly visible in strains harboring the cat-4 expression construct, but not in the empty vector control strains (Fig. 8D). At the same time, antibodies against the N. crassa catalases CAT-1, CAT-2, and CAT-3 failed to detect CAT-4 (not shown). Crude protein extracts from N. crassa wild-type mycelia grown on Vogel's sucrose medium or oleic acid-containing medium were also analyzed for the presence of CAT-4. The yeast Cta1p antibody did not recognize any protein in these samples (Fig. 8D) or in the gradient fractions (Fig. 7B), indicating that CAT-4 is not expressed or is only very weakly expressed in N. crassa under the tested conditions.
A phylogenetic analysis of catalases conducted by Johnson et al. (22) revealed that CAT-4 belongs to the family of small monofunctional catalases that are found in bacteria, animals, and fungi. Since this clade includes all known peroxisomal catalases, it was tempting to assume that CAT-4 is also a peroxisomal protein. Most peroxisomal catalases harbor a C-terminal peroxisomal targeting signal (PTS1) (15, 28, 38). However, the three C-terminal amino acid residues of CAT-4 are all acidic (DDE) and drop out of representing a PTS1 (SKL or conserved variants thereof). A PTS2 sequence (H/R-L-X5-H-L) within the N-terminal 50 amino acids is also missing. Notably, though, S. cerevisiae catalase Cta1p is imported into peroxisomes in the absence of its C-terminal targeting signal, since it additionally possesses an internal targeting signal (28). Thus, despite lacking an eye-catching PTS, it remained entirely possible that CAT-4 is targeted to microbodies.
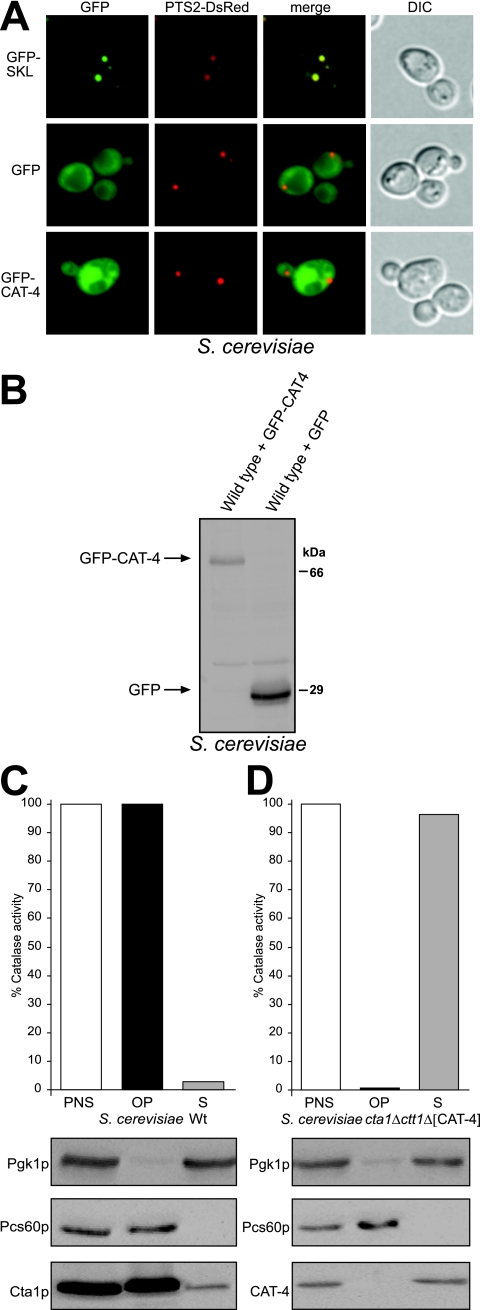
We therefore determined the subcellular localization of a GFP-CAT-4 fusion protein in an S. cerevisiae strain that expressed PTS2-DsRed, a synthetic peroxisomal marker protein (47). As expected, synthesis of GFP-SKL gave rise to a punctate staining pattern that is congruent with that of PTS2-DsRed, showing that the appended PTS1 directed GFP to peroxisomes. In contrast, diffuse fluorescence was observed when GFP-CAT-4 or GFP alone was expressed (Fig. 9A). The diffuse staining obtained with the latter protein could not have been caused by a degradation product of GFP-CAT-4, since Western blotting demonstrated the appropriate expression of the full-length fusion protein (Fig. 9B). Notably, though, the GFP-CAT-4 fusion protein was enzymatically inactive. To therefore exclude that the GFP tag compromised the localization of CAT-4, the untagged enzyme was expressed in the ctt1Δ cta1Δ mutant and its distribution between a 25,000 × g organellar pellet and the corresponding supernatant was determined. All catalase activity was recovered from the supernatant, whereas the peroxisomal matrix enzyme Pcs60p was detected in the pellet fraction (Fig. 9D). As control, yeast Cta1p was recovered from the organellar pellet fraction of a wild-type strain (Fig. 9C). Thus, CAT-4 did not possess any peroxisomal targeting information; rather it was located in the cytosol.
FIG. 9.
CAT-4 is localized to the cytosol when expressed in S. cerevisiae. (A) Fluorescence microscopy of GFP-CAT-4-expressing S. cerevisiae cells. Yeast strain yHPR251 expressing the synthetic peroxisomal marker protein PTS2-DsRed was transformed with plasmids designed to express GFP-CAT-4, GFP-SKL or GFP. Strains were grown on solid oleic acid-containing medium for 2 days and were subsequently examined for GFP and DsRed fluorescence. Colocalization with PTS2-DsRed indicates peroxisomal targeting of the GFP fusion proteins. Nomarski images demonstrate the structural integrity of the cells. (B) Expression of GFP-CAT-4. Correct expression of GFP and the full-length CAT-4 fusion protein in yeast was determined by Western blotting using anti-GFP antibodies. (C and D) Differential centrifugation. Postnuclear supernatants (PNS) of an oleic acid-induced wild-type strain (C) and the cta1Δ ctt1Δ mutant expressing CAT-4 (D) were separated into a 25,000 × g organellar pellet (OP) and a supernatant (S) fraction. Fractions were assayed (upper panels) for catalase activity and (lower panels) for the presence of cytosolic Pgk1p, peroxisomal Pcs60p, and Cta1p (C) or CAT-4 (D) by Western blot analysis. Activities measured in the PNS fractions were taken as 100%.
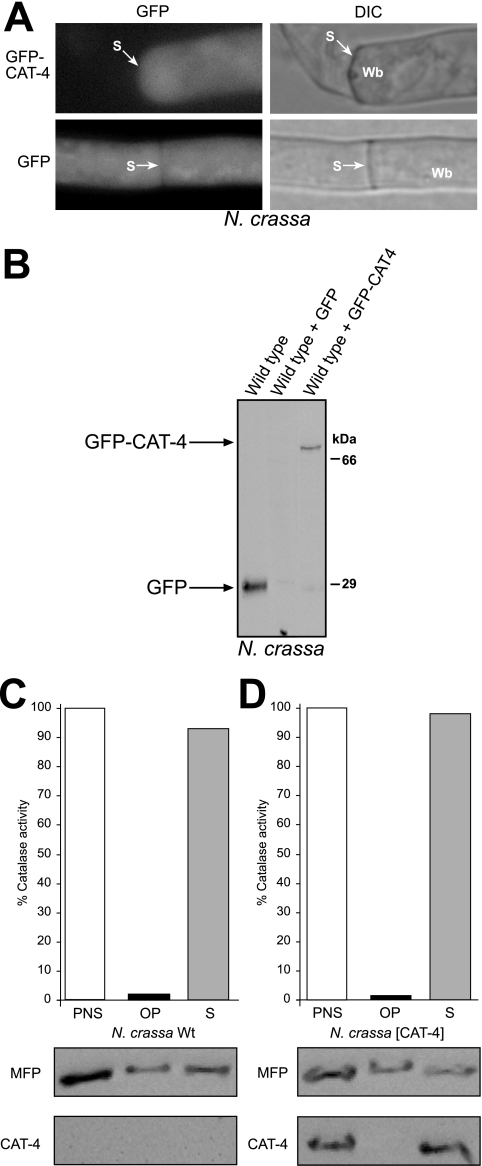
The presented localization data strongly support the notion that CAT-4 is a cytosolic enzyme; nonetheless, a remote possibility was that N. crassa maintains a distinct and unique peroxisomal import mechanism for CAT-4. A similar GFP-CAT-4 fusion protein was therefore also expressed in N. crassa (Fig. 10B). Not only GFP but also GFP-CAT-4 was uniformly distributed throughout hyphae, and Woronin bodies did not contain GFP-CAT-4 (Fig. 10A). Finally, a wild-type strain overexpressing untagged CAT-4 was generated. Its total catalase activity (70 U/mg protein), measured in a postnuclear supernatant, exceeded that of the wild-type strain (18 U/mg protein) by a factor of 4, demonstrating that expression of CAT-4 in N. crassa gave rise to an active enzyme. Subcellular fractionation of these extracts showed that CAT-4 was exclusively present in the supernatant, while a significant portion of the glyoxysomal marker protein MFP was recovered from the organellar pellet fraction (Fig. 10D). A similar distribution of MFP was obtained in the wild-type control strain (Fig. 10C). The combined results therefore demonstrated that CAT-4 is localized to the cytosol and not imported into microbodies.
FIG. 10.
CAT-4 is a cytosolic enzyme in N. crassa. (A) Localization of GFP-CAT-4 upon expression in N. crassa mycelia. Plasmids designed to express GFP-CAT-4 or GFP were integrated into the his3 locus of N. crassa strain N623. Prior to inspection, transformants were grown in minimal medium containing sucrose as the sole carbon source. The left segment of the hypha was damaged during preparation, and the cytoplasm leaked out. A Woronin body sealing the septal pore is discernible. Wb, Woronin body; S, septum. (B) Expression of GFP-CAT-4. Correct expression of GFP and the full-length CAT-4 fusion protein in N. crassa was determined by Western blotting using anti-GFP antibodies. (C and D) Differential centrifugation. Postnuclear supernatants (PNS) of an oleic acid-induced wild-type strain (C) and a wild-type strain ectopically expressing CAT-4 (D) were separated into a 25,000 × g organellar pellet (OP) and a supernatant (S) fraction. Fractions were assayed (upper panels) for catalase activity and (lower panels) for the presence of peroxisomal MFP and CAT-4 by Western blot analysis. Activities measured in the PNS fractions were taken as 100%.
Appearance of peroxisomal catalase in other filamentous fungi.
To analyze whether microbodies of other Euascomycetes also lack catalase activity, potential catalases from fungi with an available genome sequence were scrutinized for the presence of peroxisomal targeting signals. Close relatives of N. crassa from the Sordariomycete family including Fusarium graminearum and Podospora anserina do possess potential catalases of the peroxisomal family (i.e., small monofunctional catalases) with high similarity to CAT-4, and these proteins also lack obvious C-terminal PTS sequences (see Fig. S2 in the supplemental material). In contrast, one of the four catalases of the Eurotiomycete Aspergillus is a small-subunit catalase (23) with potential PTS1 sequences in all sequenced Aspergillus species (ARL in A. nidulans, A. oryzae, and A. terreus and SRL in A. fumigatus). We therefore tested whether Aspergillus indeed contains peroxisomal catalase. To that end, cell lysate of oleic acid-induced A. tamarii mycelia was separated by sucrose density gradient centrifugation and the resulting fractions were assayed for catalase activity by a spectrophotometric assay. When compared with the distribution of marker enzymes, it became evident that catalase cosedimented with the multifunctional protein (Fig. 11, upper panel). An in-gel activity assay showed that one isoform of catalase was indeed present in the peak microbody fractions (Fig. 11, lower panel). Furthermore, the genome sequences of several other Eurotiomycetes, such as Histoplasma capsulatum, Coccidioides immitis, Sclerotinia sclerotiorum, and Botrytis cinerea also revealed the presence of putative peroxisomal catalases with a PTS1 (see Fig. S2 in the supplemental material). Thus, the absence of catalase-containing microbodies is probably restricted to a few filamentous fungi of the Sordariomycetes.
FIG. 11.
Subcellular localization of catalases from Aspergillus tamariiby an in-gel activity assay. Cell lysate of oleic acid-induced wild-type A. tamarii mycelia was separated on a 30 to 60% (wt/wt) sucrose density gradient. Fractions were collected from the bottom (fraction 1) to the top (fraction 30) of the gradient and (upper panel) examined for the distribution of the following enzyme activities: fumarase (○), multifunctional β-oxidation enzyme (▴), and catalase (⧫). Densities (dotted line) of each fraction are also indicated. The lower panel shows an in-gel catalase activity assay in which equal amounts of protein (10 μg) from the indicated fractions had been separated by native PAGE. Catalase is clearly discernible in the peroxisomal fractions.
DISCUSSION
The filamentous Euascomycete N. crassa is thought to possess three types of microbodies within a single cell: glyoxysomes, Woronin bodies, and a distinct catalase-containing compartment. Here we have shown that N. crassa lacks particulate catalase. As a consequence, the existence of a peroxisomal compartment in N. crassa can be disregarded. The observation made was rather surprising in light of the fact that catalase was actually the first established marker enzyme for microbodies (10). Indeed, all catalase-containing eukaryotes examined so far also contained a peroxisomal isoform of this enzyme.
The catalase activities that were detected in oleic acid-induced N. crassa mycelia were identical to the three described catalase isoforms, CAT-1, CAT-2, and CAT-3 (33, 37). To address their subcellular localization, all three catalases were purified and used for the preparation of monospecific antibodies. These immunoglobulins did not detect any of the three catalases in the organellar fractions of sucrose density gradients; instead, they were localized to the cytosol. Electron microscopy revealed that CAT-1 additionally associated with the cell wall, as was also reported for CAT-3 (34). Thus, it appears advantageous for filamentous fungi to furnish the extracellular medium with catalase so as to detoxify peroxide already outside the mycelia.
Interestingly, also N. crassa contains one gene (cat-4) that does encode a peroxisomal type of catalase. The deduced amino acid sequence of cat-4 features consensus signatures for both protoheme binding as well as the catalase active site. We showed that CAT-4 indeed represents a bona fide catalase, as it was able to decompose hydrogen peroxide in a heterologous environment. Notably, the enzyme quickly lost its activity upon addition of its substrate, hydrogen peroxide, an effect that was not observed for yeast catalase. Several catalases are susceptible to inactivation by their own substrate, H2O2, but this can be largely prevented by bound NADPH (26). Addition of NADPH indeed stimulated the activity of CAT-4, probably by preventing it from oxidative damage. The CAT-4 sequence did not reveal any obvious peroxisomal targeting signals, suggesting that this catalase is not targeted to peroxisomes. However, a few peroxisomal matrix proteins such as yeast acyl-CoA oxidase (27, 44) lack either of the two prevalent targeting signals, PTS1 and PTS2. Also N. crassa glyoxysomes contain at least one such protein, the multifunctional enzyme (MFP) encoded by the fox-2 gene (12). It is worth noting that the orthologous catalase from S. cerevisiae can use an ill-defined internal targeting signal in lieu of its C-terminal PTS1 (28). These alternative avenues of import notwithstanding, ectopic expression of CAT-4 in N. crassa did not result in targeting to any intracellular membrane-bound structure. Furthermore, CAT-4 was also not localized to peroxisomes upon heterologous expression in S. cerevisiae.
Although it proved possible to demonstrate catalase activity for CAT-4 in both N. crassa and S. cerevisiae, we did not obtain evidence for endogenous CAT-4 being expressed in N. crassa: anti-yeast Cta1p antibodies, which did recognize CAT-4 when expressed heterologously in yeast or ectopically in N. crassa, failed to yield a signal when applied to protein extracts from wild-type mycelia grown in sucrose or oleic acid-containing medium. Furthermore, under various peroxisome-inducing conditions only CAT-1, CAT-2, and CAT-3 were discernible in native gels stained for catalase activity. Obviously, our studies still leave room for environmental conditions under which CAT-4 will be expressed. However, important for our core statement is the fact that ectopic expression of CAT-4 was feasible and this led to a cytosolic localization of the protein.
The previously reported microbody-like organelle of high density with apparent catalase activity (25, 53) is likely to coincide with the Woronin body, since spectrophotometric assays at 240 nm conducted by us and others (49) indeed measured a weak activity associated with Woronin bodies. However, several findings strongly argue against the presence of catalase in this organelle: DAB treatment of N. crassa hyphae did not reveal conclusive evidence for the presence of a catalase in Woronin bodies (or any other organelle), albeit the low sensitivity of this method would not detect very small amounts of catalase. Also native gel assays could not confirm this activity. Specific immunologic detection of the three catalase isoforms CAT-1, CAT-2, and CAT-3 showed that these proteins are not associated with any intracellular compartment. Consistent with this result, the coding sequences of CAT-1, -2, and -3 all lack PTS1 or PTS2 sequences. Finally, a GFP fusion of the novel CAT-4 was also shown to be localized to the cytosol. One plausible explanation for the spectrophotometric hydrogen peroxide breakdown could be the presence of an unidentified peroxidase associated with the Woronin body fraction. Little is known about the protein composition of this organelle. Its dominant protein, HEX-1, possessed neither catalase nor peroxidase activity when enriched by cation-exchange/gel filtration chromatography (data not shown).
Why is catalase dispensable for Neurospora microbodies? This is likely to be a direct consequence of the organellar enzyme content of this organism. The glyoxysomal β-oxidation system of N. crassa is unusual in that the first step is catalyzed by an acyl-CoA dehydrogenase instead of an acyl-CoA oxidase (25). As a consequence, hydrogen peroxide is not formed within glyoxysomes during that process. Furthermore, the H2O2-generating urate oxidase activity was localized to the cytosol. In all mammals that do express urate oxidase, this enzyme involved in purine metabolism is localized to peroxisomes (18). Even urate oxidases from Aspergillus are peroxisomal and bear PTS1 sequences, while the probable Neurospora ortholog, NCU07853.2, does not (for alignments of fungal urate oxidases, see Fig. S3 in the supplemental material). It will be interesting to see whether H2O2-producing oxidases are at all present in glyoxysomes of N. crassa. One candidate is d-amino acid oxidase, since its putative reading frame, NCU06558.2, harbors the canonical PTS1 tripeptide SKL at its C terminus. In any case, it is conceivable that due to the absence of the main sources of H2O2 production, particularly fatty acid β-oxidation, moderate amounts of H2O2 eventually produced inside glyoxysomes are tolerated even without a catalase.
In line with this perception was the observation that Aspergillus strains do contain peroxisomal catalase, since this genus exploits the typical acyl-CoA oxidase for the first step in peroxisomal β-oxidation (32, 51). This difference in catalase localization is remarkable in so far as Neurospora and Aspergillus are both members of the subphylum Pezizomycotina. A closer look at the phylogenetic tree reveals that Neurospora belongs to the class of Sordariomycetes, whereas the Aspergillus genus belongs to the Eurotiomycetes. A comparison of the putative orthologs of CAT-4 from Sordariomycetes with a sequenced genome revealed that none of these catalases possess a PTS1 signal, whereas those of the Eurotiomycetes class did. We therefore speculate that the Sordariomycetes lineage lost its peroxisomal catalase in the course of evolution because it became redundant as a consequence of reduced hydrogen peroxide production within microbodies. In light of the exceptional role of this organelle, it should be noted that the microbody-related glycosomes of trypanosomes harboring most of the glycolysis enzymes are also free of catalase. However, these parasites do not possess any catalase and use trypanothione-dependent peroxidases as an alternative system for peroxide removal (2, 45). Also the insect cell lines Sf9 and Sf21, derived from Spodoptera frugiperda pupal ovarian tissue, were reported to lack catalase (1, 29). Nonetheless, since insects do possess peroxisomal catalase at particular stages of development (31), the observations made merely reflect conditions under which peroxisomal catalase was not expressed. In the case of N. crassa, however, the catalases are intrinsically absent from microbodies and, as a consequence, peroxisomes are truly missing in this and possibly a few other closely related organisms.
Supplementary Material
Acknowledgments
We want to thank C. Schüller for the catalase-less yeast strain, M. Freitag for plasmid MF272, H. F. Tabak for the Cta1p antibody, and D. Mokranjac for the TIM-23 antibody. Special thanks go to B. Warscheid for the ESI-MS analysis.
This work was supported by Deutsche Forschungsgemeinschaft grant SFB480 (to H.R. and W.-H.K.).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Alvares, K., R. J. Widrow, G. M. Abu-Jawdeh, J. V. Schmidt, A. V. Yeldandi, M. S. Rao, and J. K. Reddy. 1992. Rat urate oxidase produced by recombinant baculovirus expression: formation of peroxisome crystalloid core-like structures. Proc. Natl. Acad. Sci. USA 89:4908-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, et al. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309:416-422. [DOI] [PubMed] [Google Scholar]
- 3.Blobel, F., and R. Erdmann. 1996. Identification of a yeast peroxisomal member of the family of AMP-binding proteins. Eur. J. Biochem. 240:468-476. [DOI] [PubMed] [Google Scholar]
- 4.Borst, P. 1986. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim. Biophys. Acta 866:179-203. [DOI] [PubMed] [Google Scholar]
- 5.Brunelli, J. P., and M. L. Pall. 1993. A series of yeast shuttle vectors for expression of cDNAs and other DNA sequences. Yeast 9:1299-1308. [DOI] [PubMed] [Google Scholar]
- 6.Chary, P., and D. O. Natvig. 1989. Evidence for three differentially regulated catalase genes in Neurospora crassa: effects of oxidative stress, heat shock, and development. J. Bacteriol. 171:2646-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross, H. S., and H. Ruis. 1978. Regulation of catalase synthesis in Saccharomyces cerevisiae by carbon catabolite repression. Mol. Gen. Genet. 166:37-43. [DOI] [PubMed] [Google Scholar]
- 9.Davies, R. H. 2000. Neurospora: contributions of a model organism, p. 283-302. Oxford University Press, Oxford, United Kingdom.
- 10.de Duve, C., and P. Baudhuin. 1966. Peroxisomes (microbodies and related particles). Physiol. Rev. 46:323-357. [DOI] [PubMed] [Google Scholar]
- 11.Erdmann, R., M. Veenhuis, D. Mertens, and W. H. Kunau. 1989. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86:5419-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fossa, A., A. Beyer, E. Pfitzner, B. Wenzel, and W. H. Kunau. 1995. Molecular cloning, sequencing and sequence analysis of the fox-2 gene of Neurospora crassa encoding the multifunctional beta-oxidation protein. Mol. Gen. Genet. 247:95-104. [DOI] [PubMed] [Google Scholar]
- 13.Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker, and N. D. Read. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:897-910. [DOI] [PubMed] [Google Scholar]
- 14.Galagan, J. E., S. E. Calvo, K. A. Borkovich, E. U. Selker, N. D. Read, D. Jaffe, W. FitzHugh, L. J. Ma, S. Smirnov, S. Purcell, B. Rehman, T. Elkins, R. Engels, S. Wang, C. B. Nielsen, J. Butler, M. Endrizzi, D. Qui, P. Ianakiev, D. Bell-Pedersen, M. A. Nelson, M. Werner-Washburne, C. P. Selitrennikoff, J. A. Kinsey, E. L. Braun, A. Zelter, U. Schulte, G. O. Kothe, G. Jedd, W. Mewes, C. Staben, E. Marcotte, D. Greenberg, A. Roy, K. Foley, J. Naylor, N. Stange-Thomann, R. Barrett, S. Gnerre, M. Kamal, M. Kamvysselis, E. Mauceli, C. Bielke, S. Rudd, D. Frishman, S. Krystofova, C. Rasmussen, R. L. Metzenberg, D. D. Perkins, S. Kroken, C. Cogoni, G. Macino, D. Catcheside, W. Li, R. J. Pratt, S. A. Osmani, C. P. DeSouza, L. Glass, M. J. Orbach, J. A. Berglund, R. Voelker, O. Yarden, M. Plamann, S. Seiler, J. Dunlap, A. Radford, R. Aramayo, D. O. Natvig, L. A. Alex, G. Mannhaupt, D. J. Ebbole, M. Freitag, I. Paulsen, M. S. Sachs, E. S. Lander, C. Nusbaum, and B. Birren. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859-868. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez, E. 1991. The C-terminal domain of plant catalases. Implications for a glyoxysomal targeting sequence. Eur. J. Biochem. 199:211-215. [DOI] [PubMed] [Google Scholar]
- 16.Gould, S. J., G. A. Keller, N. Hosken, J. Wilkinson, and S. Subramani. 1989. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108:1657-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurvitz, A., H. Rottensteiner, S. H. Kilpelainen, A. Hartig, J. K. Hiltunen, M. Binder, I. W. Dawes, and B. Hamilton. 1997. The Saccharomyces cerevisiae peroxisomal 2,4-dienoyl-CoA reductase is encoded by the oleate-inducible gene SPS19. J. Biol. Chem. 272:22140-22147. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, S., S. Fujiwara, and T. Noguchi. 2000. Evolution of urate-degrading enzymes in animal peroxisomes. Cell Biochem. Biophys. 32:123-129. [DOI] [PubMed] [Google Scholar]
- 19.Heiland, I., and R. Erdmann. 2005. Biogenesis of peroxisomes. Topogenesis of the peroxisomal membrane and matrix proteins. FEBS J. 272:2362-2372. [DOI] [PubMed] [Google Scholar]
- 20.Jacob, G. S., and W. H. Orme-Johnson. 1979. Catalase of Neurospora crassa. 1. Induction, purification, and physical properties. Biochemistry 18:2967-2975. [DOI] [PubMed] [Google Scholar]
- 21.Jedd, G., and N. H. Chua. 2000. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat. Cell Biol. 2:226-231. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, C. H., M. G. Klotz, J. L. York, V. Kruft, and J. E. McEwen. 2002. Redundancy, phylogeny and differential expression of Histoplasma capsulatum catalases. Microbiology 148:1129-1142. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki, L., and J. Aguirre. 2001. Multiple catalase genes are differentially regulated in Aspergillus nidulans. J. Bacteriol. 183:1434-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller, G. A., S. Krisans, S. J. Gould, J. M. Sommer, C. C. Wang, W. Schliebs, W. Kunau, S. Brody, and S. Subramani. 1991. Evolutionary conservation of a microbody targeting signal that targets proteins to peroxisomes, glyoxysomes, and glycosomes. J. Cell Biol. 114:893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kionka, C., and W. H. Kunau. 1985. Inducible β-oxidation pathway in Neurospora crassa. J. Bacteriol. 161:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkman, H. N., M. Rolfo, A. M. Ferraris, and G. F. Gaetani. 1999. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J. Biol. Chem. 274:13908-13914. [DOI] [PubMed] [Google Scholar]
- 27.Klein, A. T., M. van den Berg, G. Bottger, H. F. Tabak, and B. Distel. 2002. Saccharomyces cerevisiae acyl-CoA oxidase follows a novel, non-PTS1, import pathway into peroxisomes that is dependent on Pex5p. J. Biol. Chem. 277:25011-25019. [DOI] [PubMed] [Google Scholar]
- 28.Kragler, F., A. Langeder, J. Raupachova, M. Binder, and A. Hartig. 1993. Two independent peroxisomal targeting signals in catalase A of Saccharomyces cerevisiae. J. Cell Biol. 120:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurisu, M., M. Morita, Y. Kashiwayama, S. Yokota, H. Hayashi, Y. Sakai, S. Ohkuma, M. Nishimura, and T. Imanaka. 2003. Existence of catalase-less peroxisomes in Sf21 insect cells. Biochem. Biophys. Res. Commun. 306:169-176. [DOI] [PubMed] [Google Scholar]
- 30.Lametschwandtner, G., C. Brocard, M. Fransen, P. Van Veldhoven, J. Berger, and A. Hartig. 1998. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J. Biol. Chem. 273:33635-33643. [DOI] [PubMed] [Google Scholar]
- 31.Locke, M., and J. T. McMahon. 1971. The origin and fate of microbodies in the fat body of an insect. J. Cell Biol. 48:61-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maggio-Hall, L. A., and N. P. Keller. 2004. Mitochondrial β-oxidation in Aspergillus nidulans. Mol. Microbiol. 54:1173-1185. [DOI] [PubMed] [Google Scholar]
- 33.Michán, S., F. Lledias, J. D. Baldwin, D. O. Natvig, and W. Hansberg. 2002. Regulation and oxidation of two large monofunctional catalases. Free Radic. Biol. Med. 33:521-532. [DOI] [PubMed] [Google Scholar]
- 34.Michan, S., F. Lledias, and W. Hansberg. 2003. Asexual development is increased in Neurospora crassa cat-3-null mutant strains. Eukaryot. Cell 2:798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokranjac, D., S. A. Paschen, C. Kozany, H. Prokisch, S. C. Hoppins, F. E. Nargang, W. Neupert, and K. Hell. 2003. Tim50, a novel component of the TIM23 preprotein translocase of mitochondria. EMBO J. 22:816-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahm, B. H., and G. A. Marzluf. 1987. Induction and de novo synthesis of uricase, a nitrogen-regulated enzyme in Neurospora crassa. J. Bacteriol. 169:1943-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peraza, L., and W. Hansberg. 2002. Neurospora crassa catalases, singlet oxygen and cell differentiation. Biol. Chem. 383:569-575. [DOI] [PubMed] [Google Scholar]
- 38.Purdue, P. E., and P. B. Lazarow. 1996. Targeting of human catalase to peroxisomes is dependent upon a novel COOH-terminal peroxisomal targeting sequence. J. Cell Biol. 134:849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roels, F., E. Wisse, B. De Prest, and J. van der Meulen. 1975. Cytochemical discrimination between catalases and peroxidases using diaminobenzidine. Histochemistry 41:281-312. [DOI] [PubMed] [Google Scholar]
- 40.Rottensteiner, H., A. J. Kal, M. Filipits, M. Binder, B. Hamilton, H. F. Tabak, and H. Ruis. 1996. Pip2p: a transcriptional regulator of peroxisome proliferation in the yeast Saccharomyces cerevisiae. EMBO J. 15:2924-2934. [PMC free article] [PubMed] [Google Scholar]
- 41.Rottensteiner, H., A. Kramer, S. Lorenzen, K. Stein, C. Landgraf, R. Volkmer-Engert, and R. Erdmann. 2004. Peroxisomal membrane proteins contain common Pex19p-binding sites that are an integral part of their targeting signals. Mol. Biol. Cell 15:3406-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottensteiner, H., K. Stein, E. Sonnenhol, and R. Erdmann. 2003. Conserved function of Pex11p and the novel Pex25p and Pex27p in peroxisome biogenesis. Mol. Biol. Cell 14:4316-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Schäfer, A., D. Kerssen, M. Veenhuis, W.-H. Kunau, and W. Schliebs. 2004. Functional similarity between the peroxisomal PTS2 receptor binding protein Pex18p and the N-terminal half of the PTS1 receptor Pex5p. Mol. Cell. Biol. 24:8895-8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlecker, T., A. Schmidt, N. Dirdjaja, F. Voncken, C. Clayton, and R. L. Krauth-Siegel. 2005. Substrate specificity, localization, and essential role of the glutathione peroxidase-type tryparedoxin peroxidases in Trypanosoma brucei. J. Biol. Chem. 280:14385-14394. [DOI] [PubMed] [Google Scholar]
- 46.Sikora, L., and G. A. Marzluf. 1982. Regulation of L-amino acid oxidase and of D-amino acid oxidase in Neurospora crassa. Mol. Gen. Genet. 186:33-39. [DOI] [PubMed] [Google Scholar]
- 47.Stein, K., A. Schell-Steven, R. Erdmann, and H. Rottensteiner. 2002. Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol. Cell. Biol. 22:6056-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramani, S., A. Koller, and W. B. Snyder. 2000. Import of peroxisomal matrix and membrane proteins. Annu. Rev. Biochem. 69:399-418. [DOI] [PubMed] [Google Scholar]
- 49.Tenney, K., I. Hunt, J. Sweigard, J. I. Pounder, C. McClain, E. J. Bowman, and B. J. Bowman. 2000. Hex-1, a gene unique to filamentous fungi, encodes the major protein of the Woronin body and functions as a plug for septal pores. Fungal Genet. Biol. 31:205-217. [DOI] [PubMed] [Google Scholar]
- 50.Thieringer, R., and W. H. Kunau. 1991. The β-oxidation system in catalase-free microbodies of the filamentous fungus Neurospora crassa. Purification of a multifunctional protein possessing 2-enoyl-CoA hydratase, L-3-hydroxyacyl-CoA dehydrogenase, and 3-hydroxyacyl-CoA epimerase activities. J. Biol. Chem. 266:13110-13117. [PubMed] [Google Scholar]
- 51.Valenciano, S., J. R. Lucas, A. Pedregosa, I. F. Monistrol, and F. Laborda. 1996. Induction of β-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch. Microbiol. 166:336-341. [DOI] [PubMed] [Google Scholar]
- 52.Veenhuis, M., J. P. van Dijken, and W. Harder. 1976. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch. Microbiol. 111:123-135. [DOI] [PubMed] [Google Scholar]
- 53.Wanner, G., and R. R. Theimer. 1982. Two types of microbodies in Neurospora crassa. Ann. N. Y. Acad. Sci. 386:269-284. [DOI] [PubMed] [Google Scholar]
- 54.Waterham, H. R., V. I. Titorenko, P. Haima, J. M. Cregg, W. Harder, and M. Veenhuis. 1994. The Hansenula polymorpha PER1 gene is essential for peroxisome biogenesis and encodes a peroxisomal matrix protein with both carboxy- and amino-terminal targeting signals. J. Cell Biol. 127:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woodbury, W., A. K. Spencer, and M. A. Stahman. 1971. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 44:301-305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.