Abstract
Differentially expressed and immunogenic spore proteins of the Bacillus cereus group of bacteria, which includes Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis, were identified. Comparative proteomic profiling of their spore proteins distinguished the three species from each other as well as the virulent from the avirulent strains. A total of 458 proteins encoded by 232 open reading frames were identified by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry analysis for all the species. A number of highly expressed proteins, including elongation factor Tu (EF-Tu), elongation factor G, 60-kDa chaperonin, enolase, pyruvate dehydrogenase complex, and others exist as charge variants on two-dimensional gels. These charge variants have similar masses but different isoelectric points. The majority of identified proteins have cellular roles associated with energy production, carbohydrate transport and metabolism, amino acid transport and metabolism, posttranslational modifications, and translation. Novel vaccine candidate proteins were identified using B. anthracis polyclonal antisera from humans postinfected with cutaneous anthrax. Fifteen immunoreactive proteins were identified in B. anthracis spores, whereas 7, 14, and 7 immunoreactive proteins were identified for B. cereus and in the virulent and avirulent strains of B. thuringiensis spores, respectively. Some of the immunodominant antigens include charge variants of EF-Tu, glyceraldehyde-3-phosphate dehydrogenase, dihydrolipoamide acetyltransferase, Δ-1-pyrroline-5-carboxylate dehydrogenase, and a dihydrolipoamide dehydrogenase. Alanine racemase and neutral protease were uniquely immunogenic to B. anthracis. Comparative analysis of the spore immunome will be of significance for further nucleic acid- and immuno-based detection systems as well as next-generation vaccine development.
The Bacillus cereus group of bacteria consists of genetically very closely related members that include B. anthracis, B. cereus and B. thuringiensis (9, 13, 21). This genetic relatedness has led to the proposal that they should be considered a single species (13). Although genetically similar, each organism occupies a different ecological niche. B. anthracis is the causative agent of anthrax whose spores have been used in recent bioterrorist events in the United States (30). B. anthracis can also cause fatal infection in domestic livestock and wreck havoc to the economy when used as a weapon for agroterrorism. On the other hand, B. cereus is a ubiquitous, spore-forming soil bacterium and an opportunistic human pathogen which has been implicated in food poisoning (31), whereas B. thuringiensis is a specific pathogen of insects that has been used as a pesticide, although a pathogenic strain has been identified (14). All three species exhibit different phenotypes and regulatory mechanisms due to various plasmid contents and pleiotropic transcriptional regulators, such as plcR and atxA (3, 29). All produce resilient spores that are covered by an outermost integument called exosporium composed of a paracrystalline basal layer and hairlike outer region (6, 12).
Vaccines have proved to be a powerful medical intervention, and recent advances in immunoproteomics have allowed the rational design of molecularly defined vaccines. Immunoproteomic approaches, such as serological proteome analysis, have provided first vaccine candidate antigens and have led to the identification of the full set of antigens or the immunome targeted by the immune system (8, 19). The comparison of multiple proteins as well as immunomes in the B. cereus group may allow the discovery of immunogenic structural features that are shared and conserved among these pathogens. Such features can aid in the design of a broadly protective vaccine.
The currently used anthrax vaccines in the United States and United Kingdom are based on culture filtrates or secreted proteins of avirulent B. anthracis strains lacking the pXO2 plasmid (22, 37). These pXO1-containing strains produce large amounts of protective antigen (PA), which is the major component of each vaccine. However, the Russian vaccine utilizes a live nonencapsulated form of B. anthracis spores that has been reported to have a greater efficacy than either the U.S. or United Kingdom vaccines (32). Several studies have shown that this higher efficacy is due to unidentified antigens from anthrax spores that can augment the protective efficacy of PA-based vaccines (4, 5, 38). Thus, combining PA with one of the immunodominant spore antigen(s) in a formulation may ultimately lead to a highly efficacious and safer vaccine.
For the development of a next-generation anthrax vaccine, an immunoproteomic analysis of the spore antigens was performed, resulting in the identification of several immunogenic proteins from B. anthracis and other members of the B. cereus group. Some of these immunogenic spore proteins can be tested as novel vaccine candidates themselves or used for enhancing the protective efficacy of a PA-based vaccine. Comparative proteomic profiling of the spores also distinguished the different species and the pathogenic from the nonpathogenic strains of the B. cereus group.
MATERIALS AND METHODS
Preparation and isolation of Bacillus spp. spore proteins.
Spores of B. anthracis strains RA3R, A3, and 1099, B. cereus ATCC strain 14579, and B. thuringiensis strains 97-27 and ATCC 10972 were obtained by growing the Bacillus sp. cells on nutrient broth yeast extract agar (ATCC medium 763) at 30°C for 10 days. B. cereus strain 14579 and B. thuringiensis strain 97-27 were selected because their genomes have been annotated and are available from NCBI. The spores were isolated according to the method of Sylvestre et al. with modifications (34). The spores were collected by first wetting the surface of the plate with distilled water and scraping off the spores. The spores were then washed with distilled water five times, and the spore suspension was heated at 65°C for 30 min to kill the vegetative cells and then washed once more. The spores were purified by differential centrifugation for 30 min at 6,000 × g at 4°C through 50% Renografin (diatrizoate meglumine and diatrizoate sodium; Renocal-76; Bracco Diagnostic) in distilled water. The purified spores were washed three times with distilled water to remove residual Renografin. The use of a French press was omitted, and the protein extraction buffer was modified to contain 2 M thiourea instead of 8 M urea in 50 mM Tris-HCl, pH 10.00, and 2% (vol/vol) 2-mercaptoethanol. We did not use detergents and salts in the final wash to avoid losing spore surface-associated proteins, which may be important spore components, as explained previously by Liu et al. (24). An equivalent of 109 free spores was resuspended in 50 μl of extraction buffer, heated for 15 min at 90°C, and spun at 13,000 × g for 10 min. The supernatant containing the spore proteins was treated with ice-cold 10% (vol/vol) trichloroacetic acid for 30 min and spun at 13,000 × g for 25 min. The spore proteins were washed once with ice-cold acetone and dissolved with C7 resuspension buffer (Proteome Systems). The total protein concentration was determined using the Bio-Rad protein stain with bovine serum albumin as a standard.
2-DE and Western blot analysis.
Two-dimensional gel electrophoresis (2-DE) was carried out with the ElectrophoretIQ3 system (Proteome Systems). All supplies and reagents for 2-DE except for immobilized pH gradient (IPG) strips were purchased from Proteome Systems and used according to the manufacturer's instructions. Two hundred microliters (30 μg) of spore proteins was separated by isoelectric focusing (IEF) on 11-cm (pH 4 to 7) linear IPG strips. After 12 h of rehydration, the following focusing parameters were applied: 50 μA per strip, linear voltage increase over 8 h from 100 V to 10,000 V, and finally, 10,000 V for 10 h. After IEF, IPG strips were equilibrated in equilibration buffer and applied onto a 6 to 15% gradient sodium dodecyl sulfate-polyacrylamide gel. Gels were electrophoresed for 1.5 h at 500 V and stained with Sypro Ruby (Sigma-Aldrich, St. Louis, MO) for gel analysis or with ProteomIQ Blue for matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis. Three replicate two dimensional (2-D) gels for each sample were used for computer analysis using Phoretix 2D Expression software (Nonlinear Dynamics) and MALDI-TOF MS. Following automatic spot detection on triplicate gels using Phoretix 2D Expression's detection algorithm, gels were manually warped and their common spots were matched to generate averaged gels.
Immunoblotting was conducted according to the method of Towbin et al. (35). Proteins on 2-D gels were transferred to polyvinylidene difluoride (PVDF) membranes using Towbin buffer (0.025 M Trizma base in 0.192 M glycine) with 20% methanol at 100 V for 30 min. After transfer, the PVDF membrane was washed twice for 5 min each in 0.01% Tween 20 in phosphate-buffered saline (PBST) and blocked with 0.2% I-Block (Tropix) for 1 h. To identify immunogenic proteins, the PVDF membranes were washed twice with PBST for 5 min and then probed for one h with either a 1:1,000 dilution of human sera, a 1:500 dilution of goat sera, or a 1:2,000 dilution of rabbit sera. The PVDF membrane was washed twice with PBST and incubated with a 1:5,000 dilution of appropriate secondary antibody. Chemiluminescent signals were visualized using the SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce Biotechnology). Corresponding antisera from uninfected humans and animals were used as controls. Three replicate blots for each treatment were used for computer analysis using the Phoretix 2D Expression software. All immunogenic proteins that appeared in both control and treated blots were excluded from the list of immunogenic candidate proteins. Precision protein standards plugs (Bio-Rad) were included during electrophoresis as molecular weight standards. Streptactin-horseradish peroxidase conjugate (Bio-Rad) was used as a substrate for visualizing protein markers on 2-D Western blots.
In-gel trypsin digestion and MALDI-TOF MS.
Protein spots were picked, washed, and trypsin digested from 2-DE gels according to the manufacturer's instructions using the Xcise robotic workstation (Proteome Systems). Briefly, gel plugs were washed with 50 mM ammonium bicarbonate and 50% acetonitrile, dried, and treated with 1.6 μg/ml of trypsin in 50 mM ammonium bicarbonate at 37°C overnight. Tryptic peptides were applied to a MALDI-TOF MS plate in a solution of 10 mg/ml α-cyano-4-hydroxycinnamic acid in 0.1% trifluoroacetic acid and 50% acetonitrile. MS spectra (100 profiles per spectrum) were obtained using an Axima-CFR Plus MS (Shimadzu Biotech) in a positive ion reflectron mode with a source voltage of 25,000 V and laser intensity of 55%. Peptide mass fingerprints were analyzed and searched against the theoretical spectra of B. anthracis strain Ames, B. cereus ATCC 14579, or B. thuringiensis serovar Konkukian strain 97-27 using the Mascot Daemon software package (Matrix Science). The search parameters were as follows: maximum of one missed cleavage by trypsin, fixed modification of oxidized methionine, charged state of +1, and mass tolerance of ±0.25 Da. With use of these parameters and searching only the respective Bacillus sp. database, the probability-based Mowse scores greater than 48 are significant (P < 0.05). All MALDI-TOF MS identifications were run at least in duplicate.
RESULTS
Proteomic profiles of B. cereus group spores.
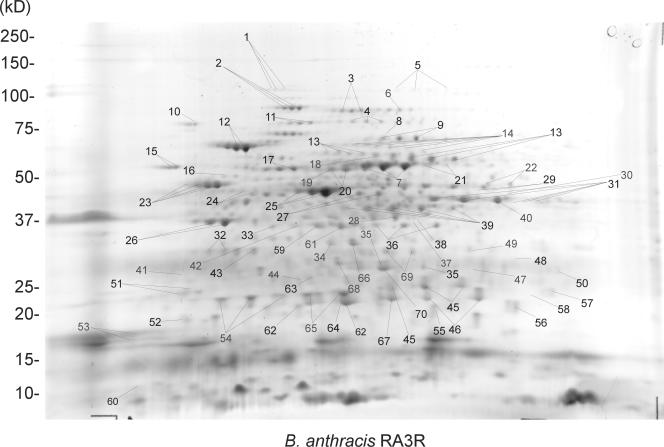
The 2-DE comparative analysis of the spore proteomes from four representative members of the B. cereus group including the avirulent B. anthracis strain RA3R, the B. cereus avirulent strain 14579, the B. thuringiensis virulent strain 97-27, and the B. thuringiensis avirulent strain 10972 is presented in Fig. 1 (see also Fig. S1, S2, and S3 in the supplemental material). A total of 1,217 protein spots were detected for all species after computer analysis of SyproRuby-stained average gels, and they were categorized into 245 for B. anthracis, 297 for B. cereus, 390 for B. thuringiensis strain 97-27, and 285 for B. thuringiensis strain 10972. Peptide mass fingerprinting resulted in the identification of 458 protein spots (46%) encoded by 232 open reading frames (ORFs) (Fig. 1; Table 1) (see Fig. S1, S2, and S3 and Tables S1, S2, and S3 in the supplemental material). Other spots were not picked because they were either very faint with low molecular weight or not very well separated at pHs 4 to 7. Computer analysis revealed that the spores of each Bacillus species can be distinguished from each other by the overall 2-DE protein patterns (Fig. 1) (see Fig. S1, S2, and S3 in the supplemental material).
FIG. 1.
Spore proteome at pH 4 to 7 of B. anthracis toxigenic and nonencapsulated avirulent strain RA3R.
TABLE 1.
Proteins identified in B. anthracis spores by using MALDI-TOF MSa
| Spot no. | Accession no. | Theoretical mass (Da) | Mowse score | % Coverage | No. of peptides matching | Protein identification |
|---|---|---|---|---|---|---|
| 1 | gi 30263563 | 99,035 | 156 | 24 | 18 | Aconitate hydratase 1 |
| 2 | gi 30260298 | 76,333 | 145 | 27 | 18 | Translation elongation factor G |
| 3 | gi 30263811 | 78,204 | 65 | 16 | 10 | Polyribonucleotide nucleotidyltransferase |
| 4 | gi 30264669 | 62,234 | 104 | 23 | 10 | Pyruvate kinase |
| 5 | gi 30261021 | 91,361 | 141 | 32 | 19 | S-layer protein EA1 |
| 6 | gi 30258721 | 87,226 | 92 | 17 | 10 | 5-Methyltetrahydropteroyltriglutamate-homocysteine methyltransferase |
| 7 | gi 30264041 | 49,452 | 91 | 24 | 9 | Dihydrolipoamide dehydrogenase (E3) |
| 8 | gi 30261304 | 70,361 | 60 | 16 | 7 | Oligoendopeptidase F |
| 9 | gi 30265362 | 59,749 | 71 | 19 | 7 | CTP synthase |
| 10 | gi 30264385 | 65,764 | 167 | 21 | 21 | Chaperone protein dnaK |
| 11 | gi 30263628 | 72,408 | 103 | 23 | 14 | Transketolase |
| 12 | gi 30260443 | 57,430 | 175 | 50 | 25 | Chaperonin, 60 kDa |
| 13 | gi 30260481 | 56,224 | 172 | 41 | 16 | Delta-1-pyrroline-5-carboxylate dehydrogenase, putative |
| 14 | gi 30260755 | 61,006 | 175 | 31 | 15 | Neutral protease |
| 15 | gi 30264541 | 47,212 | 97 | 36 | 13 | Trigger factor |
| 16 | gi 30264163 | 46,223 | 54 | 25 | 9 | Pyrimidine-nucleoside phosphorylase |
| 17 | gi 30265328 | 51,192 | 153 | 49 | 22 | ATP synthase F1, beta subunit |
| 18 | gi 30264042 | 44,900 | 72 | 36 | 12 | Dihydrolipoamide acetyltransferase (E2) |
| 19 | gi 30264939 | 50,344 | 56 | 32 | 9 | Phosphoglucose isomerase |
| 20 | gi 30260491 | 52,294 | 120 | 39 | 13 | Glutamyl-tRNA(Gln) amidotransferase, A subunit |
| 21 | gi 30263502 | 53,742 | 113 | 44 | 16 | Aldehyde dehydrogenase |
| 22 | gi 30265485 | 47,422 | 83 | 20 | 6 | Adenylosuccinate synthetase |
| 23 | gi 30265161 | 46,417 | 188 | 41 | 14 | Enolase |
| 24 | gi 30260328 | 34,933 | 68 | 21 | 7 | DNA-directed RNA polymerase, alpha subunit |
| 25 | gi 30253620 | 42,937 | 168 | 61 | 20 | Translation elongation factor Tu |
| 26 | gi 30264043 | 35,228 | 150 | 56 | 13 | Pyruvate dehydrogenase complex E1 component, beta subunit |
| 27 | gi 30264240 | 39,850 | 155 | 35 | 13 | Leucine dehydrogenase |
| 28 | gi 30263830 | 32,434 | 57 | 21 | 6 | Translation elongation factor Ts |
| 29 | gi 30260428 | 43,660 | 152 | 30 | 14 | Alanine racemase |
| 30 | gi 30265339 | 45,109 | 61 | 11 | 4 | Serine hydroxymethyltransferase |
| 31 | gi 30262916 | 40,023 | 139 | 45 | 17 | Chorismate mutase |
| 32 | gi 30265022 | 29,057 | 60 | 27 | 9 | ABC transporter, ATP-binding protein |
| 33 | gi 30264236 | 35,790 | 65 | 31 | 9 | 3-Methyl-2-oxobutanoate dehydrogenase, beta subunit |
| 34 | gi 30265359 | 30,672 | 95 | 37 | 10 | Fructose-bisphosphate aldolase, class II |
| 35 | gi 30263337 | 24,006 | 79 | 37 | 7 | Transaldolase, putative |
| 36 | gi 30260259 | 32,918 | 170 | 65 | 26 | Cysteine synthase A |
| 37 | gi 30261955 | 34,786 | 81 | 26 | 11 | l-Lactate dehydrogenase |
| 38 | gi 30263838 | 31,211 | 63 | 33 | 8 | Succinyl-CoA synthase, alpha subunit |
| 39 | gi 30265165 | 35,824 | 117 | 43 | 11 | Glyceraldehyde 3-phosphate dehydrogenase |
| 40 | gi 30264044 | 41,440 | 78 | 21 | 8 | Pyruvate dehydrogenase (E1) |
| 41 | gi 30264763 | 22,323 | 52 | 20 | 4 | tRNA binding domain protein, putative |
| 42 | gi 30264588 | 34,340 | 83 | 46 | 13 | Electron transfer flavoprotein, alpha subunit |
| 43 | gi 30265392 | 32,389 | 95 | 39 | 12 | Agmatinase, putative |
| 44 | gi 30264444 | 25,253 | 70 | 54 | 11 | MTA |
| 45 | gi 30265465 | 24,021 | 120 | 48 | 9 | Superoxide dismutase, Mn |
| 46 | gi 30263350 | 22,755 | 150 | 52 | 12 | Hypothetical protein BA3444 |
| 47 | gi 30264918 | 29,691 | 73 | 36 | 10 | Naphthoate synthase |
| 48 | gi 30261145 | 31,405 | 63 | 32 | 6 | Hypothetical protein BA1021 |
| 49 | gi 30265367 | 31,324 | 61 | 14 | 5 | 3-Hydroxyacyl-CoA dehydrogenase |
| 50 | gi 30264875 | 25,288 | 58 | 47 | 9 | Hypothetical protein BA5061 |
| 51 | gi 30260567 | 21,101 | 95 | 50 | 8 | Tellurium resistance protein |
| 52 | gi 30264449 | 17,437 | 74 | 56 | 6 | Transcription elongation factor GreA |
| 53 | gi 30261332 | 17,330 | 68 | 56 | 5 | Hypothetical protein BA1237 |
| 54 | gi 30260515 | 20,719 | 139 | 68 | 15 | Alkyl hydroperoxide reductase, subunit C |
| 55 | gi 30260290 | 18,036 | 149 | 66 | 14 | Ribosomal protein L10 |
| 56 | gi 30263075 | 18,449 | 150 | 52 | 12 | Hypothetical protein BA3129 |
| 57 | gi 30263828 | 20,679 | 109 | 51 | 8 | Ribosome recycling factor |
| 58 | gi 30264160 | 24,611 | 71 | 50 | 6 | Hypothetical protein BA4304 |
| 59 | gi 30262026 | 30,100 | 59 | 30 | 6 | NH3-dependent NAD+ synthetase |
| 60 | gi 30263790 | 9,338 | 82 | 78 | 5 | Hypothetical protein BA3920 |
| 61 | gi 30265409 | 34,726 | 70 | 20 | 6 | Phosphate acetyltransferase |
| 62 | gi 30261415 | 20,412 | 215 | 88 | 20 | PhaP protein |
| 63 | gi 30265163 | 26,437 | 92 | 42 | 12 | Triosephosphate isomerase |
| 64 | gi 30260245 | 19,691 | 76 | 35 | 8 | Stage V sporulation protein T |
| 65 | gi 30260944 | 21,649 | 95 | 44 | 9 | CotJC protein |
| 66 | gi 30265393 | 31,142 | 130 | 33 | 11 | Spermidine synthase |
| 67 | gi 30265176 | 21,390 | 182 | 53 | 19 | ATP-dependent Clp protease, proteolytic subunit ClpP |
| 68 | gi 30264164 | 29,533 | 83 | 28 | 10 | Purine nucleoside phosphorylase |
| 69 | gi 30263352 | 31,828 | 82 | 22 | 7 | Oxidoreductase, aldo |
| 70 | gi 30263832 | 28,756 | 84 | 25 | 8 | Transcriptional regulator CodY |
The spot number identifies the corresponding protein spot on the 2-DE gel as shown in Fig. 1.
A number of Bacillus spore proteins exist as charge variants with similar molecular masses but differing pIs. Each of these proteins appeared as a train of spots on 2-D gels and was noted for all the spore proteomes of B. anthracis, B. cereus, and B. thuringiensis. In B. anthracis, the most notable ones include elongation factor G, 60-kDa chaperonin, dihydrolipoamide dehydrogenase, elongation factor Tu (EF-Tu), components of the pyruvate dehydrogenase (PDH) complex, and enolase (Fig. 1). In B. cereus spores, some of the charge variants include acyl coenzyme A dehydrogenase, EF-Tu, and aldehyde dehydrogenase (see Fig. S2 in the supplemental material). In B. thuringiensis, the most prominent ones include EF-Tu, enolase, components of the PDH complex, 60-kDa chaperonin, alkylhydroperoxide reductase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phaP protein, camelysin, and Δ-1-pyrroline-5-carboxylate dehydrogenase (see Fig. S3 and S4 in the supplemental material).
Cellular functions.
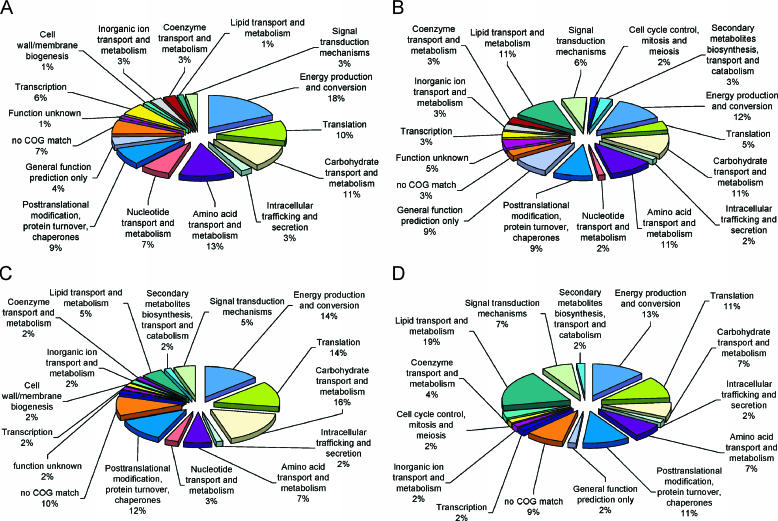
A comparative graphical representation of expressed ORFs categorized into major cellular functions in B. anthracis, B. cereus, and B. thuringiensis strains 97-27 and 10972 is shown in Fig. 2 A to D. For all of the Bacillus spores investigated, a majority of the identified proteins are involved in energy production, carbohydrate transport and metabolism, amino acid transport and metabolism, posttranslational modifications, and translation (Fig. 2 A to D). In B. cereus and B. thuringiensis strain 10792, a number of identified proteins are involved in lipid transport and metabolism (Fig. 2B and D). Since a majority of the spore proteins have metabolic functions that would typically place them in the cytoplasm, most of these proteins may have catalytic and regulatory functions associated with sporulation and germination.
FIG. 2.
Graphical representation of expressed ORFs categorized into major cellular functions for B. anthracis RA3R (A), B. cereus 14579 (B), B. thuringiensis 97-27 (C), and B. thuringiensis 10972 (D).
Biomarker proteins.
While there are a number of protein spots that are common among all the species tested, there are multiple spots unique to each species, i.e., 21 for B. anthracis, 58 for B. cereus, and 113 for B. thuringiensis (see Fig. S4A in the supplemental material). The term unique spots is based on experimentally determined location (pI and molecular weight coordinates) of these proteins on 2-D gels. A number of these spots are not encoded by unique ORFs. However, some of the unique B. anthracis spots include trigger factor, oxidoreductase, phaP protein, and hypothetical protein BA3920. It should be pointed out that despite a thorough spot picking for each species, only a few of the unique spots were identified by peptide mass fingerprinting, since a majority of them are not highly expressed and can be deciphered only by computer analysis. Functional identification of all these spots is essential for their value in species classification. Additionally, a total of 196 unique protein spots distinguished the virulent B. thuringiensis strain 97-27 from the avirulent strain 10972, which has 91 unique spots (see Fig. S4B in the supplemental material). Overall, the resulting biomarker spots can be used for quick identification of each species using 2-D polyacrylamide gel electrophoresis or for immunocapture assays.
To determine if B. anthracis spore protein profiles differ depending on the bacterial plasmid content, strains RA3R (pXO1+ pXO2−), A3 (pXO1− pXO2+), and 1099 (pXO1− pXO2−) were examined (Fig. 3A to C). A total of 166 common protein spots were found among the three strains, whereas 29, 94, and 97 protein spots distinguished strains RA3R, A3, and 1099 from each other, respectively (Fig. 3D). The difference in 2-DE spore proteomic profiles may be due not only to plasmid-encoded proteins but also to the altered expression of chromosomally encoded genes that are affected by either pXO1 or pXO2 plasmids or both plasmids.
FIG. 3.
Spore proteomes of avirulent B. anthracis toxigenic and nonencapsulated strain RA3R (pXO1+ pXO2−) (A), nontoxigenic and encapsulated strain A3 (pXO1− pXO2+) (B), and the totally cured nontoxigenic and nonencapsulated strain 1099 (pXO1− pXO2−) (C). A Venn diagram illustrating the commonly shared and unique proteins among the three strains is in D. In A to C, blue tags denote protein spots not present in RA3R but present in A3 or 1099; red tags denote protein spots present in 1099 but absent in RA3R or A3; and green tags denote protein spots absent in RA3R and A3.
Spore immunome.
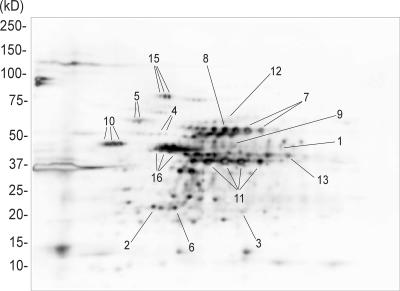
Since the endpoint users of a next-generation anthrax vaccine would be humans, proteins specifically immunoreactive to the human population would be preferable as vaccine candidates over those specifically immunoreactive to laboratory-infected animals. A total of 15 immunoreactive proteins were identified in B. anthracis strain RA3R (Fig. 4; Table 2). Notably, the most immunodominant antigens include four charge variants of GAPDH (spot 11), three charge variants of EF-Tu (spot 16), dihydrolipoamide acetyltransferase (spot 8), three charge variants of enolase (spot 10), two charge variants of Δ-1-pyrroline-5-carboxylate dehydrogenase (spot 7), alanine racemase (spot 1), the CotJC protein (spot 6), and three charge variants of elongation factor G (spot 15) (Fig. 4). Of the 15 antigens listed in Table 2, enolase and 60-kDa chaperonin have been previously identified as components of Anthrax Vaccine Adsorbed (AVA) (39). Using the same human antisera, 7, 14, and 7 immunogenic proteins were identified in B. cereus and in B. thuringiensis strains 97-27 and 10792, respectively (Table 2). Computer-assisted comparative analysis of Western blots from B. anthracis, B. cereus, and B. thuringiensis indicates that alanine racemase and neutral protease were uniquely immunogenic to B. anthracis (Table 2). Overall, the number of immunogenic proteins identified using experimentally infected goat and rabbit antisera (6 and 4, respectively) was lower than those detected using human antisera (data not shown). Two additional immunoreactive proteins, trigger factor (goat antisera) and aldehyde dehydrogenase (goat and rabbit antisera), were detected using animal antisera. This information may be invaluable for the design of veterinary vaccines. The fact that the other 13 B. anthracis immunogenic proteins are not unique does not exclude them from being used in a vaccine. In fact, these proteins may be used in a multiagent vaccine that protects against other members of the B. cereus group. Furthermore, inclusion of one or more of these newly identified immunogenic proteins in a PA-based vaccine may serve to enhance the overall immunological response and provide a greater level of protection over several life stages of B. anthracis.
FIG. 4.
Immunoblot at pH 4 to 7 of B. anthracis strain RA3R spore proteome. The blot was probed with human antisera from patients infected with cutaneous anthrax. The spot numbers correspond to the immunogenic proteins listed in Table 2.
TABLE 2.
Identified immunogenic proteins from members of the B. cereus groupa
| Protein | Presence in strain
|
|||
|---|---|---|---|---|
| Ba RA3R | Bc 14579 | Bt 9727 | Bt 10792 | |
| 1. Alanine racemase | + | |||
| 2. Alkyl hydroperoxide reductase, subunit C | + | + | + | + |
| 3. ATP-dependent Clp protease | + | + | + | + |
| 4. ATP synthase F1, beta subunit | + | + | + | + |
| 5. Chaperonin, 60 kDa | + | + | ||
| 6. CotJc protein | + | + | ||
| 7. Δ-1-pyrroline-5-carboxylate dehydrogenase | + | + | + | + |
| 8. Dihydrolipoamide acetyltransferase (E2) | + | + | ||
| 9. Dihydrolipoamide dehydrogenase (E3) | + | + | ||
| 10. Enolase | + | + | + | + |
| 11. Glyceraldehyde-3-phosphate dehydrogenase | + | + | + | |
| 12. Neutral protease | + | |||
| 13. Pyruvate dehydrogenase (E1) | + | + | ||
| 14. Ribosomal protein L7/L12 | + | + | ||
| 15. Translation EF-G | + | + | ||
| 16. Translation EF-Tu | + | + | + | + |
2-D Western blots were probed using antisera from human patients infected with cutaneous anthrax. Ba, B. anthracis; Bc, B. cereus; Bt, B. thuringiensis. The number preceding each protein corresponds to the spot number in Fig. 4.
DISCUSSION
There are numerous studies suggesting that the higher protective efficacy of the spore-based vaccine is due to the contribution of unidentified spore antigens. One study demonstrated that a single subcutaneous administration of 5 × 107 spores from an attenuated B. anthracis vaccine strain conferred complete protection to guinea pigs against a lethal challenge of wild-type anthrax spores compared to the partial protection with vaccines administered as vegetative cells (5). Another study has also shown that the administration of a combination vaccine composed of purified PA with 108 formaldehyde-inactivated spores of B. anthracis with defective lethal factor and edema factor completely protected both guinea pigs and mice from a lethal challenge with wild-type anthrax spores, in contrast to the partial protection conferred by the purified PA-based vaccine alone (4). Using immune sera from monkeys vaccinated with AVA, Welkos and Friedlander (38) have also demonstrated that unidentified anthrax spore surface antigens might be targets of AVA-induced protective immunity. The ability of monkey immune sera to enhance spore phagocytosis by murine peritoneal macrophages was in part due to the humoral immune response against the unidentified spore antigens. Based on these studies, we characterized the unidentified spore antigens of B. anthracis and other members of the B. cereus group using immunoproteomics approaches. The protein extraction protocol that we used is a modification of the method of Sylvestre et al. (34) for the extraction of the spore's exosporium. Because our extraction protocol was not validated in terms of which spore subproteome is maximally being extracted, we referred to the extracted proteins as “spore proteins” with no specific reference to the exosporium. However, it is most likely that this preparation is highly enriched for exosporial proteins.
Recently, Kudva et al. (20) identified 69 clones by immunological screening of a B. anthracis Sterne (pXO2+ pXO1−) limited expression library of putative spore surface proteins with pooled sera from human adults immunized with AVA. Of the 69 immunoreactive spore-associated proteins that Kudva et al. (20) have reported, two immunogenic proteins, i.e., alanine racemase and enolase, were found in common with this study (Table 2). Additionally, alkyl hydroperoxide reductase, subunit C, has been previously reported to be immunogenic in the B. anthracis membrane fraction (2). To our knowledge, the other 12 immunogenic proteins listed in Table 2 are novel candidate proteins for developing an improved anthrax vaccine. An immunodominant B. anthracis exosporium protein identified by Steichen et al. (33) as BclA (Bacillus collagen-like protein) was not identified by mass spectrometry in this or in a similar study (24) because this protein does not have any trypsin cleavage sites.
A number of the immunogenic spore proteins found in this study are associated with generation of metabolic energy, bacterial survival, or enzymatic processes relevant to germination. GAPDH, Clp protease, enolase, ATP synthase, and the PDH complex have associations with ATP generation. GAPDH is particularly interesting because it is currently being tested in a number of systems for development of vaccines against Edwardsiella tarda (17, 25), Streptococcus pneumoniae (16, 23), and Onchocerca volvulus (10). All of these studies suggest that the use of GAPDH alone or as a component of an anthrax vaccine formulation may similarly provide protection against B. anthracis infection. Clp protease is an ATP-dependent enzyme consisting of an ATPase specificity factor, the heat shock protein 100/clp family member ClpC, and the proteolytic subunit ClpP. Clp proteases are fundamental in many pathogenic bacteria for stress tolerance and virulence (15). The glycolytic enzyme enolase was also found to be immunogenic. A recent study has reported that enolase induced in vitro neutralizing antibodies against Chlamydia pneumoniae cells and inhibited their dissemination with a hamster model, indicating its value as a vaccine component (11). The PDH complex is composed of three enzymes, i.e., PDH (E1), dihydrolipoyl transacetylase (E2), and dihydrolipoyl dehydrogenase (E3), each of which was found to be immunogenic (Table 2). The PDH complex converts pyruvate to acetyl coenzyme A, which is subsequently used in the tricarboxylic acid cycle to generate NADH, ATP, and reduced flavin adenine dinucleotide (7). The PDH complex is known to be highly immunogenic in other bacterial species, such as Neisseria meningitidis (1), Mycoplasma capricolum (40), and Mycoplasma hyopneumoniae (28). Recently, PDH has been tested as a DNA vaccine against Mycoplasma mycoides subsp. mycoides, the causal agent of contagious bovine pleuropneumonia (27). The study showed that significant anti-M. mycoides subsp. mycoides responses were observed in mice vaccinated with clones containing the full-length genes of the PDH complex.
The identification of new immunoreactive proteins from the spores of B. anthracis, B. cereus, and B. thuringiensis adds to the list of potential targets for developing more-specific, safer, and highly efficacious vaccines. Since several of these immunoreactive proteins are common among members of the B. cereus group, it is possible to develop not only a potent anthrax vaccine but also a multiagent and multivalent vaccine against B. cereus and B. thuringiensis, where some strains are pathogenic to humans. The same immunoproteomics approach can be used for other infectious agents, including viruses, where no vaccine is currently available for treatment. One revolutionary approach is to incorporate the genes encoding these proteins into bacterial ghosts (BG), a nonliving and nonpathogenic envelope of gram-negative bacterium that is formed by the expression of the cloned bacteriophage ΦX174 E gene (26, 36). The potential of cloning and expressing genes encoding immunogenic proteins into BG has been discussed in a recent review (18). A new era in vaccine development is emerging that utilizes the advances in immunoproteomics and the BG platform technology for the design and creation of potent biodefense vaccines.
Comparative analysis of the spore proteomes from three members of the B. cereus group has also shown that it is possible to distinguish B. anthracis from B. cereus and B. thuringiensis based on their 2-DE protein signatures and the presence of spots unique to each species. This is important for differential detection and identification of each species and may lead to the development of DNA- or immuno-based detection platforms. The same approach has been used to discriminate various strains of B. anthracis that differ in their plasmid content, as well as the virulent from avirulent strains of B. thuringiensis. These data are valuable for a systems biology approach for studying the complete biology of these pathogens and making better predictions of their virulence.
Supplementary Material
Acknowledgments
We acknowledge the logistics support of Luis Puron.
This work was supported in part by grants from the U.S. Army Edgewood Chemical Biological Center.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.a'Aldeen, D. A., A. H. Westphal, K. A. De, V. Weston, M. S. Atta, T. J. Baldwin, J. Bartley, and S. P. Borriello. 1996. Cloning, sequencing, characterization and implications for vaccine design of the novel dihydrolipoyl acetyltransferase of Neisseria meningitidis. J. Med. Microbiol. 45:419-432. [DOI] [PubMed] [Google Scholar]
- 2.Ariel, N., A. Zvi, K. S. Makarova, T. Chitlaru, E. Elhanany, B. Velan, S. Cohen, A. M. Friedlander, and A. Shafferman. 2003. Genome-based bioinformatic selection of chromosomal Bacillus anthracis putative vaccine candidates coupled with proteomic identification of surface-associated antigens. Infect. Immun. 71:4563-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouillaut, L., N. Ramarao, C. Buisson, N. Gilois, M. Gohar, D. Lereclus, and C. Nielsen-Leroux. 2005. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 71:8903-8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brossier, F., M. Levy, and M. Mock. 2002. Anthrax spores make an essential contribution to vaccine efficacy. Infect. Immun. 70:661-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, S., I. Mendelson, Z. Altboum, D. Kobiler, E. Elhanany, T. Bino, M. Leitner, I. Inbar, H. Rosenberg, Y. Gozes, R. Barak, M. Fisher, C. Kronman, B. Velan, and A. Shafferman. 2000. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect. Immun. 68:4549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DesRosier, J. P., and J. C. Lara. 1984. Synthesis of the exosporium during sporulation of Bacillus cereus. J. Gen. Microbiol. 130:935-940. [Google Scholar]
- 7.Domingo, G. J., H. J. Chauhan, I. A. D. Lessard, C. Fuller, and R. N. Perham. 1999. Self-assembly and catalytic activity of the pyruvate dehydrogenase multienzyme complex from Bacillus stearothermophilus. Eur. J. Biochem. 266:1136-1146. [DOI] [PubMed] [Google Scholar]
- 8.Drake, R. R., Y. Deng, E. E. Schwegler, and S. Gravenstein. 2005. Proteomics for biodefense applications: progress and opportunities. Expert Rev. Proteomics 2:203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer, K. G., J. M. Lamonica, J. A. Schumacher, L. E. Williams, J. Bishara, A. Lewandowski, R. Redkar, G. Patra, and V. G. DelVecchio. 2004. Identification of Bacillus anthracis specific chromosomal sequences by suppressive subtractive hybridization. BMC Genomics 5:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erttmann, K. D., A. Kleensang, E. Schneider, S. Hammerschmidt, D. W. Buttner, and M. Gallin. 2005. Cloning, characterization and DNA immunization of an Onchocerca volvulus glyceraldehyde-3-phosphate dehydrogenase (OV-GAPDH). Biochim. Biophys. Acta 1741:85-94. [DOI] [PubMed] [Google Scholar]
- 11.Finco, O., A. Bonci, M. Agnusdei, M. Scarselli, R. Petracca, N. Norais, G. Ferrari, I. Garaguso, M. Donati, V. Sambri, R. Cevenini, G. Ratti, and G. Grandi. 2005. Identification of new potential vaccine candidates against Chlamydia pneumoniae by multiple screenings. Vaccine 23:1178-1188. [DOI] [PubMed] [Google Scholar]
- 12.Hachisuka, Y., K. Kojima, and T. Sato. 1966. Fine filaments on the outside of the exosporium of Bacillus anthracis spores. J. Bacteriol. 91:2382-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helgason, E., O. A. Økstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna, and A. B. Kolsto. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez, E., F. Ramisse, J. P. Ducoureau, T. Cruel, and J. D. Cavallo. 1998. Bacillus thuringiensis subsp. Konkukian (serotype H34) superinfection: case report and experimental evidence of pathogenicity in immunosuppressed mice. J. Clin. Microbiol. 36:2138-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim, Y. M., A. R. Kerr, N. A. Silva, and T. J. Mitchell. 2005. Contribution of the ATP-dependent protease ClpCP to the autolysis and virulence of Streptococcus pneumoniae. Infect. Immun. 73:730-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jomaa, M., J. M. Kyd, and A. W. Cripps. 2005. Mucosal immunisation with novel Streptococcus pneumoniae protein antigens enhances bacterial clearance in an acute mouse lung infection model. FEMS Immunol. Med. Microbiol. 44:59-67. [DOI] [PubMed] [Google Scholar]
- 17.Kawai, K., Y. Liu, K. Ohnishi, and S. Oshima. 2004. A conserved 37 kDa outer membrane protein of Edwardsiella tarda is an effective vaccine candidate. Vaccine 22:3411-3418. [DOI] [PubMed] [Google Scholar]
- 18.Khan, A. S., C. V. Mujer, T. G. Alefantis, J. P. Connolly, U. B. Mayr, P. Walcher, W. Lubitz, and V. G. DelVecchio. 2006. Proteomics and bioinformatics strategies for designing countermeasures against infectious threat agents. J. Chem. Infect. Model 46:111-115. [DOI] [PubMed] [Google Scholar]
- 19.Klade, C. S. 2002. Proteomics approaches towards antigen discovery and vaccine development. Curr. Opin. Mol. Ther. 4:216-223. [PubMed] [Google Scholar]
- 20.Kudva, I. T., R. W. Griffin, J. M. Garren, S. B. Calderwood, and M. John. 2005. Identification of a protein subset of the anthrax spore immunome in humans immunized with the anthrax vaccine adsorbed preparation. Infect. Immun. 73:5685-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Duc, M. T., M. Satomi, N. Agata, and K. Venkateswaran. 2004. gyrB as a phylogenetic discriminator for members of the Bacillus anthracis-cereus-thuringiensis group. J. Microbiol. Methods 56:383-394. [DOI] [PubMed] [Google Scholar]
- 22.Leppla, S. H., J. B. Robbins, R. Schneerson, and J. Shiloach. 2002. Development of an improved vaccine for anthrax. J. Clin. Investig. 110:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling, E., G. Feldman, M. Portnoi, R. Dagan, K. Overweg, F. Mulholland, V. Chalifa-Caspi, J. Wells, and Y. Mizrachi-Nebenzahl. 2004. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin. Exp. Immunol. 138:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, H., N. H. Bergman, B. Thomason, S. Shallom, A. Hazen, J. Crossno, D. A. Rasko, J. Ravel, T. D. Read, S. N. Peterson, J. Yates III, and P. C. Hanna. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186:164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, Y., S. Oshima, K. Kurohara, K. Ohnishi, and K. Kawai. 2005. Vaccine efficacy of recombinant GAPDH of Edwardsiella tarda against edwardsiellosis. Microbiol. Immunol. 49:605-612. [DOI] [PubMed] [Google Scholar]
- 26.Lubitz, W. 2001. Bacterial ghosts as carrier and targeting systems. Expert Opin. Biol. Ther. 1:765-771. [DOI] [PubMed] [Google Scholar]
- 27.March, J. B., C. D. Jepson, J. R. Clark, M. Totsika, and M. J. Calcutt. 2006. Phage library screening for the rapid identification and in vivo testing of candidate genes for a DNA vaccine against Mycoplasma mycoides subsp. mycoides small colony biotype. Infect. Immun. 74:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matic, J. N., J. L. Wilton, R. J. Towers, A. L. Scarman, F. C. Minion, M. J. Walker, and S. P. Djordjevic. 2003. The pyruvate dehydrogenase complex of Mycoplasma hyopneumoniae contains a novel lipoyl domain arrangement. Gene 319:99-106. [DOI] [PubMed] [Google Scholar]
- 29.Mignot, T., E. Couture-Tosi, S. Mesnage, M. Mock, and A. Fouet. 2004. In vivo Bacillus anthracis gene expression requires PagR as an intermediate effector of the AtxA signalling cascade. Int. J. Med. Microbiol. 293:619-624. [DOI] [PubMed] [Google Scholar]
- 30.Mock, M., and A. Fouet. Anthrax. 2001. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 31.Schoeni, J. L., and A. C. Wong. 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68:636-648. [DOI] [PubMed] [Google Scholar]
- 32.Shlyakhov, E. N., and E. Rubenstein. 1994. Human live anthrax vaccine in the former USSR. Vaccine 12:727-730. [DOI] [PubMed] [Google Scholar]
- 33.Steichen, C., P. Chen, J. F. Kearney, and C. L. Turnbough, Jr. 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185:1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylvestre, P., E. Couture-Tosi, and M. Mock. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169-178. [DOI] [PubMed] [Google Scholar]
- 35.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walcher, P., U. B. Mayr, C. Azimpour-Tabrizi, F. O. Eko, W. Jechlinger, P. Mayrhofer, T. Alefantis, C. V. Mujer, V. G. DelVecchio, and W. Lubitz. 2004. Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev. Vaccines 3:681-691. [DOI] [PubMed] [Google Scholar]
- 37.Wang, J. Y., and M. H. Roehrl. 2005. Anthrax vaccine design: strategies to achieve comprehensive protection against spore, bacillus, and toxin. Med. Immunol. 4:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welkos, S. L., and A. M. Friedlander. 1988. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb. Pathog. 5:127-139. [DOI] [PubMed] [Google Scholar]
- 39.Whiting, G. C., S. Rijpkema, T. Adams, and M. J. Corbel. 2004. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine 22:4245-4251. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, P. P., and A. Peterkofsky. 1996. Sequence and organization of genes encoding enzymes involved in pyruvate metabolism in Mycoplasma capricolum. Protein Sci. 5:1719-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.