Abstract
Genes of Salmonella enterica serovar Typhimurium LT2 expected to be specifically present in Salmonella were selected using the Basic Local Alignment Search Tool (BLAST) program. The 152 selected genes were compared with 11 genomic sequences of Salmonella serovars, including Salmonella enterica subsp. I and IIIb and Salmonella bongori (V), and were clustered into 17 groups by their comparison patterns. A total of 38 primer pairs were constructed to represent each of the 17 groups, and PCR was performed with various Salmonella subspecies including Salmonella enterica subsp. I, II, IIIa, IIIb, IV, VI, and V to evaluate a comprehensive DNA-based scheme for identification of Salmonella subspecies and the major disease-causing Salmonella serovars. Analysis of PCR results showed that Salmonella enterica subsp. I was critically divided from other subspecies, and Salmonella strains belonging to S. enterica subsp. I were clustered based on their serovars. In addition, genotypic relationships within S. enterica subsp. I by PCR results were investigated. Also, Salmonella signature genes, Salmonella enterica serovar Typhimurium signature genes, and Salmonella enterica subsp. I signature genes were demonstrated based on their PCR results. The described PCR method suggests a rapid and convenient method for identification of Salmonella serovars that can be used by nonspecialized laboratories. Genome sequence comparison can be a useful tool in epidemiologic and taxonomic studies of Salmonella.
Salmonellae are divided taxonomically into two species, Salmonella enterica and Salmonella bongori (V). Salmonella enterica comprises 6 subspecies: S. enterica subsp. enterica (I), S. enterica subsp. salamae (II), S. enterica subsp. arizonae (IIIa), S. enterica subsp. diarizonae (IIIb), S. enterica subsp. houtenae (IV), and S. enterica subsp. indica (VI). Salmonella is classified into more than 2,500 serovars using the Kauffmann-White scheme (25). Salmonella enterica subsp. I consists of almost 1,500 serovars (24), and most infections in warm-blooded animals are caused by Salmonella enterica subsp. I. Among S. enterica subsp. I, only a small number of Salmonella serovars (e.g., Salmonella enterica serovar Typhimurium, Salmonella enterica serovar Enteritidis, Salmonella enterica serovar Newport, Salmonella enterica serovar Typhi, Salmonella enterica serovar Paratyphi A, Salmonella enterica serovar Paratyphi C, and Salmonella enterica serovar Choleraesuis) account for most human and domestic animal Salmonella infections (26, 27). The different host ranges, diseases, and virulence potentials demonstrated by the various serovars belonging to S. enterica subsp. I (4, 13) are thought to be caused by genetic variation.
The serology of Salmonella is based on the Kauffmann-White scheme, which differentiates Salmonella serovars by the surface antigen differences of somatic (O) and flagellar (H) antigens (24). This serological method, which is a convenient and epidemiologically useful method of categorizing Salmonella, has been used to identify Salmonella serovars. At the same time, this method is labor-intensive, expensive, complicated, and time-consuming. Also, it does not provide a basis for investigating evolutionary genetic relatedness among strains. Molecular characterization of each Salmonella serovar has been reported using multilocus enzyme electrophoresis (MLEE), and many serovars were found to be represented by two or more electrophoretic types. Evolutionary trees constructed by MLEE data classified Salmonella serovars as monophyletic or polyphyletic, and these results found genotypic differences between the same Salmonella serovars and genetic distance between serovars (8). Recently, microarray methods were applied to evaluating the gene contents of Salmonella serovars. Comparative genomic hybridization using microarrays suggested that Salmonella strains of the same serovars are not always genotypically closely related and that differences are characterized at single-gene resolution. Also, a new term, “genovars,” was proposed to describe groups of strains with genetic similarity, distinguishing them from traditional Salmonella serovar classification (26).
Recently, genomic DNA sequencing projects of various Salmonella strains have been in process or completed for some serovars (12, 20, 22). These genome projects incorporate fast-capacity screening technology, such as microarray analysis, and are expected to reveal more information about Salmonella genotyping. Comparative genomics in Salmonella biology have also been initiated by genome sequencing of other related Salmonella serovars and will provide more efficient ways of identifying all of the genetic differences between closely related bacteria (13). Also, this sequence comparison will provide more information about characteristics of Salmonella serovars. PCR has become a potentially powerful alternative in microbiological diagnostics due to its simplicity, rapidity, reproducibility, and accuracy (5, 23, 32). PCR is relatively easy to perform with simple equipment in the laboratory compared to microarray analysis or other molecular methods. Also, genomic sequence comparison can be a powerful tool for probe searching (marker gene searching) and characterizing the gene contents of closely related bacterial species.
In this study, genes of S. enterica serovar Typhimurium LT2 that were expected to be specific to the Salmonella genus were selected using genomic sequence comparison. The selected gene sequences were compared with genomic sequences of 11 Salmonella strains. Primer pairs of these selected genes were constructed and used to evaluate the genomic DNA of various Salmonella serovars, including all subspecies. First, we confirmed that genomic sequence comparison patterns and PCR result patterns were comparable to determine the acceptability of applying genomic sequence comparison to a substantial experiment. Second, genomic sequence comparison results were used to identify genes that were Salmonella specific, S. enterica subsp. I specific, and S. enterica serovar Typhimurium specific to establish a comprehensive DNA-based scheme for identification of Salmonella subspecies and the major disease-causing Salmonella serovars without the need for serological testing. Also, we suggest genotypic relationships between Salmonella serovars on the basis of PCR results. These results suggest a rapid and convenient method for identification of the Salmonella serovars attainable by nonspecialized laboratories.
MATERIALS AND METHODS
Bacterial strains.
Salmonella strains used in this study are listed in Table 1. Sixteen type strains of Salmonella were collected from the American Type Culture Collection (ATCC). Forty-seven Salmonella strains were provided by Y. H. Jung of the Korea Consumer Protection Board (KCPB) (11). Thirty-nine Salmonella strains were provided by Reiner Helmuth of the Federal Institute for Risk Assessment (BFR, Molecular Biology, National Salmonella Reference Laboratory, Germany) (18). Thirty-five Salmonella strains were donated by K. H. Seo of the U.S. Food and Drug Administration (FDA, CFSAN/OPDFB) (30). Salmonella strains were inoculated in Luria-Bertani broth medium and cultured at 37°C with vigorously shaking. Non-Salmonella strains, including food-borne pathogens and Enterobacteriaceae, were collected from the ATCC and are listed in Table S4 in the supplemental material.
TABLE 1.
Salmonella strains used in this study
| Salmonella subspecies and serovar | Serogroup | Source | Strain(s) | Salmonella subspecies and serovar | Serogroup | Source | Strain(s) | |
|---|---|---|---|---|---|---|---|---|
| S. enterica subsp. I | Madelia | H | FDA | 22N | ||||
| Typhimurium | B | ATCC 19585 | LT2 | Manhattan | C2-C3 | FDA | 1293H | |
| ATCC 13311 | Mbandlaka | C1 | FDA | 37N | ||||
| ATCC 14028 | Meleagridis | E1 | FDA | 1054H | ||||
| Typhi | D1 | ATCC 33459 | Mhenohen | FDA | 2761H | |||
| Choleraesuis | C1 | ATCC 13312 | Mississippi | G | FDA | 2883H | ||
| Enteritidis | D1 | ATCC 4931 | Muenster | E1 | FDA | 1250H | ||
| Gallinarum | D1 | ATCC 9184 | Newington | FDA | 3144H | |||
| Pullorum | ATCC 9120 | Newport | C2-C3 | BFR | G07 | |||
| Paratyphi C | C1 | ATCC 13428 | Ohio | C1 | FDA | 2060H | ||
| Paratyphi B | B | ATCC 10719 | Oranienburg | C1 | FDA | 1410H | ||
| Typhimurium | B | KCPBa | S9, S15, S17, S21 | Paratyphi A | A | KCPB | S11 | |
| BFRb | G02 | Poona | G | FDA | 3417H | |||
| FDAc | DT-104 | Saintpaul | B | BFR | G09 | |||
| Enteritidis | D1 | KCPB | S25, S26, S27, S29, S32, S34, S35, S38, S39, S40, S41, S53, S54, S56, S57, S63, S64, S65, S66 | Sandow | C2-C3 | KCPB | S13 | |
| Senftenberg | E4 | BFR | G19 | |||||
| Tennessee | C1 | KCPB | S24 | |||||
| Virchow | C1 | BFR | G04 | |||||
| BFR | G01 | |||||||
| FDA | 3512H, H3353, Benson-1, ME-13, Me-14 | S. enterica subsp. II | ||||||
| S. enterica subsp. | ATCC 15793 | |||||||
| Haardt | C2-C3 | KCPB | S30, S31, S33, S36, S37 | salamae | ||||
| Virginia | C2-C3 | KCPB | S3, S5, S6, S7, S8 | 42:r:- | T | BFR | G22 | |
| Heidelberg | B | BFR | G06 | 9,12:z:z39 | D | BFR | G23 | |
| FDA | 3390H, UN-L | 48:d:z6 | Y | BFR | G24 | |||
| Infantis | C1 | KCPB | S22 | 42:b:e,n,x,z15 | T | BFR | G25 | |
| BFR | G05 | 30:l,z28:z6 | N | BFR | G26 | |||
| FDA | 1232H | |||||||
| Agona | B | KCPB | S12, S28 | S. enterica subspecies IIIa | ||||
| BFR | G10 | S. enterica subsp. | ATCC 13314 | |||||
| Bredeney | B | BFR | G13 | arizonae | ||||
| FDA | 1370H | 21:g,z51:- | L | BFR | G27 | |||
| Derby | B | FDA | 1591H | 47:r:- | X | BFR | G28 | |
| BFR | G14 | 18:z4,z32:- | K | BFR | G29 | |||
| Hadar | C2-C3 | KCPB | S2 | |||||
| BFR | G03 | S. enterica subspecies IIIb | ||||||
| Georgia | C1 | KCPB | S4, S18 | S. enterica subsp. | ATCC 43973 | |||
| Litchfield | C2-C3 | BFR | G20 | diarizonae | ||||
| FDA | 3483H | 50:z:z52 | Z | BFR | G30 | |||
| Montevideo | C1 | BFR | G17 | 47:l,v:z | X | BFR | G31 | |
| FDA | 1231H | 18:i,v:z | K | BFR | G32 | |||
| Schwarzenground | B | KCPB | S16, S19 | |||||
| Agona B | FDA | 4000H | S. enterica subsp. IV | |||||
| Anatum | E1 | FDA | 1904H | S. enterica subsp. | ATCC 43974 | |||
| Barcilly | FDA | 1955H | houtenae | |||||
| Blockley | C2-C3 | BFR | G11 | 16:z4,z32:- | I | BFR | G33 | |
| Bovismorbificans | C2-C3 | BFR | G12 | 48:g,z51:- | Y | BFR | G34 | |
| Braenderup | C1 | FDA | 10N | 11:z4,z23:- | F | BFR | G35 | |
| Brandenburg | B | BFR | G08 | |||||
| California | B | FDA | 3515H | S. enterica subsp. VI | ||||
| Cerro | K | FDA | 1325H | S. enterica subsp. indica | ATCC 43976 | |||
| Dublin | D1 | BFR | G15 | 45:a:e,n,x | W | BFR | G39 | |
| Edinburg | C1 | KCPB | S10 | 1,6,14,25:a:e,n,x | H | BFR | G40 | |
| Give E1 | E1 | FDA | 1432H | 41:b:1,7 | S | BFR | G41 | |
| Illinois | FDA | 2386H | ||||||
| Istanbul | C2-C3 | KCPB | S20 | S. bongori (V) | ||||
| Java B | FDA | 2234H | S. bongori | ATCC 43975 | ||||
| Javiana | D1 | FDA | 2080H | 44:r:- | V | BFR | G36 | |
| Joal | E1 | KCPB | S23 | 66:z65:- | BFR | G37 | ||
| Kentucky | C2-C3 | FDA | 2035 | 48:z35:- | Y | BFR | G38 | |
| Livingstone | C1 | BFR | G16 |
Genomic DNA extraction.
Cultured media of Salmonella strains were harvested in microtubes, and genomic DNA from Salmonella strains was extracted using the DNEasy tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer's manual. Concentrations of extracted DNA were measured using a UV spectrophotometer (model UV-1700; Shimadzu, Tokyo, Japan), and genomic DNA with a 1.8 to 2 ratio (A260/A280) was used. Genomic DNA from Salmonella strains was diluted in distilled water to 25 ng/μl and stored at 4°C until PCR.
Genomic sequences of Salmonella species.
Table 2 lists the 12 genomic sequences of Salmonella strains used in this study and their sources. The genomic sequencing projects of S. enterica serovar Typhimurium LT2, S. enterica serovar Typhi CT18, and S. enterica serovar Typhi Ty2 are complete (12, 20, 22), and their genomic sequences were obtained from the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/). An additional 9 genomic sequencing projects of Salmonella strains were not completed, but raw sequence data were obtained from the Sanger Institute, Washington University, and the University of Illinois. Genomic sequences of Salmonella serovar Typhimurium DT104, Salmonella serovar Typhimurium SL1344, Salmonella serovar Enteritidis PT4, Salmonella enterica serovar Gallinarum 287/91, and S. bongori 12419 were obtained from the Sanger Institute (http://www.sanger.ac.uk/Projects/Salmonella/). Genomic sequences of Salmonella enterica serovar Dublin and Salmonella enterica serovar Pullorum were obtained from the University of Illinois (http://www.salmonella.org/genomics/). Genomic sequences of S. enterica subsp. diarizonae serovar 61:1,v:1,5,(7) and Salmonella serovar Paratyphi A ATCC 9150 were obtained from the Genome Sequencing Center (GSC) at Washington University (http://genome.wustl.edu/home.cgi).
TABLE 2.
Salmonella genomic sequences used in this study
| Strain | Subspecies | Reference sequence | Genome size (kb) | Status of genome projecta | Contributor | Reference | Source |
|---|---|---|---|---|---|---|---|
| S. enterica serovar Typhimurium LT2 | I | NC_003197 | 4,857 | Finished (4,451) | R. K. Wilson (Washington University, GSC) | 20 | http://www.ncbi.nlm.nih.gov/ |
| S. enterica serovar Typhimurium DT104 | I | STmDT104.dbs (NC_004513) | 5,020 | Finishing/gap closure | Sanger Institute | http://www.sanger.ac.uk/Projects/Salmonella/ | |
| S. enterica serovar Typhimurium SL1344 | I | STmSL1344.dbs (NC_004509) | 5,091 | Finishing/gap closure | Sanger Institute | http://www.sanger.ac.uk/Projects/Salmonella/ | |
| S. enterica serovar Typhi CT18 | I | NC_003198 | 4,809 | Finished (4,949) | B. G. Barrell (Sanger Institute) | 22 | http://www.ncbi.nlm.nih.gov/ |
| S. enterica serovar Typhi Ty2 | I | NC_004631 | 4,791 | Finished (4,639) | F. R. Blattner | 12 | http://www.ncbi.nlm.nih.gov/ |
| S. enterica serovar Paratyphi A ATCC 9150 | I | SparatyphiA.txt (NC_006511) | 4,585 | Finished | Washington University (GSC) | http://www.ncbi.nlm.nih.gov/ | |
| S. enterica serovar Enteritidis PT4 | I | SePT4.dbs | 4,686 | Finishing/gap closure | Sanger Institute | http://www.sanger.ac.uk/Projects/Salmonella/ | |
| S. enterica serovar Gallinarum 287/91 | I | SG.dbs | 4,869 | Finishing/gap closure | Sanger Institute | http://www.sanger.ac.uk/Projects/Salmonella/ | |
| S. enterica serovar Dublin | I | Sdu.dbs.txt (NC_002961) | Incomplete | University of Illinois | http://www.salmonella.org/genomics/ | ||
| S.enterica serovar Pullorum | I | Spu.dbs.txt | Incomplete | University of Illinois | http://www.salmonella.org/genomics/ | ||
| S. enterica subsp. diarizonae serovar 61:1,v:1,5,(7) | IIIb | Diarizonae.txt | 3,600 | Sequence is now in shotgun | Washington University (GSC) | http://genome.wustl.edu/projects/bacterial/ | |
| S. bongori 12419 | V | SB.dbs (NC_004548) | 4,460 | Finished | Sanger Institute | http://www.sanger.ac.uk/Projects/Salmonella/ |
Numbers in parentheses indicate numbers of coding genes from the complete genome sequence.
Comparative genomics between Salmonella serovars.
A total of 4,451 gene sequences (NC_003197.ffn) of Salmonella serovar Typhimurium LT2 were submitted to the nonredundant (nr) DNA sequence NCBI database using the Basic Local Alignment Search Tool (BLAST) program (version 2.2.5) (2). BLAST outputs that matched the Salmonella genus were eliminated and the highest scored output of each 4,451 genes was selected from BLAST outputs of each gene. Based on BLAST outputs, Salmonella specific expected genes that had an nr database match score of less than 40.14 and had a matched length less than 21 bp were selected and compared to the genomic sequence of 11 Salmonella strains using the BLAST program (version 2.2.5). Each highest matched output of Salmonella specific expected genes with each Salmonella genome sequence were defined as high homology, moderate homology, and low homology, and Salmonella specific expected genes were grouped based on homology patterns with each Salmonella genomic sequence.
Primer construction and PCR conditions.
A total of 38 oligonucleotide primer pairs were constructed representing each group. Each 25 μl contained 1× EX Taq buffer (Mg2+ plus), 0.4 μM primer, 200 μM concentrations of each dNTP, 0.5 U of EX Taq DNA polymerase (TaKaRa, Shiga, Japan), and 25 ng/μl template DNA from various Salmonella serovars. PCR amplification was performed in a thermal cycler (model PC 808; ASTEC, Fukuoka, Japan) with an initial denaturation at 94°C for 3 min, followed by 30 cycles of 94°C for 45 s, annealing at the temperature listed in Table 3 for each primer pair for 30 s, 72°C for 30 s, and finishing with a final extension at 72°C for 3 min and storage at 4°C thereafter. Amplified products were electrophoresed on 1.5% agarose gels in 0.5× Tris-acetate-EDTA buffer, stained with ethidium bromide, visualized under UV irradiation, and photographed with a digital camera (COOLPIX 4300; Nikon, Tokyo, Japan).
TABLE 3.
Constructed primer pairs used in this study
| Group | Target gene (synonym) | PCR product size (bp) | Annealing temp (°C) | Primer | Sequence | Product |
|---|---|---|---|---|---|---|
| 1 | STM3098 | 423 | 65 | STM3098-f2 | 5′-TTTGGCGGCGCAGGCGATTC | Putative transcriptional regulator |
| STM3098-r2 | 5′-GCCTCCGCCTCATCAATCCG | |||||
| STM4444 | 253 | 65 | STM4444-f | 5′-ATGCCGACTGGTCGTTCCCG | Putative inner membrane protein | |
| STM4444-r | 5′-CCCACGCCGGTCTGAATTGC | |||||
| 2 | STM0349 | 249 | 65 | STM0349-f | 5′-TCGTCGGCTTGGCTTTAACC | Putative outer membrane lipoprotein |
| STM0349-r | 5′-CTGCACGCTGGGTTAACAGG | |||||
| STM4030 | 310 | 65 | STM4030-f | 5′-AAACCGTCCCACTGATGGGG | Putative cytoplasmic protein | |
| STM4031-r | 5′-CGTTAGTGCTCTGCGGCCAT | |||||
| 3 | STM1550 | 187 | 67 | STM1550-f | 5′-AGCTAAGGGAACGGCTTGAA | Putative cytoplasmic protein |
| STM1550-r | 5′-CGTGTCATTTTGTAGACGGC | |||||
| STM2235 | 177 | 65 | STM2235-f | 5′-TGCAGTCAGTGGCAATAACG | Putative phage protein | |
| STM2235-r | 5′-CGTCACCTTTAGCCATCCCA | |||||
| STM2630 | 115 | 65 | STM2630-f | 5′-CTGCCGCAAATCCATTGATG | Hypothetical protein | |
| STM2630-r | 5′-GTATTCAGCGCACTGCCTGG | |||||
| STM2744 | 141 | 65 | STM2744-f | 5′-CCGAAAGCGGCAACGTGCGT | Putative cytoplasmic protein | |
| STM2744-r | 5′-CCGCAGCATCGAAGACCACC | |||||
| STM2752 | 203 | 65 | STM2752-f | 5′-TTATTCCTCCCGGTCCCGGC | Putative glucitol-specific PTS enzyme III | |
| STM2752-r | 5′-CCCGGCGCAGTTAATCACCA | |||||
| STM2755 | 187 | 65 | STM2755-f | 5′-AGCTGCTTTTCGACGCCGGG | Putative hexulose 6 phosphate synthase | |
| STM2755-r | 5′-ACCGCCAGCATATCTGCCCC | |||||
| STM4203 | 316 | 65 | STM4203-f | 5′-CTGCCTTGCAACGTCCTGAA | Putative phage baseplate protein | |
| STM4203-r | 5′-CGCCATAACACCTCCGTTGA | |||||
| STM4214 | 109 | 65 | STM4214-f | 5′-ACGCTCGCCGACGGTCAGGA | Putative cytoplasmic protein | |
| STM4214-r | 5′-CTGGCACCAGGTGACGGCGG | |||||
| STM4497 | 310 | 63 | STM4497-f | 5′-AACAACGGCTCCGGTAATGA | Putative cytoplasmic protein | |
| STM4497-r3 | 5′-TGACAAACTCTTGATTCTGA | |||||
| STM4571 | 154 | 65 | STM4571-f | 5′-TTTGTGCAGGCCTCAGCGGG | Putative outer membrane protein | |
| STM4571-r | 5′-GGGCACTGTCATTGGGAGCA | |||||
| 4 | STM2453 | 270 | 65 | STM2453-f | 5′-TTGTATGCCCTGCGTCCAGG | Putative cytoplasmic protein |
| STM2453-r | 5′-GCTTCCTCCTGCCATCCGGA | |||||
| STM2624 | 284 | 65 | STM2624-f | 5′-CTGGTGAAAGAGCAGGGGCG | Hypothetical protein | |
| STM2624-r | 5′-GCTCCCCCCTTGTTGATGCT | |||||
| 5 | STM1269 | 425 | 65 | STM1269-f | 5′-GTGCAGCACCACTTTTGCCG | Putative chorismate mutase |
| STM1269-r | 5′-GCGCTCTCAGCCACACCATA | |||||
| STM1277 | 289 | 65 | STM1277-f | 5′-AAGCGCGTCTATTTCCCGGC | Putative cytoplasmic protein | |
| STM1277-r | 5′-GGCGAGTATCTTTAGCGGCG | |||||
| 6 | STM0538 | 288 | 65 | STM0538-f | 5′-TCTCTTCCACAGTCCCCGCT | Putative outer membrane protein |
| STM0538-r | 5′-CTGTCGCCGCTGTTTAGCCC | |||||
| STM3532 | 233 | 65 | STM3532-f | 5′-TCCGCCAGTTCCGACCATTG | Putative dihydrodipicolinate synthetase | |
| STM3532-r | 5′-CCTGCGTGCTGGTGCTGCTA | |||||
| 7 | STM4509 | 312 | 65 | STM4509-f | 5′-TGGCGTTCCGTCCTTGTCAG | Putative cytoplasmic protein |
| STM4509-r | 5′-TTGCGCCCTTATCACGACGG | |||||
| 8 | STM0305 | 155 | 65 | STM0305-f | 5′-CGGAAACAGGACGGGGCTGT | Putative cytoplasmic protein |
| STM0305-r | 5′-CCGAAGGCGCAATGGAGGAT | |||||
| STM1408 | 187 | 65 | STM1408-f | 5′-TCCTCTGCAGAACCGAGCCA | Type III secretion system apparatus protein | |
| STM1408-r | 5′-TAAGCGCTTGCGATGCTGCG | |||||
| STM1859 | 115 | 65 | STM1859-f | 5′-AACACGATGCCATTTTCAAT | Putative cytoplasmic protein | |
| STM1859-r | 5′-TTGAGGTCAGTGTGCAATTC | |||||
| STM2056 | 342 | 65 | STM2056-f | 5′-TGATGTTTATCGGCCCCAGC | Propanediol utilization protein | |
| STM2056-r | 5′-CCAGCCTGCGTAAGCCACTC | |||||
| STM3690 | 268 | 65 | STM3690-f | 5′-GAAGTCGTTGGCCGCGTTGA | Putative inner membrane lipoprotein | |
| STM3690-r | 5′-GGAGTTGTTTCCAGCGAGGC | |||||
| STM4057 | 137 | 65 | STM4057-f | 5′-GGTGGCCTCGATGATTCCCG | Putative inner membrane protein | |
| STM4057-r | 5′-CCCACTTGTAGCGAGCGCCG | |||||
| STM4071 | 167 | 65 | STM4071-f | 5′-AAGCGGTGAAGTGTGCCTGT | Putative mannose-6-phosphate isomerase | |
| STM4071-r | 5′-CGGGGTGGCTGTCATTTTCC | |||||
| STM4317 | 220 | 65 | STM4317-f | 5′-GCGAAACCCTGAACCTGCGT | Hypothetical protein | |
| STM4317-r | 5′-CGCAGTGCGGCATTAGGTGA | |||||
| STM4457 | 215 | 65 | STM4457-f | 5′-AGCAACAGCACGCTCCGTCG | Putative transposase | |
| STM4457-r | 5′-GATATGCGACGAAAGCGGCG | |||||
| 9 | STM0339 | 232 | 65 | STM0339-f | 5′-GCCCTACCCGCCACAGCATC | Putative fimbrial chaperone |
| STM0339-r | 5′-CCTGGCCTGCTTTGGGTTGA | |||||
| 10 | STM1006 | 165 | 65 | STM1006-f | 5′-TCTGATTGCGGTTACCGGGC | Excisionase |
| STM1006-r | 5′-TGCGCCTCGATCCACTGATC | |||||
| 11 | STM3752 | 165 | 65 | STM3752-f | 5′-CGGCTTGGCGTATACAGCGA | Putative cytoplasmic protein |
| STM3752-r | 5′-GCCTCCCTCCAGATACACGG | |||||
| 12 | STM0287 | 328 | 65 | STM0287-f | 5′-CGTATTTGCCTGGGGCGGAA | Putative periplasmic protein |
| STM0287-r | 5′-CGCCAGCTTCTGATCCCGTA | |||||
| 13 | STM2434 | 103 | 65 | STM2434-f | 5′-AGATATCTGCGTGGCGCGAG | Putative cytoplasmic protein |
| STM2434-r | 5′-ATCCGGGCCACTCTCCAGCA | |||||
| 14 | STM4596 | 573 | 65 | STM4596-f | 5′-ATGAAGCAGTTAAACGGCGG | Putative inner membrane protein |
| STM4596-r | 5′-GCTGCGTGAAAGCCCGGTTC | |||||
| 15 | STM2955S | 186 | 65 | STM2955-f | 5′-CTTGGCGATGAACTGCGCGA | Putative transcriptional regulator |
| STM2955-r | 5′-CTTTTCCCAGGCCTGCGGCT | |||||
| 16 | STM0409 | 170 | 65 | STM0409-f | 5′-TCGGGAAACCATGGATGGGG | Hypothetical protein |
| STM0409-r | 5′-CACCGGCAAGGACGACACGT |
Analysis of PCR results.
PCR results were scored 1 for positive results (amplified band with expected size) and 0 for negative results. Numerical taxonomy analysis of PCR results for each Salmonella strain was carried out using similarity matrices of SIMQUAL (similarity for qualitative data) and unweighted-pair group method using arithmetic means cluster analysis by the NTSYS-pc (Numerical taxonomy system using multivariate statistical program, version 2.02j; Exeter Software, Setauket, NY) program (31).
RESULTS
BLAST sequence comparison of Salmonella serovar Typhimurium LT2 genes.
A total of 4,451 genes of Salmonella enterica serovar Typhimurium LT2 (NC_003197.ffn) were submitted to the nonredundant database of NCBI using the BLAST program. One hundred fifty-two putative Salmonella-specific genes were selected from 4,451 genes (5 genes, no hits found; 147 genes, match score less than 40.14 and matched length less than 21 bp with nr database of NCBI) (see Tables S1 and S2 in the supplemental material). The 152 genes included some of the Salmonella pathogenicity island 1 and 2 genes but not rfbJ, fliC, and fljB of O antigen or H antigen, which were related with the Kauffmann-White scheme of Salmonella serovar Typhimurium (17, 33).
Sequence comparison of selected genes with various Salmonella genome sequences.
The selected 152 genes of Salmonella serovar Typhimurium LT2 were compared using BLAST with each genomic sequence of 11 Salmonella strains including S. enterica subsp. I, IIIb, and V. The 152 genes were divided into 17 groups by the BLAST output pattern of each of the 152 genes as shown in Table 4 and Table S2 in the supplemental material.
TABLE 4.
Groups of 152 genes of Salmonella enterica serovar Typhimurium LT2 based on comparison patterns between various Salmonella serovars
| Group | No. of genes | Homology witha:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. enterica subsp. I serovar Typhimurium
|
S. enterica subsp. I serovar Typhi
|
S. enterica subsp. I serovar Enteritidis PT4 | S. enterica subsp. I serovar Gallinarum 287/91 | S. enterica subsp. I serovar Pullorum | S. enterica subsp. I serovar Dublin | S. enterica subsp. I serovar Paratyphi A ATCC 9150 | S. enterica subsp. IIIb serovar Diarizonae 611,v1,5,(7) | S. bongori subsp. V 12419 | |||||
| LT2 | DT104 | SL1344 | CT18 | Ty2 | |||||||||
| 1 | 31 | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | 9 | + | + | + | + | + | + | + | + | + | + | + | − |
| 3 | 10 | + | + | + | − or = | − or = | − or = | − | − or = | − or = | − | − | − |
| 4 | 3 | + | + | + | + | + | − | − | − | − or = | − | − | − |
| 5 | 12 | + | + | + | + | + | + | + | + | − or = | + | + | + |
| 6 | 8 | + | + | + | − | − | + | + | + or = | + | − | − | − |
| 7 | 8 | + | + | + | + | + | + | + | − or = | + | + | + | + |
| 8 | 9 | + | + | + | + | + | + | + | + | + or = | + | − or = | − |
| 9 | 5 | + | + | + | + | + | + | + | − or = | + | + | + | − |
| 10 | 3 | + | + | + | + | + | − | − | − | + | − | − | − |
| 11 | 4 | + | + | + | + | + | + | + | + | − or = | + | − | − or = |
| 12 | 2 | + | + | + | + | + | + | − or = | − or = | + | − or = | − or = | − or = |
| 13 | 3 | + | + | + | + | + | + | − or = | + | − or = | + | + | + |
| 14 | 4 | + | + | + | + | + | + | + | + | + | + | − | + |
| 15 | 3 | + | + | + | + | + | − | − | − | − | + | − | − |
| 16 | 3 | + | + | + | + | + | + | + | − | − or = | + | − or = | − or = |
| 17b | 35 | ||||||||||||
| Total | 152 | ||||||||||||
+, high homology, highest matched sequence size is more than 50% of query gene; =, moderate homology, highest matched sequence size is between 20 and 50% of query gene; −, low homology, highest matched sequence size is less than 20% of query gene.
The genes of group 17 showed various comparison patterns. The data are shown in Data S2 in the supplemental material.
Several groups of 17 groups showed a subspecies- or serovar-specific expected comparison pattern as shown in Table 4. First, the 31 genes of group 1 were expected to be present in all Salmonella subspecies, and the 9 genes of group 8 were expected to specifically to be present in each S. enterica subsp. I strain. The 10 genes of group 3 were expected to be specific to Salmonella serovar Typhimurium. Also, the 9 genes of group 2 were expected to be present in all Salmonella subspecies except Salmonella bongori (i.e., Salmonella enterica signature genes). The genes in group 17 showed various comparison patterns with various Salmonella strains.
Almost 152 genes of serovar Typhimurium LT2 shared their sequences with the genomic sequence of S. enterica subsp. I, and a small number of genes were shared with S. enterica subsp. diarizonae and S. bongori and were considered genetically distant from S. enterica subsp. I, as previously reported (10).
Primer construction and PCR results.
A total of 38 primer pairs, representing each group of 152 genes, were constructed as shown in Table 3. PCR was performed with genomic DNA of various Salmonella serovars, as seen in Table S3 in the supplemental material, and the concordance of PCR results with comparison patterns of Table 4 was confirmed. Primer pairs STM3098 and STM4444 belong to group 1 and were candidate genes to amplify PCR product in all Salmonella serovars based on the results of sequence comparison. Primer pair STM3098 amplified PCR products from all Salmonella serovars from S. enterica subsp. I to VI at the expected size. Primer pair STM4444 amplified PCR products in all except S. enterica subsp. arizonae ATCC 13314. These results imply that the 31 genes of group 1 are suitable candidate genes for Salmonella signature genes. STM3098 was suggested as a specific target gene of Salmonella in this study.
The genes of group 2 were expected to be specific to S. enterica subsp. I and IIIb, and the primer pair STM0349 amplified specific PCR products with S. enterica subsp. I, II, and IIIb. Primer pair STM4030 amplified PCR product with S. enterica subsp. I and IIIb, except in some S. enterica subsp. I serovars.
The genes of group 3 were expected to be specific to S. enterica serovar Typhimurium. Among 10 genes, the primer pair STM4497 was highly specific to Salmonella serovar Typhimurium. Other primer pairs of group 3 were also relatively highly specific to Salmonella serovar Typhimurium. STM4203 and STM4214 were reported to be present in Salmonella serovar Paratyphi C and Salmonella serovar Choleraesuis by microarray results, and in this study, these genes were detected by PCR in these two serovars (10). Two primer pairs of group 4 (STM2624 and STM2453) amplified PCR products from Salmonella serovar Typhimurium and Salmonella serovar Typhi, as expected, as well as from some Salmonella serovars of S. enterica subsp. I and II.
The genes of group 8 were specific to S. enterica subsp. enterica (S. enterica subsp. I) by sequence comparison, and 9 primer pairs were constructed. Among the 9 primer pairs, STM4057 and STM0305 showed PCR products with S. enterica subsp. I, except for a few Salmonella serovars, including S. enterica subsp. I. Among 9 candidate genes for the S. enterica subsp. I signature, only the primer pair STM4057 showed specific results with S. enterica subsp. I. These PCR results demonstrated the possibility of detecting S. enterica subsp. I using primer pairs STM4057 and STM0305.
Thirty-eight primer pairs were constructed with genomic DNA of non-Salmonella strains, including food-borne pathogens and Enterobacteriaceae, and showed negative results (see Table S4 in the supplemental material).
Acceptance of sequence comparison among Salmonella serovars.
The PCR results of 38 primer sets are shown in Table S3 in the supplemental material. PCR results showed constant and reproducible results by Salmonella serovars and comparison patterns as shown in Table 4. With some primer pairs, PCR result patterns did not match the pattern of genomic sequence comparison in Table 4. For example, primer pair STM2056, which included group 8, expected to be present in S. enterica subsp. I, as shown in Table 4, was negative for Salmonella serovar Typhi and Salmonella serovar Paratyphi A. Also, with some primer pairs, PCR results were not consistent for serovar or subspecies. For example, primer pair STM2630 yielded different PCR results with 6 strains of S. enterica subsp. II (positive result, 2 strains; negative result, 4 strains). Primer pair STM2453 PCR results differed with the same serovar (Salmonella enterica serovar Heidelberg; positive result, 2 strains; negative result, 1 strain).
Subtyping of Salmonella using PCR result patterns.
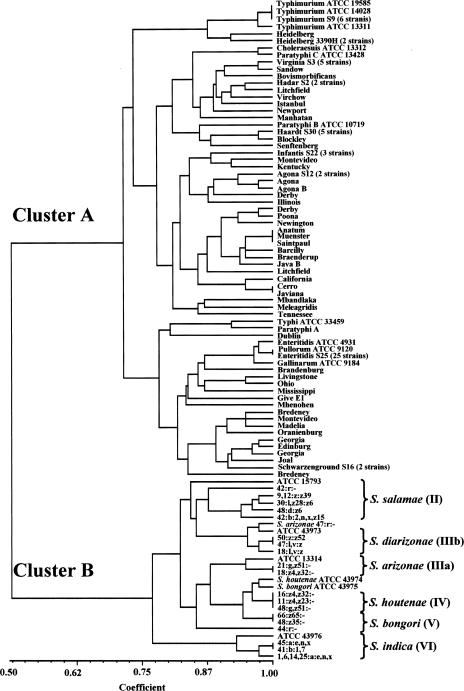
The relationship between serovars was analyzed using the NTSYS-pc program, and positive or negative PCR results were generated with the 38 primer pairs. A phylogenetic tree of various Salmonella serovars based on PCR results is shown in Fig. 1. Salmonella serovars, including S. enterica subsp. I to VI, were grouped into two clusters. One cluster (cluster A) is S. enterica subsp. I, and the other cluster (cluster B) consists of the rest of the Salmonella subspecies. These data mean that S. enterica subsp. I, which causes infections in warm-blooded animals, could be detected by PCR, which would be useful for epidemiology. Only S. enterica subsp. VI was strictly subdivided in cluster B; other subspecies were not strictly discriminated. Nine strains of S. enterica serovar Typhimurium were clustered in a narrow portion of the phylogenetic tree shown in Fig. 1. Strains in the same serovar, such as serovar Enteritidis (26 strains), serovar Heidelberg (3 strains), Salmonella enterica serovar Schwarzenground (2 strains), Salmonella enterica serovar Haardt (5 strains), Salmonella enterica serovar Virginia (5 strains), Salmonella enterica serovar Agona (3 strains), and Salmonella enterica serovar Infantis (3 strains), were genetically similar, as shown in Fig. 1. However, some strains within the same serovar, such as Salmonella enterica serovar Bredeney (2 strains), Salmonella enterica serovar Derby (2 strains), Salmonella enterica serovar Georgia (2 strains), Salmonella enterica serovar Litchfield (2 strains), and Salmonella enterica serovar Montevideo (2 strains) were not clustered. Interestingly, PCR results of certain strains in the same Salmonella serovar were not always the same patterns. This result provides further evidence that there are many genotypes in the same serovar (8, 26).
FIG. 1.
Phylogenetic tree of various Salmonella strains, including S. enterica subsp. I to VI, constructed using PCR results from 38 primer pairs by the NTSYS-pc program.
The phylogenetic tree in Fig. 1 was similar to microarray and MLEE results for Salmonella in previous studies (6-10, 16, 26-28). Salmonella serovars Enteritidis, Gallinarum, and Pullorum, which are considered avian-adapted serovars, were closely clustered, but serovar Enteritidis and serovar Pullorum were not discriminated in this study. Also, other strains were closely clustered, such as Salmonella serovar Choleraesuis with serovar Paratyphi C, serovar Heidelberg with serovar Typhimurium, serovar Montevideo with Salmonella enterica serovar Oranienburg, and serovar Typhi with serovar Paratyphi A.
DISCUSSION
Comparative genomics is an efficient way to identify all of the genetic differences between closely related bacteria (13). The Salmonella genus is a suitable model bacteria for genomic sequence comparison because more than 2,500 Salmonella serovars are very closely related and genome sequencing projects including various Salmonella serovars have been completed or are in progress. Also, the sequence data of various Salmonella serovars are publicly available. Salmonella genome sequences have been anticipated to usher in a new era of comparative genomics in Salmonella biology and are expected to provide a valuable resource to explore how and why differences arose between Salmonella serovars with different host specificities and virulence (10).
Until now, various DNA-based methods have been applied to find marker genes for specific detection of Salmonella, Salmonella serovar Typhimurium, serovar Enteritidis, and serovar Typhi, including suppression subtractive hybridization, microarray analysis, and PCR (1, 15, 17, 18, 27). In the case of suppression subtractive hybridization and microarray analysis, specific probes or genes could be highlighted, but these methods are also labor-intensive, expensive, complicated, and time-consuming. In this study, an in silico method with genome sequences of Salmonella was used to find genes specific to Salmonella. Constructed primers of selected genes were evaluated to compare the results between in silico and substantive experiments using PCR. Specific genes of Salmonella, S. enterica subsp. I, and Salmonella serovar Typhimurium were identified using genomic sequence comparison, and their specificities were evaluated across various Salmonella genomic DNAs. In addition, PCR results were accordant with genomic sequence comparison, demonstrating the effectiveness of genomic sequence comparison (Table 4; see Tables S3 and S4 in the supplemental material).
At the same time, this method has a limitation in that genomic sequence comparison is only possible when a database of genomic sequences is available. In the case of Salmonella, 12 genome sequences were used in this study. But only 3 genomic sequences (Salmonella serovar Typhimurium LT2, serovar Typhi CT18, and serovar Typhi TY2) were completed, and the genomic sequences of the other 9 strains were only available as raw sequence data. The inconsistency of these PCR results may be attributed to the fact that, in this study, genomic sequence comparison of Salmonella serovars was limited to only a few genome sequences (including S. enterica subsp. I, IIIb, and V). In addition, numerous cases of isolates of the same serovar with markedly different chromosomal genotypes have been reported (26, 29). It is impossible to characterize more than 2,500 Salmonella serovars with 12 genomic sequences. Nonetheless, efficient comparisons of 12 genomic sequences are expected to provide not only marker genes of Salmonella but also an easier approach to finding biological characteristics of Salmonella.
From the sequence comparison, the 31 genes of group 1 were expected to be present in all Salmonella subspecies. This group did not include previously reported specific target genes of Salmonella such as the invA and ompC genes (18, 21), as these genes were present not only in Salmonella but also in other closely related bacteria, such as Escherichia coli O157:H7 or Citrobacter freundii, and were eliminated through sequence comparison due to high homology with other related bacteria. In previous reports, 56 genes were suggested as Salmonella signature genes using comparison of genomic sequences with the four other enterobacteria and microarray analysis of PCR-amplified whole open reading frames of Salmonella serovar Typhimurium LT2 with 22 Salmonella strains (27). Compared with the results of genome comparison, only 4 genes (STM0699, STM2064, STM2549, and STM3098) from 31 genes of group 1 overlapped with previously reported Salmonella signature genes from microarray data. The different result of Salmonella signature genes between previous reports and this study comes from the different genome sequences used in each study. As a result, criteria for evaluating gene presence and absence were different.
In addition, the genes of group 8 were specifically expected to be present in each S. enterica subsp. I strain. In previous reports, 31 genes were suggested as a signature to S. enterica subsp. I, as they were found in strains belonging to S. enterica subsp. I but not in strains of the other subspecies using cDNA microarray analysis (26, 27). The 9 genes of group 8 were suggested as candidate S. enterica subsp. I signature genes, and only STM0305 was included in the 31 previously reported genes (26, 27). The 10 genes of group 3 were expected to be specific to Salmonella serovar Typhimurium. STM4203 and STM4214 were reported to be present not only in serovar Typhimurium but also in serovar Paratyphi C and serovar Choleraesuis. STM4497 was included in Salmonella serovar Typhimurium signature genes of STM4488 to STM4497 in a previous report (10). The 9 genes of group 2 were expected to be present in all Salmonella subspecies except Salmonella bongori (i.e., Salmonella enterica signature genes). In this group, STM1406 and STM1407 of Salmonella pathogenicity island 2 were included which were related with the type III secretion system of Salmonella (19). STM2773 (iroB), which was known as a Salmonella enterica-specific target gene, was not included in group 2 (3). The genes in group 17 showed various comparison patterns with various Salmonella strains. Among group 17, STM0894 and STM0902 (Fels-1 prophage) were only specific to the S. enterica serovar Typhimurium LT2. Fels-1 prophage was previously reported as specific to S. enterica serovar Typhimurium LT2 using microarray analysis (26). Fels-2 prophage genes (STM2716, STM2718, STM2721, STM2698, and STM2710) of group 17 were also present in other serovars, including serovar Typhimurium SL1344, but absent in serovar Typhimurium DT104, as reported previously. STM2344 and STM3736 of group 7 were previously reported as Salmonella signature genes by microarray analysis, but in this study, these genes were excluded because of low homology results with serovar Pullorum (27).
Comparative genomics between Salmonella species would provide not only genotyping and identification of Salmonella subspecies but also more information about the host specificity of Salmonella between subspecies and serovars. For example, S. enterica subsp. I signature genes might be target genes which differentiate S. enterica subsp. I that infect warm-blooded animals (including humans) from other Salmonella subspecies. Also, gene profile differences between host-specific serovars and host general serovars may give clues as to how and why the differences arose.
In an attempt to improve on serological typing using the Kauffmann-White scheme, many molecular methods have been applied to type or characterize Salmonella. But these methods have not provided enough discriminative power to resolve all Salmonella serovars. Also, these methods have been available only in a few reference laboratories. Recently, the onset of microarray and genomic sequencing technology has allowed for differences among Salmonella strains to be characterized at single-gene resolution (10, 14, 26). Microarrays appear to subdivide Salmonella with accuracy, but this method is too expensive to profile the 2,500 serovars of Salmonella and is only possible in a specialized laboratory. Further, microarray methods have disadvantages in sensitivity and scale that prevent application to field identification and detection of Salmonella and Salmonella serovar Typhimurium in the food industry. In contrast, PCR has the potential to become a powerful alternative in microbiological diagnostics due to its simplicity, rapidity, and accuracy.
In this study, 38 primer pairs were evaluated by PCR to subtype and characterize Salmonella. The phylogenetic tree in Fig. 1 generally agrees with the results of microarray analysis from several previously published reports (6-8, 26), supporting the successful application of genome sequence comparison for characterization of Salmonella strains using PCR. There were some discrepancies between this study and previous reports. For example, Salmonella serovar Enteritidis was previously observed as close to serovar Dublin with serovar Pullorum and serovar Gallinarum by MLEE and microarray results. In this study, serovar Dublin and serovar Enteritidis were in the same node but not closely clustered as shown in Fig. 1 (10). It is difficult to define a Salmonella serovar as monophyletic or polyphyletic by PCR results because of the limited number of serovars and primer pairs used in this study. However, several serovars might be monophyletic, including serovars Typhimurium, Enteritidis, Heidelberg, Virginia, Agona, and Haardt. Based on the results of this study, genomic sequence comparison can inform new microarray design to minimize the number of target genes and spots for effective genotyping and detection of bacteria. Also, in this study, we suggest target signature genes of Salmonella, Salmonella serovar Typhimurium, and S. enterica subsp. I by PCR results, providing a rapid and accurate protocol for epidemiological studies.
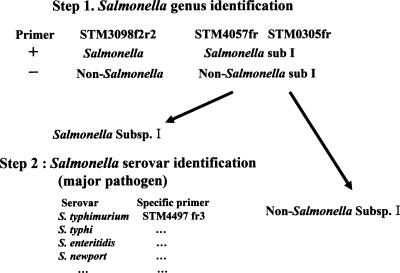
From PCR results of 38 primer pairs, we suggest a new identification scheme of the major pathogenic Salmonella strains in Fig. 2. This identification scheme consists of 2 PCR steps. The first step is Salmonella genus identification, including S. enterica subsp. I, by three primer pairs. At the results of step 1, samples are discriminated as S. enterica subsp. I or not. In the case of a positive test for S. enterica subsp. I, several sets of primers specific to major pathogens are evaluated to identify serovars. We are designing the specific primer pair of each pathogenic Salmonella serovar using genomic sequences in our laboratory. This Salmonella identification scheme needs to be evaluated with a greater variety of Salmonella serovars, including blind tests by consortium with laboratories in the other countries to examine the accuracy of Salmonella identification in epidemiological and taxonomical studies. Also, genotypic diversity within the Salmonella serovars must be considered. We are currently exploring multiplex PCR to allow simple identification of specific Salmonella serovars that cause disease.
FIG. 2.
New identification scheme of major pathogenic Salmonella strains and Salmonella spp. based on PCR results without serological testing. +, positive result; −, negative result.
In conclusion, the coding sequence region of Salmonella enterica serovar Typhimurium LT2 was compared with various Salmonella serovars, and selected genes were applied to genotyping and identification of Salmonella species. These results imply that genome sequence comparison can be successfully applied as a powerful tool for genotyping of Salmonella and can provide an easier means to detect and characterize Salmonella. In addition, we suggest target genes to differentiate between Salmonella subspecies and serovars, although these require further investigation. These methods and results can be used to expand investigations into different host ranges, distinct disease symptoms in different hosts, and specific detection of Salmonella serovars.
Supplementary Material
Acknowledgments
This work was supported by a research grant (02-PJ1-PG1-CH08-0002) from the Korea Health Industry Development Institute (KHIDI) and the Korean Ministry of Education through the Brain Korea 21 program.
We thank Reiner Helmuth and Burkhard Malorny of the Federal Institute for Risk Assessment (BFR, Molecular Biology, National Salmonella Reference Laboratory, Germany) and K. H. Seo of the U.S. Food and Drug Administration (FDA, CFSAN/OPDFB) for the kind donation of Salmonella strains.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Agron, P. G., R. L. Walker, H. Kinde, S. J. Sawyer, D. C. Hayes, J. Wollard, and G. L. Andersen. 2001. Identification by subtractive hybridization of sequence specific for Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 67:4984-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumler, A. J., F. Heffron, and R. Reissbrodt. 1997. Rapid detection of Salmonella enterica with primers specific for iroB. J. Clin. Microbiol. 35:1224-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumler, A. J., R. M. Tsolis, T. A. Ficht, and L. G. Adams. 1998. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66:4579-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belgrader, P., W. Benett, D. Hadley, J. Richards, P. Stratton, R. Mariella, and F. Milanovich. 1999. PCR detection of bacteria in seven minutes. Science 284:449-450. [DOI] [PubMed] [Google Scholar]
- 6.Beltran, P., J. M. Musser, R. Helmuth, J. J. Farmer III, W. M. Frerichs, I. K. Wachsmuth, K. Ferris, A. C. McWhorter, J. G. Wells, A. Cravioto, and R. K. Selander. 1988. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc. Natl. Acad. Sci. USA 85:7753-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltran, P., S. A. Plock, N. H. Smith, T. S. Whittam, D. C. Old, and R. K. Selander. 1991. Reference collection of strains of the Salmonella typhimurium complex from natural population. J. Gen. Microbiol. 137:601-606. [DOI] [PubMed] [Google Scholar]
- 8.Boyd, E. F., F. S. Wang, P. Beltran, S. A. Plock, K. Nelson, and R. K. Selander. 1993. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J. Gen. Microbiol. 139:1125-1132. [DOI] [PubMed] [Google Scholar]
- 9.Boyd, E. F., F. S. Wang, T. S. Whittam, and R. K. Selander. 1996. Molecular genetic relationships of the salmonellae. Appl. Environ. Microbiol. 62:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, K., S. Baker, C. C. Kim, C. S. Detweiler, G. Dougan, and S. Falkow. 2003. Genomic comparison of Salmonella enterica serovars and Salmonella bongori by use of an S. enterica serovar Typhimurium DNA microarray. J. Bacteriol. 185:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y. H., S. Y. Kim, and Y. H. Chang. 2003. Prevalence and antibiotic susceptibility of Salmonella isolated from Foods in Korea from 1993 to 2001. J. Food Prot. 66:1154-1157. [DOI] [PubMed] [Google Scholar]
- 12.Deng, W., S. R. Liou, G. Plunkett III, G. F. Mayhew, D. J. Rose, and F. R. Blattner. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 185:2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards, R. A., G. J. Olsen, and S. R. Maloy. 2002. Comparative genomics of closely related salmonellae. Trends Microbiol. 10:94-99. [DOI] [PubMed] [Google Scholar]
- 14.Garaizar, J., S. Porwollik, A. Echeita, A. Rementeria, S. Herrera, R. M. Wong, J. Frye, M. A. Usera, and M. McClelland. 2002. DNA microarray-based typing of an atypical monophasic Salmonella enterica serovar. J. Clin. Microbiol. 40:2074-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose, K., K. I. Itoh, H. Nakajima, T. Kurazono, M. Yamaguchi, K. Moriya, T. Ezaki, Y. Kawamura, K. Tamura, and H. Watanabe. 2002. Selective amplification of tyv (rfbE), prt (rfbs), viaB, and fliC genes by multiplex PCR for identification of Salmonella enterica serovars Typhi and Paratyphi A. J. Clin. Microbiol. 40:633-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, J., N. H. Smith, K. Nelson, P. B. Crichton, D. C. Old, T. S. Whittam, and R. K. Selander. 1993. Evolutionary origin and radiation of the avian-adapted non-motile salmonellae. J. Med. Microbiol. 38:129-139. [DOI] [PubMed] [Google Scholar]
- 17.Lim, Y. H., K. Hirose, H. Izumiya, E. Arakawa, H. Takahashi, and H. Watanabe. 2003. Multiplex polymerase chain reaction assay for selective detection of Salmonella enterica serovar Typhimurium. Jpn. J. Infect. Dis. 56:151-155. [PubMed] [Google Scholar]
- 18.Malorny, B., J. Hoorfar, C. Bunge, and R. Helmuth. 2003. Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl. Environ. Microbiol. 69:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus, S. L., J. H. Brumell, C. G. Pfeifer, and B. B. Finlay. 2000. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2:145-156. [DOI] [PubMed] [Google Scholar]
- 20.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 21.Olsen, J. E., S. Aabo, W. Hill, S. Notermans, K. Wernars, P. E. Granum, T. Popovic, H. N. Rasmussen, and O. Olsvik. 1995. Probes and polymerase chain reaction for detection of food-borne pathogens. Int. J. Food Microbiol. 28:1-78. [DOI] [PubMed] [Google Scholar]
- 22.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. G. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Kroghk, T. S. Larsenk, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 23.Pickup, R. W., G. Rhodes, and J. Hermon-Taylor. 2003. Monitoring bacterial pathogens in the environment: advantages of a multilayered approach. Curr. Opin. Biotechnol. 14:319-325. [DOI] [PubMed] [Google Scholar]
- 24.Popoff, M. Y. 2001. Antigenic formulas of the Salmonella serovars, 8th revision. W.H.O. Collaborating Centre for Reference and Research on Salmonella. Institute Pasteur, Paris, France.
- 25.Popoff, M. Y., J. Bockemuhl, and L. L. Gheesling. 2003. Supplement 2001 (no. 45) to the Kauffmann-White scheme. Res. Microbiol. 154:173-174. [DOI] [PubMed] [Google Scholar]
- 26.Porwollik, S., E. F. Boyd, C. Choy, P. Cheng, L. Florea, E. Proctor, and M. McClelland. 2004. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J. Bacteriol. 186:5883-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porwollik, S., R. M. Wong, and M. McClelland. 2002. Evolutionary genomics of Salmonella: gene acquisitions revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 99:8956-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves, M. W., G. M. Evins, A. A. Heiba, B. D. Plikaytis, and J. J. Farmer III. 1989. Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J. Clin. Microbiol. 27:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selander, R. K., P. Beltran, N. H. Smith, R. Helmuth, F. A. Rubin, D. J. Kopecko, K. Ferris, B. D. Tall, A. Cravioto, and J. M. Musser. 1990. Evolutionary genetic relationships of clones of Salmonella serovars that cause human typhoid and other enteric fevers. Infect. Immun. 58:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo, K. H., I. E. Valentin-Bon, R. E. Brackett, and P. S. Holt. 2004. Rapid, specific detection of Salmonella enteritidis in pooled eggs by real-time PCR. J. Food Prot. 67:864-869. [DOI] [PubMed] [Google Scholar]
- 31.Smith, M. L., and J. B. Anderson. 1989. Restriction fragment length polymorphism in mitochondrial DNAs of Armillaria: identification of north American biological species. Mycol. Res. 93:247-256. [Google Scholar]
- 32.Versalovic, J., and J. R. Lupski. 2002. Molecular detection and genotyping of pathogens: more accurate and rapid answers. Trends Microbiol. 10:S15-S21. [DOI] [PubMed] [Google Scholar]
- 33.Wyk, P., and P. Reeves. 1989. Identification and sequence of the gene for abequose synthease, which confers antigenic specificity on group B salmonellae: homology with galactose epimerase. J. Bacteriol. 171:5687-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.