Abstract
Blue light as an environmental cue plays a pivotal role in controlling the progression of the sexual life cycle in the green alga Chlamydomonas reinhardtii. Phototropin was considered a prime candidate for the blue-light receptor involved. By using the RNA interference method, knockdown strains with reduced phototropin levels were isolated. Those with severely reduced levels of this photoreceptor were partially impaired in three steps of the life cycle: in gametogenesis, the maintenance of mating ability, and the germination of zygotes. These observations suggest that phototropin is the principal sensory molecule used by this alga for the control of its life cycle by light.
Light is an environmental factor of particular importance for plants as it controls growth and development. Several classes of photoreceptors including UV- and blue-light receptors and red/far-red receptors are involved in these responses of plants to light (reviewed in ref. 1). The receptors that mediate the response to red/far-red light, the phytochromes, seem to be absent in Chlamydomonas (ref. 2 and unpublished data). However, some green algae, including Chlamydomonas reinhardtii, were shown to possess rhodopsin-like photoreceptors that respond to yellow/green light. One of these photoreceptors specifically controls photomovement responses (3).
Two photoreceptors that respond to the blue region of the electromagnetic spectrum have been defined in recent years at the molecular level in higher plants and in Chlamydomonas, the cryptochromes and the phototropins (4–9). In C. reinhardtii, a single cryptochrome gene (CPH1) has been identified (9). This gene encodes a protein that is light-labile, because the protein disappears after irradiation of the cells (G. Small, personal communication). The biological function of this cryptochrome has yet to be determined. C. reinhardtii also possesses a single phototropin gene (Phot) (8). A hallmark of the phototropins are two tandemly arranged light, oxygen, or voltage sensing (LOV) domains followed by a serine/threonine kinase domain. The LOV domains of phototropin functioned as light sensors and underwent a self-contained photocycle. This light sensing seems to occur via the formation of a stable adduct between the flavin mononucleotide chromophore and a cysteine residue conserved in both LOV domains (10, 11).
The C. reinhardtii Phot gene encodes a protein with a structure typical for that of members of the phototropin family, i.e., two LOV domains that may function in flavin mononucleotide binding and a serine/threonine kinase domain (8, 11). In contrast to the C. reinhardtii cryptochrome, phototropin did not seem to be significantly affected by light treatment because its protein levels were shown to be about the same in the dark and in the light (8).
The biological function of phototropins has been defined in higher plants, primarily by mutant studies. Two phototropin genes, PHOT1 and PHOT2, have been analyzed so far in Arabidopsis. Mutants defective in PHOT1 lack phototropic responsiveness at low but not at high fluences of unilateral blue light (12). These mutants also are impaired in the initial and rapid inhibition of hypocotyl growth after irradiation with blue light (13). Mutants with a defective PHOT2 gene exhibit a lack in the chloroplast avoidance response in strong blue light but normal chloroplast accumulation at low-intensity light (14, 15). Double mutants defective in PHOT1 and PHOT2 exhibit an impaired phototropic response under both low- and high-intensity blue light, are devoid of chloroplast relocation at both low and high light intensities (16), and, in addition, are defective in the blue-light regulation of stomatal opening (17). These results indicate that PHOT1 and PHOT2 exhibit, in part, functionally redundant roles in regulating blue-light responses.
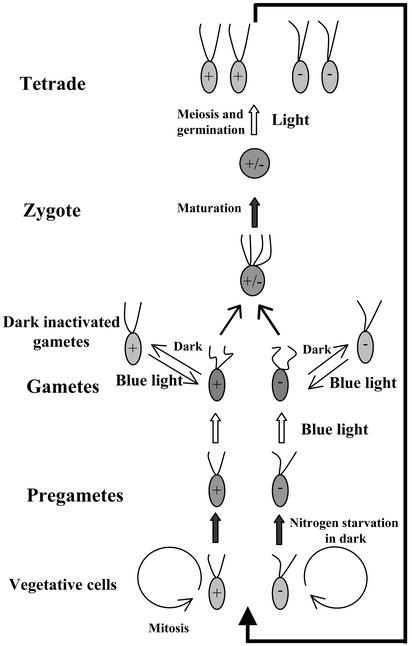
In the sexual life cycle of C. reinhardtii, gametes are generated from haploid vegetative cells, and these gametes may fuse to form diploid zygotes (Fig. 1). After a period of maturation, the zygotes are ready for meiosis and germination (18). In this cycle, light has been shown to be required at three steps: gamete formation, the maintenance of mating competence in gametes, and zygote germination (19–22). Gametogenesis requires the consecutive action of two extrinsic signals. The first signal is nitrogen starvation that, when vegetative cells are incubated in the dark, results in the formation of mating-incompetent pregametes. The second signal, blue light, causes pregametes to mature to gametes. The action spectrum of this response indicates that the photoreceptor involved has properties typical for blue-light receptors that have been defined in higher plants and various lower eukaryotes (23). Incubation of these gametes in the dark results in a rapid loss of their mating competence. These dark-inactivated gametes, by irradiation with blue light, regain their mating ability. However, although the light-induced conversion of pregametes to gametes is a slow process that requires cytoplasmic protein synthesis, the restoration of mating by light occurs rapidly and in the absence of cytoplasmic protein synthesis (20, 24). Separate signaling pathways seem to control these photoresponses. For the last step of the life cycle, i.e., zygote germination, an initiating irradiation for ≈3 h is sufficient for a quantitative induction of meiosis. Subsequent processes, i.e., meiosis and germination, may occur in the dark during the following 20 h (21). Because mutant analyses have indicated the sharing of at least one gene product between the light signaling pathways that control pregamete to gamete conversion and zygote germination, the participation of the same photoreceptor in these response pathways has been anticipated (21).
Figure 1.
Life cycle of C. reinhardtii with indication of light-dependent steps.
In C. reinhardtii, the molecular identification of a cryptochrome gene and a phototropin gene allowed us to design strategies to define the photoreceptor(s) that control the sexual life cycle. To test whether phototropin plays a role in the light control of the sexual life cycle we generated strains with reduced phototropin levels, using the RNA interference (RNAi) methodology. Here we report that diminished phototropin levels indeed affect all three light-dependent steps.
Materials and Methods
Strains and Culture Conditions.
C. reinhardtii wild-type strain CC-124 (mt−), obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC), was used throughout the experiments. CF14 (mt+), a sibling from a cross between CC-1010 (mt+) and 137C (mt−), served as the mating partner. Chlamydomonas cells were grown either in liquid or on solid (supplemented with 1.5% agar) Tris-acetate-phosphate (TAP) media (18) at 23°C in the dark or under continuous irradiation with white light (fluence rate 30 μmol⋅m−2⋅s−1) provided by fluorescent tubes (L 36W/25; Osram, Munich). Flasks with liquid cultures were incubated on a rotary shaker.
Gametogenesis.
For standard gametogenesis, liquid cultures of vegetative cells were centrifuged (2,000 × g for 5 min) and resuspended in nitrogen-free (TAP-N) medium at a density of 1 × 107 cells per ml. These cells were either incubated for 16 h in the light to generate gametes or in the dark to generate pregametes. Gametes were obtained from pregametes by exposing them to light (30 μmol⋅m−2⋅s−1). The mating ability of gametes was assayed by mixing the cells to be tested with a 3-fold excess of mature gametes of opposite mating type. These were generated by resuspending vegetative cells grown on plates in TAP-N medium at a density of 1–2 × 107 cells per ml, followed by an incubation with continuous light for 16–24 h. After incubation in the dark for 1 h, the percentage of gametes was determined as described (24). Reductions in light intensity were achieved by using combinations of copper screens (23). For an increase in light intensity, the distance between the fluorescent tubes and the cultures was reduced.
Protein Extraction and Immunoblot Analyses.
Cells were sedimented by centrifugation (3,000 × g for 5 min) and resuspended in 0.1 M DTT/0.1 M Na2CO3. Then, 0.66 vol of 5% SDS/30% sucrose was added. In cases where the lysates were too viscous, samples were sonicated. Homogenization of the suspensions was achieved by rapid shaking at room temperature for 20 min. The protein concentration was determined by staining with amido black, using BSA as a standard (25). After separation of the proteins by SDS/PAGE (26), they were transferred to poly(vinylidene difluoride) (PVDF) membranes (Hybond-P, Amersham Biosciences). Peroxidase-conjugated anti-rabbit serum (Sigma) was used to detect the primary Abs directed against C. reinhardtii phototropin (8). For signal detection we used the enhanced chemiluminescence system (Amersham Biosciences).
RNA Isolation and Northern Blot Hybridization.
Total RNA was isolated and processed for blotting and hybridization as described (27, 28). The GLE gene probe (29) and the CBLP gene, encoding a Chlamydomonas Gβ-like polypeptide (30), were kindly provided by Y. Matsuda (Kobe University, Kobe, Japan) and M. Wettern (Technische Universität, Braunschweig, Germany), respectively.
Nucleic Acid Manipulations and Transformation.
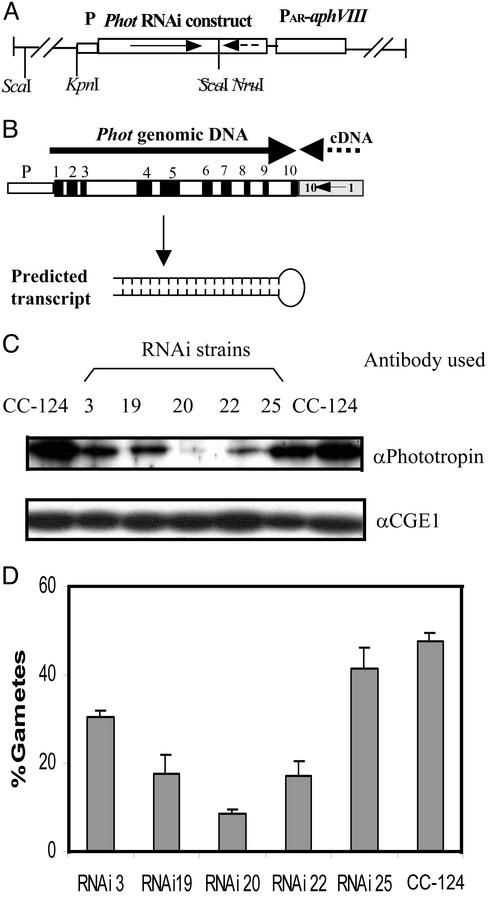
Genomic DNA that includes the C. reinhardtii Phot gene (8) was cloned as a 10.6-kb SalI fragment into vector pBluescript II SK(+) (Stratagene). To generate the RNAi construct, the plasmid was cut with KpnI and ScaI. ScaI cuts within exon 10. A fragment of 6.7 kb (containing ≈1.7 kb of the promoter and exons 1–10 of Phot) was inserted into plasmid AV394090, which contains the complete Phot cDNA (31). Before the insertion, plasmid AV394090 was cut with KpnI and NruI, deleting exons 11–13 and the 3′ UTR of Phot cDNA. NruI also cuts within exon 10, 156 bp upstream from the ScaI site. Into the blunt-ended BamHI site upstream from the Phot cDNA, the aphVIII gene, driven by the HSP70A-RBCS2 fusion promoters (32), was inserted. The aphVIII gene was isolated as a PvuII fragment from plasmid pIS103 (33). The resulting construct (pCB1136) is shown in Fig. 2A. In all constructs, the newly formed junctions were subjected to sequencing. Before transformation, the plasmid was linearized with ScaI.
Figure 2.
Reduction in phototropin levels by using the RNAi technique. (A) The plasmid used for transformation. (B) Phot construct generated for a reduction of in vivo phototropin levels. Black boxes represent exons, white boxes represent introns, and the cDNA is indicated by a stippled line. The orientations of the gene segments are indicated by arrows. The predicted structure of the resulting RNA is given below the construct. (C) Relative amounts of phototropin in different transformants as compared with wild-type strain CC-124. For this assay, an Ab directed against Chlamydomonas phototropin (8) was used. For a loading control, an Ab that reacts with the cochaperone CGE1 (47) was used. (D) Assay of transformants that exhibited reduced levels of phototropin for pregamete to gamete conversion. Pregametes of strain CC-124 and various transformants of CC-124 harboring the RNAi construct were generated by incubation in TAP-N for 16 h in the dark. Pregametes were then irradiated for 90 min with white light at a fluence rate of 0.64 μmol⋅m−2⋅s−1. Then the cells were mated and the percentage of gametes was calculated as described in Materials and Methods.
To prepare the cells for transformation, the cell wall was removed by treatment with autolysin that was produced as described (18). The DNA was introduced into the cells by using the glass bead method (34). Transformants were selected on plates with 10 μg/ml paromomycin (Sigma) and tested for phototropin levels by Western blot analyses.
Zygote Germination.
For the generation of zygotes with lowered phototropin levels we used a strategy in which either one or both mating partners harbored the RNAi construct. To generate Phot RNAi strains of mating type mt+, we crossed the RNAi20 strain (mt−) with CF14 (mt+) by using the standard protocol (18). Among the progeny of tetrads, we selected mt+ clones that grew on paromomycin and assayed them for their phototropin levels. One of the progeny strains, RNAi20(+), which showed very low levels of phototropin, was selected as mating partner for the RNAi20(−) strain.
To produce zygotes, gametes were generated according to our standard protocol, and cells after 2 h of mating were diluted and plated on TAP plates with 4% agar. These plates were illuminated for 24 h and then transferred to the dark for 5 days. The bulk of vegetative cells was then removed with a razor blade. To kill the remaining vegetative cells, the plates were treated with chloroform vapors for 30 sec. These two steps were performed in the dark. Subsequently, the plates were illuminated for 1–5 h with white light (fluence rate 30 μmol⋅m−2⋅s−1). After this irradiation, the plates were transferred back into the dark, and 11 days later, the number of germinated and nongerminated zygotes was counted by using a dissecting microscope. In this assay system, only single zygotes, either germinated or nongerminated, were observed on the plates.
Results
Because Chlamydomonas mutants defective in Phot are not available, we applied the RNAi technology to generate strains with reduced phototropin levels. To achieve the desired double-stranded RNA level that fluctuates in parallel with the endogenous Phot RNA, a fragment of Phot cDNA covering exons 1–10 was linked in inverse orientation to the corresponding genomic fragment under control of the endogenous Phot promoter (Fig. 2B). We surmised that after excision of the introns, a self-complementary hairpin RNA might be formed (Fig. 2B). The resulting hairpin RNA would be a perfect inverted repeat of 1.4 kb with a linker of 156 bases between the sense and antisense regions. Such structures were reported to be efficient in triggering posttranscriptional gene silencing in plants (35, 36).
The aphVIII gene (conferring resistance to paromomycin) was cloned (Fig. 2A) into the construct with the modified Phot gene, which may be used as a selection gene in C. reinhardtii (32). Paromomycin-resistant transformants of wild-type strain CC-124 were screened for their Phot levels. Eight of 80 transformants exhibited reduced levels of phototropin, although the levels varied in individual clones (Fig. 2C). In one transformant (RNAi20), the Phot levels were reduced to <10% from that of wild type.
Five transformants were assayed for their gamete-forming potential. For this purpose, pregametes were exposed to white light of low fluence rate (0.64 μmol⋅m−2⋅s−1) for 90 min. As shown in Fig. 2D, all transformants tested exhibited a decrease in gamete formation, and its degree approximately correlated with the level of Phot protein detected by specific Abs. These data suggest that the reduction in phototropin levels was the cause for the decrease in pregamete to gamete conversion observed. Strain RNAi20, the principal transformant used in subsequent experiments, was subjected to additional tests. In Southern blot experiments we determined that this strain harbored a single complete copy of the transgene (data not shown). The stability of the phenotype, i.e., reduced phototropin levels, was confirmed by a cross with strain CF14. Among nine paromomycin-resistant products analyzed, eight exhibited a reduction in phototropin levels similar to that seen in the parental RNAi20 strain; one showed an intermediate level (data not shown).
Assay of the RNAi20 strain for its behavior during gametogenesis confirmed that, as the parental strain, it strictly depends on light for the formation of sexually competent gametes. If gametogenesis was performed with continuous irradiation, no significant differences as compared with the parental strain were observed.
Effect of Phot Knockdown on the Kinetics and Fluence Dependence of Pregamete to Gamete Conversion.
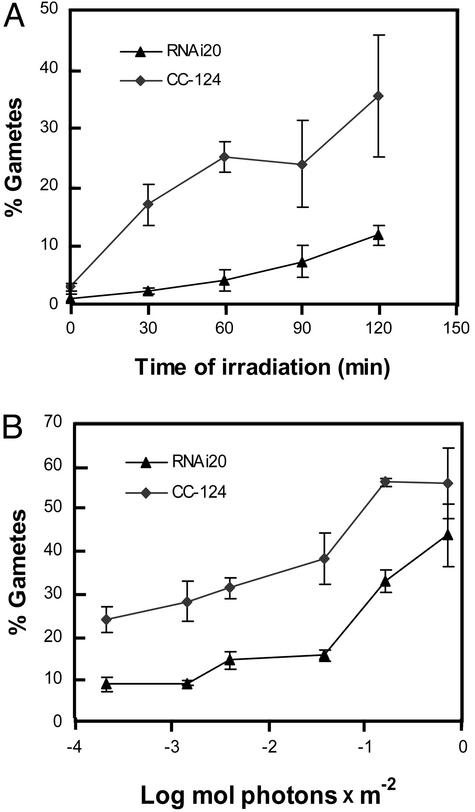
Because the fluence rate determined the rate of pregamete differentiation (24), a reduction in photoreceptor molecules involved was expected to affect the kinetics of pregamete to gamete conversion. After irradiation with low-intensity light (0.64 μmol⋅m−2⋅s−1), the RNAi20 strain indeed exhibited a delayed and reduced increase in the percentage of gametes as compared with wild type (Fig. 3A). Similar observations were made with strain RNAi19 (data not shown).
Figure 3.
Test of strain RNAi20 for pregamete to gamete conversion. (A) Kinetics of pregamete to gamete conversion. Pregametes of the wild-type (CC-124) and the RNAi20 strains were generated by incubation in TAP-N medium for 16 h in the dark. The fluence rate of white light used was 0.64 μmol⋅m−2⋅s−1. (B) Fluence response curves for pregamete to gamete conversion of strain RNAi20 and wild type. Pregametes were irradiated for 90 min with white light of the fluences indicated. Generation of pregametes and assay for gametes after irradiation were done as described in Fig. 2.
A different illustration of the RNAi20 phenotype is shown in Fig. 3B. Here, the percentage of gametes generated from pregametes after irradiation with white light for 90 min is plotted against the total fluence applied, which ranged (in photons) from ≈2 × 10−4 to 0.8 mol/m2. With the RNAi20 strain, a reduced level of pregamete to gamete conversion over this fluence range was observed. However, at a higher fluence, i.e., >0.1 mol/m2, an increase in gamete formation was still observed in the RNAi20 strain, whereas in the wild type the increase leveled off. A fluence response of this type was expected for strains with a reduced number of photoreceptor molecules. From the combined data (Figs. 2 and 3) we draw the conclusion that phototropin seems to be the photoreceptor that controls the blue-light-dependent step in the sexual differentiation of this green alga.
A Reduction of Phot Affects Gene Expression.
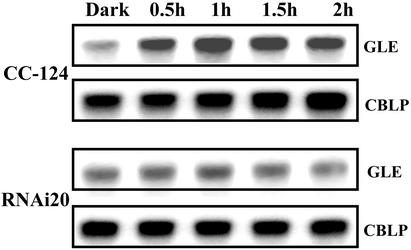
In the late phase of gametogenesis a specific up-regulation of various genes in response to irradiation was observed (37, 38). To test whether a reduction in phototropin may affect this gene expression we assayed the mRNA levels of gene GLE encoding the metalloprotease autolysin that digests the cell wall of gametes before cell fusion (29). The GLE mRNA levels increased when wild-type pregametes were irradiated. In the RNAi20 strain, such an increase was not observed (Fig. 4), suggesting that for the light induction of GLE, phototropin serves as a photoreceptor. A lack of GLE-encoded autolysin, however, may not account for the reduction in pregamete to gamete conversion (assayed by zygote formation) observed for the RNAi20 strain (Fig. 3A), because we have shown that a light-induced increase in GLE mRNA is not a prerequisite for successful mating (37).
Figure 4.
Expression of gene GLE (encoding gametic lytic enzyme) during pregamete to gamete conversion at the RNA level. Samples were taken from the cultures of the experiment shown in Fig. 3A. Hybridization with gene CBLP served as a loading control.
Involvement of Phototropin in Maintaining Mating Competence.
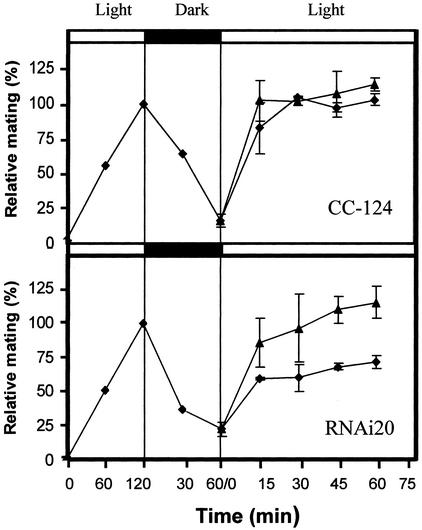
Incubation of gametes in the dark results in a loss of their mating ability. Reillumination of these dark-inactivated gametes rapidly restores their mating competence. For this step, 450-nm-wavelength light was shown to be most effective (20). To test a possible function of phototropin in this process, gametes of the RNAi20 strain were generated from pregametes and then incubated in the dark for 60 min. This treatment resulted in a distinct loss in mating ability (Fig. 5). Subsequently, the cultures were divided and exposed to white light of low (fluence rate 0.45 μmol⋅m−2⋅s−1) and medium intensities (120 μmol⋅m−2⋅s−1). As shown in Fig. 5, the RNAi20 strain, when compared with wild-type cells at the low intensity, exhibited a distinctly reduced rate in the reappearance of mating-competent gametes. This defect was alleviated when a higher intensity was applied. Similar results were observed with a transformant harboring a different RNAi construct (data not shown). We conclude that phototropin also seems to be the photoreceptor involved in the restoration of mating ability of gametes that have been inactivated by dark incubation.
Figure 5.
Reactivation of dark-inactivated gametes after illumination. Pregametes of CC-124 and RNAi20 were generated by incubation in TAP-N medium for 16 h in the dark. These pregametes were converted into gametes by irradiation with white light for 120 min at a fluence rate of 120 μmol⋅m−2⋅s−1. The percentage of gametes observed after this incubation (values for CC-124 and RNAi20 were 41.5% and 30.5%, respectively) was set at 100%. These gametes were inactivated by incubation in the dark for 1 h. The dark-inactivated gametes were then reexposed to white light of the following two fluence rates: 0.45 μmol⋅m−2⋅s−1 (⧫) and 120 μmol⋅m−2⋅s−1 (▴).
Involvement of Phototropin in the Control of Zygote Germination.
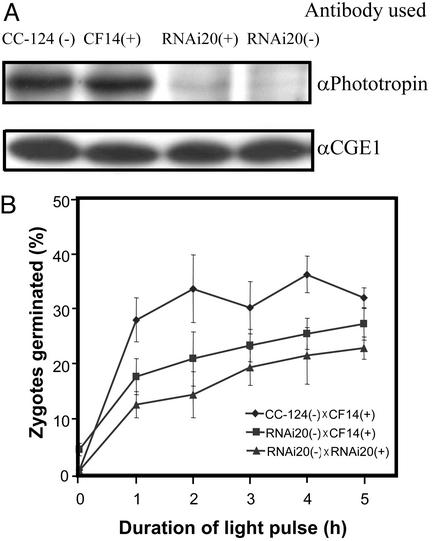
Light was shown to trigger a program that, within ≈20 h after start of illumination, resulted in meiosis and germination of C. reinhardtii zygotes (21). To test whether the photoreceptor involved may be phototropin, we generated zygotes from crosses of two wild-type strains, the wild-type strain and RNAi20 and two strains that were derived from RNAi20. Analysis of the phototropin levels from gametes of strains used for zygote generation showed that both mt+ and mt− RNAi20 strains exhibited distinctly reduced concentrations of the photoreceptor (Fig. 6A).
Figure 6.
Light-induced germination of zygotes generated from gametes with reduced phototropin levels. (A) Phototropin levels in gametes used for the generation of zygotes. (B) Germination of zygotes induced by irradiation with white light of a fluence rate of 30 μmol⋅m−2⋅s−1. Zygotes were generated on plates following the protocol given in Materials and Methods. The percentage of zygotes germinated was calculated by dividing the number of zygotes that formed colonies by the total number of zygotes (germinated and nongerminated). Zygotes generated by the mating of two wild-type strains, of wild type and the RNAi20 strain, and of two RNAi20 strains were assayed. The data given represent the average from five independent experiments.
Zygotes resulting from these three crosses were exposed to light at a fluence rate of 30 μmol⋅m−2⋅s−1 for 1–5 h. At this light intensity an optimal difference among the three types of zygotes was observed. After a subsequent incubation for 11 days in the dark the percentage of zygotes that had germinated was determined (Fig. 6B). Zygotes generated from two RNAi20 parents showed the lowest rate of germination whereas zygotes from the cross between an RNAi20 strain (−) and wild type (+) exhibited an intermediate rate. Because the degree of zygote germination is distinctly lower when gametes with reduced phototropin levels were used for their generation, we suggest that this photoreceptor also mediates the germination of zygotes in response to light.
Discussion
Light plays a key role in the sexual development of C. reinhardtii. Although asexual propagation may occur in the absence of light with acetate serving as sole source of energy and carbon, the sexual life cycle absolutely depends on the signaling function of light (39). Here, we demonstrate a crucial role for phototropin in controlling progression of the life cycle by using strains with strongly reduced levels of this photoreceptor. In such strains we show that three light-dependent steps are impaired: the conversion of mating-incompetent pregametes to mature gametes (Fig. 3), the reactivation of gametes inactivated by dark incubation (Fig. 5), and the germination of zygotes (Fig. 6).
Although higher plant phototropins seem to be involved mostly in the light control of short-term responses like stomata opening or chloroplast movements, the Chlamydomonas phototropin seems to control primarily developmental processes. In higher plants, such responses, e.g., deetiolation and floral initiation, when elicited by blue light, are controlled by cryptochromes (40). A possible reason for the involvement of phototropin in controlling the sexual life cycle in Chlamydomonas is its stability in the light (8). Stability of the photoreceptor is essential in cases where light input over an extended period is required to elicit a response. Because the C. reinhardtii cryptochrome, just like the cry2 protein of Arabidopsis, is rapidly degraded after exposure of the organisms to light (ref. 41; G. Small, personal communication), this photoreceptor seems unsuitable to mediate the light-induced conversion of pregametes to gametes and zygote germination (both processes require irradiation for at least 1 h).
It may at a first glance seem surprising that a reduction in phototropin levels to <10% still permits a clearly measurable response at low fluence rates (Fig. 3). This observation may be accounted for by the exquisite sensitivity of the blue-light response observed during pregamete to gamete conversion. Thus, already at a blue-light fluence of 10−11 mol (photons)/m2 applied over 90 min, a conversion of pregametes to gametes was observed (42). It should be stressed, however, that we cannot rule out the participation of another photoreceptor in these responses, although a second phototropin gene seems not to be present in the C. reinhardtii genome (8).
Up to now little has been known about the signaling cascade(s) that link phototropin to the physiological responses downstream of blue-light perception. In higher plants this signaling seems to involve membrane-associated activities such as the uptake of Ca2+ (43), a concept supported by the recent discovery of a blue-light-activated Ca2+-permeable channel (44). In addition, there is evidence for a role of phototropin in blue-light-induced membrane depolarization (13).
In C. reinhardtii, mutant analyses of the phototropin-dependent light-signaling pathway that controls gamete formation revealed a role for a transport protein of unknown specificity; its absence results in light independence of gamete formation (45). Studies at the biochemical level have identified a protein kinase C-like activity and a tyrosine kinase (20, 46). Does light signaling during gametogenesis and zygote germination use the same or different pathways? Genetic analyses suggest that some components may be shared, whereas others are different. Among the shared components, besides the photoreceptor, is the product of gene LRG4. This conclusion was deduced from studies showing that a mutant defective in LRG4 forms gametes in the absence of light; in addition, zygotes homozygous for lrg4 may germinate in the dark (21). However, other members of the signaling pathway required for pregamete to gamete conversion like the products of LRG1 and LRG3 seem not to be involved in light signaling during zygote germination (21).
Because the reactivation of dark-inactivated gametes does not require protein synthesis, it has been hypothesized that blue light activates proteins of the flagella involved in sexual agglutination by some chemical modification (20). Consistent with this hypothesis is the observation that phototropin is associated not only with the cell body but also found in the flagellum (unpublished data). This finding opens up the opportunity to analyze the consequences of phototropin activation in flagella, i.e., a system of reduced complexity.
Acknowledgments
We thank Michael Schroda for helpful comments and a critical reading of the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (to C.F.B.).
Abbreviations
- TAP-N
nitrogen-free Tris-acetate-phosphate
- RNAi
RNA interference
References
- 1.Neff M M, Fankhauser C, Chory J. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- 2.Bonenberger J, Salomon M, Formanek H, Busl T, Rüdiger W. J Plant Physiol. 1994;144:346–350. [Google Scholar]
- 3.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti A M, Bamberg E, Hegemann P. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad M. Curr Opin Plant Biol. 1999;2:230–235. doi: 10.1016/S1369-5266(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 5.Batschauer A. Planta. 1998;206:479–492. doi: 10.1007/s004250050425. [DOI] [PubMed] [Google Scholar]
- 6.Briggs W R, Beck C F, Cashmore A R, Christie J M, Hughes J, Jarillo J A, Kagawa T, Kanegae H, Liscum E, Nagatani A, et al. Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs W R, Christie J M. Trends Plant Sci. 2002;7:204–210. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- 8.Huang K, Merkle T, Beck C F. Physiol Plant. 2002;115:613–622. doi: 10.1034/j.1399-3054.2002.1150416.x. [DOI] [PubMed] [Google Scholar]
- 9.Small G D, Min B, Lefebvre P A. Plant Mol Biol. 1995;28:443–454. doi: 10.1007/BF00020393. [DOI] [PubMed] [Google Scholar]
- 10.Salomon M, Eisenreich W, Dürr H, Schleicher E, Knieb E, Massey V, Rüdiger W, Müller F, Bacher A, Richter G. Proc Natl Acad Sci USA. 2001;98:12357–12361. doi: 10.1073/pnas.221455298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasahara M, Swartz T E, Olney M A, Onodera A, Mochizuki N, Fukuzawa H, Asamizu E, Tabata S, Kanegae H, Takano M, et al. Plant Physiol. 2002;129:762–773. doi: 10.1104/pp.002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liscum E, Briggs W R. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folta K M, Spalding E P. Plant J. 2001;26:471–478. doi: 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- 14.Jarillo J A, Gabrys H, Capel J, Alonso J M, Ecker J R, Cashmore A R. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 15.Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Kagawa T, Kasahara M, Swartz T E, Christie J M, Briggs W R, Wada M, Okada K. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 18.Harris E H. The Chlamydomonas Sourcebook. San Diego: Academic; 1989. [Google Scholar]
- 19.Treier U, Fuchs S, Weber M, Warkarchuk W W, Beck C F. Arch Microbiol. 1989;152:572–577. [Google Scholar]
- 20.Pan J M, Haring M A, Beck C F. Plant Physiol. 1997;115:1241–1249. doi: 10.1104/pp.115.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloeckner G, Beck C F. Genetics. 1995;141:937–943. doi: 10.1093/genetics/141.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito T, Inoue M, Yamada M, Matsuda Y. Plant Cell Physiol. 1998;39:8–15. [Google Scholar]
- 23.Weissig H, Beck C F. Plant Physiol. 1991;97:118–121. doi: 10.1104/pp.97.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck C F, Acker A. Plant Physiol. 1992;98:822–826. doi: 10.1104/pp.98.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popov N, Schmitt S, Matthices H. Acta Biol Germ. 1975;31:1441–1446. [PubMed] [Google Scholar]
- 26.Laemmli U K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Von Gromoff E, Treier U, Beck C F. Mol Cell Biol. 1989;9:3911–3918. doi: 10.1128/mcb.9.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegener D, Beck C F. Plant Mol Biol. 1991;16:937–946. doi: 10.1007/BF00016066. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita T, Fukuzawa H, Shimada T, Saito R, Matsuda Y. Proc Natl Acad Sci USA. 1992;89:4693–4697. doi: 10.1073/pnas.89.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Kampen J, Nielaender U, Wettern M. J Plant Physiol. 1994;143:756–758. [Google Scholar]
- 31.Asamizu E, Nakamura Y, Sato S, Fukuzawa H, Tabata S. DNA Res. 1999;6:369–373. doi: 10.1093/dnares/6.6.369. [DOI] [PubMed] [Google Scholar]
- 32.Schroda M, Blöcker D, Beck C F. Plant J. 2000;21:121–131. doi: 10.1046/j.1365-313x.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- 33.Sizova I, Fuhrmann M, Hegemann P. Gene. 2001;277:221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 34.Kindle K L. Proc Natl Acad Sci USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuhrmann M, Stahlberg A, Govorunova E, Rank S, Hegemann P. J Cell Sci. 2001;114:3857–3863. doi: 10.1242/jcs.114.21.3857. [DOI] [PubMed] [Google Scholar]
- 36.Smith N A, Sing S P, Wang M-B, Stoutjesdijk P A, Green A G, Waterhouse P M. Nature. 2000;407:319–320. doi: 10.1038/35030305. [DOI] [PubMed] [Google Scholar]
- 37.Von Gromoff E, Beck C F. Mol Gen Genet. 1993;241:415–421. doi: 10.1007/BF00284695. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez H, Haring M A, Beck C F. Mol Gen Genet. 1999;261:267–274. doi: 10.1007/s004380050966. [DOI] [PubMed] [Google Scholar]
- 39.Beck C F, Haring M A. Int Rev Cytol. 1996;168:259–302. [Google Scholar]
- 40.Lin C. Trends Plant Sci. 2000;5:337–342. doi: 10.1016/s1360-1385(00)01687-3. [DOI] [PubMed] [Google Scholar]
- 41.Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore A R. Proc Natl Acad Sci USA. 1998;95:2686–2690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buerkle S, Gloeckner G, Beck C F. Proc Natl Acad Sci USA. 1993;90:6981–6985. doi: 10.1073/pnas.90.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baum G, Long J C, Jenkins G I, Trewavas A J. Proc Natl Acad Sci USA. 1999;96:13554–13559. doi: 10.1073/pnas.96.23.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P. Proc Natl Acad Sci USA. 2003;100:1456–1461. doi: 10.1073/pnas.0333408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dame G, Gloeckner G, Beck C F. Plant J. 2002;31:577–587. doi: 10.1046/j.1365-313x.2002.01379.x. [DOI] [PubMed] [Google Scholar]
- 46.Pan J M, Haring M A, Beck C F. Plant Physiol. 1996;112:303–309. doi: 10.1104/pp.112.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroda M, Vallon O, Whitelegge J P, Beck C F, Wollman F-A. Plant Cell. 2001;13:2823–2839. doi: 10.1105/tpc.010202. [DOI] [PMC free article] [PubMed] [Google Scholar]