Abstract
Studies showed that specific probiotics might provide therapeutic benefits in inflammatory bowel disease. However, a rigorous screening of new probiotics is needed to study possible adverse interactions with the host, particularly when intended for administration to individuals with certain health risks. In this context, the objective of this study was to investigate the role of three lactobacilli (LAB) on intestinal inflammation and bacterial translocation using variations of the mouse model of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced acute colitis. We first compared the in vitro ability of LAB to survive gastrointestinal tract (GIT) conditions and their ability to persist in the GIT of mice following daily oral administration. As a control, we included a nonprobiotic Lactobacillus paracasei strain, previously isolated from an endocarditis patient. Feeding high doses of LAB strains to healthy and to TNBS-treated mice did not induce any detrimental effect or abnormal translocation of the bacteria. Oral administration of Lactobacillus salivarius Ls-33 had a significant preventive effect on colitis in mice, while Lactobacillus plantarum Lp-115 and Lactobacillus acidophilus NCFM did not. None of the three selected LAB strains translocated to extraintestinal organs of TNBS-treated mice. In contrast, L. paracasei exacerbated colitis under severe inflammatory conditions and translocated to extraintestinal organs. This study showed that evaluations of the safety and functionality of new probiotics are recommended. We conclude that not all lactobacilli have similar effects on intestinal inflammation and that selected probiotics such as L. salivarius Ls-33 may be considered in the prevention or treatment of intestinal inflammation.
Inflammatory bowel diseases (IBD), including Crohn's disease and ulcerative colitis, are chronic immune-mediated diseases in which endogenous bacteria are thought to play an important role, as suggested by numerous clinical observations and experimental studies summarized in recent reviews (13, 38). Recent studies have also shown that some bacterial strains or mixtures may have the capacity to promote or reduce intestinal inflammation (19, 27). This evidence has led to an increased use of probiotic preparations in the therapy of IBD that usually contain lactobacilli, bifidobacteria, or Escherichia coli strains (8, 14, 18). However, many therapeutic trials make limited allowances for (i) the optimal frequency and route of probiotic administration, (ii) possible adverse effects in diseased or debilitated patients, and (iii) rational bacterial selection, e.g., through preliminary in vitro and in vivo studies of relevant functional characteristics. Dose and frequency of administration may depend on the degree of persistence of the bacteria in the gastrointestinal tract (GIT), which itself will depend on certain properties that can easily be measured in vitro (resistance to proteolytic enzymes, low pH, and high bile concentrations). In order to further select the strain with the most promising prophylactic or therapeutic effect on intestinal inflammation, it is important to select strains in a relevant in vivo animal model such as the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model, which mimics severe colonic inflammation described for patients with IBD (10, 28).
Most lactobacilli (LAB) have a remarkable record of safety and have been consumed by humans for decades. However, the possible involvement of certain LAB strains was described in cases of sepsis, endocarditis, or bacteremia, mostly in association with a severe underlying disease or detrimental condition (21, 35, 36). In view of these facts, the safety of potential probiotic microorganisms in these conditions should be assessed individually (44). Bacterial translocation (BT) is most likely the first step in the possible passage of viable (indigenous) bacteria to sterile body sites (3, 42) and is thus important for sepsis, endocarditis, and bacteremia caused by the commensal flora. In a healthy host, BT is a highly regulated, physiological event that occurs continuously at a low rate. When the integrity of the intestinal barrier is disturbed or when the immune system is not able to confine an infection, pathogenic or commensal bacteria can reach the bloodstream and cause septicemia (3). Consequently, when considering probiotic applications for ulcerative colitis or Crohn's disease patients, it is recommended that the safety of the probiotic used be verified by assessing its potential to translocate not only in healthy conditions but also in conditions with injured intestinal mucosa (42).
We selected Lactobacillus plantarum Lp-115, Lactobacillus salivarius Ls-33, and Lactobacillus acidophilus NCFM as probiotic candidates based on their technological as well as various in vitro immunomodulating properties (9, 10). The complete genome of strain NCFM was recently published (1), and the strain was extensively studied in vitro as well as in multiple human studies (reviewed in reference 37). We have also included Lactobacillus paracasei subsp. paracasei YS8866441, previously isolated from a patient with infective endocarditis (15), as a nonprobiotic strain. This strain was received through the Prosafe EU research project (http://www.biomatnet.org/secure/FP5/S1597.htm). In this study, we first evaluated some properties of these LAB strains believed to be important for survival in the GIT in vitro and compared the results with their actual ability to survive and persist in the GIT of mice after daily intragastric (IG) administration. The safety of the strains was assessed using healthy mice. Furthermore, we evaluated the potential of the IG administered live strains to prevent inflammation in TNBS-treated mice and compared their potential risk of BT in two distinct experiments with various levels of colitis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Five Lactobacillus strains were used in this study: L. plantarum NCIMB8826, isolated from human saliva (National Collection of Industrial and Marine Bacteria, Aberdeen, United Kingdom) and used as a reference strain; three strains from Danisco USA, Inc. (L. plantarum Lp-115, isolated from plant material, L. salivarius Ls-33, isolated from an unknown source, and L. acidophilus NCFM, isolated from human feces); and the nonprobiotic reference strain L. paracasei subsp. paracasei YS8866441, isolated from a blood culture of a patient with infective endocarditis (15), kindly provided by H. Goossens, UIA, Antwerp, Belgium. Lactobacillus strains were grown at 37°C in MRS medium (Difco, Becton Dickinson, MD) without shaking. Strain YS8866441 is available from the LMG Culture Collection (Belgium) as LMG 23554.
For detection purposes, the Lactobacillus strains were electrotransformed with plasmid pNZYR, encoding resistance to chloramphenicol (10 μg/ml; Sigma-Aldrich, St. Quentin Fallavier, France). L. plantarum and L. salivarius were electrotransformed as described previously by Josson et al. (17), and L. acidophilus was electrotransformed as described previously by Walker et al. (45). It was verified that subculturing of the antibiotic-resistant variant did not lead to plasmid loss, even after approximately 100 generations (10 subcultures) on nonselective MRS medium. The electrotransformed antibiotic-resistant strains were L. plantarum NCIMB8826(pNZYR), L. plantarum Lp-115(pNZYR), L. salivarius Ls-33(pNZYR), and L. acidophilus NCFM(pNZYR). L. paracasei YS8866441 was naturally resistant to vancomycin (100 μg/ml; Sigma-Aldrich) and fucidic acid (50 μg/ml; Sigma-Aldrich).
In vitro resistance to GIT conditions.
Simulation of gastric and small intestinal transit tolerance was tested through resistance to pepsin (3 mg/ml; Sigma) and pancreatin USP (1 mg/ml; Sigma) in phosphate-buffered saline (PBS), pH 2 and 8, respectively, as described previously (5). Resistance of strains to proteolytic enzymes was assessed in terms of viability counts, enumerated at different time intervals, and expressed as a percentage of viable bacteria after enzyme exposure compared to the initial population (100%).
Tolerance to bile acids was tested by inoculation of fresh cultures into MRS broth enriched with 0.3% (wt/vol) Oxgall (Sigma-Aldrich) as described previously (6). Growth curves were plotted, and the time required to obtain an optical density at 600 nm of 0.3 was determined for both the negative control and cultures supplemented with bile salts. The time difference (d) was defined as the growth delay significant for the inhibition caused by the presence of bile salts: if d < 15 min, the strains are classified as bile salt resistant; if 15 min < d < 40 min, the strains are classified as bile salt tolerant; if 40 min < d < 60 min, the strains are classified as low bile salt tolerant (6).
Adhesion to Caco-2 cells.
Adhesion of bacteria to Caco-2 cells was assessed as described previously (24). Briefly, cells were routinely grown in Dulbecco's modified Eagle medium (DMEM; Gibco BRL, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (Gibco), 1% (vol/vol) nonessential amino acids (Gibco), 1% (vol/vol) l-glutamine (Gibco), and 20 μg/ml of streptomycin and penicillin. Monolayers of Caco-2 cells were prepared, seeded at a concentration of 1.2 × 105 cells/ml, and used at postconfluence after 15 days of culturing. Bacteria in stationary phase were harvested by centrifugation, washed twice, and resuspended to a concentration of 108 CFU/ml in nonsupplemented DMEM (Gibco). The growth medium of Caco-2 monolayers was aspirated, the cells were washed with PBS, and, subsequently, 1 ml of bacterial DMEM suspension was transferred onto the Caco-2 monolayers. The plates were incubated at 37°C for 90 min, the bacterial suspension was then aspirated, and the Caco-2 monolayers were washed twice before Tween 80 (0.04%; Sigma-Aldrich) was added to resuspend the bacterial cells. The bacterial suspension was then enumerated as described above. The adhesion of strains to Caco-2 cells was expressed as a percentage of viable bacteria compared to their initial population in the DMEM suspension. E. coli strain TG1 was used as a positive control, and the nonadhesive L. plantarum strain V299 adh− was used as a negative control as described previously (24).
Preparation of bacterial strains and administration to mice.
Bacterial strains were grown to an optical density at 600 nm of 3 to 4 (early stationary phase), harvested by centrifugation, washed with PBS, and resuspended at 1011 CFU/ml in 0.2 M NaHCO3 buffer containing 1% glucose, and mice received 1010 CFU intragastrically.
Animals.
Animal experiments were performed in an accredited establishment (number A59107; animal facility of the Institut Pasteur de Lille, France) according to guidelines of the French government (number 86/609/CEE). Seven-week-old female BALB/c mice were purchased from Iffa Credo (L'Arbresle, France) and kept under filter-top hoods.
In vivo persistence of LAB in the GIT of mice.
Groups of five animals received a daily dose of 1010 CFU of live L. plantarum NCIMB8826(pNZYR), L. plantarum Lp-115(pNZYR), L. salivarius Ls-33(pNZYR), L. acidophilus NCFM(pNZYR), or L. paracasei YS8866441 intragastrically for four consecutive days. Fecal samples were collected daily, pooled, and mechanically homogenized in MRS medium at 100 mg of feces/ml. Dilutions were plated onto the selective media described above and incubated before enumeration. No chloramphenicol- or vancomycin/fucidic acid-resistant bacteria were detected in noninoculated mice. The persistence experiment was repeated three times for each strain.
Safety assessment of LAB in healthy and TNBS-treated mice.
Groups of 10 mice received carbonate buffer or 1010 CFU of live L. plantarum Lp-115(pNZYR), L. salivarius Ls-33(pNZYR), L. acidophilus NCFM(pNZYR), or L. paracasei YS8866441 per day for five consecutive days. Using a standardized mouse model of TNBS-induced acute colitis as previously described (10), colitis was induced on day 5 by intrarectal administration of TNBS (Fluka, Saint Quentin Fallavier, France) at doses varying from 120 to 150 mg/kg of body weight mixed in 50% ethanol.
Healthy mice (no TNBS) and mice treated with TNBS were sacrificed on day 7 by cervical dislocation. “TNBS-positive control” mice received NaHCO3 buffer before TNBS treatment, while “treated” mice received bacteria before TNBS treatment. Mice were weighed prior to TNBS administration and at sacrifice. Mortality rate, colonic damage, and inflammation scores were assessed 48 h after TNBS administration according to the Wallace criteria as described previously (10). These criteria for macroscopic scoring (score range, 0 to 10) reflect the level of inflammation, the thickening of the colon mucosa, and the extent of ulceration. The activity of colonic tissue myeloperoxidase (MPO), a marker of polymorphonuclear neutrophil primary granules, was determined as previously described, with slight modifications (4). Briefly, tissue strips were suspended in potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (pH 6.0) and then homogenized using a Polytron homogenizer. After centrifugation, MPO activity in supernatants was determined. One unit of MPO was defined as the amount needed to degrade 1 μmol of hydrogen peroxide in 1 min at 25°C, and MPO from human neutrophils was used as a standard.
The mesenteric lymph nodes (MLNs), spleen, liver, and kidneys of each individual mouse were aseptically removed and immediately placed in one-quarter-strength Ringer's solution. The samples were mechanically homogenized. Selective enumeration of the four Lactobacillus antibiotic-resistant strains was performed by plating individually homogenized organs onto MRS agar containing the respective antibiotic. Aerobic gram-positive bacteria were cultured on MRS agar.
Rep-PCR protocol.
Repetitive element PCR (Rep-PCR) DNA fingerprinting using a single oligonucleotide primer, (GTG)5, was performed on DNA extracted from the antibiotic-resistant strains found to translocate in the organs and the original administered strains as previously described (12).
Statistical analysis.
After necropsy, dead mice (due to too-severe TNBS-induced colitis) were scored at 8. Statistical significance between different groups of mice was evaluated by the Mann-Whitney U test. Differences were considered significant at a P value of <0.05 or <0.01.
RESULTS
In vitro and in vivo resistance of LAB to gastrointestinal tract conditions.
Survival in the GIT and association with the host epithelium were examined in vitro by testing the resistance of each strain to pepsin, pancreatin, and bile and by testing the ability to adhere to Caco-2 cells (Table 1). All strains were resistant to 20 min of pepsin exposure except L. paracasei YS8866441, but after 1 h, only L. acidophilus NCFM and L. plantarum Lp-115 exhibited a significantly higher level of survival than the reference strain, L. plantarum NCIMB8826. All lactobacilli retained viability or increased in number after 2 h of exposure to pancreatin, except for strain L. plantarum Lp-115. Strains were classified as resistant or tolerant to bile salts, except for L. paracasei YS8866441. Strains exhibited a moderate adhesion to Caco-2 cells that was not considered significantly different from that of L. plantarum NCIMB8826. The only statistical difference was observed between L. salivarius Ls-33 and L. plantarum Lp-115. Fifty-seven percent of the E. coli TG1 cells adhered to Caco-2 cells, while the nonadhering L. plantarum strain V299 adh− resulted in an adhesion percentage of only 2.1%.
TABLE 1.
Survival of lactobacilli in the presence of proteolytic enzymes, bile tolerance, and microbial adhesion to Caco-2 cellsa
| Strain | % Viable bacteria ± SEM
|
Bile tolerance | ||||||
|---|---|---|---|---|---|---|---|---|
| Pepsin
|
Pancreatin
|
Caco-2 adhesion | ||||||
| 0 min | 20 min | 60 min | 0 min | 60 min | 120 min | |||
| NCIMB8826 | 100 ± 0 | 65.5 ± 15.5 | 1.3 ± 1 | 100 ± 0 | 107 ± 13.3 | 127 ± 7.5 | 15.6 ± 2.4 | Resistant |
| Ls-33 | 100 ± 0 | 95.5 ± 29 | 4.4 ± 3 | 100 ± 0 | 177 ± 18** | 162 ± 7.5** | 21.2 ± 1.8 | Tolerant |
| Lp-115 | 100 ± 0 | 105.5 ± 40 | 15.5 ± 7** | 100 ± 0 | 89 ± 6 | 87 ± 1** | 11.4 ± 1.2 | Resistant |
| NCFM | 100 ± 0 | 88 ± 13 | 39 ± 15** | 100 ± 0 | 116 ± 40 | 128 ± 37 | 18.2 ± 2.4 | Tolerant |
| YS8866441 | 100 ± 0 | 0 ± 0** | 0 ± 0 | 100 ± 0 | 130 ± 10 | 223 ± 15** | ND | Sensitive |
Data are expressed as percentages of viable bacteria compared to their initial population. Differences were considered significant compared to the NCIMB8826 reference strain (**, P ≤ 0.01). Experiments were done in duplicate, and values are means ± standard errors of the means (SEM). ND, not determined.
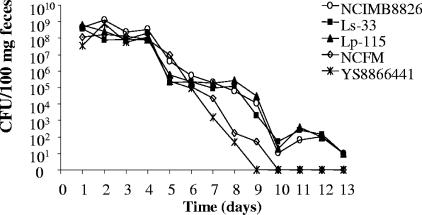
The persistence of the strains in the mouse GIT was investigated (Fig. 1). L. plantarum NCIMB8826(pNZYR), Ls-33(pNZYR), and Lp-115(pNZYR) were recovered until day 13 in the feces. L. acidophilus NCFM(pNZYR) and L. paracasei YS8866441 counts dropped earlier and faster (days 8 and 9, respectively).
FIG. 1.
Fecal counts of L. plantarum NCIMB8826(pNZYR), L. plantarum Lp-115(pNZYR), L. salivarius Ls-33(pNZYR), L. paracasei subsp. paracasei YS8866441, and L. acidophilus NCFM(pNZYR) administered intragastrically for four consecutive days (days 1 to 4) in mice (n = 5). Data are the arithmetic means of results from three separate experiments (CFU/100 mg feces).
Safety evaluation of LAB in healthy mice.
In healthy mice, IG administration of L. plantarum Lp-115(pNZYR), L. salivarius Ls-33(pNZYR), L. acidophilus NCFM(pNZYR), and L. paracasei YS8866441 did not show any potential adverse effect on mouse activity, weight, and colon inflammation (Table 2). MPO levels remained very low and did not significantly differ from those of the buffer-treated control group. In contrast, the MPO levels in the colonic tissues of TNBS-treated mice were significantly higher (P < 0.01) and reflect the levels of neutrophil recruitment. None of the four LAB strains were isolated from cultures of MLNs, spleen, liver, and kidneys of mice in the different groups, indicating that repeated ingestion of high doses of either LAB strain did not induce abnormal BT or dissemination in healthy mice.
TABLE 2.
Effects of intragastric administration of carbonate buffer, L. salivarius Ls-33(pNZYR), L. plantarum Lp-115(pNZYR), L. acidophilus NCFM(pNZYR), and L. paracasei YS8866441 on healthy micea
| Buffer or strain | No. of mice | Activity score ± SEM | % Variation in wt (day 7-day 1) ± SEM | Colon inflammation | LAB translocation in different organsb | MPO (IU) ± SEMc |
|---|---|---|---|---|---|---|
| NaHCO3 | 5 | 3 ± 0 | 4.4 ± 2 | No | ND | 0.011 ± 0.0017** |
| Ls-33 | 5 | 3 ± 0 | 3.4 ± 2.6 | No | No | 0.017 ± 0.0022** |
| Lp-115 | 5 | 3 ± 0 | 2.7 ± 2.3 | No | No | 0.018 ± 0.0026** |
| NCFM | 5 | 3 ± 0 | 2.4 ± 1 | No | No | 0.014 ± 0.0003** |
| YS8866441 | 5 | 3 ± 0 | 6.6 ± 5.9 | No | No | 0.014 ± 0.0003** |
| TNBS controld | 5 | ND | −11.3 ± 4.5 | Yes | ND | 0.55 ± 0.032 |
One TNBS-positive control group (120 mg/kg) was added to the study (mice received phosphate buffer instead of bacteria and received TNBS).Values are means ± SEM. ND, not determined.
Detection level in MLN, spleen, liver, and kidneys, ≥1 CFU/organ.
Expressed in international units. **, differences were considered significant at a P value of ≤0.01 compared to the TNBS control group.
Mean Wallace score, 5 ± 0.9.
Safety evaluation of LAB in TNBS-treated mice (strong colitis).
We found no significant difference between the protection capacity of the wild-type and that of the respective antibiotic-resistant variants in TNBS-treated mice (data not shown).
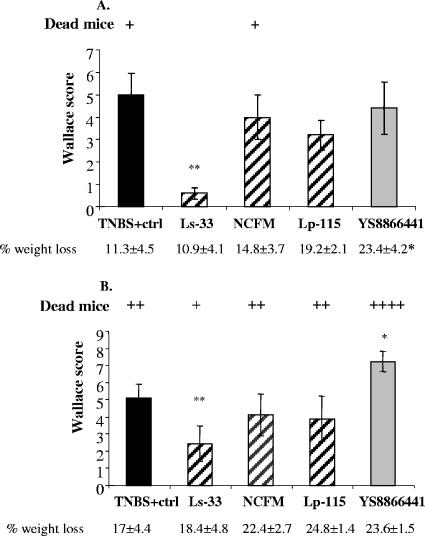
A TNBS dose of 120 mg/kg body weight resulted in strong colitis associated with colon inflammation, weight loss, and mortality (Fig. 2A). IG administration of L. salivarius Ls-33 in TNBS-treated mice resulted in a significant reduction of the inflammatory score but without any significant reduction of weight loss. In contrast, no significant improvement of colitis was observed in the groups fed L. acidophilus NCFM, L. plantarum Lp-115, and L. paracasei YS8866441. Interestingly, there was a significant increase in weight loss only for the YS8866441 group. None of the four LAB strains were isolated from cultures of MLNs, spleen, liver, and kidneys of mice in the different groups.
FIG. 2.
Effects of IG administration of L. salivarius Ls-33(pNZYR), L. plantarum Lp-115(pNZYR), L. acidophilus NCFM(pNZYR), and L. paracasei YS8866441 on macroscopic damage induced by two distinct doses of TNBS: (A) 120 mg/kg and (B) 150 mg/kg body weight. Results are expressed as mean Wallace scores of the group (n = 10) ± SEM (significantly different from the corresponding TNBS-control group [*, P < 0.05; **, P < 0.01]). Body weight variation is expressed as mean values of the percentage of weight loss of each group ± SEM between day 7 (sacrifice) and day 5 (TNBS). Mortality corresponds to numbers of individual dead mice (+).
Safety assessment of LAB in TNBS-treated mice (very strong colitis).
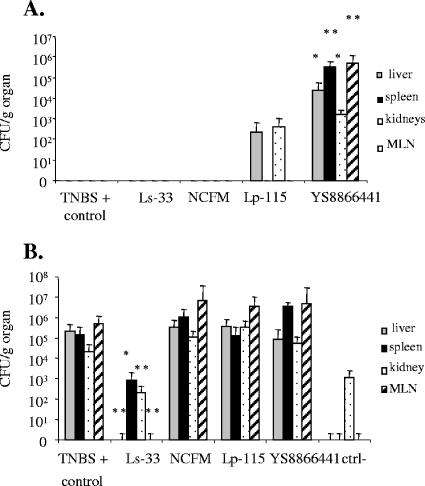
IG administration of L. salivarius Ls-33 in TNBS-treated mice still resulted in a significant reduction in the inflammatory score compared to scores of the TNBS-positive control group (Fig. 2B). However, no improvement of colitis was observed for groups fed L. acidophilus NCFM and L. plantarum Lp-115. In contrast, IG administration of strain YS8866441 exacerbated colon inflammation and increased the mortality rate. Bacterial translocation was also found (Fig. 3). Strains Ls-33 and NCFM were not recovered from cultures of MLNs, spleen, liver, or kidneys of TNBS-treated mice. Strain Lp-115 was found at low levels in the liver and kidney of only one mouse, without any particular negative effect on the mouse (activity score, weight loss, and colon inflammation). In contrast, YS8866441 was recovered in high numbers from the liver and MLNs of 80% of the mice and from the spleen and kidneys of all mice. Translocation of YS8866441 to extraintestinal organs was correlated with increased weight loss and colon inflammation. The presumed Lp-115 and YS8866441 strains isolated from mice were found to be identical to the strains administered, as shown by Rep-PCR (data not shown). Furthermore, the BT of gram-positive aerobic bacteria (bacilli and lactobacilli growing on MRS agar) from total endogenous flora was also determined (Fig. 3B). In healthy mice, low numbers of bacteria were found, reflecting the normal basal translocation level. In the TNBS-positive control group, the translocation level of part of the endogenous flora was much higher in all organs but was found to be significantly reduced in all organs after IG administration of Ls-33. NCFM and Lp-115 did not significantly reduce bacterial translocation of the endogenous flora.
FIG. 3.
Bacterial translocation in liver, spleen, kidney, and MLNs of TNBS-treated mice (150 mg/kg; very strong colitis) IG administered Ls-33(pNZYR), NCFM(pNZYR), Lp-115(pNZYR), YS8866441, or carbonate buffer (TNBS+ control) (n = 10 mice/group). Carbonate buffer was administered to a negative control group of healthy mice (ctrl−) (significantly different from the corresponding TNBS-positive control group [*, P < 0.05; **, P < 0.01]). (A) Translocation of specific administered LAB strains (enumerated on MRS agar plus the respective antibiotic). (B) Translocation of gram-positive bacteria from total endogenous flora (enumerated on MRS agar). Results presented (numbers of CFU/g of individual organ) are the arithmetic means ± standard errors of the means.
DISCUSSION
According to the definition by the World Health Organization (7), a probiotic strain is “a live microorganism which, when administered in adequate amounts, confers a health benefit on the host.” Potential probiotic applications are numerous but often lack clinical practicalities as well as proper selection procedures that allow clinicians and microbiologists to safely promote unrestricted recommended use in severely ill or debilitated patients (39). The aim of this study was to evaluate some aspects of the safety and functionality of three probiotic lactobacilli by using in vitro techniques and variations of the mouse model of TNBS-induced in vivo colitis. To this purpose, in our study, we have also included the nonprobiotic Lactobacillus paracasei strain YS8866441, previously isolated from a patient with endocarditis.
We first compared the persistence of the LAB in the mouse GIT to that of L. plantarum NCIMB8826 because its survival and persistence in mouse and human GITs was well documented (33, 43). Our results showed that LAB strains from different origins were able to persist for more than a week in the GIT of mice. All strains showed some resistance to the in vitro-tested conditions and adhered to Caco-2 cells, except L. paracasei YS8866441, which was shown to persist in mice despite its in vitro sensitivity to bile salts and acid. These results indicate that in vitro tests cannot always be predictive of the in vivo behavior of strains. Similar observations have been made by other authors who showed that strains of lactobacilli, which have a documented ability to survive and reproduce in the human gut, scored poorly when challenged in vitro. Furthermore, when data obtained for different strains by in vitro experiments are compared, it appears that the lactobacilli of the Lactobacillus casei group of species are the most sensitive (11, 16). In a second step, we have shown that oral administration of these LAB strains to healthy mice did not induce adverse effects or abnormal translocation of the bacteria administered.
We then compared the prophylactic capacity of LAB in a mouse model of TNBS-induced acute colitis and investigated the potential risk of bacterial translocation using the same mouse model. Although the relationship of the model of TNBS-induced colitis to human disease is imperfect, hapten-induced colitis displays Crohn's disease-like features, notably, transmural mononuclear inflammation, lymphocyte infiltration, and a Th1-dominated cytokine profile (30). Ls-33 is the only strain that had significant anti-inflammatory effects under both conditions tested, while L. paracasei YS8866441 exacerbated the inflammatory score under severe inflammatory conditions and translocated to extraintestinal organs. These results confirmed previous findings that probiotic activities are largely strain specific and that not all lactobacilli have similar effects on intestinal inflammation (9, 10). Other probiotics were shown to have anti-inflammatory effects on experimental colitis (20, 22, 25, 31, 34), but very few studies have actually demonstrated strain-specific responses for different Lactobacillus strains in the model of TNBS-induced colitis (10). Our results also indicate that in vitro adherence to Caco-2 cells and in vivo persistence in the GIT do not correlate directly with anti-inflammatory effects.
It is likely that multiple properties contribute to the anti-inflammatory nature of a particular strain like L. salivarius Ls-33 in the mouse model of TNBS-induced colitis used, but the exact mechanisms have yet to be established. The selection of Ls-33 was based on previous in vitro studies that showed its ability to induce high levels of anti-inflammatory cytokines together with low levels of proinflammatory cytokines after stimulation of immunocompetent cells (9, 26). The in vitro anti-inflammatory capacity seems to be closely correlated with its in vivo protection capacity, suggesting that Ls-33 could downregulate an early Th1 proinflammatory immune response evoked by the colonic instillation of TNBS. Inhibition of bacterial translocation and reinforcement of barrier function could also contribute indirectly to the observed anti-inflammatory properties of L. salivarius Ls-33. Similar observations have been made by Llopis et al., who showed that L. casei administration exerts a protective effect by preventing barrier disruption by TNBS, as translocation of bacteria to extraintestinal organs was reduced in rats colonized with L. casei (22). It has also been shown that a mixture of probiotic bacteria, in addition to decreasing proinflammatory cytokines, reinforces barrier function by the secretion of soluble factors that enhance barrier integrity and by the regulation of tight junctions (23, 32). Two other L. salivarius subsp. salivarius strains have been shown to have anti-inflammatory effects in two different models of experimental colitis (25, 34). Attenuation of colitis in both cases was associated with a reduced ability to produce proinflammatory cytokines at the mucosal level. Sheil et al. challenged the conventional hypothesis of probiotics by administering L. salivarius UCC118 subcutaneously to interleukin-10 knockout mice (40). The anti-inflammatory effect of subcutaneous administration was not specific, as it was also seen in a murine model of arthritis, suggesting that probiotics have more than a local anti-inflammatory effect (9, 40).
We have shown for the first time a detrimental effect of an L. paracasei strain in extreme experimental conditions of intestinal inflammation. We needed these conditions of inflammation to exacerbate the risk of bacterial translocation and in which the safety of strains could be challenged. While these extreme conditions in mice may mimic the integrity of an impaired human intestinal barrier to some extent, these conditions are probably too severe to allow proper conclusions on the bacterial efficiency in colitis protection in mice and hence on the efficiency in humans. We previously established (10) the optimal experimental settings by using doses varying from 100 to 125 mg/kg to accurately compare the various protective capacities of LAB strains. Using this standardized mouse model of TNBS-induced colitis, it has previously been shown that L. casei BL23 and L. paracasei IPL111 were able to reduce 69 and 48% of colon inflammation, respectively, compared to the nontreated TNBS control group (26). These two strains were considered “protective,” in contrast to L. paracasei strain YS8866441, illustrating that taxonomically related strains can have substantially different behaviors in response to TNBS-induced inflammation.
Our results have shown that Ls-33, NCFM, and Lp-115 have an acceptable safety profile, while certain risks may exist for YS8866441 under conditions of extensive mucosal damage. We selected L. paracasei YS8866441 as a positive control in our bacterial translocation experiments out of 12 other L. paracasei and Lactobacillus rhamnosus strains isolated from endocarditis patients or patients with bacteremia (2, 15) (results not shown). Only L. paracasei YS8866441 and another L. rhamnosus strain were found to exacerbate intestinal inflammation and translocate to different extraintestinal organs under conditions of extensive mucosal damage, indicating that (i) LAB strains very rarely translocate actively to extraintestinal organs and (ii) further studies may be necessary to identify the parameters or properties that allow a LAB strain to cross the intestinal mucosal barrier and/or persist in the extraintestinal organs or circulation.
As more studies involving the administration of probiotics to critically ill patients are done, the safety of LAB should be evaluated under extreme conditions, and preferably, clinicians and microbiologists should give priority to strains with a proven safety profile, remaining vigilant concerning the use of nonscreened LAB in such patients (41). This is especially important in light of recent demonstrations that under metabolic stress, enteric epithelia will react even towards members of the host's own commensal flora by increased interleukin-8 production, loss of barrier function, and increased translocation (29). These host-related effects could further exacerbate severe intestinal inflammatory conditions, as observed with L. paracasei YS8866441, and might lead to unexpected in vivo translocation towards extraintestinal organs and may hence explain its initial isolation from a critically ill patient (15). The strain-related differences observed remain to be clarified.
Our results showed that an evaluation of the safety and functionality of new probiotics in healthy as well as in extreme conditions is recommended. The study supports the use of selected probiotic strains, such as L. salivarius Ls-33, as possible candidates in the prevention or treatment of intestinal mucosa inflammation, as it was the only strain that could significantly attenuate colitis and reduce the translocation of the endogenous flora. Our study thus provides new insights into the efficacy of Ls-33 in suppressing acute mucosal inflammation, and our efforts should now be directed to study the exact mechanisms explaining its strain-specific anti-inflammatory capacity.
Acknowledgments
This work was supported by funds from Danisco, France, and Institut Pasteur de Lille.
We thank Yvonne Roussel for providing plasmid pNZYR. We thank Benoit Foligné for the MPO measurements. We also warmly thank Geert Huys of the Laboratory of Microbiology, Ghent University, Belgium, for kindly performing the Rep-PCR experiments.
REFERENCES
- 1.Altermann, E., W. M. Russell, M. A. Azcarate-Peril, R. Barrangou, B. L. Buck, O. McAuliffe, N. Souther, A. Dobson, T. Duong, M. Callanan, S. Lick, A. Hamrick, R. Cano, and T. R. Klaenhammer. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 102:3906-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpi, M., M. Vancanneyt, J. Swings, and J. J. Leisner. 2003. Six cases of Lactobacillus bacteraemia: identification of organisms and antibiotic susceptibility and therapy. Scand. J. Infect. Dis. 35:404-408. [DOI] [PubMed] [Google Scholar]
- 3.Berg, R. D. 1999. Bacterial translocation from the gastrointestinal tract. Adv. Exp. Med. Biol. 473:11-30. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, P. P., D. A. Priebat, R. D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 78:206-209. [DOI] [PubMed] [Google Scholar]
- 5.Charteris, W. P., P. M. Kelly, L. Morelli, and J. K. Collins. 1998. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 84:759-768. [DOI] [PubMed] [Google Scholar]
- 6.Chateau, N., A. M. Deschamps, and A. H. Sassi. 1994. Heterogeneity of bile salts resistance in the Lactobacillus isolates of a probiotic consortium. Lett. Appl. Microbiol. 18:42-44. [Google Scholar]
- 7.FAO/WHO. 2001. Report of a Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization and Food and Agriculture Organization of the United Nations, London, Ontario, Canada.
- 8.Fedorak, R. N., and K. L. Madsen. 2004. Probiotics and the management of inflammatory bowel disease. Inflamm. Bowel Dis. 10:286-299. [DOI] [PubMed] [Google Scholar]
- 9.Foligné, B., C. Grangette, and B. Pot. 2005. Probiotics in IBD: mucosal and systemic routes of administration may promote similar effects. Gut 54:727-728. [PMC free article] [PubMed] [Google Scholar]
- 10.Foligné, B., S. Nutten, L. Steidler, V. Dennin, D. Goudercourt, A. Mercenier, and B. Pot. 2006. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis. Sci. 51:390-400. [DOI] [PubMed] [Google Scholar]
- 11.Fonden, R., R. Bjorneholm, and K. Ohlson. 2000. Lactobacillus F19—a new probiotic strain. Poster presented at the 4th Workshop of the PROBEMO-FAIR CT 96-1028 Project Functional Foods for EU Health in 2000.
- 12.Gevers, D., G. Huys, and J. Swings. 2001. Applicability of rep-PCR fingerprinting for identification of Lactobacillus species. FEMS Microbiol. Lett. 205:31-36. [DOI] [PubMed] [Google Scholar]
- 13.Gill, H. S., and F. Guarner. 2004. Probiotics and human health: a clinical perspective. Postgrad. Med. J. 80:516-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart, A. L., A. J. Stagg, and M. A. Kamm. 2003. Use of probiotics in the treatment of inflammatory bowel disease. J. Clin. Gastroenterol. 36:111-119. [DOI] [PubMed] [Google Scholar]
- 15.Harty, D. W. S., M. Patrikakis, and K. W. Knox. 1993. Identification of Lactobacillus strains isolated from patients with infective endocarditis and comparison of their surface-associated properties with those of other strains of the same species. Microb. Ecol. Health Dis. 6:191-201. [Google Scholar]
- 16.Jacobsen, C. N., V. R. Nielsen, A. E. Hayford, P. L. Moller, K. F. Michaelsen, A. Paerregaard, B. Sandstrom, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josson, K., T. Scheirlinck, F. Michiels, C. Platteeuw, P. Stanssens, H. Joos, P. Dhaese, M. Zabeau, and J. Mahillon. 1989. Characterization of a gram-positive broad-host-range plasmid isolated from Lactobacillus hilgardii. Plasmid 21:9-20. [DOI] [PubMed] [Google Scholar]
- 18.Kruis, W., P. Fric, J. Pokrotnieks, M. Lukas, B. Fixa, M. Kascak, M. A. Kamm, J. Weismueller, C. Beglinger, M. Stolte, C. Wolff, and J. Schulze. 2004. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuisma, J., S. Mentula, H. Jarvinen, A. Kahri, M. Saxelin, and M. Farkkila. 2003. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment. Pharmacol. Ther. 17:509-515. [DOI] [PubMed] [Google Scholar]
- 20.Lamine, F., H. Eutamene, J. Fioramonti, L. Bueno, and V. Theodorou. 2004. Colonic responses to Lactobacillus farciminis treatment in trinitrobenzene sulphonic acid-induced colitis in rats. Scand. J. Gastroenterol. 39:1250-1258. [DOI] [PubMed] [Google Scholar]
- 21.Land, M. H., K. Rouster-Stevens, C. R. Woods, M. L. Cannon, J. Cnota, and A. K. Shetty. 2005. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115:178-181. [DOI] [PubMed] [Google Scholar]
- 22.Llopis, M., M. Antolin, F. Guarner, A. Salas, and J. R. Malagelada. 2005. Mucosal colonisation with Lactobacillus casei mitigates barrier injury induced by exposure to trinitronbenzene sulphonic acid. Gut 54:955-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madsen, K., A. Cornish, P. Soper, C. McKaigney, H. Jijon, C. Yachimec, J. Doyle, L. Jewell, and C. De Simone. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580-591. [DOI] [PubMed] [Google Scholar]
- 24.Maragkoudakis, P. A., G. Zoumpopoulou, C. Miaris, G. Kalantzopoulos, B. Pot, and E. Tsakalidou. 2006. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 16:189-199. [Google Scholar]
- 25.McCarthy, J., L. O'Mahony, L. O'Callaghan, B. Sheil, E. E. Vaughan, N. Fitzsimons, J. Fitzgibbon, G. C. O'Sullivan, B. Kiely, J. K. Collins, and F. Shanahan. 2003. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 52:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercenier, A., P. Hols, Y. Roussel, G. Perez-Martinez, J. Buesa, M. Wilks, G. Pozzi, E. Remaut, L. Morelli, C. Grangette, V. Monedero, E. Palumbo, B. Foligne, L. Steidler, and S. Nutten. 2004. Screening and construction of probiotic strains with enhanced protective properties against intestinal disorders. Microb. Ecol. Health Dis. 16:86-95. [Google Scholar]
- 27.Mimura, T., F. Rizzello, U. Helwig, G. Poggioli, S. Schreiber, I. C. Talbot, R. J. Nicholls, P. Gionchetti, M. Campieri, and M. A. Kamm. 2004. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris, G. P., P. L. Beck, M. S. Herridge, W. T. Depew, M. R. Szewczuk, and J. L. Wallace. 1989. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795-803. [PubMed] [Google Scholar]
- 29.Nazli, A., P. C. Yang, J. Jury, K. Howe, J. L. Watson, J. D. Soderholm, P. M. Sherman, M. H. Perdue, and D. M. McKay. 2004. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 164:947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neurath, M., I. Fuss, and W. Strober. 2000. TNBS-colitis. Int. Rev. Immunol. 19:51-62. [DOI] [PubMed] [Google Scholar]
- 31.Osman, N., D. Adawi, S. Ahrne, B. Jeppsson, and G. Molin. 2004. Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Dig. Dis. Sci. 49:320-327. [DOI] [PubMed] [Google Scholar]
- 32.Otte, J. M., and D. K. Podolsky. 2004. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G613-G626. [DOI] [PubMed] [Google Scholar]
- 33.Pavan, S., P. Desreumaux, and A. Mercenier. 2003. Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria. Clin. Diagn. Lab. Immunol. 10:696-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peran, L., D. Camuesco, M. Comalada, A. Nieto, A. Concha, M. P. Diaz-Ropero, M. Olivares, J. Xaus, A. Zarzuelo, and J. Galvez. 2005. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J. Gastroenterol. 11:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salminen, M. K., H. Rautelin, S. Tynkkynen, T. Poussa, M. Saxelin, V. Valtonen, and A. Jarvinen. 2004. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 38:62-69. [DOI] [PubMed] [Google Scholar]
- 36.Salminen, M. K., S. Tynkkynen, H. Rautelin, M. Saxelin, M. Vaara, P. Ruutu, S. Sarna, V. Valtonen, and A. Jarvinen. 2002. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 35:1155-1160. [DOI] [PubMed] [Google Scholar]
- 37.Sanders, M. E., and T. R. Klaenhammer. 2001. The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J. Dairy Sci. 84:319-331. [DOI] [PubMed] [Google Scholar]
- 38.Shanahan, F. 2004. Host-flora interactions in inflammatory bowel disease. Inflamm. Bowel Dis. 10:S16-S24. [DOI] [PubMed] [Google Scholar]
- 39.Shanahan, F. 2003. Probiotics: a perspective on problems and pitfalls. Scand. J. Gastroenterol. Suppl.:34-36. [DOI] [PubMed] [Google Scholar]
- 40.Sheil, B., J. McCarthy, L. O'Mahony, M. W. Bennett, P. Ryan, J. J. Fitzgibbon, B. Kiely, J. K. Collins, and F. Shanahan. 2004. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut 53:694-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snelling, A. M. 2005. Effects of probiotics on the gastrointestinal tract. Curr. Opin. Infect. Dis. 18:420-426. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg, S. M. 2003. Bacterial translocation: what it is and what it is not. Am. J. Surg. 186:301-305. [DOI] [PubMed] [Google Scholar]
- 43.Vesa, T., P. Pochart, and P. Marteau. 2000. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 14:823-828. [DOI] [PubMed] [Google Scholar]
- 44.von Wright, A. 2005. Regulating the safety of probiotics—the European approach. Curr. Pharm. Des. 11:17-23. [DOI] [PubMed] [Google Scholar]
- 45.Walker, D. C., K. Aoyama, and T. R. Klaenhammer. 1996. Electrotransformation of Lactobacillus acidophilus group A1. FEMS Microbiol. Lett. 138:233-237. [DOI] [PubMed] [Google Scholar]