Abstract
The accumulation of compatible solutes was studied in the hyperthermophilic bacterium Aquifex pyrophilus as a function of the temperature and the NaCl concentration of the growth medium. Nuclear magnetic resonance analysis of cell extracts revealed the presence of α- and β-glutamate, di-mannosyl-di-myo-inositol phosphate, di-myo-inositol phosphate, and an additional compound here identified as 1-glyceryl-1-myo-inosityl phosphate. All solutes accumulated by A. pyrophilus are negatively charged at physiological pH. The intracellular levels of di-myo-inositol phosphate increased in response to supraoptimal growth temperature, while α- and β-glutamate accumulated in response to osmotic stress, especially at growth temperatures below the optimum. The newly discovered compound, 1-glyceryl-1-myo-inosityl phosphate, appears to play a double role in osmo- and thermoprotection, since its intracellular pool increased primarily in response to a combination of osmotic and heat stresses. This work also uncovered the nature of the unknown compound, previously detected in Archaeoglobus fulgidus (L. O. Martins et al., Appl. Environ. Microbiol. 63:896-902, 1997). The curious structural relationship between diglycerol phosphate (found only in Archaeoglobus species), di-myo-inositol phosphate (a canonical solute of hyperthermophiles), and the newly identified solute is highlighted. This is the first report on the occurrence of 1-glyceryl-1-myo-inosityl phosphate in living systems.
The accumulation of low-molecular-mass organic compounds (compatible solutes) is a common strategy among organisms isolated from saline environments to cope with osmotic stress (4). Hyperthermophiles, organisms adapted to grow at high temperature (near 100°C), are unable to cope with high salinity (more than ∼7% NaCl). Many, however, thrive in niches of hot seawater and often also use organic solutes for osmoregulation. Compatible solutes of hyperthermophiles belong broadly to the same classes of compounds used by mesophiles, i.e., sugars, amino acids, and polyols, but hyperthermophiles tend to accumulate solutes that are rarely or never encountered in mesophiles, such as di-myo-inositol phosphate, diglycerol phosphate, mannosylglycerate, and derivatives of these three compounds (8, 20, 21). Interestingly, solutes of hyperthermophiles are generally negatively charged, while mesophiles accumulate primarily neutral or zwitterionic molecules. These trends indicate that compatible solutes in hyperthermophiles may play more complex roles than in mesophiles and are probably part of the strategies used by these organisms to protect cellular structures against heat damage.
Recent research efforts, especially in the last decade, have noticeably expanded our knowledge about the nature and accumulation profiles of compatible solutes in hyperthermophiles (21). However, the number of hyperthermophilic organisms examined thus far is relatively low and insufficient to provide a clear picture of the chemical diversity and physiological roles of compatible solutes in organisms adapted to high temperature. The poor growth yields of many hyperthermophiles and the demand for peculiar culture conditions are the main reasons underlying the slow progress.
Within the domain Bacteria, hyperthermophiles are classified in two orders: the Thermotogales and the Aquificales. There is already considerable information about compatible solutes in the order Thermotogales, including stress response patterns in several species (14), but nothing is known, in this respect, in the order Aquificales, probably the deepest lineage in the bacterial phylogenetic tree (5, 10). Therefore, to extend our knowledge on the strategies of osmo- and thermoadaptation in hyperthermophiles, we decided to study the organic solute pool of Aquifex pyrophilus as a function of the temperature and salinity of the growth medium.
Aquifex pyrophilus is a microaerophilic hyperthermophile isolated from hot marine sediments in Iceland. In respect to metabolic traits, these bacteria are strict chemolithoautotrophs using molecular hydrogen, thiosulfate, or elemental sulfur as electron donors and oxygen (at low concentration) and nitrate as electron acceptors (10). This work led to the discovery of a new compatible solute, 1-glyceryl-1-myo-inosityl phosphate, which occurs in the two species of the genus Aquifex as well as in the unrelated organism Archaeoglobus fulgidus. In every case, the intracellular content of this compound increased primarily in response to a combination of osmotic and heat stresses.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Aquifex pyrophilus strain Kol5α (DSM 6858) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany. The organism was grown in modified SME medium (DSM 534). Seed cultures were initially produced in 160-ml glass Wheaton-type vials with a 20-mm internal neck diameter and containing 20 ml of sterile growth medium. Vials were fitted with 10-mm-depth butyl rubber stoppers (Bellco) and crimp sealed. The headspace was then gassed for 5 min with oxygen-free nitrogen followed by hydrogen-carbon dioxide gas mix (80:20) for a further 5 min, and adjusted to 1 bar overpressure. The oxygen content was raised to approximately 0.5% by the injection of an appropriate volume of filtered atmospheric air. Cultures were inoculated with a 5% inoculum and then incubated at 85°C and monitored for growth by direct cell counting using a Thoma chamber. Scale up of seed cultures was achieved using an equivalent procedure employing Duran-type bottles (45-mm internal diameter, 1- to 5-liter volumes) fitted with 4-valve PTFE screw-threaded caps (Omnifit). Seed cultures routinely remained viable for a period of 1 to 2 weeks following storage at 4°C.
Biomass samples were produced by preparing 4-liter quantities of medium in 20-liter polypropylene bottles (Nalgene) which had been modified to permit pH control (set point pH 6.8) and gas exchange. The overall salinity of the medium was adjusted to the required concentration by the addition of an appropriate quantity of NaCl. Cultures were grown within a high-temperature oven (Sanyo Gallenkamp) at the required temperature, with agitation provided by two metered gas streams, hydrogen-carbon dioxide (80:20) and filter-sterilized atmospheric air (0.1 total vessel volume per minute) passing through P100 sintered glass thimbles (Radleys). Culture growth was monitored by direct cell counting, as described above. Cells were harvested by centrifugation at 10,000 × g for 20 min, transferred to storage tubes, and stored frozen at below −70°C until the extraction of organic solutes.
Extraction and quantification of intracellular solutes and cell protein determination.
Organic solutes were extracted twice with boiling 80% ethanol as previously described (14). Freeze-dried ethanolic extracts were dissolved in water and cleansed of lipid components through chloroform addition and subsequent centrifugation to separate and collect the aqueous phase. The resulting extracts were freeze-dried and resuspended in D2O for nuclear magnetic resonance (NMR) analysis. Quantification of organic compounds was performed by 1H NMR and 31P NMR (see below). The protein content of the cells was determined by the Bradford assay (3) after treatment with 1 M NaOH (100°C, 10 min) and neutralization with 1 M HCl.
NMR spectroscopy.
All spectra were acquired on a DRX500 spectrometer (Bruker, Rheinstetten, Germany). Typically, 1H NMR spectra were acquired with water presaturation, using a 60° flip angle and a repetition delay of 5 s. Proton chemical shifts were relative to 3-(trimethylsilyl)propanesulfonic acid (sodium salt) designated at 0.015 ppm.
13C NMR spectra were recorded at 125.77 MHz using a 5-mm carbon selective probe head. Typically, spectra were acquired with a repetition delay of 1 s and a 75° flip angle. 31P NMR spectra were recorded at 202.45 MHz on the same spectrometer using a broadband inverse detection 5-mm probe head. Proton decoupling was applied in both cases during the acquisition time only, using the wideband alternating-phase low-power technique for zero-residue splitting sequence. Chemical shifts were referenced with respect to (trimethylsilyl)propanesulfonic acid for 13C and external 85% H3PO4 for 31P.
For quantification purposes, 1H NMR spectra were acquired with a repetition delay of 60 s. Formate was added as an internal concentration standard. When possible, quantification was also carried out using 31P NMR. In this case, a repetition delay of 30 s was employed and 1 mM EDTA was added to the sample. Inorganic phosphate was used as an internal concentration standard.
Two-dimensional spectra were performed using standard Bruker pulse programs. Phase-sensitive nuclear Overhauser effect spectroscopy, proton-homonuclear shift correlation spectroscopy, and total-correlation spectroscopy were acquired collecting 4,096 (t2) by 512 (t1) data points, while in the heteronuclear spectra, 4,096 (t2) by 256 (t1) data points were collected. In the 1H-13C heteronuclear multiple-quantum coherence spectra (HMQC) (1), a delay of 3.5 ms was used for evolution of 1JCH, and in the heteronuclear multiple-bond connectivity spectrum, a delay of 73.5 ms was used for evolution of long-range couplings. The 1H-31P HMQC was acquired using a delay of 41.6 ms for the evolution of JPH constants.
Purification of 1-glyceryl-1-myo-inosityl phosphate in Archaeoglobus fulgidus extracts.
A. fulgidus (strain VC-16) biomass was obtained from a 300-liter fermentation at 83°C and 1.8% NaCl. Cells were broken in a French pressure cell at 3.3 MPa; the resulting lysate was dialysed against 50 mM Tris-HCl buffer, pH 7.5, containing 10 mM MgCl2, 200 μM phenylmethylsulfonyl fluoride, and 1 mM EDTA. The dialysis buffer containing the intracellular organic solutes was freeze-dried. The resulting powder was treated 4 times with boiling 100% ethanol, after which the insoluble fraction was extracted twice with boiling 80% ethanol. 1H NMR analysis revealed that the unidentified compound (1-glyceryl-1-myo-inosityl phosphate) was only present in the 80% ethanol extract, which also contained diglycerol phosphate, di-myo-inositol phosphate, and Tris-HCl. This preparation was applied to an anion-exchange resin (QAE-Sephadex A-25; Pharmacia, Uppsala, Sweden) and developed with a linear gradient of sodium carbonate buffer (5 mM to 1 M, pH 9.8). Fractions containing the unknown compound, as judged by NMR, were pooled, freeze-dried, and desalted in an activated cation-exchange resin (Dowex 50W-X8; Bio-Rad) and eluted with distilled water. Fractions were degassed under a vacuum, and the pH was adjusted to 5 with 1 M KOH. The new compound was further purified by gel filtration (Sephadex-G10; Pharmacia, Uppsala, Sweden). Two partially purified samples, containing the desired solute as the major component, were obtained: one had di-myo-inositol phosphate as a contaminant, while the other had diglycerol phosphate. These fractions were judged to be suitable for structural determination by NMR.
Mass spectrometry.
Mass spectra were acquired on an LCQ advantage ion-trap mass spectrometer from ThermoFinnigan (San Jose, CA) equipped with an electrospray ionization interface operated in the negative mode. Samples were injected at 300°C and −33 V in 50% methanol-0.1% formic acid.
RESULTS
Identification of organic solutes in Aquifex pyrophilus.
The identification of organic solutes in the extracts of A. pyrophilus was accomplished by 1H and 31P NMR spectral analysis. Whenever necessary, these identifications were confirmed through the acquisition of 13C spectra and subsequent comparison of the observed and the published chemical shifts.
The spectra revealed the presence of five compounds: α-glutamate, β-glutamate, di-mannosyl-di-myo-inositol phosphate, di-myo-inositol phosphate, and another compound with spectral features identical to those of a solute previously detected in A. fulgidus extracts but never identified. This compound had been referred to as an “unidentified isomer of di-myo-inositol phosphate” based on the similarity of the proton resonances due to the inositol moieties in the two compounds (9, 15). The chemical nature remained unknown mainly because this is a minor solute in A. fulgidus. In contrast, in A. pyrophilus grown at 88°C and 4% NaCl, the unidentified compound was the major organic solute, and it was possible to perform its identification by NMR spectral analysis without resorting to any purification step.
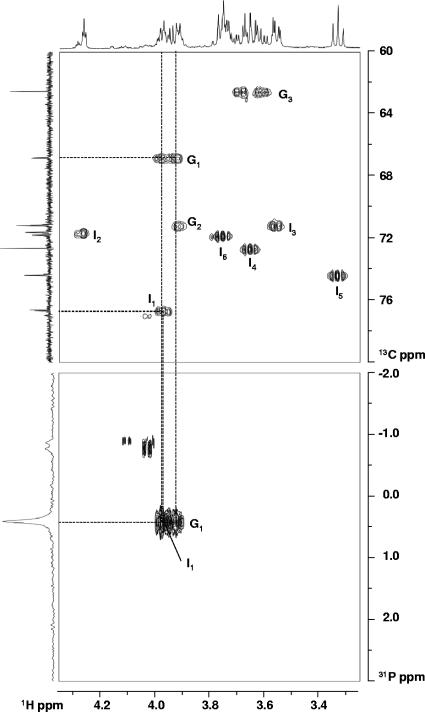
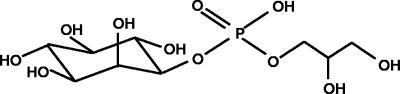
The 31P resonance at 0.4 ppm (Table 1) is typical of a phosphodiester compound and its coupling pattern revealed a doublet of triplets with coupling constants of 7.8 and 6.2 Hz, indicating that the phosphate group is esterified with two distinct moieties. 13C-1H and 31P-1H HMQC spectra revealed (Fig. 1) the correlation between the phosphate signal and two partially overlapping signals in the proton spectrum. This correlation is further confirmed by the carbon-phosphorous splitting that can be observed in the carbon signals at 77.0 and 67.1 ppm, assigned to C1 of the glycerol and inositol moieties, respectively. The total-correlation spectroscopy spectrum showed that these two signals belong to two different spin systems of three and six members. According to the 13C-1H HMQC spectra, all carbon signals of this compound are in the region typical of primary alcohols. The coupling patterns and chemical shift values in carbon and proton spectra show that these moieties are glycerol and myo-inositol. We conclude that the structure of the unknown compound is 1-glyceryl-1-myo-inosityl phosphate (Fig. 2). The combined analysis of the 1H proton-homonuclear shift correlation spectroscopy and 13C-1H HMQC spectra allowed the complete assignment of all of the proton and carbon resonances (Table 1).
TABLE 1.
NMR parameters of 1-glyceryl-1-myo-inosityl-phosphate
| Moiety | 13C NMR δ (ppm) |
1H NMR
|
31P NMR
|
||
|---|---|---|---|---|---|
| δ (ppm) | nJH,H (Hz) | δ (ppm) | nJP,X (Hz) | ||
| Inositol | |||||
| C1 | 77.0 | 3.97 | 3J1,2 = 2.75 | 2JP,C = 6.24 | |
| C2 | 71.9 | 4.27 | 3J2,3 = 2.75 | ||
| C3 | 71.4 | 3.56 | 3J3,4 = 9.92 | ||
| C4 | 72.9 | 3.66 | 3J4,5 = 9.60 | ||
| C5 | 74.6 | 3.34 | 3J5,6 = 9.31 | ||
| C6 | 72.1 | 3.76 | 3J6,1 = 10.0 | ||
| Glycerol | |||||
| C1 | 67.1 | 3.98/3.94 | NDa | 2JP,C = 5.76 | |
| C2 | 71.5 | 3.92 | 3J2,3a = 4.13 3J2,3b = 6.20 | 3JP,C = 6.72 | |
| C3 | 62.8 | 3.69/3.61 | 2J3a,3b = 12.40 | ||
| Phosphate | 0.4 | ||||
ND, not determined.
FIG. 1.
1H-13C HMQC (top) and 1H-31P HMQC (bottom) correlation spectra of 1-glyceryl-1-myo-inositol phosphate in the ethanolic extract of Aquifex pyrophilus grown at 88°C and 4% NaCl. The assignments are indicated next to each signal. G, glycerol moiety; I, inositol moiety.
FIG. 2.
Molecular representation of 1-glyceryl-1-myo-inosityl phosphate. The depicted structure shows the inositol moiety in the l configuration (as found in di-myo-inositol phosphate isolated from other hyperthermophiles) (6, 25). The stereochemical configuration of the newly discovered compound has not been established.
The partially purified preparations of A. fulgidus were spiked with the extract of A. pyrophilus cells grown at 88°C and 4% NaCl. We observed a perfect match between the signals, thus establishing that 1-glyceryl-1-myo-inosityl phosphate is the previously unidentified compound and that it occurs both in A. pyrophilus and in A. fulgidus.
The identity of the compound was confirmed by mass spectrometry. The mass spectrum of the extract of A. pyrophilus grown at 88°C and 4% NaCl revealed one major signal with an m/z of 332.88 (the expected molecular mass of 1-glyceryl-1-myo-inosityl phosphate is 332.9 g · mol−1) and smaller-intensity peaks corresponding to other solutes in the extract. As expected from the NMR data, a signal with an m/z of 332.88 also occurred in the partially purified preparations of A. fulgidus together with signals at m/z of 244.9 and 420.8 corresponding to diglycerol phosphate and di-myo-inositol phosphate, respectively.
Effect of temperature and salt stress on the accumulation of organic solutes in Aquifex pyrophilus.
The total amount of compatible solutes in A. pyrophilus was fairly independent of the growth temperature, with values between 1.3 and 1.6 μmol · mg of protein−1, in the range of temperatures examined, 80 to 88°C (Table 2). However, the relative contribution of the several solutes varied greatly. At 80°C, glutamate (α and β forms) dominated the solute pool, while the phosphodiesters (di-myo-inositol phosphate, 1-glyceryl-1-myo-inosityl phosphate, and di-mannosyl-di-myo-inositol phosphate) represent only about a third of the total pool. As the growth temperature increased, the phosphate derivatives, di-myo-inositol phosphate, and 1-glyceryl-1-myo-inosityl phosphate, became dominant, accounting for 88% of the total solute pool, while the level of glutamate decreased sharply. In response to a rise from 2% to 4% in the NaCl concentration of the medium, the total solute pool tripled; however, the major cause responsible for this increase was different according to the temperature at which the salt stress was applied. At 80°C, an increase in salinity caused a widespread rise in the level of all compounds, with a more pronounced effect on the level of glutamate (α and β forms). In contrast, when the same salinity stress was applied at 88°C, the most pronounced rise was observed in the levels of the phosphodiesters, with particular emphasis for 1-glyceryl-1-myo-inosityl phosphate, which reached levels of 2.27 μmol · mg of protein−1 (about half of the total solute pool) (Table 2).
TABLE 2.
Accumulation of compatible solutes in Aquifex pyrophilus grown at different temperatures and NaCl concentrations
| Growth temp (°C) | NaCl concn (%) | Amt of solute (μmol/mg protein)
|
|||||
|---|---|---|---|---|---|---|---|
| DIPa | DMDIPb | GIPc | β-Glud | α-Glue | Total | ||
| 80 | 2 | 0.10 | 0.02 | 0.41 | 0.72 | 0.36 | 1.61 |
| 85 | 2 | 0.18 | 0.03 | 0.62 | 0.52 | 0.25 | 1.60 |
| 88 | 2 | 0.48 | 0.02 | 0.63 | 0.08 | 0.07 | 1.28 |
| 80 | 4 | 0.13 | 0.47 | 1.23 | 2.66 | 1.00 | 5.49 |
| 88 | 4 | 0.41 | 0.12 | 2.27 | 1.12 | 0.52 | 4.44 |
DIP, di-myo-inositol-1,1′-phosphate.
DMDIP, di-mannosyl-di-myo-inositol-1,1′-phosphate.
GIP, 1-glyceryl-1-myo-inosityl phosphate.
β-Glu, β-glutamate.
α-Glu, α-glutamate.
Di-mannosyl-di-myo-inositol phosphate was generally a minor compound not appearing to respond clearly to any of the applied stresses. At the optimal salinity, the level of di-myo-inositol phosphate strongly increased with the temperature, while both forms of glutamate nearly disappeared and the level of 1-glyceryl-1-myo-inosityl phosphate was fairly constant.
DISCUSSION
The profiles of organic solute accumulation as a function of the growth temperature and NaCl concentration have been studied in several thermophiles and hyperthermophiles isolated from marine environments (7, 9, 12, 13, 14, 15, 16). These data suggest that solutes in hyperthermophiles tend to play specialized roles. Some, like mannosylglycerate and diglycerol phosphate, have a primary role in osmoadaptation, whereas di-myo-inositol phosphate is used mainly in thermoadaptation (21).
The pool of organic solutes in A. pyrophilus comprises five compounds: α-glutamate, β-glutamate, di-myo-inositol phosphate (DIP), di-mannosyl-di-myo-inositol phosphate, and the newly discovered compound, 1-glyceryl-1-myo-inosityl phosphate (GIP). In the absence of the solutes typically used in hyperthermophiles for osmoadaptation (mannosylglycerate and diglycerol phosphate), A. pyrophilus uses glutamate (mainly the β-isomer) and GIP for osmotic adjustment. Interestingly, glutamate plays a major role in osmoadaptation in the absence of heat stress but is replaced by GIP at temperatures above the optimum. This behavior resembles that of Rhodothermus marinus in which the role of mannosylglyceramide during osmoadaptation at moderate temperatures is replaced by mannosylglycerate under heat stress (24). In contrast, diglycerol phosphate (DGP) is used for osmoadaptation in A. fulgidus in the full range of growth temperatures.
It is interesting that all solutes detected in A. pyrophilus bear a negative charge, a finding that corroborates the strong correlation between thermophily and preference for charged solutes and further supports the view that these solutes may be implicated in thermoprotection. In addition, the preponderance of phosphodiester compounds in the solute pool of this hyperthermophile is noteworthy, contrasting with the fact that phosphodiester compounds were never reported to play a role in stress adaptation in mesophiles.
The total amount of solutes in A. pyrophilus did not increase at supraoptimal temperatures, a result atypical of marine hyperthermophiles but already observed in species of the genus Thermococcus (12). In agreement with the general trend of preferential accumulation of DIP in response to heat stress, the level of this solute in A. pyrophilus increased considerably above the optimal temperature, but the contribution of GIP for thermoprotection should not be underestimated because the level of this compound was greater than that of DIP under all of the stressful conditions examined.
In contrast to α-glutamate, which is used in many organisms during low-level osmotic adaptation, the accumulation of β-glutamate is rather uncommon, with only one occurrence reported among bacteria from hot environments (14). However, this solute was found to accumulate in several mesophilic marine bacteria and (hyper)thermophilic methanogenic archaea (8, 18, 19), showing a strong dependence on the salinity of the medium. β-Glutamate occurs along with the α-isomer in all the (hyper)thermophiles known to accumulate this β-amino acid (Methanococcus igneus, Methanococcus thermolithotrophicus, Methanococcus jannaschii, and Thermotoga neapolitana) (7, 14, 19). The same joint occurrence was observed in A. pyrophilus, with the β-isomer playing the preponderant role.
The occurrence of GIP as a cell metabolite has not been reported earlier. Being a phosphodiester of inositol and glycerol, it resembles a chimera of DIP and DGP. Moreover, as DIP accumulates mainly in response to heat, while the level of DGP responds consistently to increased salinity, it is interesting that GIP also seems to play a mixed physiological role, its level increasing strongly when osmotic and heat stresses are imposed simultaneously.
DIP has never been found in mesophiles, but it is the most common solute in extreme hyperthermophiles and its role as a protector of cell components in vivo has been often suggested (20, 21, 22). However, assays to determine the degree of stabilization rendered by DIP on isolated enzymes produced contradictory results (2, 23). On the other hand, the beneficial effect of DGP as a protein thermoprotectant is well documented (11, 17). In this respect, it would be interesting to ascertain the performance of GIP, a hybrid structure of DIP and DGP, but the difficulty in obtaining the required amounts of this solute from natural sources precludes that intent for the time being. We are currently attempting to obtain the needed amounts by chemical synthesis.
The present work also established the identity of the “unidentified isomer of DIP” earlier detected in cell extracts of the hyperthermophilic archaeon A. fulgidus by Martins et al. (15). Therefore, the information on its accumulation profile reported by Gonçalves et al. (9) can now be interpreted in the light of this identification. GIP displays, in A. fulgidus, an accumulation pattern similar to that found in A. pyrophilus insofar as its level increased noticeably when a combination of osmotic and heat stresses was applied. In A. pyrophilus, however, GIP was the major solute used for osmotic balance at growth temperatures above the optimum, while this prominent role was played by a different solute (DGP) in A. fulgidus.
Besides A. pyrophilus and A. fulgidus, we verified the presence of GIP in cells of Aquifex aeolicus, kindly supplied by H. Huber and R. Huber at the University of Regensberg, Germany (unpublished results). It is remarkable to find that bacterial species of the genus Aquifex and archaeal species of the genus Archaeoglobus have in common the ability to synthesize this rare solute, GIP. Given the long phylogenetic distance between the two genera and the apparent scarce distribution of GIP, the occurrence of lateral gene transfer could be a plausible hypothesis to explain these findings, taking into account that these hyperthermophilic organisms populate similar habitats. However, a sound conclusion cannot be put forward without knowledge of the genes and enzymes implicated in the respective biosynthetic pathway. This is the purpose of current work in our lab.
Acknowledgments
This work was supported by the European Commission, 5th and 6th Framework Programme contracts QLK3-2000-00640, and COOP-CT-2003-508644, FEDER, and POCTI, Portugal (project A004/2005, action V.5.1, SFRH/BPD/11511/2002 to P.L., SFRH/BD/5076/2001 to L.G., and SFRH/BD/25539/2005 to M.V.R.).
We acknowledge Ana Coelho from the mass spectrometry service at ITQB for performing the mass spectra. We thank H. Huber and R. Huber for kindly supplying A. aeolicus biomass.
REFERENCES
- 1.Bax, A., and M. F. Summers. 1986. 1H and 13C assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 108:2093-2094. [Google Scholar]
- 2.Borges, N., A. Ramos, N. D. H. Raven, R. J. Sharp, and H. Santos. 2002. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209-216. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brown, A. D. 1978. Compatible solutes and extreme water stress in eukaryotic micro-organisms. Adv. Microb. Physiol. 17:181-242. [DOI] [PubMed] [Google Scholar]
- 5.Burggraf, S., G. J. Olsen, K. O. Stetter, and C. R. Woese. 1992. A phylogenetic analysis of Aquifex pyrophilus. Syst. Appl. Microbiol. 15:352-356. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L., E. T. Spiliotis, and M. F. Roberts. 1998. Biosynthesis of di-myo-inositol-1,1′-phosphate, a novel osmolyte in hyperthermophilic archaea. J. Bacteriol. 180:3785-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciulla, R. A., S. Burggraf, K. O. Stetter, and M. F. Roberts. 1994. Occurrence and role of di-myo-inositol-1,1′-phosphate in Methanococcus igneus. Appl. Environ. Microbiol. 60:3660-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Costa, M. S., H. Santos, and E. A. Galinski. 1998. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 61:117-153. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves, L. G., R. Huber, M. S. da Costa, and H. Santos. 2003. A variant of the hyperthermophiles Archaeoglobus fulgidus adapted to grow at high salinity. FEMS Microbiol. Lett. 218:239-244. [DOI] [PubMed] [Google Scholar]
- 10.Huber, R., T. Wilharm, D. Huber, A. Trincone, S. Burggraf, H. König, R. Rachel, I. Rockinger, H. Fricke, and K. O. Stetter. 1992. Aquifex pyrophilus gen. nov., sp. nov., represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst. Appl. Microbiol. 15:340-351. [Google Scholar]
- 11.Lamosa, P., A. Burke, R. Peist, R. Huber, M. Y. Liu, G. Silva, C. Rodrigues-Pousada, J. LeGall, C. Maycock, and H. Santos. 2000. Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from the hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 66:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamosa, P., L. O. Martins, M. S. da Costa, and H. Santos. 1998. Effects of temperature, salinity, and medium composition on compatible solute accumulation by Thermococcus spp. Appl. Environ. Microbiol. 64:3591-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins, L. O., and H. Santos. 1995. Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus furiosos in response to salinity and temperature. Appl. Environ. Microbiol. 61:3299-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins, L. O., L. S. Carreto, M. S. da Costa, and H. Santos. 1996. New compatible solutes related to di-myo-inositol-phosphate in members of the order Thermotogales. J. Bacteriol. 178:5644-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins, L. O., R. Huber, H. Huber, K. O. Stetter, M. S. da Costa, and H. Santos. 1997. Organic solutes in hyperthermophilic Archaea. Appl. Environ. Microbiol. 63:896-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neves, C., M. S. da Costa, and H. Santos. 2005. Compatible solutes of the hyperthermophile Palaeococcus ferrophilus: osmoadaptation and thermoadaptation in the order Thermococcales. Appl. Environ. Microbiol. 71:8091-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pais, T. M., P. Lamosa, W. dos Santos, J. Legall, D. L. Turner, and H. Santos. 2005. Structural determinants of protein stabilization by solutes. The importance of the hairpin loop in rubredoxins. FEBS J. 272:999-1011. [DOI] [PubMed] [Google Scholar]
- 18.Robertson, D. E., D. Noll, and M. F. Roberts. 1992. Free amino acid dynamics in marine methanogens. J. Biol. Chem. 267:14893-14901. [PubMed] [Google Scholar]
- 19.Robertson, D. E., M. F. Roberts, N. Belay, K. O. Stetter, and D. R. Boone. 1990. Occurrence of β-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl. Environ. Microbiol. 56:1504-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos, H., and M. S. da Costa. 2001. Organic solutes from thermophiles and hyperthermophiles. Methods Enzymol. 334:302-315. [DOI] [PubMed] [Google Scholar]
- 21.Santos, H., P. Lamosa, N. Borges, T. Q. Faria, and C. Neves. The physiological role, biosynthesis and mode of action of compatible solutes from (hyper)thermophiles. In C. Gerday and N. Glandorf (ed.), Physiology and biochemistry of extremophiles, in press. ASM Press, Washington, D.C.
- 22.Scholz, S., J. Sonnenbichler, W. Schäfer, and R. Hensel. 1992. Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett. 306:239-242. [DOI] [PubMed] [Google Scholar]
- 23.Shima, S., D. A. Herault, A. Berkessel, and R. K. Thauer. 1998. Activation and thermostabilization effects of cyclic 2,3-diphosphoglycerate on enzymes from the hyperthermophilic Methanopyrus kandleri. Arch. Microbiol. 170:469-472. [DOI] [PubMed] [Google Scholar]
- 24.Silva, Z., N. Borges, L. O. Martins, R. Wait, M. S. da Costa, and H. Santos. 1999. Combined effect of the growth temperature and salinity of the medium on the accumulation of compatible solutes by Rhodothermus marinus and Rhodothermus obamensis. Extremophiles 3:163-172. [DOI] [PubMed] [Google Scholar]
- 25.Van Leeuwen, S. H., G. A. van der Marel, R. Hensel, and J. H. van Boom. 1994. Synthesis of L,L-di-myo-inositol-1,1′-phosphate: a novel inositol phosphate from Pyrococcus woesei. Recl. Trav. Chim. Pays Bas 113:335-336. [Google Scholar]