Abstract
We recently showed that expressing an H2O-NADH oxidase in Saccharomyces cerevisiae drastically reduces the intracellular NADH concentration and substantially alters the distribution of metabolic fluxes in the cell. Although the engineered strain produces a reduced amount of ethanol, a high level of acetaldehyde accumulates early in the process (1 g/liter), impairing growth and fermentation performance. To overcome these undesirable effects, we carried out a comprehensive analysis of the impact of oxygen on the metabolic network of the same NADH oxidase-expressing strain. While reducing the oxygen transfer rate led to a gradual recovery of the growth and fermentation performance, its impact on the ethanol yield was negligible. In contrast, supplying oxygen only during the stationary phase resulted in a 7% reduction in the ethanol yield, but without affecting growth and fermentation. This approach thus represents an effective strategy for producing wine with reduced levels of alcohol. Importantly, our data also point to a significant role for NAD+ reoxidation in controlling the glycolytic flux, indicating that engineered yeast strains expressing an NADH oxidase can be used as a powerful tool for gaining insight into redox metabolism in yeast.
There is growing demand worldwide for wines containing lower levels of alcohol. Today, however, wine-making practices favor the production of wines with high flavor intensity, prepared from fully matured grapes. In some cases, the juice obtained from such grapes contains very high sugar concentrations, resulting in wines with excessive levels of alcohol. This situation is antithetical to current public health policies and to the prevention of drunkenness. Furthermore, the high ethanol concentration may affect wine quality, for example, by altering the volatility of aroma compounds (1).
Currently, several physical processes are available for producing wines containing less alcohol, all of them involving the selective extraction of ethanol based on volatility or diffusion (20). Despite their efficacy, these procedures are expensive and difficult to perform, and they can also affect the flavor balance through the loss of aroma compounds. One biological alternative would be to use yeast strains that give low ethanol yields, a method that promises to be both faster and less expensive. Several attempts have been made through genetic engineering to reduce the ethanol yield of Saccharomyces cerevisiae by diverting sugar metabolism into by-products other than ethanol. For example, yeast strains producing more glycerol and less ethanol have been obtained by overexpressing GPD1, a gene that codes for glycerol 3-phosphate dehydrogenase (GPDH) (16, 17, 18, 21). Another strategy has been to express lactate dehydrogenase in yeast, resulting in the simultaneous conversion of pyruvate into ethanol and lactate (4, 5). Still other approaches have been based on the removal of fermentable sugar from the grape must; this can be achieved, for example, by using a glucose oxidase before fermentation to catalyze the oxidation of glucose to form gluconolactone in the presence of molecular oxygen (20) or by using a yeast strain producing the same enzyme during fermentation (15).
A major constraint that is not fully satisfied by these approaches, however, is the need to obtain a significant reduction in the ethanol yield without causing a direct or indirect accumulation of undesirable by-products in the wine. Since the amount of sugar to be diverted per percent of reduced alcohol is high (16.8 g/liter), it is probably more realistic to redirect the carbon flux toward multiple metabolites rather than a single by-product, the accumulation of which may affect the sensory properties of the wine at high levels (e.g., lactic or gluconic acid).
We recently reported a novel strategy for reducing the ethanol yield in yeast based on cofactor engineering (11). In this approach, an H2O-forming NADH oxidase is expressed in S. cerevisiae, leading to a marked decrease in the intracellular NADH pool. In contrast to previous approaches, this strategy directly affects numerous oxidoreduction reactions participating in many different metabolic pathways, resulting in a unique metabolite redistribution pattern. During the fermentation of synthetic must under microaerobic conditions, we observed that the ethanol yield of the strain expressing the NADH oxidase was reduced by 15% compared to that of the wild-type strain. At the same time, however, the strain exhibited altered growth under wine fermentation conditions and was unable to ferment more than 100 g/liter glucose. These limitations were shown to be largely due to the accumulation of high levels of acetaldehyde resulting from the alcohol dehydrogenase reduced flux through the (ADH) reaction (11).
As a 15% reduction in the yield of ethanol from glucose would be highly valuable for the production of wine with reduced ethanol content, an important question remaining after these studies was whether the effects of NADH oxidase expression could be controlled in a way that would limit its negative impact on fermentation performance. In particular, as molecular oxygen is the substrate of the NADH oxidase-catalyzed reaction, we wanted to examine the impact of oxygen on the flux distribution and to determine the minimal amount of oxygen that needs to be transferred in order to obtain a metabolic shift and to reduce ethanol production. In the present study, we have thus characterized the NADH oxidase-expressing strain with regard to its oxygen requirement, examining the impacts of different oxygen transfer rates on the strain's physiology and metabolic redistribution. Based on these results, we have designed a two-step fermentation procedure in which the oxidase expression phase is uncoupled from the growth phase. Finally, the relevant fermentation products formed during and after glucose depletion, as well as the specific rates of glucose consumption, oxygen consumption, and ethanol formation, were determined for both the wild-type and the NADH oxidase-expressing strains.
MATERIALS AND METHODS
Strains and growth conditions.
The Saccharomyces cerevisiae yeast strain V5 (MATa ura3) was derived from a champagne wine yeast. Strain V5noxE was previously described (11) and contains the noxE gene from Lactoccocus lactis under the control of the TDH3 promoter (encoding glyceraldehyde 3-phosphate dehydrogenase) integrated in the URA3 locus. The S. cerevisiae strains were grown in YPD medium (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose).
Fermentation conditions.
Fermentation experiments were carried out on synthetic MS medium as described previously (11). Anaerobic batch fermentations were carried out at 28°C in fermentors with a working volume of 1 liter and equipped with fermentation locks, with continuous magnetic stirring at 500 rpm. These conditions give fermentation kinetics similar to those obtained under enological conditions at pilot scale (2). Microaerobic batch fermentations were performed in a 2-liter bioreactor (SGI, France) with a working volume of 1 liter. Various microoxygenation conditions were obtained by using aeration rates between 5 and 45 ml/min for each culture and by maintaining agitation at 500 rpm.
The two-step fermentation process was carried out by sparging the bioreactor with argon gas at 17 ml/min throughout the exponential growth phase. The bioreactor was then sparged with air at 17 ml/min from the beginning of the stationary phase (28 h later) until the end of the fermentation.
The dissolved oxygen was measured using an INGOLD Clark electrode. The mass transfer coefficient was determined using the gassing-out method as previously described (6). The maximal oxygen transfer rate (OTR), the oxygen uptake rate, and the specific oxygen consumption were calculated as previously described (11). The off gas passed through a cooled condenser to avoid stripping. Samples were withdrawn with a syringe. Fermentations were characterized by fermentation progress, expressed as 1 − S/S0 (where S is the glucose concentration and S0 is the initial glucose concentration).
Analytical methods.
Cells were counted using an electronic particle counter (Coulter-Counter Coultronics, ZBI model) fitted with a probe with a 100-μm aperture. Glucose, glycerol, ethanol, and acetate were analyzed by high-pressure liquid chromatography using an HPX-87H ion exclusion column (Bio-Rad). Acetoin and 2,3-butanediol were measured by gas chromatography as described previously (16). The acetaldehyde concentration was determined enzymatically according to the method of Lundquist (14).
Calculation of specific metabolic rates.
The specific rates of metabolite consumption and formation (qi) were estimated from plots of metabolite concentration (Ci) and cell number (Cx) versus time, according to the following equation: qi = (dCi/dt)/Cx, where dt is a derivate of time and dCi is a derivate of metabolite concentration. The value of dCi/dt was estimated by deriving a polynomial function fitted to plots of Ci versus time using Sigmaplot software (SPSS Inc.).
RESULTS
Impacts of various oxygen transfer rates on a strain expressing an H2O-forming NADH oxidase.
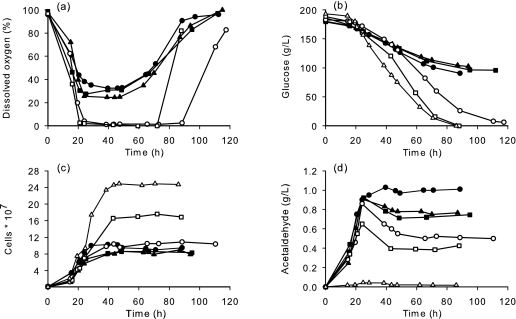
We recently showed that expressing L. lactis NADH oxidase in S. cerevisiae reduces the ethanol yield during fermentation under microaerobic conditions (11). The reduced ethanol production in these cells was coupled to an increased formation of acetaldehyde, which reached high levels (1 g/liter) early in the process and had undesirable effects on the growth and fermentation rate. In this previous study, oxygen was supplied at a maximal transfer rate of 20 mg/liter/h, which corresponded to the limiting conditions for the control strain, V5. As oxygen is the substrate of the reaction catalyzed by NADH oxidase, however, it is possible that the oxygen requirement of the engineered V5noxE strain might differ from that of the control strain. We therefore decided to perform a careful analysis of the oxygen requirement of this strain in order to (i) determine the minimal level of microoxygenation required to obtain a metabolic shift toward reduced ethanol production and (ii) assess the possibility of limiting the undesirable effects of oxidase expression by fine tuning the oxygen supply. The oxygen requirement of the noxE strain was analyzed by performing batch cultivations on MS medium with maximal oxygen transfer rates ranging from 0 to 20 mg/liter/h. Growth, glucose consumption, and metabolite production were measured at different time points of the fermentation (Fig. 1).
FIG. 1.
Oxygen consumption (a), glucose consumption (b), growth (c), and acetaldehyde production (d) of V5noxE during batch cultivation under anaerobic conditions (▵) and microaerobic conditions at OTRs of 20 mg/liter/h (•), 14 mg/liter/h (▪), 11 mg/liter/h (▴), 9 mg/liter/h (○), and 5 mg/liter/h (□).
The biomass yield and metabolic pattern obtained for strain V5noxE in the absence of oxygen was similar to that of the wild-type strain (data not shown), indicating the absence of oxidase activity under these conditions. Based on this result, we can conclude that the Lactococcus lactis NADH oxidase has no electron acceptor alternative to molecular oxygen when expressed in S. cerevisiae, unlike what has been observed when the same gene is overexpressed in Lactococcus lactis (3).
At a maximal OTR of 20 mg/liter/h, corresponding to the rate used in our previous study (11), oxygen was never depleted in the medium and remained above 35% of the saturation value (Fig. 1a). Decreasing the oxygen transfer rate to 14 or 11 mg/liter/h did not change the profile of oxygen consumption (Fig. 1a). At lower oxygen transfer rates (9 and 5 mg/liter/h), however, all of the supplied oxygen was consumed within the first 20 h, and the dissolved oxygen concentration remained close to zero until the end of the fermentation.
Glucose consumption and biomass formation were also affected by the level of microoxygenation. Under nonlimiting oxygenation conditions (20, 14, or 11 mg/liter/h), V5noxE was unable to degrade more than half of the initial amount of sugar, and biomass formation was also strongly reduced (Fig. 1b and c). In contrast, at oxygen transfer rates of 9 and 5 mg/liter/h (limiting conditions), V5noxE was able to complete the fermentation (Fig. 1b). We previously suggested that the reduced biomass formation and fermentation arrest of V5noxE at an OTR of 20 mg/liter/h was at least partly due to a large accumulation of acetaldehyde. Indeed, under nonlimiting conditions, acetaldehyde accumulated to a high level (1 g/liter) and remained high throughout the stationary phase (Fig. 1d), with marked effects on the biomass and glucose consumption. Interestingly, at an oxygen transfer rate of 9 mg/liter/h, a similarly high level of acetaldehyde was attained during the growth phase, similarly resulting in a 60% reduction in the biomass relative to what is observed under anaerobic conditions (Fig. 1c and d). Under these conditions, however, the acetaldehyde concentration decreased to 0.5 g/liter during the stationary phase, and glucose consumption was increased (Fig. 1b and d). With an even lower oxygen transfer rate (5 mg/liter/h), the maximal accumulation of acetaldehyde was 0.65 g/liter, the biomass was only partially reduced (by 30%) relative to anaerobic conditions, and glucose consumption was not affected (Fig. 1b, c, and d). There is thus a good correlation between the level of acetaldehyde, the formation of biomass, and fermentation performance, confirming earlier observations (11, 21).
The decrease in the acetaldehyde concentration that was observed when oxygen was supplied at 5 and 9 mg/liter/h started when the oxygen was depleted from the growth medium (Fig. 1a and d). When oxygen became limiting, the specific oxygen uptake rate and the oxygen yield were fixed by the oxygen supply, i.e., the OTR, meaning that these values were markedly lower than they were under nonlimiting microoxygenation conditions (Table 1). Under oxygen-limiting conditions, it may be assumed that the NADH oxidase activity was lower than under nonlimiting conditions and that the amount of NADH available for NADH-dependent reactions was consequently higher. In agreement with this, the yield and rate of ethanol production were higher at low OTRs than at high OTRs (Table 1), indicating a higher flux through the alcohol dehydrogenase reaction. On the other hand, the glycerol yields were similar under the five different microoxygenation conditions (around 0.034 g/g at the same fermentation progress, 0.5) and in all cases lower than the yield obtained when the NADH oxidase was not active (0.051 g/g) (Table 1). The acetoin and 2,3-butanediol yields were reduced with smaller amounts of transferred oxygen. The percentages of acetoin converted to 2,3-butanediol, which gives an indication of the flux through the 2,3-butanediol dehydrogenase (BDH) reaction, were similar for the five different microoxygenation conditions and were dramatically reduced compared to the percentages observed under anaerobic conditions (Table 1). Together, these data indicate that the two reactions catalyzed by GPDH and BDH are more sensitive to a small decrease in NADH (i.e., low-OTR versus anaerobic conditions) than was the reaction catalyzed by ADH.
TABLE 1.
Product yields and specific rates of glucose consumption, oxygen consumption, and ethanol formation during microaerobic and anaerobic batch cultivation of V5noxE
| OTR (mg/liter/h) | Fermentation progress | Product yield (g of product formed or oxygen consumed/g glucose consumed)a,b
|
Amt of acetoin converted in 2,3-butanediol (%)d | Specific ratea,e
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxygenc | Ethanol | Glycerol | Pyruvate | Acetoin | 2,3-Butanediol | Glucose consumption (mg/108 cells/h) | Ethanol formation (mg/108 cells/h) | Oxygen uptake (μg/108 cells/h) | |||
| 20 | 0.5 | 7.9 (0.0) | 0.40 (0.00) | 0.032 (0.01) | 0.004 (0.00) | 0.063 (0.005) | 0.011 (0.000) | 16 | 1.42 (0.02) | 0.57 (0.01) | 11.2 (0.03) |
| 14 | 0.5 | 8.1 | 0.40 | 0.035 | 0.005 | 0.061 | 0.010 | 14 | 1.39 | 0.55 | 10.7 |
| 11 | 0.5 | 7.8 | 0.39 | 0.032 | 0.006 | 0.059 | 0.011 | 16 | 1.41 | 0.58 | 10.9 |
| 9 | 0.5 | 4.1 | 0.46 | 0.033 | 0.005 | 0.045 | 0.008 | 15 | 2.35 | 1.12 | 8.44 |
| 0.97 | 4.4 | 0.47 | 0.020 | 0.001 | 0.044 | 0.008 | 16 | ||||
| 5 | 0.4 | 1.6 | 0.46 | 0.034 | 0.006 | 0.031 | 0.006 | 16 | 2.07 | 1.01 | 2.79 |
| 1 | 1.6 | 0.47 | 0.021 | 0.001 | 0.026 | 0.010 | 28 | ||||
| Anaerobic | 0.5 | 0 | 0.48 (0.000) | 0.051 (0.001) | 0.002 (0.000) | 0 | 0.005 (0.001) | 100 | 1.51 (0.01) | 0.75 (0.02) | 0 |
| 1 | 0 | 0.49 (0.000) | 0.032 | 0.001 (0.000) | 0 | 0.004 (0.000) | 100 | ||||
Values in parentheses are standard deviations calculated from two independent experiments.
Only some metabolites are shown. The carbon recovery (including acetate, acetaldehyde, succinate, 2-oxoglutarate, 2-hydroxyglutarate, CO2, and biomass) was between 105 and 115%.
Values are in mg of oxygen consumed per g of glucose consumed.
Calculated as 100 × {[2,3-butanediol]/([acetoin] + [2,3-butanediol])}.
Average of values during the stationary phase.
The specific rates of glucose consumption and ethanol formation were reduced by 6% and 24%, respectively, under nonlimiting conditions compared to the rates under conditions in which NADH oxidase did not operate. This strong decrease in the specific rate of ethanol formation matches our previous observation that the ethanol yield is strongly reduced at high OTRs. Interestingly, at OTRs of 5 and 9 mg/liter/h, the specific rates of glucose consumption were increased by 37% and 56%, respectively, relative to the rates observed under anaerobic conditions, and the specific rates of ethanol formation were enhanced accordingly. This indicates a positive effect of the NADH oxidase and/or oxygen on the glycolytic flux.
Impact of oxygen on yeast metabolism.
At a maximal oxygen transfer rate of 20 mg/liter/h, which was the rate used in our previous study (11), the oxygen was completely consumed by the control strain. In contrast, the dissolved-oxygen concentration never fell below 35% of the saturation value during cultivation of the NADH oxidase-expressing strain (Fig. 1a). Because of this, the noxE cells were exposed to higher oxygen concentrations, which may have in itself affected the metabolic network. In order to dissociate the effect of NADH oxidase from that of oxygen, we compared the relative impacts of oxygen transfer rates of 20 mg/liter/h and 36 mg/liter/h, corresponding to limiting and nonlimiting oxygenation conditions, respectively, for strain V5. When supplied at 36 mg/liter/h, oxygen remained at around 20% of the saturation value in the medium. Under these conditions, the biomass, ethanol, and by-product yields were the same as those obtained under limiting conditions (20 mg/liter/h) (Table 2). Similarly, the specific rates of glucose consumption and ethanol production by V5 at 36 mg/liter/h were comparable to those obtained at an OTR of 20 mg/liter/h, as well as during anaerobic fermentation of V5noxE (Tables 1 and 2). These data indicate that a continuous supply of oxygen under the conditions used in this study has no impact on yeast metabolism. The specific oxygen uptake rates and oxygen yields were very close at 20 and 36 mg/liter/h, indicating that the maximal oxygen demand was satisfied at 20 mg/liter/h (Table 2). These values were, however, 1.4- and 2-fold lower, respectively, than that obtained with V5noxE under nonlimiting conditions (Tables 1 and 2), demonstrating that the NADH oxidase activity induces higher oxygen consumption. Together, these data show that the metabolic changes observed in the V5noxE strain are solely due to the activity of NADH oxidase.
TABLE 2.
Product yields, biomass formation, dissolved oxygen, and specific rates of glucose consumption, oxygen consumption, and ethanol formation during microaerobic batch cultivation of V5
| OTR (mg/liter/h) | Product yield (g of product formed or oxygen consumed/g glucose consumed)a
|
Biomass formation (107 cells/ml) | Dissolved oxygenc (% of saturation value) | Specific rated
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oxygenb | Ethanol | Glycerol | Acetaldehyde | Acetate | Glucose consumption (mg/108 cells/h) | Ethanol formation (mg/108 cells/h) | Oxygen uptake (μg/108 cells/h) | |||
| 20 | 3.8 (0.0) | 0.47 (0.00) | 0.027 (0.02) | 0.0031 (0.001) | 0.013 (0.001) | 30.5 (1.5) | 0 | 1.48 (0.03) | 0.69 (0.01) | 7.15 (0.02) |
| 36 | 4.1 (0.0) | 0.47 (0.01) | 0.028 (0.00) | 0.0029 (0.001) | 0.012 (0.002) | 32.5 (1.5) | 20 | 1.49 (0.02) | 0.68 (0.02) | 7.71 (0.03) |
Values in parentheses are standard deviations calculated from two independent experiments.
Values are in mg of oxygen consumed per g of glucose consumed.
Minimal value reached during the culture.
Average of values during the stationary phase.
Behavior of V5noxE in a two-step process.
Our data indicated that although decreasing oxygen transfer leads to a gradual recovery of growth and fermentation performance in V5noxE cells, the effect on ethanol production is lost as soon as oxygen becomes limiting in the medium (Fig. 1 and Table 1). When oxygen was transferred at a continuous rate in these cells, the major side effect of NADH oxidase activity was decreased biomass resulting from the accumulation of acetaldehyde during the growth phase. During grape must fermentation, nutrients, and particularly nitrogen, are rapidly depleted from the medium, causing growth arrest. Since most of the sugar (70%) is consumed by nongrowing cells, it was possible to limit the expression of the NADH oxidase to the stationary phase, thereby alleviating the undesirable effects on growth while maintaining a significant effect on ethanol yield.
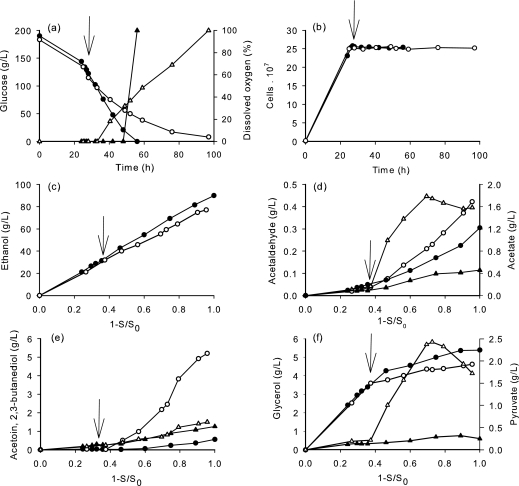
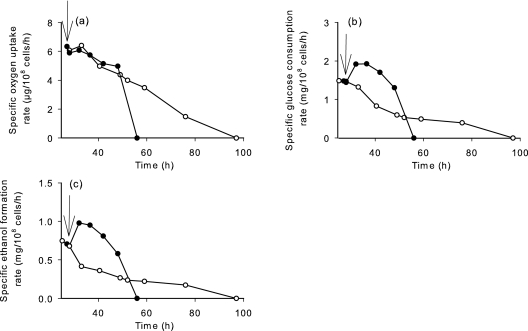
We therefore designed a two-step fermentation process involving a first anaerobic phase in which the reaction catalyzed by NADH oxidase was prevented, followed by a second, microaerobic phase that was initiated after growth arrest. The traces of oxygen that might be used by the oxidase were eliminated by sparging the medium with argon before and after inoculation in order to maintain the dissolved oxygen at 0% of saturation value. Once the cells had reached the stationary phase, after 28 h of fermentation (25 × 107 cells/ml), the argon was replaced by air and oxygen was supplied at a maximum OTR of 20 mg/liter/h until the end of fermentation. The concentration of dissolved oxygen, growth, glucose consumption, and formation of ethanol and by-products were determined over the course of the fermentation (Fig. 2), and the specific rates of oxygen and glucose consumption and ethanol production were calculated (Fig. 3).
FIG. 2.
Impacts of NADH oxidase on growth, glucose consumption, oxygen consumption, and formation of metabolites during batch cultivation with oxygen supply during stationary phase (↓, beginning of oxygen delivery; OTR = 20 mg/liter/h) of the S. cerevisiae control strain (V5; filled symbols) and V5 expressing NADH oxidase (V5noxE; open symbols). (a) Glucose consumption (circles) and dissolved oxygen (triangles). (b) Cell population. (c) Ethanol. (d) Acetate (circles) and acetaldehyde (triangles). (e) Acetoin (circles) and 2,3-butanediol (triangles). (f) Glycerol (circles) and pyruvate (triangles). One representative of two experiments is shown.
FIG. 3.
Specific rate of glucose consumption (a), specific oxygen uptake rate (b), and specific rate of ethanol production (c) during batch cultivation with oxygen supply during stationary phase (↓, beginning of oxygen delivery; OTR = 20 mg/liter/h) of the S. cerevisiae control strain (V5; •) and V5 expressing NADH oxidase (V5noxE; ○).
During the anaerobic phase, the behavior of V5noxE was identical to that of V5, as expected from the absence of molecular oxygen, which is the substrate of the NADH oxidase reaction (Fig. 2). During the microaeration phase, however, all of the oxygen was consumed by the wild-type strain, while it remained mainly nonlimiting for strain V5noxE (Fig. 2a). Under these conditions, the NADH oxidase was functional and caused a significant rerouting of metabolism. The ethanol yield of V5noxE was reduced from 0.47 g/g to 0.41 g/g during the microaeration phase (Fig. 2c), producing a final ethanol yield of 0.44 g/g. As expected from the oxidase-catalyzed reaction, the production of acetaldehyde increased as soon as oxygen was provided. However, this increase was strongly limited (maximal concentration, 0.45 g/liter) compared to when oxygen was provided at the beginning of the fermentation (Fig. 1). Interestingly, the V5noxE strain was still able to consume essentially all of the sugar (Fig. 2a).
The formation of oxidized products (acetate and acetoin) was largely favored, but to a lesser extent than with continuous microaeration. The formation of 2,3-butanediol was unaffected, indicating a limited flux through the BDH reaction in the noxE strain, and the production of glycerol was slightly reduced (Fig. 2d to f). Remarkably, a large accumulation of pyruvate was observed immediately after the beginning of oxygenation in V5noxE, reaching 2.3 g/liter at the end of the process (Fig. 2f). This phenomenon was not observed during the continuous microaeration process (Table 1).
The specific oxygen uptake rate of V5noxE was 6 μg/108 cells/h, which decreased progressively until sugar consumption was complete (Fig. 3a). The oxygen consumption yield reached 6.4 mg/g glucose consumed, which was very similar to that obtained with continuous oxygen transfer at an OTR of 20 mg/liter/h, although the average specific oxygen uptake rate was twofold lower (i.e., 4.9 μg/108 cells/h versus 11.2 μg/108 cells/h) (Fig. 3 and Table 1). This indicates that NADH oxidase uses the same amount of oxygen under these conditions as when oxygen is supplied from the beginning of the process, although it does so more slowly. The specific rates of glucose consumption and ethanol production decreased immediately after aeration and then increased in the wild-type strain (Fig. 3b and c) but remained 2- and 2.5-fold lower, respectively, in V5noxE than in V5. As a result, it took 100 h before all the glucose was consumed in V5noxE cells compared to 56 h in the wild-type strain. The reduction in the glucose consumption rate for V5noxE may account for the lower oxygen uptake rate that was observed in the two-step process relative to the continuous process.
DISCUSSION
A main objective of this work was to investigate the potential for developing an engineered wine yeast strain expressing NADH oxidase to decrease the ethanol yield during wine fermentation. We first analyzed the impacts of a range of continuous oxygen transfer rates on metabolic networks. Yeast metabolism was differently affected depending on the oxygen transfer rate. At high OTRs, expression of the NADH oxidase resulted in a 15% reduction in the ethanol yield, but fermentation was incomplete. In contrast, at low OTRs, the ethanol yield was not affected and all of the glucose was consumed. Evidence that the NADH oxidase was functional in these cells comes from the decreased glycerol formation and the reduced flux through the BDH reaction. However, the oxidase might work at a lower level than at high OTRs, as evidenced by the lower oxygen uptake rates. The absence of an impact on ethanol formation cannot be explained in terms of affinity for NADH, since the Km of ADH for NADH (Adh1p, 110 μM) is higher than those of GPDH and BDH (23 μM and 55 μM, respectively) (10, 22).
We previously reported that V5noxE exhibits a higher intracellular NAD+ concentration (about 40%) than the control strain when grown under continuous microaeration at 20 mg/liter/h (11). When the oxygen transfer rate diminishes, we can assume that the NAD+ pool is gradually decreased but is still higher than in the absence of NADH oxidase activity. NADH reoxidation (9, 13), as well as sugar transport, glycolytic enzyme activity, and ATP hydrolysis (7, 12, 19), might play a role in controlling the glycolytic flux. At high OTRs, we showed that the specific rate of ethanol formation was strongly reduced, while that of glucose consumption remained essentially the same as under anaerobiosis, where NADH oxidase activity is absent. This allowed us to assume that the NAD+ surplus generated by the NADH oxidase activity permitted a glycolytic flux to be sustained close to what is observed when ADH is fully functional. At lower OTRs, the ethanol yield was almost unaffected, whereas the flux through ADH was lower than under anaerobic conditions, as indicated by the accumulation of acetaldehyde and acetoin. Interestingly, under these conditions, the specific rates of glucose consumption and ethanol formation were increased to similar extents, suggesting that the limited ADH-catalyzed reaction is compensated for by an increased glycolytic flux. Again, this is in agreement with the idea that NADH oxidase has the capacity to sustain the glycolytic demand for NAD+.
Together, these data highlighted the limits of such continuous microaeration processes using yeast expressing an NADH oxidase for the production of low-alcohol beverages. For this reason, we developed a two-step process based on an anaerobic growth phase followed by a microaeration phase with nongrowing cells. This process was able to reduce the ethanol yield by 7% compared to the wild-type while avoiding undesirable effects on growth and glucose consumption. The addition of oxygen to the noxE strain during the stationary phase resulted in a sharp decrease in the specific rates of glucose consumption and ethanol formation, with average values about threefold lower than those obtained during continuous microaeration at 20 mg/liter/h. The rapid and strong accumulation of pyruvate, together with increased amounts of acetaldehyde, suggests that there was a sudden limitation in the level of ADH when the glycolytic flux was relatively high. This is consistent with the possibility that the ability of NADH oxidase to meet the demand for NAD+ for glycolysis becomes insufficient, causing a progressive decrease in the glycolytic flux. However, the possibility that other factors, e.g., inhibition of glycolysis by pyruvate (8), contribute to the reduced rate of glycolysis under these conditions cannot be entirely excluded. It is unlikely, however, that the effects are due to an inhibitory effect of acetaldehyde, which accumulated to the same level as when the glycolytic flux was high.
Finally, compared to continuous microoxygenation (11), the two-step process described here might be valuable for producing wines with ethanol contents reduced by about 1%. However, compared to processes run under standard anaerobic conditions, the production of oxidized metabolites (acetaldehyde, acetate, and acetoin) was globally favored (data not shown). While some effects were due to oxygen, the major redistribution of metabolites at the acetaldehyde node was a specific effect of oxidase expression. In particular, the production of acetoin was increased 10-fold in the oxidase-expressing strain compared to the wild-type strain under microaerobic conditions. Since the formation of oxidized products might be unfavorable in wine, the impact of these modifications on the sensory properties of wine should be carefully analyzed. Also, our results indicate that the reoxidation of NADH is not the only component in the intricate regulatory system employed by cells for adjusting the rates of central metabolic pathways, such as glycolysis, to appropriate levels. Nevertheless, this study demonstrates that in addition to its ability to reduce the ethanol content in wine, the noxE strain may offer an interesting system for further investigating the control exerted by NADH reoxidation on glycolysis.
Acknowledgments
This work was supported by INRA and the Bourgogne region.
REFERENCES
- 1.Athes, V., M. Pena y Lillo, C. Bernard, R. Perez-Correa, and I. Souchon. 2004. Comparison of experimental methods for measuring infinite dilution volatilities of aroma compounds in water/ethanol mixtures. J. Agric. Food Chem. 52:2021-2027. [DOI] [PubMed] [Google Scholar]
- 2.Bely, M., J. M. Sablayrolles, and P. Barre. 1990. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 70:246-252. [Google Scholar]
- 3.Bongers, R. S., M. H. Hoefnagel, and M. Kleerebezem. 2005. High-level acetaldehyde production in Lactococcus lactis by metabolic engineering. Appl. Environ. Microbiol. 71:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dequin, S., E. Baptista, and P. Barre. 1999. Acidification of grape must by Saccharomyces cerevisiae wine yeast strains genetically engineered to produce lactic acid. Am. J. Enol. Vitic. 50:45-50. [Google Scholar]
- 5.Dequin, S., and P. Barre. 1994. Mixed lactic acid-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei L(+)-LDH. Biotechnology 12:173-177. [DOI] [PubMed] [Google Scholar]
- 6.Dursun, G., A. Özer, M. Elibol, and D. Özer. 1999. Mass transfer characteristics of a fermentation broth in a reactor: co-current downflow contacting reactor. Process Biochem. 34:133-137. [Google Scholar]
- 7.Elbing, K., C. Larsson, R. M. Bill, E. Albers, J. L. Snoep, E. Boles, S. Hohmann, and L. Gustafsson. 2004. Role of hexose transport in control of glycolytic flux in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 70:5323-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flikweert, M. T. 1999. Physiological roles of pyruvate decarboxylase in Saccharomyces cerevisiae. Ph.D. thesis. Delft University of Technology, Delft, The Netherlands.
- 9.Flikweert, M. T., J. P. van Dijken, and J. T. Pronk. 1997. Metabolic responses of pyruvate decarboxylase-negative Saccharomyces cerevisiae to glucose excess. Appl. Environ. Microbiol. 63:3399-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez, E., M. R. Fernandez, C. Larroy, L. Sola, M. A. Pericas, X. Pares, and J. A. Biosca. 2000. Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product. Disruption and induction of the gene. J. Biol. Chem. 275:35876-35885. [DOI] [PubMed] [Google Scholar]
- 11.Heux, S., R. Cachon, and S. Dequin. 2006. Cofactor engineering in Saccharomyces cerevisiae: expression of a H2O-forming NADH oxidase and impact on redox metabolism. Metab. Eng. 8:303-314. [DOI] [PubMed] [Google Scholar]
- 12.Larsson, C., I. L. Pahlman, and L. Gustafsson. 2000. The importance of ATP as a regulator of glycolytic flux in Saccharomyces cerevisiae. Yeast 16:797-809. [DOI] [PubMed] [Google Scholar]
- 13.Liu, L., Y. Li, G. Du, and J. Chen. 2006. Redirection of the NADH oxidation pathway in Torulopsis glabrata leads to an enhanced pyruvate production. Appl. Microbiol. Biotechnol. 11:1-9. [DOI] [PubMed] [Google Scholar]
- 14.Lundquist, F. 1974. Acetaldehyd: Bestimmung mit Aldehyd-dehydrogenase, p. 1509-1513. In H. U. Bermeyer (ed.), Methods of enzymatic analysis. Academic Press, Inc., New York, N.Y.
- 15.Malherbe, D. F., M. Du Toit, R. R. Cordero Otero, P. Van Rensburg, and I. S. Pretorius. 2003. Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Appl. Microbiol. Biotechnol. 61:502-511. [DOI] [PubMed] [Google Scholar]
- 16.Michnick, S., J. L. Roustan, F. Remize, P. Barre, and S. Dequin. 1997. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 13:783-793. [DOI] [PubMed] [Google Scholar]
- 17.Nevoigt, E., R. Pilger, E. Mast-Gerlach, U. Schmidt, S. Freihammer, M. Eschenbrenner, L. Garbe, and U. Stahl. 2002. Genetic engineering of brewing yeast to reduce the content of ethanol in beer. FEMS Yeast Res. 2:225-232. [DOI] [PubMed] [Google Scholar]
- 18.Nevoigt, E., and U. Stahl. 1996. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331-1337. [DOI] [PubMed] [Google Scholar]
- 19.Pearce, A. K., K. Crimmins, E. Groussac, M. J. Hewlins, J. R. Dickinson, J. Francois, I. R. Booth, and A. J. Brown. 2001. Pyruvate kinase (Pyk1) levels influence both the rate and direction of carbon flux in yeast under fermentative conditions. Microbiology 147:391-401. [DOI] [PubMed] [Google Scholar]
- 20.Pickering, G. H. 2000. Low- and reduced-alcohol wine: a review. J. Wine Res. 11:129-144. [Google Scholar]
- 21.Remize, F., J. L. Roustan, J. M. Sablayrolles, P. Barre, and S. Dequin. 1999. Glycerol overproduction by engineered Saccharomyces cerevisiae wine strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microbiol. 65:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teusink, B., J. Passarge, C. A. Reijenga, E. Esgalhado, C. C. van der Weijden, M. Schepper, M. C. Walsh, B. M. Bakker, K. van Dam, H. V. Westerhoff, and J. L. Snoep. 2000. Can yeast glycolysis be understood in terms of in vitro kinetics of the constituent enzymes? Testing biochemistry. Eur. J. Biochem. 267:5313-5329. [DOI] [PubMed] [Google Scholar]