Abstract
A triphenyltin (TPT)-decomposing strain, Pseudomonas aeruginosa CGMCC 1.860, was screened out. It secreted an unknown TPT-decomposing factor into the medium, later shown to be pyochelin, even in the presence of 100 μM iron. To our knowledge, this is the first report of organotin decomposition by pyochelin.
Organotin compounds are ubiquitous in the environment and have a wide range of industrial and agricultural applications (7). However, they are toxic and harmful to a variety of nontarget organisms (see, e.g., references 10 and 16). Without a doubt, it is important to remove organotins from the environment.
It has previously been reported that some microalgae were capable of degrading organotin, but their degradation rate was very low (14). Information on the bacterial degradation of organotin compounds is still severely limited (7, 9). This work investigated the decomposition of triphenyltin chloride (TPT) by microorganisms, and interestingly, pyochelin (PCH) [2-(2-o-hydroxy phenyl-2-thiazolin-4-yl)-3-methylthiazolidine-4-carboxylic acid], different from pyoverdine (PVD), was found as a new organotin-decomposing factor.
Chemicals.
TPT (95%), diphenyltin dichloride (DPT) (98%), and monophenyltin trichloride (MPT) (96%) were purchased from Aldrich Chemical Co.
Seventeen strains belonging to Pseudomonas and Burkholderia genera, obtained from the China General Microbiological Culture Collection Center (Beijing, People's Republic of China), were used for screening for TPT-decomposing bacteria. The TPT decomposition activity was measured by monitoring the decrease of TPT concentrations and the increase of DPT and MPT levels using high-performance liquid chromatography (HPLC) as described previously (12). Shimadzu (Kyoto, Japan) HPLC equipment (LC-10ATVP) with a UV-Vis detector and a reversed-phase column (Kromasil C18) (250- by 4.6-mm internal diameter, 5 μm) was used. The mobile phase consisted of methanol-acetate acid-water (60:10:30 [vol/vol/vol]) containing 1 mM dithiothreitol. The flow rate was 0.75 ml/min, and the UV detection wavelength was 257 nm. Among all the strains, none of them were able to utilize TPT as a sole carbon source to support growth, but all of them were able to grow in the M9 medium with sodium succinate (4 g/liter) as a carbon source supplemented with 200 μM TPT. Pseudomonas aeruginosa CGMCC 1.860, a fluorescent pseudomonad with the greatest ability to decompose TPT (data not shown), was chosen for the following experiments.
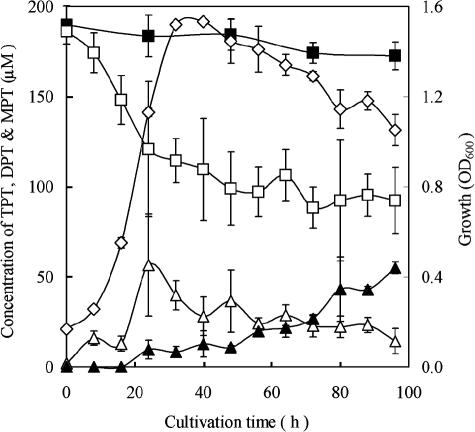
The kinetics of TPT (200 μM) decomposition by P. aeruginosa CGMCC 1.860 are shown in Fig. 1. TPT rapidly decomposed during log-phase growth and reduced to about 40% of the initial amount within 36 h, and it decreased slowly in the stationary phase (Fig. 1). The accumulation of DPT reached a maximal concentration of 50 μM at 24 h and then slowly decreased. The concentration of MPT increased slowly during 96 h of incubation. This strain showed a decomposition ability similar to that of other Pseudomonas strains (7), and it had a relatively higher organotin-decomposing capacity than reported for other strains previously, such as Alteromonas sp. strains (6, 9). Methanol used to dissolve TPT did not inhibit either the growth of bacteria or TPT decomposition with the concentration used (data not shown), and no decomposition occurred in the control (Fig. 1). These facts indicate that the TPT decomposition was done by the microorganism.
FIG. 1.
Decomposition of TPT by P. aeruginosa CGMCC 1.860 in M9 medium supplemented with TPT (200 μM). The concentrations of TPT (□), DPT (▵), and MPT (▴) were determined by HPLC with a UV detector at 257 nm, and the growth (⋄) was measured as the optical density at 600 nm (OD600). ▪, TPT level for control without inoculation. There were triplicate samples for each condition.
Further experiments on TPT decomposition by the screened strain were conducted. First of all, resting cells and cell-free supernatants were prepared to investigate whether the intracellular or extracellular decomposition of TPT occurred. At the beginning, the resting cells were resuspended in Tris-HCl buffer. TPT (200 μM) was added in the resting cells or supernatants on a shaker (120 rpm) for 3 days. There was only a slight decrease in the TPT level (about 6%) in the case of resting cells. In contrast, the supernatant exhibited a much higher capacity for TPT decomposition (at 40%), indicating that there was a TPT-decomposing compound(s) secreted by the bacteria into the medium.
As reported previously, under conditions of iron starvation, P. aeruginosa can secret a low-molecular-weight compound with high iron-binding affinity known as PVD (2, 12). PVD produced by Pseudomonas chlororaphis was demonstrated to decompose TPT (7, 8). In our work, the PVD obtained from the supernatant of P. aeruginosa CGMCC 1.860 by solid-phase extraction (8) could decompose TPT (data not shown).
According to the literature (18), under iron-sufficient conditions (100 μM), the production of PVD would be absolutely inhibited. Initially, it was supposed that PVD was the sole TPT-decomposing factor, and if sufficient ferric ions were added into the medium, the activity of TPT decomposition by the bacterium would decrease or disappear due to the inhibition of PVD production by Fe3+. Here, the stock solution of FeCl3 (20 mM) was sterilized by passage through membrane filters and was added to cooled media just prior to inoculation (4). The production of PVD in the medium in the presence of 100 μM FeCl3 was detected spectrophotometrically by measuring the A400 (11) or by measuring fluorescence at an excitation wavelength of 405 nm and an emission wavelength of 455 nm (3, 11). Although the bacterium grew slightly better in the presence of Fe3+ than it did without Fe3+, the A400 and fluorescence at 455 nm in the medium with Fe3+ were very low compared to those in medium without Fe3+ (Table 1). The results indicated that PVD production was completely inhibited in the presence of Fe3+ in medium, which is in agreement with previous work (17). However, interestingly, the concentration of TPT decreased from 186.8 to 122.8 μM in the presence of 100 μM Fe3+. It is obvious that TPT decomposition still occurred during the bacterial growth in the medium containing 100 μM FeCl3, although PVD production was inhibited (Table 1). The facts suggest that there was another unknown TPT-decomposing factor secreted by P. aeruginosa into the medium that is different from PVD.
TABLE 1.
Optical densities of cell-free supernatants at 600 nm (for growth) and 400 nm (for PVD) and fluorescent valuea
| Supernatant | OD600 ± SD | A400 ± SD | Fluorescent value |
|---|---|---|---|
| Control | 0.032 ± 0.002 | 0.031 ± 0.001 | 24 ± 0.3 |
| With FeCl3 | 0.80 ± 0.063 | 0.032 ± 0.001 | 11 ± 0.3 |
| Without FeCl3 | 0.63 ± 0.034 | 0.94 ± 0.006 | 624 ± 1.3 |
The absorbance at 400 nm or optical density at 600 nm (OD600) was measured using a UV-Vis spectrophotometer, and the fluorescent value at an excitation wavelength at 405 nm and at an emission wavelength of 455 nm was determined by using a fluorescent spectrophotometer. The control was the medium containing FeCl3.
The extract of the cell-free supernatant (16 liters) with ethyl acetate was found to possess high TPT decomposition activity (data not shown), and it was applied onto a Silica Gel G thin-layer chromatography (TLC) plate with chloroform-acetic acid-ethanol (95:5:2.5 [vol/vol/vol]) as the development solvent. Four fractions were eluted with methanol and dried, and their TPT decomposition activities were then detected. Fraction 3 exhibited a relatively higher capacity for TPT decomposition than other fractions (Table 2), suggesting that it contained a TPT-decomposing factor, and this fraction was further purified by thin-layer chromatography with chloroform-acetic acid-ethanol (95:5:5 [vol/vol/vol]). The purity of PCH was over 99.6%, as determined by HPLC with the normalization method. The chemical structure of the finally purified TPT-decomposing factor was identified by mass spectrometry (MS) and nuclear magnetic resonance (NMR) (1H and 13C NMR) (data not shown); its high-resolution electron ionization mass spectrum contained major peaks at 220.0649 (C11H12N2OS) and 191.0361 (C10H9NOS), and the high-resolution electrospray ionization mass spectrum showed a molecular ion peak at m/z 325.0659 [M + H] (C12H16N2O3S2). Those data indicate that this new TPT-decomposing factor is PCH, with a molecular weight of 324.1 (Fig. 2), which is a kind of siderophore (6). In addition, investigations on its chemical properties confirmed that it had UV absorption, fluorescent absorption, Rf, and color reactions identical to those of PCH reported previously (1, 4, 5, 19, 20). Furthermore, the purified PCH was used to decompose TPT. In 24 h, the TPT concentration decreased, and the decomposition products DPT and MPT were detected (Table 3). Benzene, another decomposition product, was also detected by HPLC in additional experiments (data not shown). Furthermore, the molecular ion peak of m/z 396.1 [PCH + Sn + Na] in the electrospray ionization-MS spectrum of the reaction mixture (at 24 h) was seen (data not shown), which indicated the formation of the PCH-Sn complex, and the data suggested that PCH could decompose TPT to inorganic tin and chelate the tin. The above-described results confirmed the TPT-decomposing capacity of PCH.
TABLE 2.
TPT decomposition by extract fractions of TLCa
| TLC fraction | Organotin concn (μM)
|
||
|---|---|---|---|
| TPT | DPT | MPT | |
| Control | 80 ± 3.6 | 1.2 ± 0.58 | 1.9 ± 0.23 |
| 1 | 77 ± 4.2 | 2.5 ± 0.21 | 2.6 ± 0.37 |
| 2 | 76 ± 3.9 | 3.8 ± 0.55 | 3.4 ± 0.48 |
| 3 | 55 ± 4.6 | 10 ± 1.6 | 18 ± 3.3 |
| 4 | 84 ± 6.7 | 1.1 ± 0.27 | 0.58 ± 0.14 |
The extract was obtained from extraction of cell-free supernatants with ethyl acetate. The mixture of each fraction with TPT (80 μM) in Tris-HCl (pH 8.0) was kept at 40°C for 12 h. The control was 80 μM TPT in the same buffer.
FIG. 2.
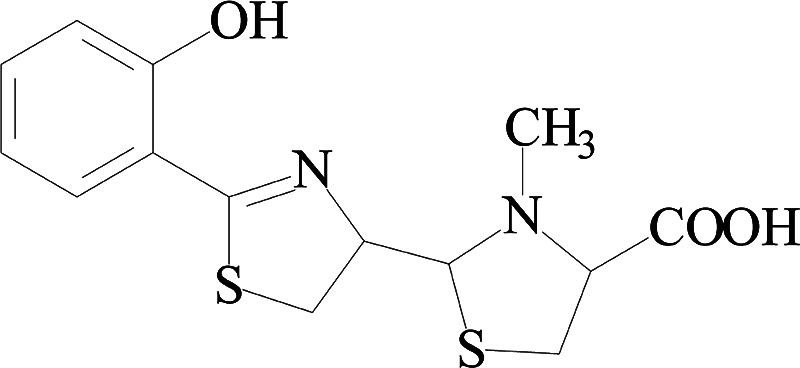
Structure of a novel TPT-degrading factor, which was identified as PCH by MS and NMR.
TABLE 3.
TPT decomposition by PCHa
| Sample | Organotin concn (μM)
|
|||||
|---|---|---|---|---|---|---|
| TPT
|
DPT
|
MPT
|
||||
| 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | |
| Without PCH | 80 ± 2.7 | 77 ± 3.8 | 0.89 ± 0.21 | 1.2 ± 0.73 | 3.5 ± 0.78 | 3.5 ± 0.48 |
| With PCH | 65 ± 3.3 | 61 ± 4.2 | 3.6 ± 0.40 | 2.7 ± 0.25 | 10 ± 3.2 | 15 ± 2.7 |
PCH (50 μM) and TPT (80 μM) were mixed in Tris-HCl (50 mM, pH 8.0), and the reaction mixture (total volume of 0.5 ml) was kept in the dark at 40°C for 12 and 24 h, respectively. Samples without the addition of PCH were used as a control.
In supplementary experiments, 100 μM Fe3+ and 200 μM TPT were added alone or together into the culture medium to investigate their effects on PCH biosynthesis by P. aeruginosa CGMCC 1.860 (data not shown). The PCH production titer was 24.76 μM for the control (without both Fe3+ and TPT), and it was decreased to 0.83 μM with the sole addition of Fe3+. It is apparent that Fe3+ remarkably inhibited the PCH synthesis. This is in agreement with a previous report, where the PCH production was significantly (but not completely) repressed in the presence of Fe3+ (19). However, it is different from other reports, which claimed a complete repression of PCH synthesis by P. aeruginosa PAO1 at 10 μM Fe3+ (4, 20). The reason for this may be related to the different physiologies of different strains. In the case with the sole addition of TPT, the PCH level detected was 29.69 μM, slightly higher than that of the control. This indicates that TPT had a slight effect on PCH production. When both Fe3+ and TPT were added into the culture medium, the PCH titer reached 3.29 μM, fourfold more than that with only Fe3+ added, suggesting that the addition of TPT significantly enhanced PCH biosynthesis under iron-rich conditions. It seems that TPT could have an iron-limiting effect on P. aeruginosa, which could have caused the increase of PCH accumulation. Similarly, other researchers also claimed that lead could stimulate the siderophore yield under conditions of an excess of iron (13), although the mechanism is yet unclear. Given that the TPT level was slightly reduced (about 6%) in resting cells, a small amount of TPT might be taken up by the cells and might affect cellular physiology and metabolism. As reported previously, in the presence of iron, the ferric uptake regulator (Fur) binds to the promoter of pvdS for PVD and pchR for PCH, which leads to the repression of PVD and PCH biosynthesis (15). We speculate that a certain amount of TPT absorbed into the cells might bind to Fur and reduce its affinity for pchR but not for pvdS, which accordingly would result in the increased synthesis of PCH but not of PVD.
In conclusion, this work demonstrated that P. aeruginosa CGMCC 1.860 could decompose TPT and that it secreted a new TPT-decomposing factor identified as PCH when it was grown in medium containing 100 μM Fe3+, in contrast to data reported previously (7). To the best of our knowledge, there have been no reports on the decomposition function of PCH and specifically the decomposition capacity of organometallic compounds. Further work on the decomposition mechanism is under way in our laboratory.
Acknowledgments
Financial support from the National Natural Science Foundation of China (NSFC project no. 20225619) is acknowledged.
We also thank Zhong Li of the Shanghai Key Laboratory of Chemical Biology, ECUST, for his advice on chemical structure analyses.
REFERENCES
- 1.Ankenbauer, R. G., T. Toyokuni, A. Staley, K. Rinehart, Jr., and C. D. Cox. 1988. Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J. Bacteriol. 170:5344-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman, T. J., C. D. Cox, B. L. Edeker, and B. E. Britigan. 1990. Possible role of bacterial siderophores in inflammation: iron bound to the Pseudomonas siderophore pyochelin can function as a hydroxyl radical catalyst. J. Clin. Investig. 86:1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox, C. D., and P. Adams. 1985. Siderophore activity of pyoverdine for Pseudomonas aeruginosa. Infect. Immun. 48:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox, C. D., and R. Graham. 1979. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J. Bacteriol. 137:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, C. D., K. L. Rinehart, M. L. Moore, and J. C. Cook. 1981. Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 78:4256-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukagawa, T., S. Suzuki, K. Fukunaga, T. Suzuki, and K. Takima. 1992. Isolation and characterization of tributyltin chloride resistant marine Vibrio. FEMS Microbiol. Lett. 93:83-86. [DOI] [PubMed] [Google Scholar]
- 7.Inoue, H., O. Takimura, H. Fuse, K. Murakami, K. Kamimura, and Y. Yamaoka. 2000. Degradation of triphenyltin by a fluorescent pseudomonad. Appl. Environ. Microbiol. 66:3492-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue, H., O. Takimura, K. Kawaguchi, T. Nitoda, H. Fuse, K. Murakami, and Y. Yamaoka. 2003. Tin-carbon cleavage of organotin compounds by pyoverdine from Pseudomonas chlororaphis. Appl. Environ. Microbiol. 69:878-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai, S., Y. Kurokawa, H. Harino, and M. Fukushima. 1998. Degradation of tributyltin by a bacterial strain isolated from polluted river water. Environ. Pollut. 102:259-263. [Google Scholar]
- 10.Landmeyer, J. E., T. L. Tanner, and B. E. Watt. 2004. Biotransformation of tributyltin to tin in freshwater river-bed sediments contaminated by an organotin release. Environ. Sci. Technol. 38:4106-4112. [DOI] [PubMed] [Google Scholar]
- 11.Meyer, J. M., and M. A. Abdallah. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J. Gen. Microbiol. 107:319-328. [Google Scholar]
- 12.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 13.Rachid, D., and B. Ahmed. 2005. Effect of iron and growth inhibitors on siderophores production by Pseudomonas fluorescens. Afr. J. Biotechnol. 4:697-702. [Google Scholar]
- 14.Reader, S., and E. Pelletier. 1992. Biosorption and degradation of butyltin compounds by the marine diatom Skeletonema costatum and the associated bacterial community at low temperature. Bull. Environ. Contam. Toxicol. 48:599-607. [DOI] [PubMed] [Google Scholar]
- 15.Reimmann, C., L. Serino, M. Beyeler, and D. Haas. 1998. Dihydroaeruginoic acid synthetase and pyochelin synthetase, products of the pchEF genes, are induced by extracellular pyochelin in Pseudomonas aeruginosa. Microbiology 144:3135-3148. [DOI] [PubMed] [Google Scholar]
- 16.Sidharthan, M., K. S. Young, L. H Woul, P. K. Soon, and H. W. Shin. 2002. TBT toxicity on the marine microalga Nannochloropsis oculata. Mar. Pollut. Bull. 45:177-180. [DOI] [PubMed] [Google Scholar]
- 17.Soderquist, C. J., and D. G. Crosby. 1980. Degradation of triphenyltin hydroxide in water. J. Agric. Food Chem. 28:111-117. [Google Scholar]
- 18.Stintzi, A., and J. M. Meyer. 1994. Search for siderophores in microorganisms. Microbes Better Living 35:169-176. [Google Scholar]
- 19.Visca, P., A. Ciervo, V. Sanfilippo, and N. Orsi. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J. Gen. Microbiol. 139:1995-2001. [DOI] [PubMed] [Google Scholar]
- 20.Visca, P., G. Colotti, L. Serino, D. Verzili, N. Orsi, and E. Chiancone. 1992. Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Appl. Environ. Microbiol. 58:2886-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]