Abstract
Autoinducer 2 (AI-2) quorum sensing was shown before to regulate the virulence of Vibrio harveyi towards the brine shrimp Artemia franciscana. In this study, several different pathogenic V. harveyi, Vibrio campbellii, and Vibrio parahaemolyticus isolates were shown to produce AI-2. Furthermore, disruption of AI-2 quorum sensing by a natural and a synthetic brominated furanone protected gnotobiotic Artemia from the pathogenic isolates in in vivo challenge tests.
Bacteria belonging to the species Vibrio harveyi and the closely related Vibrio campbellii and Vibrio parahaemolyticus are important pathogens in the intensive rearing of mollusks, finfish, and shrimp (1, 7, 10, 11, 14, 19, 25). The traditional control of bacterial disease in aquaculture relies on the use of antibiotics (3, 21). However, the frequent use of these compounds, in many cases even when pathogens are not evident, has led to the development and spread of resistance (3, 10, 13, 23, 24). Therefore, there is an urgent need for alternative control techniques. Disruption of quorum sensing, bacterial cell-to-cell communication by means of small signal molecules, has been suggested as a new anti-infective strategy for aquaculture (4).
The quorum sensing system of V. harveyi has been shown to consist of three channels (8). Recently, we found that the autoinducer 2 (AI-2)-mediated channel of the system regulates the virulence of the bacterium towards the brine shrimp Artemia franciscana in vivo (5). Interestingly, halogenated furanones were found to interfere with AI-2 quorum sensing in Escherichia coli (16, 17) and were shown before to block quorum sensing-regulated extracellular toxin production in V. harveyi, resulting in a reduced toxicity of cell-free culture fluids to Penaeus shrimp (12). Therefore, in this study, we aimed at investigating whether these halogenated furanones could reduce the virulence of V. harveyi and closely related bacteria in our model system with gnotobiotic Artemia franciscana.
Detection of AI-2 production by the pathogenic isolates.
In a first experiment, we aimed at detecting AI-2 production by the different pathogenic V. harveyi, V. campbellii, and V. parahaemolyticus isolates. The isolates tested are described in Table 1. Cell-free culture fluids of the isolates were prepared, and autoinducers were detected by a bioluminescent reporter assay as described before (22). The autoinducer receptor double mutant JMH597 (sensor HAI-1−, sensor AI-2+, sensor CAI-1−) (8) was used as a reporter strain. The culture fluids of all isolates significantly induced bioluminescence in the reporter strain (data not shown), indicating that they all produced AI-2. The detection of AI-2 in cell-free culture supernatants of V. harveyi and V. parahaemolyticus confirms the report of Bassler et al. (2), who used the HAI-1 receptor mutant BB170 as a reporter strain. To the best of our knowledge, this is the first report mentioning AI-2 production by V. campbellii.
TABLE 1.
Bacterial strains used in this studya
| Strain | Relevant features and/or synonyms | Reference(s) or source |
|---|---|---|
| V. campbellii | ||
| LMG21361 | = CAIM415 = Z1; isolated from seawater from shrimp (Litopenaeus spp.) broodstock tank, Mexico | 7, 20 |
| LMG21362 | = CAIM333 = M1; isolated from seawater from shrimp (Litopenaeus spp.) broodstock tank, Mexico | 7, 20 |
| LMG21363 | = CAIM372 = PN9801; isolated from the lymphoid organ of diseased shrimp (Penaeus spp.) juveniles, Philippines | 7, 20 |
| LMG22888 | = CAIM416 = Z2; isolated from seawater from shrimp (Litopenaeus spp.) broodstock tank, Mexico | 7, 20 |
| LMG22889 | = CAIM417 = Z3; isolated from seawater from shrimp (Litopenaeus spp.) broodstock tank, Mexico | 7, 20 |
| LMG22890 | = CAIM395 = STD3-131; isolated from diseased shrimp (Litopenaeus spp.) postlarvae, Ecuador | 7, 20 |
| LMG22895 | = CAIM223; isolated from the hepatopancreas of diseased shrimp (Litopenaeus spp.), Mexico | Bruno Gomez-Gil |
| V. harveyi | ||
| LMG22891 | = CAIM88; isolated from the hemolymph of shrimp (Litopenaeus spp.), Mexico | Bruno Gomez-Gil |
| LMG22893 | = CAIM148; isolated from the hemolymph of diseased shrimp (Penaeus spp.), Mexico | Bruno Gomez-Gil |
| LMG22894 | = CAIM151; isolated from the hemolymph of diseased shrimp (Penaeus spp.), Mexico | Bruno Gomez-Gil |
| BB120 | 2 | |
| V. parahaemolyticus | ||
| CAIM170 | Isolated from the hemolymph of diseased shrimp (Penaeus spp.), Mexico | Bruno Gomez-Gil |
LMG, Laboratory of Microbiology Collection (Ghent University, Ghent, Belgium); CAIM, Collection of Aquacultural Important Micro-organisms (CIAD/Mazatlán Unit for Aquaculture, Mazatlán, Mexico).
Disruption of AI-2 quorum sensing in V. harveyi by the natural furanone.
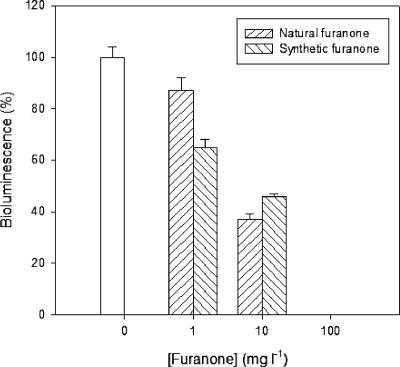
A second in vitro experiment aimed at determining whether the natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone could disrupt AI-2 quorum sensing in V. harveyi (Fig. 1), using bioluminescence as a representative of other quorum sensing-regulated phenotypes. The furanone, synthesized as described previously (18), blocked bioluminescence of the V. harveyi receptor double mutant JMH597 (sensor HAI-1−, sensor AI-2+, sensor CAI-1−) in a concentration-dependent way (Fig. 2). Importantly, halogenated furanones were shown before not to block bioluminescence in a constitutive system (6), indicating that the biochemical function of the Lux proteins is not affected. Our data confirm the report by Ren et al. (17), who determined the impact of the furanone on HAI-1 and AI-2 quorum sensing by using single mutants with a mutation in the AI-2 or HAI-1 receptor protein, which thus were still responsive to both HAI-1 and CAI-1 and both AI-2 and CAI-1, respectively (the CAI-1-mediated channel was not yet discovered at that time).
FIG. 1.
Structures of the natural and synthetic furanones used in this study.
FIG. 2.
Bioluminescence of the V. harveyi reporter strain JMH597 (sensor HAI-1−, sensor AI-2+, sensor CAI-1−) as a function of the concentration of the natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone and the synthetic furanone (5Z)-4-bromo-5-(bromomethylene)-2(5H)-furanone. The luminescence without the addition of furanone was set at 100% (white bar), and the other samples were normalized accordingly. The error bars represent the standard deviations of three replicates.
In vivo protection of Artemia from the pathogenic isolates by the natural furanone.
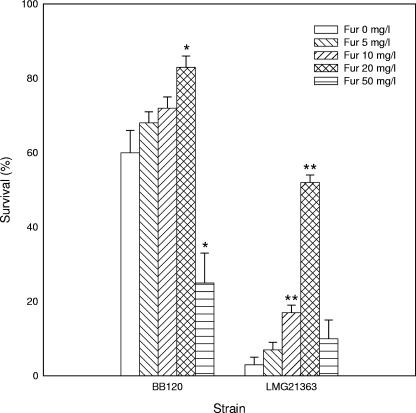
We previously showed that the virulence of V. harveyi BB120 towards Artemia is regulated by the AI-2-mediated channel of its quorum sensing system (5), which we showed here to be blocked by the natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Consequently, we investigated whether the natural furanone could protect gnotobiotic Artemia from pathogenic V. campbellii, V. harveyi, and V. parahaemolyticus in in vivo challenge tests. Challenge tests were performed as described in the work of Defoirdt et al. (5). In a first test, we aimed at determining the best dosage of the natural furanone, and therefore, we investigated the impact of the furanone added in different concentrations (5, 10, 20, and 50 mg/liter) on the virulence of the opportunistic strain V. harveyi BB120 (causing mortality only in nauplii cultured under suboptimal conditions [5]) and the virulent strain V. campbellii LMG21363. The survival of Artemia was proportional to the concentration of furanone for the lower concentrations (5 to 20 mg/liter), whereas high mortality was noted after treatment with 50 mg/liter of furanone (Fig. 3). For both pathogens, the furanone significantly enhanced survival of the nauplii when added at 20 mg/liter (P < 0.05). The protection was complete for V. harveyi BB120 (no significant difference in survival from unchallenged nauplii), whereas significant mortality still occurred in furanone-treated nauplii challenged with the more virulent V. campbellii LMG21363 (P < 0.01). In further challenges, we investigated whether the natural furanone, at 20 mg/liter, could offer some protection against the different pathogenic isolates. The compound significantly reduced mortality in Artemia for all isolates except for LMG22889, where differences were not significant (Table 2). The protection was complete for all V. harveyi strains and the V. parahaemolyticus strain, whereas for V. campbellii LMG21361, LMG22888, and LMG22889 there was still significant mortality in furanone-treated Artemia (P < 0.05) in the first experiment and for strain LMG21363 there was still significant mortality in furanone-treated Artemia in both experiments (P < 0.05).
FIG. 3.
Percent survival of Artemia after 48 h of challenge with V. harveyi BB120 and V. campbellii LMG21363, with and without the natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone (Fur). The error bars represent the standard errors of three replicates. The survival of unchallenged nauplii was 83% ± 3%. *, significantly different from the treatment with the same pathogen and without furanone (P < 0.05); **, significantly different from the treatment with the same pathogen and without furanone (P < 0.01).
TABLE 2.
Percent survival of Artemia (means ± standard errors of three replicates) after 48 h of challenge with different V. campbellii, V. harveyi, and V. parahaemolyticus isolates, with and without the natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone (20 mg/liter)
| Treatment | Expt 1
|
Expt 2
|
||
|---|---|---|---|---|
| − Furanone | + Furanoned | − Furanone | + Furanoned | |
| V. campbelliia | ||||
| LMG21361 | 43 ± 4 | 62 ± 4* | 43 ± 3 | 80 ± 3** |
| LMG21362 | 32 ± 4 | 75 ± 3** | 38 ± 3 | 72 ± 4** |
| LMG21363 | 18 ± 4 | 58 ± 4** | 12 ± 4 | 55 ± 5** |
| LMG22888 | 48 ± 4 | 63 ± 2* | 48 ± 6 | 77 ± 6* |
| LMG22889 | 50 ± 3 | 63 ± 4 | 48 ± 4 | 62 ± 6 |
| LMG22890 | 38 ± 3 | 67 ± 4** | 45 ± 6 | 70 ± 5* |
| LMG22895 | 52 ± 6 | 73 ± 4* | 50 ± 5 | 83 ± 2** |
| V. harveyib | ||||
| BB120 | 52 ± 4 | 87 ± 3** | 60 ± 3 | 78 ± 2** |
| LMG22891 | 62 ± 4 | 83 ± 3* | 53 ± 6 | 73 ± 2* |
| LMG22893 | 55 ± 3 | 78 ± 2** | 57 ± 3 | 75 ± 3* |
| LMG22894 | 48 ± 4 | 75 ± 5* | 43 ± 4 | 72 ± 6* |
| V. parahaemolyticusc | ||||
| CAIM170 | 55 ± 6 | 82 ± 4* | 57 ± 2 | 78 ± 4* |
Survival of unchallenged nauplii was 85% ± 3% in the first experiment and 75% ± 3% in the second experiment.
Survival of unchallenged nauplii was 77% ± 2% in the first experiment and 83% ± 3% in the second experiment.
Survival of unchallenged nauplii was 85% ± 5% in the first experiment and 83% ± 6% in the second experiment.
*, significantly different from treatment with the same pathogen and without furanone (P < 0.05); **, significantly different from treatment with the same pathogen and without furanone (P < 0.01).
Effect of the natural furanone on growth of the vibrios in vitro and in vivo.
There was no effect of the natural furanone on growth of V. harveyi BB120 and V. campbellii LMG21363 in liquid growth medium as well as in the Artemia challenge tubes (data not shown), indicating that the protection offered by the natural furanone was not due to growth inhibition of the pathogens. We used plate counts of the Artemia culture water as an indicator of growth of the pathogens in vivo since it was not feasible to determine the bacterial concentration in/on infected Artemia. Our data are in accordance with those reported by Manefield et al. (12), who showed that there was no effect on the growth of V. harveyi strain 47666-1 for concentrations up to 200 μM (≈62 mg/liter) of the same furanone compound.
In vitro and in vivo disruption of AI-2 quorum sensing by the synthetic furanone.
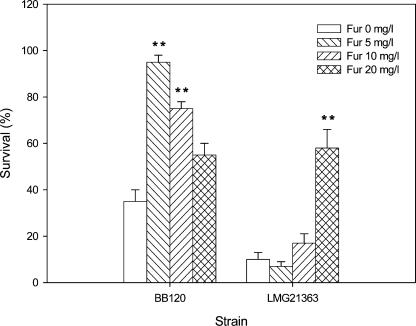
We also tested the quorum sensing-disrupting potential of the synthetic furanone (5Z)-4-bromo-5-(bromomethylene)-2(5H)-furanone (Fig. 1), which as far as we know is the compound with the highest quorum sensing-disrupting activity reported to date. The furanone, synthesized as described before (18), did not have a higher activity towards the V. harveyi quorum sensing system in vitro since it blocked bioluminescence at similar concentrations as did the natural furanone (Fig. 2). Finally, the impact of the synthetic furanone, added in different concentrations (5, 10, and 20 mg/liter), on the virulence of V. harveyi BB120 and V. campbellii LMG21363 towards gnotobiotic Artemia was investigated. The compound completely protected the Artemia nauplii from V. harveyi BB120 at 5 mg/liter, whereas 20 mg/liter was needed to obtain protection from the virulent V. campbellii strain (Fig. 4). As was the case for the natural furanone, the protection against the virulent strain was not complete since significant mortality still occurred in furanone-treated nauplii (P < 0.01). For V. harveyi BB120, the survival with 5 mg/liter was the highest. The survival decreased proportionally to the furanone concentration for higher concentrations, indicating that the compound is slightly more active and more toxic than the natural furanone. The concentration needed to disrupt the V. harveyi quorum sensing system is comparable to that in the report by Hentzer et al. (9), who mentioned a partial or complete suppression of the production of virulence factors in Pseudomonas aeruginosa in the presence of 2.5 mg/liter of the same synthetic furanone as used in this study. Rasch et al. (15), in contrast, found that the compound protected rainbow trout (Oncorhynchus mykiss) from Vibrio anguillarum at much lower concentrations (≈2.5 μg/liter). However, these authors mentioned that the effect that they observed might have been due to interaction of the furanone with the fish host rather than the V. anguillarum quorum sensing system. The fact that the furanones protected the Artemia nauplii from the pathogenic isolates in our in vivo tests at concentrations similar to those needed to block quorum sensing-regulated bioluminescence in V. harveyi in vitro indicates that the effect that we observed was probably due to quorum sensing disruption rather than interaction with the shrimp, although we cannot exclude the latter possibility.
FIG. 4.
Percent survival of Artemia after 48 h of challenge with V. harveyi BB120 and V. campbellii LMG21363, with and without the synthetic furanone (5Z)-4-bromo-5-(bromomethylene)-2(5H)-furanone (Fur). The error bars represent the standard errors of three replicates. The survival of unchallenged nauplii was 85% ± 3%. **, significantly different from the treatment with the same pathogen and without furanone (P < 0.01).
Detailed experimental procedures can be found in the supplemental material.
Supplementary Material
Acknowledgments
We thank Bonnie Bassler for generously providing us with V. harveyi BB120 and JMH597 and Bruno Gomez-Gil for helpful suggestions in the selection of pathogenic isolates. Special thanks go to Greet Dewaele for critically reading the manuscript.
This work was supported by the “Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen” (IWT grant no. 33205) and the “Fonds voor Wetenschappelijk Onderzoek” (project no. G0230.02N).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alvarez, J. D., B. Austin, A. M. Alvarez, and H. Reyes. 1998. Vibrio harveyi: a pathogen of penaeid shrimps and fish in Venezuela. J. Fish Dis. 21:313-316. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabello, F. C. 2006. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8:1137-1144. [DOI] [PubMed] [Google Scholar]
- 4.Defoirdt, T., N. Boon, P. Bossier, and W. Verstraete. 2004. Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture 240:69-88. [Google Scholar]
- 5.Defoirdt, T., P. Bossier, P. Sorgeloos, and W. Verstraete. 2005. The impact of mutations in the quorum sensing systems of Aeromonas hydrophila, Vibrio anguillarum and Vibrio harveyi on their virulence towards gnotobiotically cultured Artemia franciscana. Environ. Microbiol. 7:1239-1247. [DOI] [PubMed] [Google Scholar]
- 6.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Gil, B., S. Soto-Rodriguez, A. Garcia-Gasca, A. Roque, R. Vazquez-Juarez, F. L. Thompson, and J. Swings. 2004. Molecular identification of Vibrio harveyi-related isolates associated with diseased aquatic organisms. Microbiology 150:1769-1777. [DOI] [PubMed] [Google Scholar]
- 8.Henke, J. M., and B. L. Bassler. 2004. Three parallel quorum sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 186:6902-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffensen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steineberg, S. Kjelleberg, N. Høiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karunasagar, I., R. Pai, G. R. Malahti, and I. Karunasagar. 1994. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture 128:203-209. [Google Scholar]
- 11.Lavilla-Pitogo, C. R., E. M. Leaño, and M. G. Paner. 1998. Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture 164:337-349. [Google Scholar]
- 12.Manefield, M., L. Harris, S. A. Rice, R. de Nys, and S. Kjelleberg. 2000. Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl. Environ. Microbiol. 66:2079-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriarty, D. J. W. 1998. Disease control in shrimp aquaculture with probiotic bacteria. In C. R. Bell, M. Brylinsky, and P. Johnson-Green (ed.), Microbial biosystems: new frontiers. Proceedings of the Eighth International Symposium on Microbial Ecology, Halifax, Canada. [online.] http://ag.arizona.edu/azaqua/tilapia/tilapia_shrimp/moriarty.pdf
- 14.Pass, D. A., R. Dybadahl, and M. M. Manion. 1987. Investigations into the causes of mortality in the pearl oyster, Pinctata maxima (Jamson), in western Australia. Aquaculture 65:149-169. [Google Scholar]
- 15.Rasch, M., C. Buch, B. Austin, W. J. Slierendrecht, K. S. Ekmann, J. L. Larsen, C. Johansen, K. Riedel, L. Eberl, M. Givskov, and L. Gram. 2004. An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). Syst. Appl. Microbiol. 27:350-359. [DOI] [PubMed] [Google Scholar]
- 16.Ren, D., L. Bedzyk, R. W. Ye, S. M. Thomas, and T. K. Wood. 2004. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol. Bioeng. 88:630-642. [DOI] [PubMed] [Google Scholar]
- 17.Ren, D., J. Sims, and T. K. Wood. 2001. Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ. Microbiol. 3:731-736. [DOI] [PubMed] [Google Scholar]
- 18.Ren, D., and T. K. Wood. 2004. (5Z)-4-Bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone reduces corrosion from Desulfotomaculum orientis. Environ. Microbiol. 6:535-540. [DOI] [PubMed] [Google Scholar]
- 19.Roque, A., J. F. Turnbull, G. Escalante, B. Gomez-Gil, and M. V. Alday-Sanz. 1998. Development of a batch challenge for the marine shrimp Penaeus vannamei Boone, 1931. Aquaculture 169:283-290. [Google Scholar]
- 20.Soto-Rodriguez, S. A., A. Roque, M. L. Lizarraga-Partida, A. L. Guerra-Flores, and B. Gomez-Gil. 2003. Virulence of luminous vibrios to Artemia franciscana nauplii. Dis. Aquat. Org. 53:231-240. [DOI] [PubMed] [Google Scholar]
- 21.Subasinghe, R. P., M. G. Bondad-Reantaso, and S. E. McGladdery. 2001. Aquaculture development, health and wealth, p. 167-191. In R. P. Subasinghe, P. Bueno, M. J. Phillips, C. Hough, S. E. McGladdery, and J. R. Arthur (ed.), Aquaculture in the third millennium. Technical proceedings of the Conference on Aquaculture in the Third Millennium. NACA, Bangkok, Thailand, and FAO, Rome, Italy.
- 22.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo, J. W. P., A. Suwanto, and C. Laa Poh. 2000. Novel β-lactamase genes from two environmental isolates of Vibrio harveyi. Antimicrob. Agents Chemother. 44:1309-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teo, J. W. P., T. M. C. Tan, and C. Laa Poh. 2002. Genetic determinants of tetracycline resistance in Vibrio harveyi. Antimicrob. Agents Chemother. 46:1038-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, X. H., and B. Austin. 2000. Pathogenicity of Vibrio harveyi to salmonids. J. Fish Dis. 23:93-102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.