Abstract
In Colletotrichum lagenarium, which is the causal agent of cucumber anthracnose, PEX6 is required for peroxisome biogenesis and appressorium-mediated infection. To verify the roles of peroxisome-associated metabolism in fungal pathogenicity, we isolated and functionally characterized ICL1 of C. lagenarium, which encodes isocitrate lyase involved in the glyoxylate cycle in peroxisomes. The icl1 mutants failed to utilize fatty acids and acetate for growth. Although Icl1 has no typical peroxisomal targeting signals, expression analysis of the GFP-Icl1 fusion protein indicated that Icl1 localizes in peroxisomes. These results indicate that the glyoxylate cycle that occurs inside the peroxisome is required for fatty acid and acetate metabolism for growth. Importantly, in contrast with the pex6 mutants that form nonmelanized appressoria, the icl1 mutants formed appressoria that were highly pigmented with melanin, suggesting that the glyoxylate cycle is not essential for melanin biosynthesis in appressoria. However, the icl1 mutants exhibited a severe reduction in virulence. Appressoria of the icl1 mutants failed to develop penetration hyphae in the host plant, suggesting that ICL1 is involved in host invasion. The addition of glucose partially restored virulence of the icl1 mutant. Heat shock treatment of the host plant also enabled the icl1 mutants to develop lesions, implying that the infection defect of the icl1 mutant is associated with plant defense. Together with the requirement of PEX6 for appressorial melanization, our findings suggest that peroxisomal metabolic pathways play functional roles in appressorial melanization and subsequent host invasion steps, and the latter step requires the glyoxylate cycle.
Fungal spores are the infectious propagules responsible for initiating infection as well as disease dissemination. In the initial stage of infection, the spores of phytopathogenic fungi on the plant surface must be under nutrient-limited conditions, and therefore, infection-related cellular events need to be promoted through the metabolism of storage compounds. However, metabolic pathways used by pathogens during infection are poorly understood. In a part of phytopathogenic fungi, asexual spores called conidia germinate and develop a specific infection structure called an appressorium that enables fungal penetration of the host plant (4). Colletotrichum lagenarium, the causal agent of cucumber anthracnose, forms appressoria that are darkly pigmented with melanin on the surface of its host plants (2). C. lagenarium also forms melanized appressoria on artificial surfaces, such as glass, in the absence of external nutrients, indicating that the fungus is able to develop appressoria by utilizing storage compounds of conidium cells. Conidia of C. lagenarium contain large numbers of lipid bodies (12), and rapid degradation of lipid bodies occurs during appressorium formation and subsequent penetration of the host plant, implying that lipolysis provides the compounds required for appressorium formation and function (35). This idea is supported by the finding that the cyclic AMP signaling pathway is required for appressorium function and proper lipolysis in C. lagenarium and the rice blast fungus Magnaporthe grisea (25, 35, 30).
We previously identified ClaPEX6 (hereafter referred to as PEX6) encoding a protein involved in peroxisome biogenesis through analysis of nonpathogenic mutants of C. lagenarium generated by plasmid insertional mutagenesis (12). Knockout analysis of the PEX6 gene demonstrated that it is required for fungal pathogenicity in C. lagenarium. The pex6 deletion mutant formed small appressoria with severely reduced melanization that failed to generate penetration hyphae. Melanin is a secondary metabolite required for appressorium function in fungal pathogens, such as Colletotrichum and Magnaporthe species (10, 14). These data suggest that peroxisomal metabolic functions are required for appressorium functionality, especially for appressorial melanization. However, it remains to be elucidated how peroxisomal metabolic pathways contribute to the pathogenicity of C. lagenarium. Peroxisomes typically contain enzymes for reactions involving molecular oxygen and for metabolizing hydrogen peroxide. Another important feature of peroxisomes is the site of β-oxidation of fatty acids. The pex6 mutant of C. lagenarium consistently failed to utilize fatty acids for vegetative growth, suggesting that the mutant had a defect in the β-oxidation of fatty acids in its peroxisomes (12).
Utilization of lipids as the sole or main carbon source presupposes a requirement of the glyoxylate cycle to provide hexose residues for nucleotide, cell wall, and amino acid biosynthesis. Enzymes involved in the glyoxylate cycle are suggested to localize in peroxisomes in fungi, including Yarrowia lipolytica (13, 32, 33), whereas Saccharomyces cerevisiae isocitrate lyase (ICL1), a key component of the glyoxylate cycle, is solely cytosolic, even under growth conditions that induce peroxisome proliferation (29). The significance of the glyoxylate cycle for fungal pathogenicity was first underscored by the requirement of isocitrate lyase for the virulence of the pathogenic yeast Candida albicans in mouse (15). Additionally, in the pathogenic bacterium Mycobacterium tuberculosis, disruption of the isocitrate lyase gene attenuated bacterial persistence and virulence in immunocompetent mice (16). It has also been reported that ICL1 is required for pathogenicity in Leptosphaeria maculans, a pathogen of canola, and the rice blast fungus M. grisea (11, 34) and that malate synthase, another key enzyme of the glyoxylate cycle, is required for pathogenicity of the wheat pathogen Stagonospora nodorum (20).
In this report, to investigate roles of the glyoxylate cycle in C. lagenarium, we isolated and functionally characterized the C. lagenarium ICL1 gene, which encodes isocitrate lyase. We show that ICL1-encoded isocitrate lyase is required for the utilization of fatty acids and acetate for growth and that this enzyme distinctly localizes in peroxisomes during fungal infection. Importantly, in contrast to the pex6 mutant, icl1 knockout mutants formed appressoria that were highly pigmented with melanin, suggesting that the glyoxylate cycle in peroxisomes is not essential for appressorial melanization. However, the icl1 mutant exhibited reduced virulence on its host cucumber plant, suggesting that ICL1 in C. lagenarium is involved in fungal infection.
MATERIALS AND METHODS
Fungal strains, media, transformation, and DNA analysis.
C. lagenarium strain 104-T (stock culture of the Laboratory of Plant Pathology, Kyoto University) was used as the wild-type strain. All C. lagenarium cultures were maintained on potato dextrose agar (PDA) media (3.9% [wt/vol]; Difco, Detroit, MI) at 24°C in the dark. Protoplast preparation and transformation of C. lagenarium were performed according to a method described previously (28). Hygromycin- and bialaphos-resistant transformants were selected on regeneration medium containing 100 μg/ml hygromycin B (Wako Pure Chemicals, Osaka, Japan) and 250 μg/ml bialaphos (kindly provided by Nobuyuki Fuchigami, Meiji Seika, Japan). Minimal medium consisted of 6 g/liter NaNO3, 0.52 g/liter KCl, 0.52 g/liter MgSO4 · 7H2O, 1.52 g/liter KH2PO4, 0.001% thiamine, and 0.1% trace elements supplemented with 10 g/liter glucose. The sole carbon source media contained 1.6% yeast nitrogen base without amino acids (Difco) and 1% NH4NO3, and pH was adjusted to 6 with Na2HPO4. The fatty acid medium had 0.5% (vol/vol) Tween 80 added to it. The glucose and acetate media contained 2% glucose and 50 mM sodium acetate, respectively. Total DNA of C. lagenarium was isolated from mycelia using the DNeasy plant mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Restriction enzyme digestion, cloning, plasmid isolation, and gel electrophoresis were performed according to the manufacturer's instructions and standard methods (18). Genomic DNA gel blot analysis of C. lagenarium was performed as described previously (26). A 2.0-kb fragment containing the entire ICL1 gene was amplified by PCR with the primers ICL1spS1 (5′-GTCTTATCCGTCTTCTCTGCCGTC-3′) and ICL1spAS1(5′-CGTACATACAGCTCAGGTGCATATCC-3′), and the amplified fragment was used as a probe. DNA probes were labeled with digoxigenin-dUTP (Roche Diagnostics Japan) with the BcaBEST digoxigenin labeling kit (Takara, Ohtsu, Japan).
Plasmid constructs.
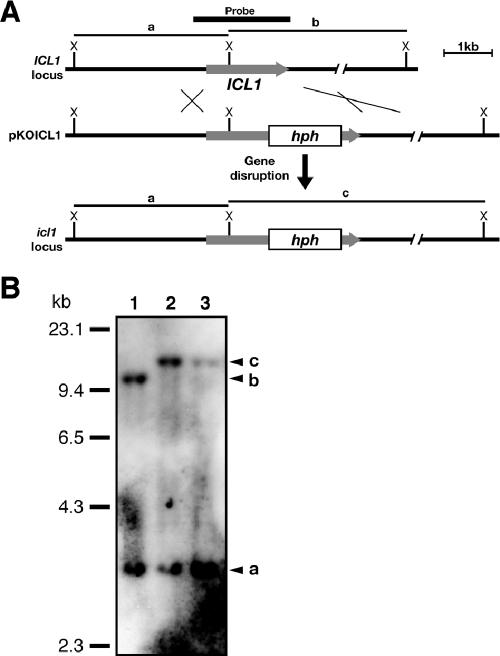
ICL1 was mutated using an adaptation of the in vitro transposon tagging procedure previously described (8). A cosmid clone, pD4SNF1, containing ICL1 was used as the target. A gene disruption vector, pKOICL1, was constructed by mobilizing the modified Tn7 transposable element containing the hygromycin-phosphotransferase gene cassette (the GPS-HYG transposon) into pD4SNF1 in vitro (see Fig. 2A). The sequences of the transposon-inserted regions were determined using primers TN7L (5′-ATAATCCTTAAAAACTCCATTTCCACCCCT-3′) and TN7R (5′-ACTTTATTGTCATAGTTTAGATCTATTTTG-3′). In pKOICL1, the transposon was inserted into the ICL1 sequence at nucleotide 1314 (amino acid residue 419) (Fig. 1). For analysis of the subcellular localization of Icl1, the plasmid pHGFPICL1, carrying the GFP-ICL1 fusion gene, was constructed. Expression of GFP-ICL1 was controlled by a 221-bp short promoter region of the melanin gene SCD1 because expression of green fluorescent protein (GFP) under the SCD1 short promoter resulted in constitutive GFP fluorescence at all fungal stages examined (28). pCB16EGFPSP was previously constructed by the introduction of enhanced green fluorescent protein (EGFP) and the SCD1 short promoter into pCB1636, containing the hph gene (22, 28). The SCD1 terminator region was amplified with primers SD1THS and SD1TCA (12). The amplified product was digested with HindIII and ClaI and introduced into pCB16EGFPSP, which resulted in pCB16EGFPSPST. The entire ICL1 open reading frame was amplified from pD4SNF1 with the primers ICL1FSB (5′-CGGGATCCCCGCGAACAACATGGTCAACT-3′) and ICL1FASE (5′-CGGCGGAATTCCTAGTGGAATTGGTCCTCCGT-3′). ICL1FSB and ICL1FASE contain a BamHI site and EcoRI site, respectively. The amplified product was digested with BamHI and EcoRI and introduced into pCB16EGFPSPST to produce pHGFPICL1. For introduction of pHGFPICL1 into the icl1 mutant DIC1, pHGFPICL1 and pCB1531 carrying a bialaphos-resistant gene (22) were cotransformed into DIC1, and bialaphos-resistant transformants were isolated. Among them, the transformants carrying the GFP-ICL1 fusion gene were selected by PCR analysis. For expression of mRFP1 carrying PTS1, the entire open reading frame of mRFP1 was amplified from mRFP1 in pRSET1b with primers MRFPXKZ (5′-GCC CTCTAGACCAGACACAATGGCCTCCTCCGAGGACGTCATC-3′) and MRFPPTS1B (5′-GGCGGATCCTTACAGCTTCGAGGCGCCGGTGGAGTGGCGGCC-3′). pBATP was previously constructed by the introduction of the SCD1 promoter and terminator regions into pCB1531 (12). The amplified product was digested with XbaI and BamHI and introduced into pBATP. The resultant plasmid was designated pBATPMRPTS1. Each PCR amplification was performed with KOD Plus DNA polymerase (Toyobo, Tsuruga, Japan) according to the manufacturer's instructions. pBATPMRPTS1 was introduced into the wild-type strain 104-T, and the transformant MRPTS1 expressing mRFP1 was isolated.
FIG. 2.
Gene disruption of ICL1. (A) ICL1 locus and the ICL1 gene disruption vector pKOICL1. pKOICL1 was generated by insertion of the GPS-HYG transposon carrying a hygromycin phosphotransferase (hph) gene into ICL1 in the genomic clone pD4SNF1 containing ICL1. (B) DNA gel blot analysis of the icl1 mutant. Genomic DNAs were isolated from the wild-type strain 104-T (lane 1) and the icl1 mutant strains DIC1 and DIC2 (lanes 2 and 3). Genomic DNAs were digested with XhoI. The blot was hybridized with a 2.0-kb fragment containing ICL1, indicated by the black bar in panel A.
FIG. 1.
The C. lagenarium ICL1 gene, which encodes isocitrate lyase. Sequence alignment of C. lagenarium (C.l.) isocitrate lyase with that of N. crassa (N.c.) and M. grisea (M.g.). Amino acid sequences were aligned using the Clustal W program (31). Identical amino acids are indicated as white letters on black background, similar residues are indicated on gray background, and gaps introduced for alignments are indicated by hyphens. The GPS-HYG insertion site is indicated by an arrow.
Pathogenicity tests.
Conidia were collected from 7-day-old PDA cultures and suspended in water, 1 mM glucose, or 1 mM sucrose solutions to a concentration of approximately 5 × 105 conidia per ml. Conidial suspensions (15 μl each) were drop inoculated onto detached leaves or cotyledons of cucumber (Cucumis sativus L. ‘Suyo’). For inoculation through wounded sites, conidial suspension was spotted onto the wounded sites by a 26G1/2 needle. To give plants heat shock, detached cucumber cotyledons were dipped into distilled water at 50°C for 30 s. Subsequently, the icl1 mutant was inoculated onto the heat-treated cotyledon. As a control, cotyledons were dipped into distilled water at 25°C for 30 s before inoculation. We performed each pathogenicity test described above at least three times and obtained similar results from the repeated experiments.
Assay for appressorium formation and penetration.
Infection-related morphogenesis, including germination and appressorium formation on glass, was observed as described previously (28). Nitrocellulose membranes prepared from Visking cellulose tubing were used as a model substrate (14). For the assay on penetration into the membrane, conidial suspensions were poured onto pieces of the membrane placed in petri dishes. Samples were incubated for 2 days and observed under light microscopy. To assess appressorium formation and subsequent invasion of the host plant, conidial suspensions were spotted onto the lower epidermis of cucumber cotyledons. The epidermal layers of inoculated cotyledons were peeled off and observed under light microscopy. To observe intracellular penetration hyphae, the epidermal layers were stained with lactophenol aniline blue (26).
Fluorescent microscopy.
To assess papilla formation, the nonhost plant Arabidopsis thaliana Col-0 was inoculated with a conidial suspension of the icl1 mutant (5 × 105 conidia per ml), as previously reported (19). Inoculated plants were placed in a plant growth box (CUL-JAR300; Iwaki, Japan) at 25°C for 24 h. To determine the presence of callose deposits, samples were stained with aniline blue as described by Adam and Somerville (1). Material was mounted on a slide in 50% glycerol and examined with a fluorescence microscope (Zeiss Axioskop; Germany) with Zeiss filter set 02 (excitation, 365 nm; dichroic, 395 nm; emission, 420 nm). To stain lipid bodies, conidia were incubated in the presence of 10 μg/ml carpropamid solution on glass slides (9, 28) and stained with 2.5 μg/ml Nile red solution (7). Nile Red fluorescence, mRFP1 fluorescence, and EGFP fluorescence were observed using an Olympus FluoView FV500 confocal microscope (Olympus Optical Co., Tokyo, Japan) equipped with an argon laser, an He:Ne laser, and a 60× Plan Apo (1.4 numerical aperture) oil immersion objective lens. The samples were excited with the argon laser for EGFP and with the He:Ne laser for Nile red and mRFP1. We used a dichroic mirror, DM488/543, a beam splitter, SDM560, and two emission filters, BA505-525 for EGFP and BA560IF for Nile red and mRFP1.
Nucleotide sequence accession number.
Accession no. AB246699 in the GenBank database contains the DNA sequence for the ICL1 gene from C. lagenarium.
RESULTS
Isolation and knockout analysis of the ICL1 gene in C. lagenarium.
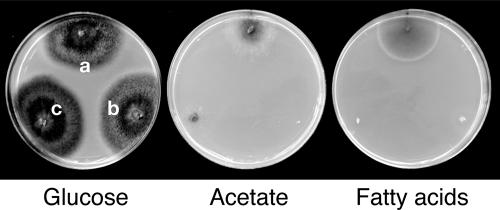
To understand the role of the glyoxylate cycle in fungal pathogenesis of C. lagenarium, we characterized the ICL1 gene encoding isocitrate lyase, one of the principal enzymes of the glyoxylate cycle. The C. lagenarium ICL1 gene was identified by sequence analysis of a genomic clone, pD4SNF1. pD4SNF1 was originally isolated from the cosmid library of C. lagenarium as a cosmid containing a gene homologous to S. cerevisiae SNF1, known as a factor essential for the response to glucose starvation (M. Asakura and Y. Takano, unpublished results). The entire nucleotide sequence of the ICL1 gene in pD4SNF1 was determined. The ICL1 gene sequence contained an open reading frame composed of 546 amino acids with two introns (Fig. 1 and data not shown). The deduced amino acid sequence of Icl1 has high homology with those of the isocitrate lyases Acu3 (84% identity) and Icl1 (82% identity) of Neurospora crassa and M. grisea, respectively (6, 34). To investigate the role of ICL1 in C. lagenarium, we performed knockout analysis. We constructed pKOICL1 for gene disruption of ICL1 using an in vitro transposon tagging procedure (see Materials and Methods) (8). pKOICL1 was introduced into the wild-type strain, 104-T, and hygromycin-resistant transformants were obtained. First, icl1 strains were screened by PCR analysis against genomic DNA of six obtained transformants. The PCR screening suggested that ICL1 is disrupted in all tested transformants (data not shown). In the two transformants DIC1 and DIC2 of the six transformants, gene disruption of ICL1 was confirmed by DNA gel blot analysis (Fig. 2). The wild-type strain 104-T had the 3.2-kb XhoI fragment and 10.5-kb XhoI fragment. The DNA gel blot analysis using the ICL1 gene did not detect any additional fragments, implying that there were no structural homologs of ICL1 in C. lagenarium. Transformants DIC1 and DIC2 possessed the 3.2-kb XhoI fragment and lost the 10.5-kb fragment but commonly had the 12-kb XhoI fragment. These results demonstrated that ICL1 was disrupted in DIC1 and DIC2. These icl1 strains grew normally on PDA, although growth rates of the mutants were slightly lower than that of the wild-type strain (Table 1). The icl1 mutants also showed reduction in growth rate on minimal media (data not shown). Mycelial colonies of the icl1 mutants on PDA were darkly melanized, as seen in the wild-type strain (data not shown). The number of conidia formed on culture of the icl1 mutants grown on PDA was similar to that produced by the wild-type strain (Table 1). However, the icl1 mutants failed to grow on fatty acids as a sole carbon source, which was also observed in the pex6 mutants, whereas the icl1 mutants grew normally on glucose medium (Fig. 3). These results suggest that ICL1 encodes an isocitrate lyase that is essential for fatty acid metabolism in C. lagenarium. The icl1 mutants also failed to grow on acetate medium, whereas the pex6 mutants showed slight growth on this medium (Fig. 3).
TABLE 1.
Characteristics of the icl1 mutantsa
| Strain | Genotype | Colony diam (mm) | Total no. of conidia (106) | % of germinating conidiab | % of germinating conidia that formed appressoriac |
|---|---|---|---|---|---|
| 104-T | ICL1 | 35.3 ± 0.2 | 35.5 ± 10.9 | 94.2 ± 0.2 | 87.1 ± 2.8 |
| DIC1 | icl1 | 31.2 ± 0.1 | 30.5 ± 8.2 | 93.6 ± 1.7 | 79.5 ± 0.7 |
| DIC2 | icl1 | 30.8 ± 0.1 | 27.4 ± 5.9 | 96.3 ± 1.8 | 85.6 ± 4.8 |
| GIC1 | icl1:GFP-ICL1 | 35.2 ± 0.2 | 35.7 ± 8.6 | 96.7 ± 1.0 | 94.3 ± 0.6 |
Each strain was grown on PDA medium for 7 days. Each value represents the mean ± standard deviation from three independent experiments.
Percentage of conidia that germinated after incubation of glass slides at 24°C for 10 h. At least 200 conidia were examined for each experiment.
Percentage of germinating conidia that formed appressoria after incubation for 10 h. At least 200 germinating conidia were examined for each experiment.
FIG. 3.
The icl1 mutant is unable to utilize acetate or fatty acids as the sole carbon source for growth. Growth abilities of the wild-type strain, 104-T, the icl1 strain, DIC1, and the pex6 strain, DPE1, were assessed on glucose, acetate, and fatty acid media as sole carbon sources. The tested strains were incubated for 24 days. a, 104-T; b, DIC1; c, DPE1.
The ICL1-encoded isocitrate lyase localizes in peroxisomes.
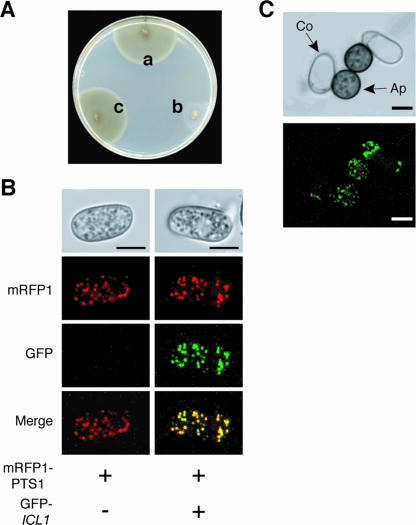
Peroxisomal matrix proteins generally possess peroxisomal targeting signals (PTSs) (21). Most peroxisomal matrix proteins possess PTS1 at the C terminus, whereas some carry PTS2 at the N terminus. Analysis of the amino acid sequence of Icl1 from C. lagenarium suggests that it does not have a typical PTS1 or PTS2. To investigate the intracellular location of Icl1 in C. lagenarium, we performed expression analysis of the GFP-Icl1 fusion protein. The GFP-ICL1 fusion gene, with the C terminus of GFP fused to ICL1, was constructed. To assess functionality of this fusion gene, the GFP-ICL1 fusion gene was introduced into the icl1 mutant DIC1. As a result, the transformant GIC1 carrying the GFP-ICL1 fusion gene showed normal growth on fatty acids, unlike the parental strain DIC1, suggesting that the fusion gene was functional (Fig. 4A). We investigated the subcellular location of GFP-Icl1 in conidium cells of the strain GIC1. The GFP-Icl1 signals formed punctate green dots (data not shown), whereas expression of intact GFP resulted in a diffuse green fluorescence (data not shown), implying that GFP-Icl1 was localized in the peroxisomes. We also observed punctate dots of GFP-Icl1 signals in vegetative mycelia grown on PDA (data not shown). We introduced the GFP-ICL1 gene into the strain MRPTS1, which expresses monomeric red fluorescent protein (mRFP1) that possesses PTS1 at its C terminus (3). In the transformants, which contained two fusion genes, colocalization of mRFP1-PTS1 and GFP-Icl1 was observed (Fig. 4B). This result indicates that Icl1 is localized in the peroxisomes in conidia of C. lagenarium, despite Icl1 having no typical PTS. We also investigated the cellular localization of GFP-Icl1 during appressorium formation and found that GFP-Icl1 was localized in the peroxisomes of appressorium cells, suggesting that Icl1 is localized in peroxisomes in the infection structure of C. lagenarium (Fig. 4C). We investigated localization of Icl1 in vegetative hyphae grown on media containing glucose or fatty acid as a sole carbon source and found that Icl1 was localized in peroxisomes inside vegetative hyphae under both conditions (data not shown).
FIG. 4.
ICL1-encoded isocitrate lyase localizes in the peroxisomes of C. lagenarium. (A) The GFP-ICL1 fusion gene complemented growth of the icl1 mutant on fatty acids. The GFP-ICL1 fusion gene was introduced into the icl1 mutant DIC, and the transformant GIC possessing the fusion gene was isolated. The tested strains were grown on fatty acid medium for 25 days. a, the wild-type strain, 104-T; b, the icl1 mutant, DIC1; c, the complemented strain, GIC1, with the GFP-ICL1 gene. (B) Subcellular localization of GFP-Icl1 in conidia. The GFP-ICL1 fusion gene was introduced into the MRPTS1 strain, which expresses mRFP1-PTS1. mRFP1 and GFP fluorescence was investigated in preincubated conidia of the MRPTS1 strain (left panels) and the GFP-ICL1-introduced transformant of MRPTS1 (right panels). The GFP-Icl1 fusion protein colocalized with mRFP1-PTS1. Bars = 5 μm. (C) Localization of GFP-Icl1 in appressoria. For induction of appressorium formation, conidia of GIC1 were incubated on glass. The fluorescence of GFP-Icl1 was investigated after 6 h of incubation when conidia formed melanized appressoria. Ap, appressorium; Co, conidium. Bars = 5 μm.
The glyoxylate cycle is not essential for appressorial melanization and lipolysis.
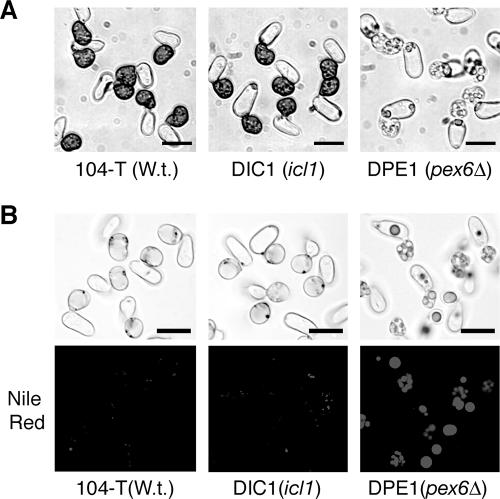
We subsequently investigated germination and appressorium formation of the icl1 mutants on glass at 2 h, 4 h, 6 h, 8 h, and 12 h after incubation and found that the mutants germinated and developed appressoria as did the wild-type strain (Fig. 5A, Table 1, and data not shown). The icl1 mutants also germinated and developed appressoria on host plant cucumber, as did the wild-type strain (data not shown). Thus, ICL1 is not essential for germination and appressorium morphogenesis in C. lagenarium. Notably, appressoria formed by the icl1 mutants were darkly pigmented with melanin, in contrast to the nonmelanized small appressoria formed by the mutant disrupted in the peroxin Pex6 involved in peroxisome biogenesis (Fig. 5A). These results suggest that ICL1 is dispensable for appressorial melanization, and the defect of the pex6 mutant in appressorial melanization is not related primarily to the glyoxylate cycle in peroxisomes. Malonyl coenzyme A (CoA) derived from acetyl-CoA is used for biosynthesis of dihydroxynaphthalene melanin of C. lagenarium (5). Rapid lipolysis during appressorium formation suggests that a large supply of fatty acids is subjected to β-oxidation in peroxisomes. Based on the finding that the glyoxylate cycle is not essential for appressorial melanization, it is likely that the β-oxidation of fatty acids in peroxisomes produces acetyl-CoA that is transported into the cytosol and utilized for melanin biosynthesis in appressorial cells. In addition to the difference in appressorial melanization, we found a difference in lipid metabolism in appressoria between the pex6 mutants and the icl1 mutants. Conidia of C. lagenarium contain large numbers of lipid bodies that can be stained with Nile red (7), and lipid bodies are highly degraded during appressorium formation (35). Because Nile red is not efficiently incorporated into melanized appressoria, conidia were incubated with the melanin biosynthesis inhibitor, carpropamid (9), and lipid bodies inside the nonmelanized appressoria were then stained with Nile red (35). The Nile red assay demonstrated that appressoria of the pex6 mutants possessed a large amount of lipid bodies, unlike the wild-type strain (Fig. 5B). Large lipid bodies frequently remained in conidium cells without moving to appressorial cells in the pex6 mutants. This suggests that PEX6 is involved in the mobilization and metabolism of lipid bodies in C. lagenarium. In contrast, the icl1 mutants degraded lipid bodies, as did the wild-type strain (Fig. 5B). This result suggests that disruption of the ICL1-encoded isocitrate lyase has no strong effects on lipolysis during the infection-related morphogenesis of C. lagenarium.
FIG. 5.
ICL1 is not essential for appressorial melanization and lipolysis. (A) Appressoria formed by the icl1 and pex6 mutants. Conidial suspensions from the wild-type strain, 104-T, the icl1 strain, DIC1, and the pex6 strain, DPE1, were spotted on glass and incubated at 24°C for 16 h. Bars = 10 μm. (B) Lipid bodies in appressoria of the wild-type strain, icl1 mutants, and pex6 mutants. Conidia of each strain were incubated on glass with the melanin biosynthesis inhibitor, carpropamid, for 24 h, and lipid bodies were stained with Nile red. 104-T, wild-type (W.t.) strain; DIC1, icl1 strain; DPE1, pex6 strain. Bars = 10 μm.
The glyoxylate cycle is required for fungal virulence.
Pathogenicity of the icl1 mutants was assayed by drop inoculation of conidial suspension onto detached cucumber leaves and cotyledons. Lesions were formed at most of the sites inoculated with the wild-type strain. In contrast, the icl1 mutant DIC1 significantly reduced the frequency of lesion formation when they were inoculated on cucumber leaves. However, DIC1 often formed lesions in cucumber leaves, whereas the pex6 mutant DPE1 did not form any lesions (12; data not shown). Reduction in lesion formation was more obvious when DIC1 was inoculated on cucumber cotyledons (Fig. 6A). We obtained similar results from the inoculation experiments of the other icl1 mutant DIC2 (data not shown). These results suggest that the icl1 mutants have a defect in the fungal infection process; however, they do not lose pathogenicity completely. The introduction of the GFP-ICL1 fusion gene restored the defect of the icl1 mutant DIC1, i.e., the strain GIC1 carrying the fusion gene formed lesions on cucumber cotyledons as did the wild-type strain (Fig. 6A). These results demonstrate that ICL1 is involved in fungal pathogenicity of C. lagenarium. Subsequently, postinvasion mycelial growth, termed invasive growth, of the icl1 mutants inside cucumber tissue was assessed. Invasive growth ability was assessed by inoculation of conidial suspension on wound sites of the host plant. The melanin-deficient pks1 mutant, ALB1, was inoculated as a positive control because this strain lacks appressorial penetration ability; however, it can grow invasively inside plants (24). In the invasive growth assay for cucumber, the icl1 mutants formed lesions, as did the pks1 mutants (Fig. 6B), suggesting that ICL1 is dispensable for invasive growth inside plant tissue.
FIG. 6.
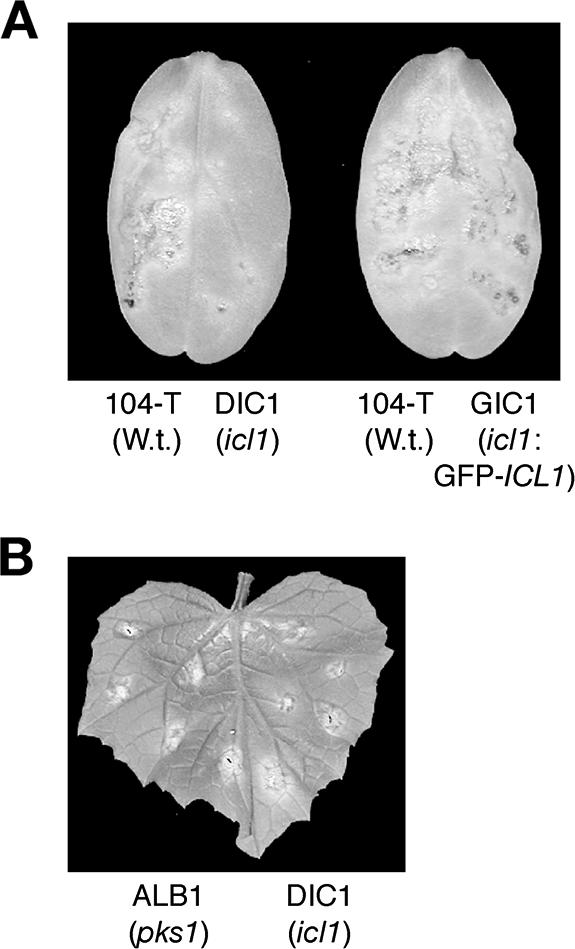
ICL1 is required for fungal virulence of C. lagenarium. (A) Pathogenicity test of the icl1 mutants. Conidial suspensions of tested strains were spotted onto detached cucumber cotyledons. On the left half of the cotyledons, the wild-type (W.t.) strain 104-T was inoculated as a positive control. On the right half, the icl1 mutant DIC1 (the left cotyledon) or the complemented strain GIC1 carrying GFP-ICL1 (the right cotyledon) was inoculated. Inoculated plants were incubated at 25°C for 8 days. (B) Inoculation assay of a wounded cucumber leaf. Conidial suspensions of DIC1 were inoculated on the right half of the cucumber leaf. The albino mutant ALB1 (pks1) was inoculated as a control on the left half of the cucumber leaf. The inoculated plant was incubated at 25°C for 6 days.
Requirement of ICL1 for appressorium-mediated host invasion.
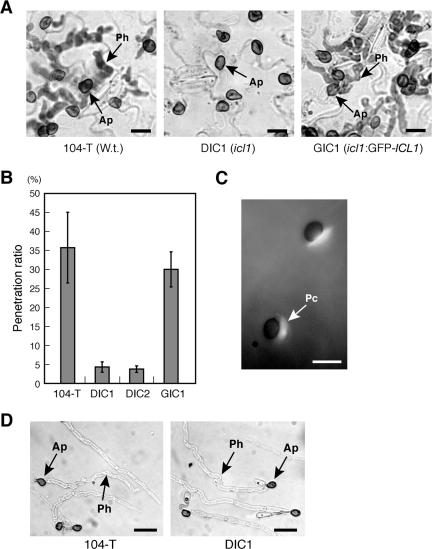
As mentioned above, the icl1 mutants developed melanized appressoria like the wild-type strain, and ICL1 is dispensable for invasive growth. Therefore, it would be expected that the icl1 mutants have a defect in the appressorium-mediated penetration stage of infection. Consistent with this, a penetration assay with cucumber cotyledons revealed that the icl1 mutants failed to generate penetration hyphae in the host plant (Fig. 7A and B). When the wild-type strain was inoculated on cucumber cotyledons, 35.7% ± 9.2% (mean ± standard deviation) of appressoria generated intracellular penetration hyphae at 4 days postinoculation (dpi). In contrast, the penetration frequencies of the icl1 strains, DIC1 and DIC2, at 4 dpi were 4.3% ± 1.4% and 3.7% ± 0.9%, respectively. The penetration frequency of DIC1 was still low at 10 dpi (data not shown). Furthermore, the penetration frequency of GIC1, the complementation strain with GFP-ICL1, was 30.0% ± 4.6%, suggesting that GIC1 formed penetration hyphae as did the wild type (Fig. 7A and B). These results indicate that ICL1 is involved in appressorium-mediated invasion of the host plant. C. lagenarium forms appressoria on the nonhost plant, Arabidopsis thaliana; however, it fails to generate penetration hyphae accompanied with strong elicitation of papilla formation (19). We have shown that papillae are not induced by the C. lagenarium mutant 82335, which has a defect in the formation of penetration pegs, suggesting that the penetration peg is a possible inductive cue for papilla formation (19). To assess whether the icl1 mutant was able to develop a penetration peg, we inoculated the icl1 mutant of C. lagenarium on Arabidopsis and investigated the deposition of papillary callose. Papillary callose formed beneath the appressoria of the icl1 mutant (Fig. 7C). Thus, it appears that the icl1 mutants retain the ability to develop penetration pegs. Consistent with this result, the icl1 mutants penetrated the artificial membranes, nitrocellulose membranes, as did the wild-type strain, suggesting that the icl1 mutants retain basic appressorial penetration functions, including the ability to form a penetration peg and subsequent penetration hyphae (Fig. 7D).
FIG. 7.
The icl1 mutants have a defect in appressorium-mediated host invasion. (A) The icl1 mutants failed to generate intracellular penetration hyphae into cucumber cotyledons. Conidial suspensions of each strain were inoculated on the lower surface of cucumber cotyledons, and cotyledons were incubated for 4 days. Bars = 10 μm. Ap, appressorium; Ph, penetration hypha. (B) Results of a quantitative assay for appressorial penetration. In each experiment, at least 100 appressoria were examined and counted to calculate the percentage of penetration hyphae formed. Means and standard deviations were calculated from three independent experiments. (C) Deposition of papillary callose under appressoria formed by the icl1 mutant. The icl1 mutant was inoculated on the nonhost plant, A. thaliana. At 1 dpi, callose deposits in papillae were stained with aniline blue. Bar = 10 μm. Pc, papillary callose. (D) Results of an appressorial penetration assay with nitrocellulose membranes. A conidial suspension of each strain was inoculated on nitrocellulose membranes and incubated for 2 days. Ap, appressorium; Ph, penetration hypha. Bars = 20 μm.
Appressorium-mediated host invasion and the glyoxylate cycle.
Scytalone, an intermediate in melanin biosynthesis, restored appressorial melanization in the C. lagenarium pex6 mutants; however, it did not restore pathogenicity of pex6 mutants (12). In contrast, the addition of glucose partially restored pathogenicity as well as appressorial melanization in the pex6 mutants (12). This suggests that pex6 mutants have other pathogenicity-related defects in addition to the defect in appressorial melanization. Based on this, we assessed the effects of glucose on the virulence of icl1 mutants. When we inoculated the icl1 mutants on cucumber cotyledons in the presence of 1 mM glucose, the icl1 mutants increased lesion formation (Fig. 8A). Partial remediation of virulence in the icl1 mutant was also observed in the presence of 1 mM sucrose (Fig. 8A). The finding that glucose and sucrose partially restored virulence of the icl1 mutants implies that gluconeogenesis plays a role in host invasion via the glyoxylate cycle. Recently, we characterized the APH1 gene, which is required for appressorium-mediated host invasion of C. lagenarium, and showed that heat shock treatment of cucumber leaves enabled the aph1 mutant to infect (27). We assessed the effect of heat shock treatment on the infection ability of the icl1 mutant and found that heat shock treatment permitted the icl1 mutant to form lesions in common with the aph1 mutant (Fig. 8B).
FIG. 8.
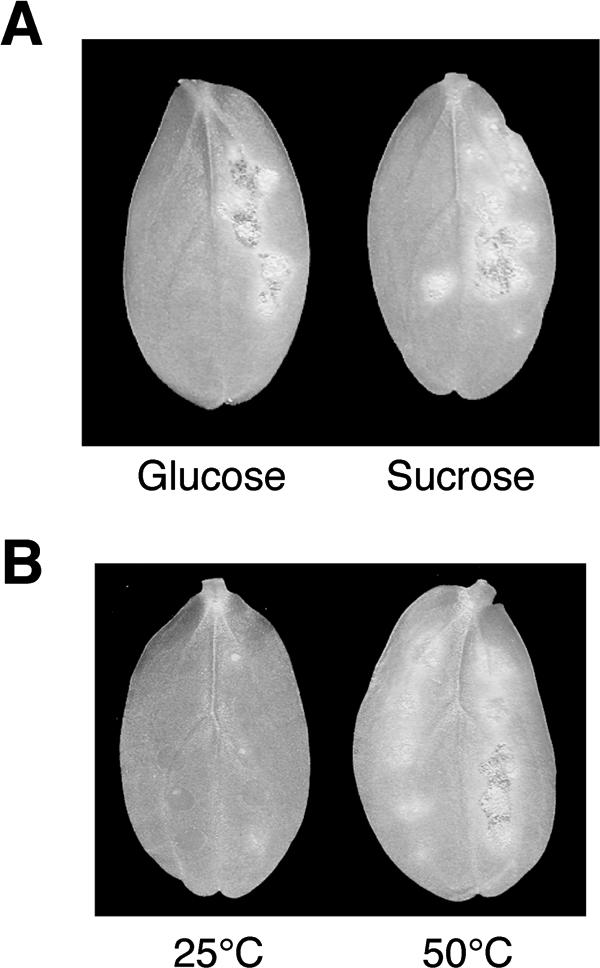
Restoration of virulence in icl1 mutants. (A) Pathogenicity of the icl1 mutants was partially remedied by the addition of saccharides. Conidia of the icl1 mutant DIC1 suspended in 1 mM glucose or sucrose solution were drop inoculated on the right half of cucumber cotyledons. The left halves of cucumber cotyledons were inoculated with conidia of DIC1 suspended in water. Inoculated plants were incubated at 25°C for 8 days. (B) Heat shock treatment of the host plant enabled the icl1 mutant to infect. Cucumber cotyledons pretreated at 50°C for 30 s were inoculated with conidial suspensions of the icl11 mutant. Inoculated plants were incubated at 25°C for 6 days. As a control, cotyledons were treated at 25°C for 30 s before inoculation.
DISCUSSION
Genomic Southern blot analysis suggested that there are no structural homologs of ICL1 in C. lagenarium. Consistent with this, Magnaporthe grisea, related to Colletotrichum species, has a single isocitrate lyase gene in the whole-genome sequence. Also, the icl1 mutants of C. lagenarium failed to utilize fatty acid and acetate, suggesting that it is unlikely that there is no second isocitrate lyase in C. lagenarium and that disruption of ICL1 causes inactivation of the glyoxylate cycle.
Colocalization of the GFP-Icl1 fusion proteins with mRFP1-PTS1 indicated that the ICL1-encoded isocitrate lyase is localized in peroxisomes; however, the amino acid sequence of C. lagenarium Icl1 revealed that Icl1 has neither typical PTS1 nor PTS2. The isocitrate lyases of N. crassa and M. grisea also lack typical PTS1 and PTS2 (Fig. 1) (6, 34). However, density gradient centrifugation analysis implied that isocitrate lyases of N. crassa and Aspergillus nidulans are located in peroxisomes (13, 33), suggesting that isocitrate lyases of filamentous fungi are commonly localized in peroxisomes. The mechanism for targeting C. lagenarium Icl1 to peroxisomes remains unclear. Icl1 could be transported into peroxisomes by a piggyback mechanism, i.e., transportation via interaction with a protein carrying PTS. Alternatively, Icl1 might possess an unidentified signal sequence that is used to target peroxisomes. In contrast with Icl1 of C. lagenarium, isocitrate lyases of S. cerevisiae are cytosolic, even under growth conditions that induce peroxisome proliferation (29). In C. lagenarium, the icl1 mutant is unable to grow on acetate medium, whereas the pex6 mutant grows slightly on this medium (Fig. 3). Icl1 of the pex6 mutant might locate in the cytosol and partially function in the glyoxylate cycle. However, the growth ability of the pex6 mutant on acetate medium was lower than that of the wild-type strain. Thus, it is likely that the localization of Icl1 in peroxisomes is important for the efficiency of the glyoxylate cycle in C. lagenarium.
The β-oxidation of fatty acids is a well-conserved metabolic process that degrades fatty acids to acetyl-CoA. It is likely that rapid lipolysis produces fatty acids, and the fatty acids that are generated are subjected to β-oxidation in peroxisomes to produce acetyl-CoA during appressorium formation in C. lagenarium (12, 35). Functional analysis of PEX6 revealed that it plays a role in peroxisomal metabolic function for appressorium-mediated infection, especially the melanization of appressoria (12). In contrast, the icl1 mutants formed melanized appressoria, suggesting that the glyoxylate cycle is not essential for appressorial melanization in C. lagenarium. This finding suggests that acetyl-CoA, generated by β-oxidation of fatty acids, is transported from peroxisomes into the cytosol via carnitine acetyltransferase and is utilized for melanin biosynthesis in appressoria. Consistent with this idea, PTH2, which encodes a putative carnitine acetyltransferase, is required for full pathogenicity of M. grisea, although detailed analysis of PTH2 has not yet been performed (23). However, we cannot currently exclude the possibility that the glyoxylate cycle partially contributes to appressorial melanization in C. lagenarium. The pex6 mutants did not undergo efficient lipolysis during infection-related morphogenesis, suggesting that peroxisomal function is involved in the regulation of lipid metabolism in the fungal infection process. However, in contrast to the pex6 mutant, impaired lipolysis was not observed in the icl1 mutant, suggesting that disruption of Icl1 involved in the glyoxylate cycle did not have an obvious effect on the regulation of lipolysis. We consider that the β-oxidation pathway for fatty acids is associated with the regulation of lipolysis during appressorium formation; however, it remains unclear how this metabolic pathway affects lipolysis.
The icl1 mutants of M. grisea exhibited delayed germination and appressorium development in comparison with the wild-type strain (34). In contrast, the C. lagenarium icl1 mutant germinated and formed appressoria without delay. Contribution of the glyoxylate cycle to infection-related morphogenesis seems to be uncommon among these pathogens. The glyoxylate cycle provides a means for cells to assimilate two-carbon compounds into the tricarboxylic acid cycle and channel these via gluconeogenesis to generate glucose. The icl1 mutants of C. lagenarium have a defect in appressorium-mediated invasion of the host plant, and the addition of glucose or sucrose partially restored virulence of the icl1 mutants. Based on these findings, we are currently considering that gluconeogenesis mediated by the glyoxylate cycle is probably important for appressorium-mediated host invasion.
The abilities of the icl1 mutant (i) to elicit papilla on A. thaliana and (ii) to penetrate into cellophane suggest that appressoria formed by the mutants retain the basic functions required for penetration. Also, a pathogenicity test of the icl1 mutant on heat-treated cucumber suggests that the impaired virulence in the icl1 mutant that we observed is likely to be associated with defense responses of the host plant. Together with partial remediation of pathogenicity in the presence of glucose, it is possible that the icl1 mutant has a defect in cell wall biosynthesis that is related to the protection of fungal cells from plant defense responses. We recently reported that the APH1 gene of C. lagenarium encodes a tRNA methylase required for appressorium-mediated host invasion (27). Heat shock treatment restored virulence of the aph1 mutant, in common with the icl1 mutant, although it remains to be elucidated how Aph1 functions in the host invasion step. Additionally, C. lagenarium formed melanized appressoria on the nonhost plant, A. thaliana; however, it failed to generate penetration hyphae, suggesting that nonhost resistance blocks appressorium-mediated invasion (19). In contrast to C. lagenarium, it is likely that the adapted Colletotrichum higginsianum suppresses preinvasion resistance of A. thaliana (19). Based on these findings, fungal factors that resist and/or suppress plant defense must be critical for successful appressorium-mediated host invasion.
In this report, we have shown that the glyoxylate cycle is required for virulence of the fungal plant pathogen C. lagenarium. It was recently reported that two isocitrate lyases (ICL1 and ICL2) are jointly required for virulence of M. tuberculosis, the etiologic agent of leprosy and tuberculosis (17). Importantly, a dual-specific isocitrate lyase inhibitor blocked the growth of M. tuberculosis on fatty acids and in macrophages, implicating glyoxylate inhibitors as new drug candidates for the treatment of tuberculosis. Our analysis, including phenotypic comparison of the icl1 mutant with the pex6 mutant, strongly suggests pivotal roles for other aspects of peroxisomal metabolism in fungal phytopathogenicity in addition to the glyoxylate cycle. Further functional analysis of other peroxisomal matrix proteins, such as enzymes involved in the β-oxidation of fatty acids, will be important to understand the multiple roles of peroxisomes in fungal pathogenicity. This analysis will also provide useful information for the development of new drugs against plant and animal pathogens.
Acknowledgments
We thank Roger Y. Tsien for providing the mRFP1 gene. We also thank Junko Yamauchi for construction of pBATPMRPTS1, Eriko Oshiro for construction of pCB16EGFPSPST, and Kazuyuki Mise for valuable discussion.
This work was supported in part by a Grant-in-Aid (15780035) for Young Scientists (B), a Grant-in-Aid (13306005) for Scientific Research (A) from the Japan Society of the Promotion of Science, and a Grant-in-Aid (12146202) for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Adam, L., and S. C. Somerville. 1996. Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 9:341-356. [DOI] [PubMed] [Google Scholar]
- 2.Agrios, G. N. 2004. Plant pathology, 5th ed., p. 487-498. Academic Press, San Diego, Calif.
- 3.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emmett, R. W., and D. G. Parbery. 1975. Appressoria. Annu. Rev. Phytopathol. 13:147-167. [Google Scholar]
- 5.Fujii, I., Y. Mori, A. Watanabe, Y. Kubo, G. Tsuji, and Y. Ebizuka. 2000. Enzymatic synthesis of 1,3,6,8-tetrahydroxynaphthalene solely from malonyl coenzyme A by a fungal iterative type I polyketide synthase PKS1. Biochemistry 39:8853-8858. [DOI] [PubMed] [Google Scholar]
- 6.Gainey, L. D., I. F. Connerton, E. H. Lewis, G. Turner, and D. J. Ballance. 1992. Characterization of the glyoxysomal isocitrate lyase genes of Aspergillus nidulans (acuD) and Neurospora crassa (acu3). Curr. Genet. 21:43-47. [DOI] [PubMed] [Google Scholar]
- 7.Greenspan, P., E. P. Mayer, and S. D. Fowler. 1985. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100:965-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer, L., K. Adachi, M. V. Montenegro-Chamorro, M. M. Tanzer, S. K. Mahanty, C. Lo, R. W. Tarpey, A. R. Skalchunes, R. W. Heiniger, S. A. Frank, B. A. Darveaux, D. J. Lampe, T. M. Slater, L. Ramamurthy, T. M. DeZwaan, G. H. Nelson, J. R. Shuster, J. Woessner, and J. E. Hamer. 2001. Gene discovery and gene function assignment in filamentous fungi. Proc. Natl. Acad. Sci. USA 98:5110-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori, T., K. Kurahashi, S. Kagabu, J. Konze, and U. Kraatz. 1994. KTU3616: a novel fungicide for rice blast control, p. 517-524. In Abstract of Brighton Crop Protection Conference: Pests and Diseases. The British Crop Protection Council, Brighton, England.
- 10.Howard, R. J., and B. Valent. 1996. Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 50:491-512. [DOI] [PubMed] [Google Scholar]
- 11.Idnurm, A., and B. J. Howlett. 2002. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot. Cell 1:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, A., Y. Takano, I. Furusawa, and T. Okuno. 2001. Peroxisomal metabolic function is required for appressorium-mediated plant infection by Colletotrichum lagenarium. Plant Cell 13:1945-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kionka, C., and W.-H. Kunau. 1985. Inducible β-oxidation pathway in Neurospora crassa. J. Bacteriol. 161:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo, Y., and I. Furusawa. 1991. Melanin biosynthesis: prerequisite for successful invasion of the plant host by appressoria of Colletotrichum and Pyricularia, p. 205-217. In G. T. Cole and H. C. Hoch (ed.), The fungal spore and disease initiation in plants and animals. Plenum Publishing, New York, N.Y.
- 15.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 16.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 17.Munoz-Elias, E. J., and J. D. McKinney. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11:638-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook, J., E. F., Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Shimada, C., V. Lipka, R. O'Connell, T. Okuno, P. Schulze-Lefert, and Y. Takano. 2006. Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol. Plant-Microbe Interact. 19:270-279. [DOI] [PubMed] [Google Scholar]
- 20.Solomon, P. S., R. C. Lee, T. J. Wilson, and R. P. Oliver. 2004. Pathogenicity of Stagonospora nodorum requires malate synthase. Mol. Microbiol. 53:1065-1073. [DOI] [PubMed] [Google Scholar]
- 21.Subramani, S. 1993. Protein import into peroxisomes and biogenesis of the organelle. Annu. Rev. Cell Biol. 9:445-478. [DOI] [PubMed] [Google Scholar]
- 22.Sweigard, J., F. Chumley, A. Carroll, L. Farrall, and B. Valent. 1997. A series of vectors for fungal transformation. Fungal Genet. Newsl. 44:52-55. [Google Scholar]
- 23.Sweigard, J. A., A. M. Carroll, L. Farrall, F. G. Chumley, and B. Valent. 1998. Magnaporthe grisea pathogenicity genes obtained through insertional mutagenesis. Mol. Plant-Microbe Interact. 11:404-412. [DOI] [PubMed] [Google Scholar]
- 24.Takano, Y., T. Kikuchi, Y. Kubo, J. E. Hamer, K. Mise, and I. Furusawa. 2000. The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant-Microbe Interact. 13:374-383. [DOI] [PubMed] [Google Scholar]
- 25.Takano, Y., K. Komeda, K. Kojima, and T. Okuno. 2001. Proper regulation of cyclic AMP-dependent protein kinase is required for growth, conidiation, and appressorium function in the anthracnose fungus Colletotrichum lagenarium. Mol. Plant-Microbe Interact. 14:1149-1157. [DOI] [PubMed] [Google Scholar]
- 26.Takano, Y., Y. Kubo, C. Kawamura, T. Tsuge, and I. I. Furusawa. 1997. The Alternaria alternata melanin biosynthesis gene restores appressorial melanization and penetration of cellulose membranes in the melanin-deficient albino mutant of Colletotrichum lagenarium. Fungal Genet. Biol. 21:131-140. [PubMed] [Google Scholar]
- 27.Takano, Y., N. Takayanagi, H. Hori, Y. Ikeuchi, T. Suzuki, A. Kimura, and T. Okuno. 2006. A gene involved in modifying transfer RNA is required for fungal pathogenicity and stress tolerance of Colletotrichum lagenarium. Mol. Microbiol. 60:81-92. [DOI] [PubMed] [Google Scholar]
- 28.Takano, Y., E. Oshiro, and T. Okuno. 2001. Microtubule dynamics during infection-related morphogenesis of Colletotrichum lagenarium. Fungal Genet. Biol. 34:107-121. [DOI] [PubMed] [Google Scholar]
- 29.Taylor, K. M., C. P. Kaplan, X. Gao, and A. Baker. 1996. Localization and targeting of isocitrate lyases in Saccharomyces cerevisiae. Biochem. J. 319:255-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thines, E., R. W. Weber, and N. J. Talbot. 2000. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titorenko, V. I., J. J. Smith, R. K. Szilard, and R. A. Rachubinski. 1998. Pex20p of the yeast Yarrowia lipolytica is required for the oligomerization of thiolse in the cytosol and for its targeting to the peroxisome. J. Cell Biol. 142:403-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenciano, S., J. R. De Lucas, A. Pedregosa, I. F. Monistrol, and F. Laborda. 1996. Induction of β-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch. Microbiol. 166:336-341. [DOI] [PubMed] [Google Scholar]
- 34.Wang, Z. Y., C. R. Thornton, M. J. Kershaw, L. Debao, and N. J. Talbot. 2003. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 47:1601-1612. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi, J., N. Takayanagi, K. Komeda, Y. Takano, and T. Okuno. 2004. cAMP-PKA signaling regulates multiple steps of fungal infection cooperatively with Cmk1 MAP kinase in Colletotrichum lagenarium. Mol. Plant-Microbe Interact. 17:1355-1365. [DOI] [PubMed] [Google Scholar]