Abstract
Bacterial biofilms were exposed to electrolysis by making the steel substratum an electrode in a circuit including a 6-V battery. These treatments resulted in killing (2.1-log reduction) and removal (4.0-log reduction) of viable cells at the anode and cathode, respectively, within a few minutes.
One alternative strategy for controlling microbial biofilms is to generate an antimicrobial or antibiofilm substance locally at the attachment surface (6). There are at least two attractive aspects of this approach. First, the antimicrobial agent is generated at the site of its target. The bulk fluid does not have to be loaded with the antimicrobial, reducing chemical costs and environmental impact. Second, the antimicrobial is produced exactly where its action is likely to have the greatest impact. Agents targeted to the attachment surface may be better able to promote removal of the biofilm instead of just killing. Especially for reactive agents that have difficulty penetrating to the bottom of a biofilm when delivered from the fluid bathing the film, in situ generation of the antimicrobial at the base of the biofilm would be effective. An example of this strategy is the work of Wood and coworkers (9, 10) who synthesized polymer surfaces containing cobalt or copper catalysts that generate reactive oxygen species at the substratum underneath the biofilm.
Another means of generating antimicrobial agents at a surface is through electrolysis. Electrolysis of water can generate local pH changes which may affect bacteria or their matrix polymers. When chloride ions are present, electrolysis can generate free chlorine, a powerful antimicrobial agent. Matsunaga and coworkers have demonstrated the potential of this approach to remove and inactivate marine biofouling bacteria (2-5). Their results also show that biofouling control can be achieved without perturbing the bulk fluid pH or imparting a chlorine residual to the bulk fluid.
The purpose of the research reported in this article was to investigate the effects of electrolysis on biofilms formed by Staphylococcus epidermidis. This microorganism was chosen because of its medical relevance and because it forms thick biofilms in in vitro reactor systems. Experiments were conducted in the presence of chloride ion to allow for the electrolytic generation of free chlorine at the cathode.
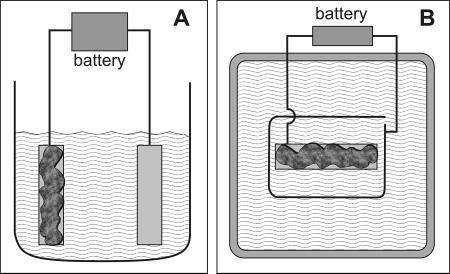
Biofilms of Staphylococcus epidermidis ATCC 35984 were grown on stainless steel coupons in drip flow reactors (7, 12) at 37°C with provision of 1/10-strength tryptic soy broth at 10 ml h−1. Two-day-old biofilms were removed from the drip flow reactor chamber, placed in either of two vessels, and incorporated into an electrical circuit (Fig. 1). The first vessel used was a 500-ml beaker filled with sterile 10 g liter−1 sodium chloride so that the metal coupons were completely submerged. The circuit was completed by clamping the other ends of the cables to their respective positions on a 6-volt battery for a certain length of time, between 10 s and 5 min. The biofilm-covered coupon was connected as the anode (to the negative terminal of the battery) in some experiments and as the cathode (to the positive terminal of the battery) in other experiments. At the end of the treatment period, the biofilm-covered coupon was lifted from the NaCl solution with ethanol-sterilized tweezers and rinsed by slowly pipetting 2 ml of deionized water over each side of the coupon. The specimen was then either scraped and plated or stained and examined microscopically as described below.
FIG. 1.
Diagram of systems used for electrolytic treatment of biofilms. The coupon covered with biofilm (dark gray) and a clean coupon or wire (light gray) were immersed in a beaker (A, side view) or open dish (B, top view) containing 10 g liter−1 NaCl. The two metal pieces were connected to a 6-V battery with small jumper cables.
For some of the experiments, the biofilm-covered coupon was placed, biofilm up, at the center of a 9-in. Pyrex dish. This vessel was used especially for making short movies of the electrolysis treatment by suspending a digital camera above the dish.
Viable cells were enumerated by serial dilution and plating. Intact biofilms were stained for respiratory activity with a 0.2% solution of 5-cyano-2,3-ditolyltetrazolium chloride (CTC), counterstained with a 10 μg ml−1 solution of 4′,6′-diamidino-2-phenylindole (DAPI), cryoembedded, sectioned, and examined by epifluorescence microscopy with a Nikon E800 microscope using a 10× dry objective and a 450/50-nm excitation filter, a 480-nm dichroic mirror, and a 630/30-nm emission filter for CTC and a 360/40-nm excitation filter, a 400-nm dichroic mirror, and a 460/50-nm emission filter for DAPI.
Staphylococcus epidermidis formed dense, cream-colored biofilms in the drip flow reactor after 2 days of growth. These biofilms contained approximately 2 × 109 viable cells per cm2 prior to treatment. Untreated biofilms were 360 ± 80 μm thick, as measured from microscopic examination of several frozen sections.
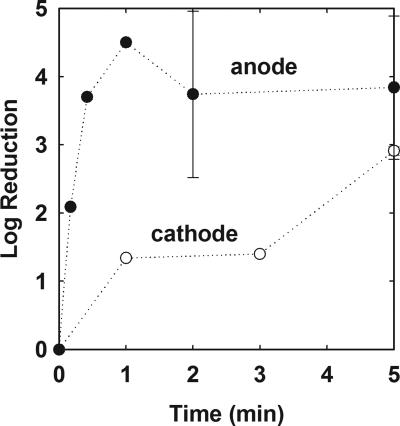
When a biofilm-covered steel slide was connected as the anode in an electrical circuit with a 6-V potential, biofilm rapidly sloughed from the surface. Biofilm sloughing was accompanied by the copious evolution of gas bubbles from the metal surface (visit http://www.erc.montana.edu/Res-Lib99-SW/Movies/2006/06-M001.htm to view a video of this treatment). After approximately 30 seconds of treatment, most of the biofilm had been visibly removed from the surface. The log reduction in the number of surface-associated viable cells after 30 seconds of treatment as the anode corresponded to a decrease of 4 orders of magnitude (Fig. 2). Extending the treatment period did not further decrease the number of viable cells. The mean log reduction for treatments of 0.5 to 5 min was 3.99 ± 0.64. The decrease in viable cells, comparing controls (n = 6) and treatments (n = 7), was statistically significant (P = 3 × 10−6).
FIG. 2.
Reduction in biofilm viable cell numbers during electrolytic treatment of biofilms connected as the anode (•) or cathode (○). The error indicated is the standard deviation.
When a biofilm-covered steel slide was connected as the cathode in an electrical circuit with a 6-V potential, release of biomass from the surface was less apparent. Most of the biomass remained attached to the slide, and the evolution of gas bubbles was not as strong as it was at the anode. Rather, the solution near the slide was discolored in tones ranging from green to yellow-brown. The loss of viable cells was slower when a coupon was treated as the cathode compared to when it was treated as the anode (Fig. 2). After 1 min of treatment as the cathode, the log reduction was 1.4, and this rose to approximately a 3-log reduction after 5 min. The decrease in viable cells, comparing controls (n = 6) and treatments (n = 3), was borderline statistically significant (P = 0.06).
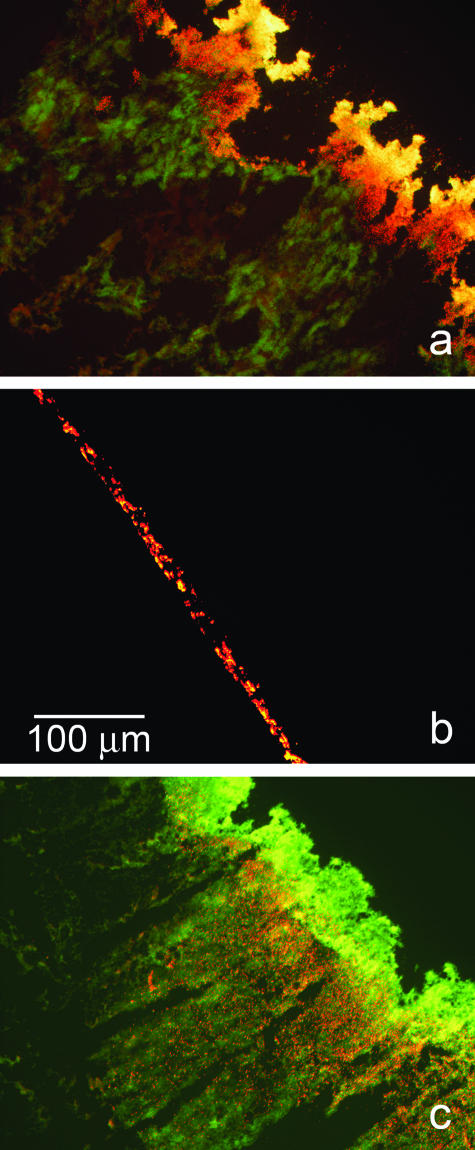
These effects on the biofilm were qualitatively confirmed at the microscale by staining biofilms with the respiratory indicator CTC and examining frozen sections microscopically. This stain deposits red fluorescent crystals at locations of active respiration. Untreated control biofilms exhibited intense red staining (Fig. 3a). Biofilms treated as the anode were largely removed from the surface, leaving only a trace of biomass (Fig. 3b). Some of the residual biomass in these specimens did stain red, indicating respiratory potential. In biofilms treated as the cathode, thick regions of biomass remained intact and could be seen in frozen sections (Fig. 3c). These specimens had much less red color than the untreated control, indicating a significant loss of respiratory activity. What residual respiratory activity could be visualized seemed to be localized to an interior stratum just below the surface of the biofilm. Cells at the base of the biofilm were mostly inactive, as were cells in the layer adjacent to the bulk fluid interface.
FIG. 3.
Patterns of respiratory activity in S. epidermidis biofilms visualized by staining with the respiratory indicator CTC (red) and the DNA dye DAPI (green). These are frozen cross sections that are representative of the patterns seen in duplicate experiments. In each panel, the substratum was formerly closer to the lower left corner and the biofilm-bulk fluid interface was closer to the upper right corner. Red color indicates that highly actively respiring cells are present. Yellow color, obtained by the overlap of red and green, indicates the presence of cells with an intermediate level of respiratory activity. Green color, indicating the presence of DNA but absence of CTC staining, reveals regions of very low respiratory activity. (a) Untreated control; (b) biofilm treated as the anode; (c) biofilm treated as the cathode.
The effects of electrical currents on S. epidermidis biofilms observed in this investigation can be interpreted by considering the electrolytic reactions occurring. At the anode, the relevant reaction is 2H2O + 2e−→2OH− + H2. Thus, at the anode, one predicts an increase in pH and the evolution of hydrogen gas. At the cathode, the important reaction is likely to be H2O + Cl−→HOCl + H+ + 2e−. At the cathode, one predicts a decrease in pH and the generation of the potent antimicrobial hypochlorous acid.
We hypothesize that the increase in pH near the anode leads to alkaline hydrolysis of the polysaccharide matrix of the biofilm. Removal of biofilm from the surface is probably aided by hydrogen gas bubbles physically pushing biomass away from the substratum. The pH increase may also kill some of the bacteria. Dilute sodium hydroxide has been reported elsewhere to weaken and remove biofilms formed by S. epidermidis (11).
We hypothesize that the generation of hypochlorous acid at the cathode is responsible for most of the loss of viable cells that occurs in biofilms treated as the cathode. We further suggest that a reduction in pH is not conducive to hydrolysis of the particular polymers that comprise the matrix of these biofilms. This feature, along with less gas production at the cathode, could explain why less biofilm is removed than when a specimen is treated as the anode.
A decrease in pH is probably responsible for the obvious corrosion occurring at the cathode. The discoloration of the solution that was observed in our experiments was likely due to the release of Fe2+ and Fe3+ from the metal substratum. Corrosion is an issue that would need to be addressed to make this technology practicable. Potential solutions include plating the cathode with an inert metal (e.g., gold) or using conductive polymers or paints.
Our results are in general agreement with those of Matsunaga and coworkers (2-5) and of van der Borden et al. (8). The electrical enhancement of antibiotic action against biofilms, dubbed the “bioelectric effect,” must be distinct from the results reported in this article, because the bioelectric effect produces no inactivation or removal of biofilm in the absence of added antimicrobial agent (1).
Acknowledgments
This work was supported by an award from the W. M. Keck Foundation.
We thank Haluk Beyenal for his review of the manuscript.
REFERENCES
- 1.Blenkinsopp, S. A., A. E. Khoury, and J. W. Costerton. 1992. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 58:3770-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim, T.-Y., T. Murakami, M. Tsuboi, K. Yamashita, and T. Matsunaga. 2003. Preparation of a colored conductive paint electrode for electrochemical inactivation of bacteria. Biotechnol. Bioeng. 81:299-304. [DOI] [PubMed] [Google Scholar]
- 3.Matsunaga, T., T. Nakayama, H. Wake, M. Takahashi, M. Okochi, and N. Nakamura. 1998. Prevention of marine biofouling using a conductive paint electrode. Biotechnol. Bioeng. 59:374-378. [DOI] [PubMed] [Google Scholar]
- 4.Nakasono, S., N. Nakamura, K. Sode, and T. Matsunaga. 1992. Electrochemical disinfection of marine bacteria attached on a plastic electrode. Bioelectrochem. Bioenerg. 27:191-198. [Google Scholar]
- 5.Okochi, M., T.-K. Lim, N. Nakamura, and T. Matsunaga. 1997. Electrochemical disinfection of drinking water using an activated-carbon-fiber reactor capable of monitoring its microbial fouling. Appl. Microbiol. Biotechnol. 47:18-22. [DOI] [PubMed] [Google Scholar]
- 6.Stewart, P. S., G. A. McFeters, and C.-T. Huang. 2000. Biofilm control by antimicrobial agents, p. 373-405. In J. D. Bryers (ed.), Biofilms II: process analysis and applications. Wiley-Liss, New York, N.Y.
- 7.Stewart, P. S., J. Rayner, F. Roe, and W. M. Rees. 2001. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 91:525-532. [DOI] [PubMed] [Google Scholar]
- 8.van der Borden, A. J., H. van der Werf, H. C. van der Mei, and H. J. Busscher. 2004. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl. Environ. Microbiol. 70:6871-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood, P., M. Jones, M. Bhakoo, and P. Gilbert. 1996. A novel strategy for control of microbial biofilms through generation of biocide at the biofilm-surface interface. Appl. Environ. Microbiol. 62:2598-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood, P., D. E. Caldwell, E. Evans, M. Jones, D. R. Korber, G. M. Wolfhaardt, M. Wilson, and P. Gilbert. 1998. Surface-catalyzed disinfection of thick Pseudomonas aeruginosa biofilms. J. Appl. Microbiol. 84:1092-1098. [DOI] [PubMed] [Google Scholar]
- 11.Xavier, J. B., C. Picioreanu, S. Abdul Rani, M. C. M. van Loosdrecht, and P. S. Stewart. 2005. Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix—a modelling study. Microbiology 151:3817-3832. [DOI] [PubMed] [Google Scholar]
- 12.Zheng, Z., and P. S. Stewart. 2004. Growth limitation of Staphylococcus epidermidis in biofilms contributes to rifampin tolerance. Biofilms 1:31-35. [Google Scholar]