Abstract
The use of freeze-dried kefir coculture as a starter in the production of feta-type cheese was investigated. Maturation of the produced cheese at 4°C was monitored for up to 70 days, and the effects of the starter culture, the salting method, and the ripening process on quality characteristics were studied. The use of kefir coculture as a starter led to increased lactic acid concentrations and decreased pH values in the final product associated with significantly higher conversion rates compared to salted rennet cheese. Determination of bacterial diversity at the end of the ripening process in salted kefir and rennet cheeses by denaturing gradient gel electrophoresis technology, based on both DNA and RNA analyses, suggested a potential species-specific inhibition of members of the genera Staphylococcus and Psychrobacter by kefir coculture. The main active microbial associations in salted kefir cheese appeared to be members of the genera Pseudomonas and Lactococcus, while in salted rennet cheese, Oxalobacteraceae, Janthinobacterium, Psychrobacter, and Pseudomonas species were noted. The effect of the starter culture on the production of aroma-related compounds responsible for cheese flavor was also studied by the solid-phase microextraction-gas chromatography-mass spectrometry technique. Kefir coculture also appeared to extend the shelf life of unsalted cheese. Spoilage of kefir cheese was observed on the 9th and 20th days of preservation at 10 and 5°C, respectively, while spoilage in the corresponding rennet cheese was detected on the 7th and 16th days. Microbial counts during preservation of both types of unsalted cheese increased steadily and reached similar levels, with the exception of staphylococci, which were significantly lower in unsalted kefir cheese. All types of cheese produced with kefir as a starter were approved and accepted by the panel during the preliminary sensory evaluation compared to commercial feta-type cheese.
Feta cheese, one of the most significant and popular dairy products in Greece, with its characteristic, slightly acid, salty taste, pleasant organoleptic properties, and worldwide acceptance, is a soft, white cheese usually ripened in brine. Traditionally, feta cheese was prepared by either thermized or raw ewe's milk on small family-owned premises with elementary equipment by using only rennet and without the addition of any starter cultures. Its characteristic flavor and texture were developed by the action of the natural lactic acid microflora of milk. Nowadays, most feta cheese is produced from ewe's milk or a mixture of ewe's milk and goat's milk in commercial cheese dairies with yogurt culture for lactic acid production, followed by addition of rennet for completion of precipitation. However, variations in the production of feta cheese such as the use of bovine milk or the involvement of treatments like filtration, salting, and preservation at 4°C are observed in the production of cheeses with local names. The produced cheeses are consumed after a 2-month ripening period. This maturation period is necessary for the sanitation of cheese products, especially those made from raw milk.
Microbial hazards have acquired substantial economical, ethical, and legal importance in the food industry, and a variety of food additives, along with strict preservation processes, are used to suppress spoilage and pathogenic microorganisms and to prolong the shelf life of foods. Various psychrotropic bacterial species, such as members of the genera Enterobacter, Pseudomonas, Alcaligenes, Acinetobacter, and Proteus (32), have been associated with cheese spoilage, while Escherichia coli, Listeria monocytogenes, Salmonella spp., and Staphylococcus aureus constitute the main pathogens occurring in cheeses (3, 12, 15, 56). Recently, there has been increasing pressure on food manufacturers either to completely remove chemical preservatives from their products or to adopt more “natural” alternatives for the maintenance or extension of foods' shelf life. Biopreservation with certain starter cultures in food manufacture is an attractive tool to reduce the use of food preservatives and to monitor microbial hazards (27, 29).
Consequently, an upsurge of interest in the use of suitable starter cultures in cheese production has occurred. Many researchers have proposed a variety of cultures suitable for use as starters, including bifidobacteria (9) and Lactococcus (34, 41, 46, 57), Lactobacillus (37, 41), Leuconostoc (41), and Enterococcus (41) species.
Kefir is a consortium of microbes that is mainly used in the production of the low-alcohol, traditional Russian drink kefir, where milk constitutes the initial fermenting substrate. This mixed culture consists of various yeasts (Kluyveromyces, Candida, Saccharomyces, and Pichia spp.); various lactic acid bacteria of the genera Lactobacillus, Lactococcus, and Leuconostoc; and acetic acid bacteria (20, 43, 70). Yeasts and lactic acid bacteria coexist in a symbiotic association and are responsible for an acid-alcoholic fermentation. Lactic acid bacteria that exist in kefir grains have attracted a lot of attention because of their ability to inhibit the development of spoilage and pathogenic microorganisms, either by the production of lactic acid or by the expression of antimicrobial agents (54). In addition, consumption of kefir has been linked with a variety of health benefits (11, 19, 42). This mixed culture is able to utilize lactose, and therefore whey, a waste of negligible cost rich in lactose, could be used as raw material for kefir production (30, 38). Kefir coculture has also been used as a starter in white pickled cheese production (24), and its use in baking has also been proposed (52).
All of the above studies were performed with wet kefir cultures. However, this technology is incompatible with commercial needs because of the physical status of the culture. In this study, the use of freeze-dried kefir cultures, a technology which accommodates its use by the commercial sector, was evaluated in the production of feta-type cheese. Data suggesting the improvement of the quality characteristics of the final product and the extension of its shelf life are presented.
MATERIALS AND METHODS
Production of freeze-dried kefir coculture.
Kefir coculture isolated from a commercial kefir drink was used in the present study. It was grown on a synthetic medium consisting of 4% lactose, 0.4% yeast extract, 0.1% (NH4)2SO4, 0.1% KH2PO4, and 0.5% MgSO4 · 7H2O at 30°C. The synthetic medium was sterilized at 130°C for 15 min prior use. Pressed wet-weight cells (∼0.5 to 1.0 g dry weight) were prepared and used directly in aerobic fermentations of whey for further production of kefir coculture.
A kefir coculture produced by aerobic fermentations of whey was resuspended in fermented whey, which was used as a cryoprotecting agent, and the whole was then frozen to −45°C. The frozen samples were freeze-dried overnight at 5 × 102 Pa and −45°C in a freeze-drying system (Freezone 4.5; Labconco).
Cheese making.
In this study, cheese was prepared with commercial pasteurized bovine milk. Cheese containing a freeze-dried kefir coculture, designated kefir cheese, was produced with milk heated at 37°C which, after the addition of freeze-dried kefir coculture along with commercial rennet (0.01%), was left undisturbed for 2 h for curd formation. Subsequently, the curd was cut into squares (∼1 cm), left undisturbed for 10 min, and then cloth filtered overnight at room temperature (18 to 22°C) for complete whey removal. Addition of freeze-dried kefir coculture 30 min before or 30 min after rennet addition was evaluated along with the effects of the initial freeze-dried kefir coculture concentration and the salting method. Three methods of salting were tested. (i) The surface of cheese samples (100 g each) was rubbed with 7 g of salt, (ii) cheese samples were immersed in a 10% (wt/vol) brine solution, and (iii) the surface of cheese samples (100 g) was rubbed with 7 g of salt and after 12 h the cheese samples were immersed in a 7% (wt/vol) brine solution. Cheese without kefir coculture, designated rennet cheese, was also produced for comparison. Ripening of the produced cheeses was monitored at 4 to 6°C for 70 days. All treatments were carried out in triplicate, and mean values are presented (the standard deviation for all values was about ±5% in most cases). Duplicate samples from each treatment were collected at various intervals and analyzed for lactic acid, ethanol, residual sugar, and volatile by-products.
In addition, unsalted rennet cheese and unsalted kefir cheese made with 1 g freeze-dried kefir culture/liter of milk were produced. Addition of freeze-dried kefir was carried out prior to rennet addition. The effect of freeze-dried kefir starter coculture on preservation time was evaluated macroscopically and by sensory tests at 25, 15, and 5°C.
Chemical analysis.
Cheese samples, 20 g each, were macerated with warm water (40°C) to produce a total volume of 210 ml. Each sample was then filtered, and the filtrate was used for lactic acid, ethanol, and residual-sugar determination (36).
Lactic acid was determined by titration with 0.1 N NaOH with phenolphthalein as an indicator (36). Residual sugar (lactose, glucose, and galactose) was determined by high-performance liquid chromatography with a Shimadzu chromatograph with an SCR-101N stainless steel column, an LC-9A pump, a CTO-10A oven at 60°C, and a RID-6A refractive-index detector. Triple-distilled water was used as the mobile phase at a flow rate of 0.8 ml/min, and 1-butanol was used as an internal standard (IS). A volume of 0.5 ml of cheese filtrate and 2.5 ml of a 1% (vol/vol) solution of 1-butanol were diluted to 50 ml, so that the actual concentration of 1-butanol was 0.05% (vol/vol). Forty microliters of the final solution was then injected directly into the column. Residual-sugar concentrations were calculated by using standard curves prepared with at least seven standard solutions by correlating the ratio of residual-sugar peak areas divided by 1-butanol peak areas to residual-sugar concentrations.
Ethanol was determined by gas chromatography (GC) with a Porapac S column. Nitrogen was used as the carrier gas at 40 ml/min. The column temperature was settled at 120 to 170°C at a rate of 10°C/min. The temperatures of the injector and flame ionization detector were 210 and 220°C, respectively. For ethanol determination, a total volume of 2 μl of each sample was injected directly into the column and the concentration of ethanol was determined with standard curves. 1-Butanol was used as an IS at a concentration of 0.5% (vol/vol).
Solid-phase microextraction (SPME)-GC-mass spectrometry (MS) analysis.
The volatile by-product composition of salted cheese samples ripened for 30 days at 4 to 6°C was studied by SPME-GC-MS analysis. Grated cheese samples (∼7 g each) were placed into a 20-ml headspace vial fitted with a Teflon-lined septum sealed with an aluminum crimp seal through which an SPME syringe needle (bearing a 2-cm fiber coated with 50/30-mm divinylbenzene-carboxen on polydimethylsiloxane bonded to a flexible fused silica core; Supelco, Bellefonte, PA) was introduced. The container was then kept at 80°C with a thermostat for 30 to 35 min (7). The absorbed volatile analytes were then analyzed by GC-MS (Shimadzu GC-17A, MS QP5050, capillary column Supelco CO Wax-10 60m, 0.32-mm inside diameter, 0.25-μm film thickness). Helium was used as the carrier gas (linear velocity of 1.5 ml/min). The oven temperature was set at 35°C for 3 min, followed by a temperature gradient of 5°C/min to 110°C and then 10°C/min to 240°C. A final extension was applied at 240°C for 10 min. The injector was operated in splitless mode. The injector and detector temperatures were 280°C and 250°C, respectively. The mass spectrometer was operated in the electron impact mode with the electron energy set at 70 eV. Identification was done by comparison with standard compounds and data obtained from NIST107, NIST21, and SZTERP libraries. For semiquantification of volatile compounds, methyl octanoate (Sigma-Aldrich, Poole, United Kingdom) diluted in pure ethanol was used as an IS at various concentrations (1.25, 12.5, 125, and 1,250 μg/kg of cheese) (63). The volatile compounds were quantified by dividing the peak areas of the compounds of interest by the peak area of the IS and multiplying this ratio by the initial concentration of the IS (expressed in micrograms per kilogram). The peak areas were measured from the full-scan chromatograph by using the total ion current. Each determination was carried out in triplicate, and the mean data are presented (the standard deviation for all values was about ±10% in most cases).
Microbiological analysis.
Representative 10-g portions of duplicate samples taken from the cheese interior were blended with 90 ml of sterilized Ringer solution (1/4 strength) and subjected to serial dilutions.
The following microbiological analyses were performed: (i) determination of total aerobic counts on plate count agar (Fluka 70188) at 30°C for 48 h, (ii) enumeration of coliforms on violet red bile agar (Fluka 70188) after incubation at 30°C for 24 h, (iii) enumeration of enterobacteria after incubation on violet red bile glucose agar (Fluka 70189) at 37°C for 24 h, (iv) enumeration of yeasts and molds after incubation on malt agar (Fluka 70145) (pH adjusted to 4.5 by sterile solution of 10% lactic acid) at 30°C for 48 h, (v) enumeration of staphylococci after incubation on Baird Parker egg yolk tellurite medium (Fluka 11705) at 37°C for 48 h and confirmation by a positive coagulase test, (vi) enumeration of lactococci (gram positive, catalase negative) after incubation on M-17 agar (Fluka 63016) at 37°C for 48 h, (vii) enumeration of lactobacilli (gram positive, catalase negative) after incubation on acidified MRS agar (Fluka 69964) at 37°C for 48 h anaerobically (Anerocult C anaerobic jar; Merck), and (viii) enumeration of Salmonella sp. bacteria after incubation on brilliant green agar (Fluka 70134) at 37°C for 48 h. All incubations were further extended for up to 120 h, but no extra colonies were observed. Gram staining and catalase tests were performed for confirmation of lactic acid bacteria. Results are presented as the log of the mean number of CFU on solid-medium culture plates containing between 30 and 300 colonies per g of cheese.
Molecular techniques.
DNA and RNA extractions from cheese samples (0.5 g each), PCR and reverse transcription (RT)-PCR amplification of the bacterial 16S rRNA V3 region, and denaturing gradient gel electrophoresis (DGGE) analysis were conducted as described previously by Griffiths et al. (25). DGGE bands were subsequently reamplified and cloned into E. coli cells with a TOPO TA cloning kit for sequencing (Invitrogen, Paisley, United Kingdom). Three recombinant colonies for each band were picked for sequencing analysis. Both strands of DGGE bands cloned into E. coli cells were sequenced with a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) and analyzed on an ABI PRISM 3700 DNA analyzer (Applied Biosystems). Phylogenetic affiliation was determined by comparison of nucleotide sequences obtained in this study with sequences in the GenBank database (8) by using the BLASTn facility (1) of the National Center for Biotechnology Information.
Preliminary sensory evaluation.
Kefir cheese samples were tested for their sensory characteristics and compared to commercial feta-type cheese produced with bovine milk. Samples of approximately 25 g of cheese ripened for 30 days were presented. Sensory evaluation was conducted by 14 laboratory members (7 previously trained and 7 untrained) using locally approved protocols. The panel was asked to score cheeses on a 0-to-10 scale (0, unacceptable; 10, exceptional) for attributes grouped into three categories, aroma, taste, and flavor. Panelists used water to wash their palates between samples and were unaware of the identities of the samples they tasted (62). Significance was established at P < 0.05. Results were analyzed for statistical significance by analysis of variance (ANOVA). Duncan's multiple-range test was used to determine significant differences among results (coefficients, ANOVA tables, and significance [P < 0.05] were computed with Statistica v.5.0).
Cheese spoilage was determined macroscopically and by sensory tests. A scoring scale with three categories was used. Class 1 corresponded to high-quality cheese without any off odor or off flavor, class 2 corresponded to cheese that had slight off odors or off flavors but was still acceptable, and class 3 corresponded to cheese of unacceptable quality. The shelf life limit was defined as the point at which 50% of the panelists rejected the cheese samples.
Experimental design and statistical analysis.
In the experiments conducted, the effects of the method of addition of the freeze-dried kefir starter coculture, the initial concentration of the starter culture, the salting method used, and the ripening process were studied. Furthermore, the effect of the freeze-dried kefir starter coculture on physicochemical parameters and the effect of the preservation time during ripening of unsalted cheeses at various temperatures were studied and a microbiological analysis was also done. The experiments were designed and analyzed statistically by ANOVA. Duncan's multiple-range test was used to determine significant differences among results (coefficients, ANOVA tables, and significance [P < 0.05] were computed with Statistica v.5.0).
RESULTS
Physicochemical characteristics of feta-type cheese produced by freeze-dried kefir coculture.
The physicochemical parameters of the salted kefir cheeses and the salted rennet cheese are summarized in Table 1.
TABLE 1.
Physicochemical characteristics and preliminary sensory evaluations of feta-type cheese produced by freeze-dried kefir coculture during ripening at 4 to 6°C
| Analysis and maturation period (days) | Surface-salted rennet cheese (7 g salt/100 g cheese) | Surface-salted cheese (7 g salt/100 g cheese) with following amt of freeze-dried kefir/liter milk added:
|
Cheese ripened in 10% brine with 1.0 g freeze-dried kefir/liter milk addeda | Surface-salted cheese (7 g salt/100 g cheese) in 7% brine with 1.0 g freeze-dried kefir/liter milk addeda | Commercial feta-type cheese | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0 ga | 1.0 gb | 0.5 ga | 2.0 ga | 5.0 ga | |||||
| Lactose (g/100 g cheese) | |||||||||
| 0 | 3.18 ± 0.2 | Trc | 1.13 ± 0.05 | 1.25 ± 0.05 | Tr | Tr | Tr | Tr | |
| 1 | 3.09 ± 0.2 | Tr | 0.98 ± 0.05 | 0.46 ± 0.05 | Tr | Tr | Tr | Tr | |
| 4 | 3.05 ± 0.2 | Tr | 0.68 ± 0.05 | 0.38 ± 0.02 | Tr | Tr | Tr | Tr | |
| 15 | 2.89 ± 0.2 | Tr | 0.56 ± 0.05 | 0.32 ± 0.02 | Tr | Tr | Tr | Tr | |
| 30 | 2.44 ± 0.2 | Tr | 0.24 ± 0.02 | Tr | Tr | Tr | Tr | Tr | |
| 70 | 0.94 ± 0.05 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | |
| Glucose (g/100 g cheese) | |||||||||
| 0 | 0.13 ± 0.01 | 0.57 ± 0.04 | 1.16 ± 0.05 | 0.58 ± 0.05 | 0.38 ± 0.2 | 0.50 ± 0.02 | 0.95 ± 0.05 | 0.75 ± 0.05 | |
| 1 | Tr | Tr | 1.10 ± 0.05 | Tr | Tr | Tr | Tr | Tr | |
| 4 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | |
| 15 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | |
| 30 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | |
| 70 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | |
| Galactose (g/100 g cheese) | |||||||||
| 0 | 0.16 ± 0.01 | 1.25 ± 0.05 | 1.11 ± 0.05 | 1.00 ± 0.05 | 1.23 ± 0.1 | 1.55 ± 0.1 | 1.34 ± 0.1 | 1.41 ± 0.1 | |
| 1 | Tr | 0.79 ± 0.05 | 1.00 ± 0.05 | 0.78 ± 0.05 | 0.70 ± 0.05 | 0.87 ± 0.05 | 0.77 ± 0.05 | 0.99 ± 0.05 | |
| 4 | Tr | 0.67 ± 0.05 | 0.87 ± 0.05 | 0.72 ± 0.05 | 0.52 ± 0.02 | 0.42 ± 0.02 | 0.21 ± 0.01 | 0.65 ± 0.05 | |
| 15 | Tr | 0.58 ± 0.02 | 0.77 ± 0.05 | 0.65 ± 0.05 | 0.38 ± 0.02 | Tr | 0.19 ± 0.01 | 0.22 ± 0.01 | |
| 30 | Tr | 0.29 ± 0.02 | 0.49 ± 0.02 | 0.40 ± 0.05 | Tr | Tr | Tr | Tr | |
| 70 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | |
| Lactic acid (g/100 g cheese) | |||||||||
| 0 | 0.07 ± 0.02 | 0.54 ± 0.05 | 0.32 ± 0.02 | 0.18 ± 0.02 | 0.34 ± 0.04 | 0.43 ± 0.03 | 0.36 ± 0.02 | 0.50 ± 0.02 | |
| 1 | 0.07 ± 0.02 | 0.83 ± 0.04 | 0.86 ± 0.05 | 0.54 ± 0.04 | 0.72 ± 0.05 | 1.00 ± 0.05 | 0.43 ± 0.03 | 0.79 ± 0.05 | |
| 4 | 0.11 ± 0.02 | 0.86 ± 0.05 | 0.94 ± 0.05 | 0.72 ± 0.05 | 0.90 ± 0.05 | 1.04 ± 0.05 | 0.61 ± 0.03 | 0.83 ± 0.05 | |
| 15 | 0.14 ± 0.02 | 0.90 ± 0.05 | 0.97 ± 0.05 | 0.83 ± 0.05 | 1.04 ± 0.05 | 1.15 ± 0.05 | 0.43 ± 0.03 | 0.90 ± 0.05 | |
| 30 | 0.18 ± 0.02 | 1.04 ± 0.05 | 0.90 ± 0.05 | 0.97 ± 0.05 | 1.03 ± 0.05 | 1.37 ± 0.05 | 0.43 ± 0.03 | 0.94 ± 0.05 | |
| 70 | 0.36 ± 0.02 | 0.97 ± 0.05 | 0.86 ± 0.05 | 0.90 ± 0.05 | 0.94 ± 0.05 | 1.12 ± 0.05 | 0.36 ± 0.02 | 0.97 ± 0.05 | |
| pH | |||||||||
| 0 | 6.71 ± 0.1 | 5.24 ± 0.1 | 5.55 ± 0.1 | 5.81 ± 0.1 | 5.61 ± 0.1 | 5.13 ± 0.1 | 5.53 ± 0.1 | 5.39 ± 0.1 | |
| 1 | 6.65 ± 0.1 | 4.97 ± 0.1 | 5.12 ± 0.1 | 5.08 ± 0.1 | 5.13 ± 0.1 | 4.86 ± 0.1 | 5.05 ± 0.1 | 5.05 ± 0.1 | |
| 4 | 6.50 ± 0.1 | 4.90 ± 0.1 | 4.87 ± 0.1 | 5.01 ± 0.1 | 5.05 ± 0.1 | 4.76 ± 0.1 | 5.24 ± 0.1 | 4.97 ± 0.1 | |
| 15 | 6.50 ± 0.1 | 4.87 ± 0.1 | 4.90 ± 0.1 | 4.98 ± 0.1 | 4.73 ± 0.1 | 4.70 ± 0.1 | 5.31 ± 0.1 | 4.87 ± 0.1 | |
| 30 | 6.34 ± 0.1 | 4.82 ± 0.1 | 5.08 ± 0.1 | 4.86 ± 0.1 | 4.89 ± 0.1 | 4.66 ± 0.1 | 5.30 ± 0.1 | 4.82 ± 0.1 | |
| 70 | 5.76 ± 0.1 | 5.11 ± 0.1 | 5.35 ± 0.1 | 5.23 ± 0.1 | 5.61 ± 0.1 | 5.63 ± 0.1 | 5.33 ± 0.1 | 4.80 ± 0.1 | |
| Ethanol (g/100 g cheese) | |||||||||
| 0 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.001 | |
| 1 | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.04 ± 0.001 | 0.10 ± 0.005 | 0.32 ± 0.02 | 0.04 ± 0.001 | 0.03 ± 0.001 | |
| 4 | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.06 ± 0.002 | 0.42 ± 0.02 | 0.50 ± 0.02 | 0.07 ± 0.005 | 0.04 ± 0.001 | |
| 15 | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.001 | 0.10 ± 0.005 | 0.10 ± 0.005 | 0.04 ± 0.001 | 0.03 ± 0.001 | |
| 30 | 0.01 ± 0.001 | 0.02 ± 0.001 | Tr | 0.03 ± 0.001 | 0.07 ± 0.005 | 0.07 ± 0.005 | 0.04 ± 0.001 | 0.03 ± 0.001 | |
| 70 | 0.06 ± 0.001 | 0.04 ± 0.002 | 0.06 ± 0.002 | 0.04 ± 0.002 | 0.06 ± 0.005 | 0.06 ± 0.005 | 0.05 ± 0.002 | 0.02 ± 0.001 | |
| Sensory evaluation | |||||||||
| 30 | 6.93 ± 1.49 | 7.86 ± 1.29 | 6.79 ± 1.76 | 7.86 ± 1.10 | 7.43 ± 1.45 | 6.90 ± 0.96 | 5.79 ± 1.53 | 7.82 ± 1.75 | 7.64 ± 1.65 |
Freeze-dried kefir coculture was added 30 min prior to rennet addition.
Freeze-dried kefir coculture was added 30 min after rennet addition.
Tr, compound present at <0.001% (trace).
Addition of a freeze-dried kefir starter coculture, the initial concentration of the freeze-dried kefir coculture, the salting method used, and the ripening process affected all of the parameters studied (P < 0.01). Strong interactions between the method of freeze-dried kefir coculture addition and the ripening process (P < 0.05 for pH and P < 0.01 for the rest of the parameters) and between the initial freeze-dried kefir coculture and the ripening process (P < 0.01) affecting all parameters were observed. Likewise, a strong interaction between the salting method and the ripening process (P < 0.01) affecting all parameters except pH was also observed.
Addition of the freeze-dried kefir starter coculture prior to rennet addition resulted in higher lactic acid concentrations, lower residual-sugar levels, and a lower pH on day 0 of ripening compared to addition of the freeze-dried kefir starter coculture after rennet addition. At the beginning of the ripening process, lactic acid content, pH, and residual sugar in kefir cheese produced by addition of the starter culture prior to rennet addition were 0.54%, 5.24, and 1.82%, respectively, while the corresponding values in kefir cheese produced by addition of the starter culture after rennet addition were 0.32%, 5.55, and 3.4%, respectively. However, in kefir cheese produced by addition of the starter culture after rennet addition, lactose was further metabolized and almost equal amounts of lactic acid (about 1%) were produced at the end of the ripening process (Table 1), although the final pH of the latter was 5.35, which was statistically significantly higher compared to the final pH (5.11) of the former. Kefir cheese produced with amounts of starter culture greater than 2 g/liter of milk generally contained higher concentrations of ethanol. In those cheese samples, the maximum ethanol content (up to 0.5%) was observed on the 4th day of ripening and gradually decreased to significantly lower values (0.06%) during the ripening process. In general, ethanol content ranged in low levels in all cases. Rennet cheese contained greater amounts of lactose and had a statistically significantly lower lactic acid content at the end of the ripening process compared to surface-salted kefir cheeses, up to 0.94% and 0.36%, respectively. As a consequence, the final pH (5.76) was significantly higher. Ripening in brine with no surface presalting resulted in a statistically significantly lower lactic acid concentration (0.36%) and a higher pH (5.33) at the end of the process compared to the other two salting methods tested (Table 1). This could be attributed to the facts that the cheese which was not surface salted lacked the necessary hardness and was easily grated and therefore extraction of the produced lactic acid by the brine solution might have occurred. In contrast, the lowest pH value (4.80) was reported in surface-presalted kefir cheese ripened in brine.
Determination of biodiversity in salted kefir and rennet cheeses.
Surface-salted kefir cheese produced by the freeze-dried kefir coculture at an initial concentration of 1 g/liter of milk was subjected to analysis by DGGE after a 70-day ripening period in order to determine differences in bacterial flora compared to rennet cheese. Assays were based on both DNA and RNA in order to discriminate the main active species. DGGE analysis of PCR and RT-PCR products targeting the bacterial 16S rRNA V3 region was performed with DNA and RNA templates, respectively, derived from the same cheese sample. Assays were conducted in duplicate, and identical profiles were obtained for the same cheese type (Fig. 1, lanes 1 and 2, 3 and 4, 5 and 6, and 7 and 8). Sequence determination of the separated bands revealed considerable shifts in the final bacterial diversity of the two cheese products (Table 2). Members of the genera Staphylococcus and Psychrobacter, as well as forest soil bacterial strain 2S2.B6 (accession no. AY043581), were not detected in cheese fermented with kefir as a coculture. Staphylococcus spp. were not detected as active members in rennet cheese, as revealed by the RNA-based analysis, but more likely constituted a main microbial culture during the production process. On the other hand, members of the genus Lactococcus were detected as an active population only in kefir cheese. Pseudomonas, Lactobacillus, and Janthinobacterium species existed as active members in both types of cheese. The main active microbial population in kefir cheese appeared to be members of the genera Pseudomonas and Lactococcus, while in rennet cheese this activity was more likely equally allocated among Oxalobacteraceae, Janthinobacterium, Psychrobacter, and Pseudomonas species. Of note, differences in detecting bacterial associations based on RNA and DNA suggest the preeminence of the RNA-based approach, which appeared to be less selective and possibly more sensitive.
FIG. 1.
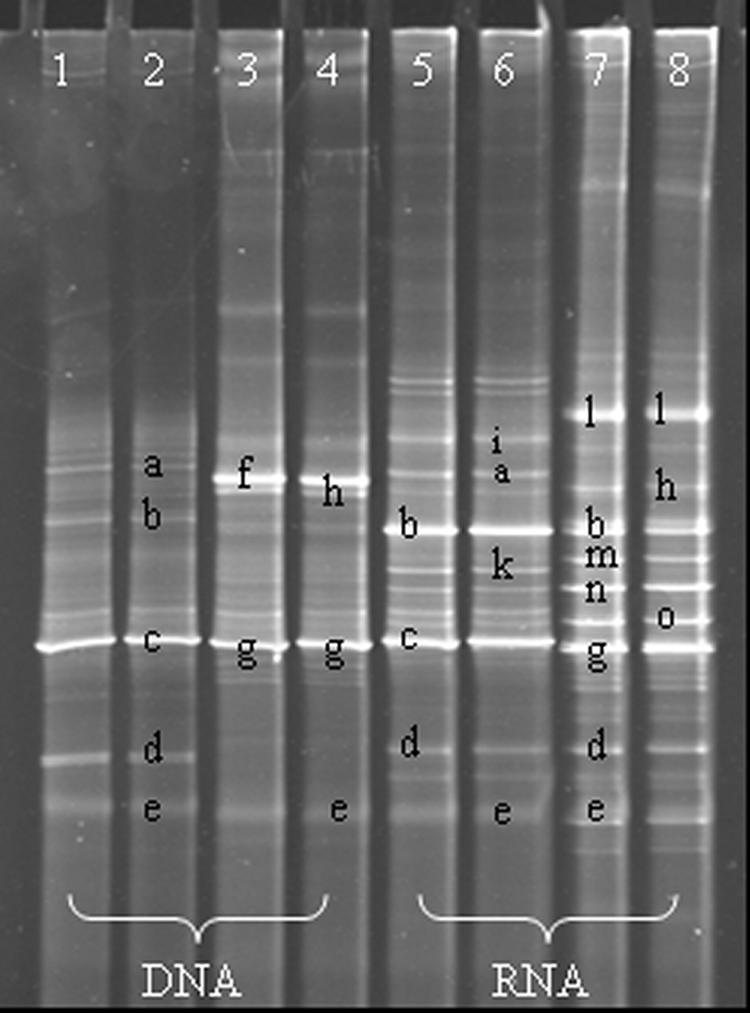
DGGE fingerprint representing PCR- and RT-PCR-amplified 16S rRNA V3 fragments from total-community DNAs and RNAs derived from kefir and rennet cheeses. For each type of cheese and each type of analysis, two replicate profiles from two independent nucleic acid extracts are displayed. Lanes: 1 and 2, DNA-based analysis of kefir cheese; 3 and 4, DNA-based analysis of rennet cheese; 5 and 6, RNA-based analysis of kefir cheese; 7 and 8, RNA-based analysis of rennet cheese. All bands marked by letters were subjected to sequence determination. The same designation is used only for bands that showed more than 99% identity.
TABLE 2.
Phylogenetic affiliations of bacterial associations in kefir and rennet cheeses based on DNA and RNA analyses and the corresponding band(s) in the DGGE profile
| Closest relative(s) | Kefir cheese
|
Rennet cheese
|
DGGE band(s) | ||
|---|---|---|---|---|---|
| DNA | RNA | DNA | RNA | ||
| Members of Pseudomonas genus | +a | + | + | a, b, k | |
| Lactococcus lactis | + | + | c | ||
| Members of Janthinobacterium genus | + | + | + | d, n | |
| Members of Lactobacillus genus | + | + | + | + | e |
| Members of Staphylococcus genus | + | f | |||
| Members of Psychrobacter genus | + | + | g, h, m | ||
| Members of Lactococcus genus | + | i | |||
| Members of Oxalobacteraceae family | + | l | |||
| Forest soil bacterial strain 2S2.B6b | + | o | |||
Aroma-related compounds.
For evaluation of aromatic profiles, kefir cheese samples were analyzed by an SPME-GC-MS technique and compared to rennet cheese and commercial feta-type cheese produced with bovine milk. Only salted cheeses ripened for at least 30 days, a period necessary for aroma development, were analyzed. Kefir cheese produced with 1 g of the freeze-dried kefir coculture/liter of milk was used because of the high-quality characteristics (low pH, high lactic acid content, and improved cheese texture) of products produced by initial cultures at concentrations in the range of 0.5 to 1.0 g/liter of milk. Semiquantitative results of the volatile-compound analysis are presented in Table 3. More compounds were detected in kefir cheese samples compared to commercial feta-type cheese and rennet cheese. A total of 68 compounds were detected, of which 32 were found in surface-salted kefir cheese and in commercial feta-type cheese, 33 were found in surface-salted kefir cheese ripened in brine, and 10 were found in rennet cheese. The most important compounds identified were esters, organic acids, alcohols, and carbonyl compounds.
TABLE 3.
Aroma-related compounds isolated in kefir, rennet, and commercial feta-type cheeses after ripening for 30 days at 4 to 6°C by the SPME-GC/MS technique
| Compound | Retention index (Kovats) | Concn (μg/kg cheese) in:
|
|||
|---|---|---|---|---|---|
| Rennet cheese | Surface-salted cheese containing 1 g freeze-dried kefir/liter milk | Surface-salted cheese containing 1 g freeze-dried kefir/liter milk, ripened in 7% brine | Commercial feta-type cheese | ||
| Esters | |||||
| Ethyl acetate | 866 | NDe | ND | 27a | 8a |
| 2-Propenyl acetate | 876 | Trb,c | ND | ND | ND |
| Ethyl butanoate | 977 | ND | ND | Tra | ND |
| Propyl butanoate | 1,067 | ND | 4a | ND | ND |
| Ethenyl formate | 1,090 | ND | ND | ND | Trb |
| Ethyl octanoate | 1,371 | ND | Tra | Tra | ND |
| Isopentyl hexanoate | 1,437 | ND | Tra | ND | ND |
| Ethyl decanoate | 1,563 | ND | 4a | 6a | Tra |
| 3-Methylbutyl octanoate | 1,597 | ND | Tra | ND | ND |
| 2-Phenylethyl acetate | 1,725 | ND | Tra | 4a | 3a |
| Ethyl dodecanoate | 1,777 | ND | ND | ND | 2b |
| Ethyl tridecanoate | 1,787 | ND | ND | 2b | ND |
| Ethyl tetradecanoate | 1,978 | ND | 2b | Trb | 2b |
| n-Butyl tetradecanoate | 2,089 | ND | Trb | ND | ND |
| Organic acids | |||||
| 3-Methylbutanoic acid | 1,639 | ND | Trb | ND | ND |
| Propanedioic acid | 1,774 | ND | ND | ND | Trb |
| Hexanoic acid | 1,937 | ND | ND | 6a | 2a |
| Heptanoic acid | 2,014 | ND | ND | Trb | ND |
| Octanoic acid | 2,036 | Tra | 3a | 11a | Tra |
| Nonanoic acid | 2,138 | ND | ND | 2a | 2a |
| n-Decanoic acid | 2,221 | ND | ND | 5a | 10a |
| Dodecanoic acid | >2,400 | ND | 14a | 8a | 31a |
| Tetradecanoic acid | >2,400 | ND | ND | 2b | 6b |
| Alcohols | |||||
| Methanol | <800 | ND | ND | Tra | ND |
| Ethanol | 905 | >10,000a | >10,000a | >10,000a | >10,000a |
| 2-Methyl-1-propanol | 1,036 | ND | 9a | Tra | ND |
| 1-Butanol | 1,078 | ND | ND | ND | Tra |
| 3-Methyl-1-butanol | 1,128 | Tra | 30a | 8a | Tra |
| 2-Heptanol | 1,227 | Tra | ND | ND | ND |
| 1,3-Butanediol | 1,511 | ND | 2a | ND | ND |
| 4-Butoxy-1-butanol | 1,592 | ND | Trb | ND | ND |
| 3-(Methylthio)-1-propanol | 1,608 | ND | Trb | ND | ND |
| Benzyl alcohol | 1,807 | ND | Trb | Trb | ND |
| Phenyl ethanol | 1,811 | 10a | 113a | 18a | Tra |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 1,916 | ND | ND | ND | Trb |
| 4-Methylphenol | 2,003 | ND | ND | Tra | ND |
| 2-Ethylphenol | 2,012 | ND | ND | Tra | ND |
| Carbonyl compounds | |||||
| Acetaldehyde | <800 | ND | ND | 7a | 15a |
| Acetone | 847 | Tra | ND | ND | ND |
| 2,3-Butanedione | 935 | ND | 3a | ND | ND |
| 2-Pentanone | 942 | ND | ND | ND | 3b |
| 2-Methylpentanal | 974 | ND | 3b | ND | ND |
| 2-Heptanone | 1,113 | Tra | ND | Tra | Tra |
| 2-Nonanone | 1,318 | Tra | ND | ND | ND |
| Furfural | 1,410 | ND | ND | Trb | ND |
| Benzaldehyde | 1,458 | ND | Tra | Tra | ND |
| 2-Undecanone | 1,519 | ND | 3b | Trb | Trb |
| Phenylacetaldehyde | 1,524 | ND | Trb | ND | ND |
| Dodecanal | 1,616 | ND | Tra | ND | ND |
| 3-Methyl-[5H]-furanone | 1,626 | ND | ND | Trb | Trb |
| Butyraldehyde | 1,731 | ND | Trb | ND | ND |
| 2-Tridecanone | 1,742 | ND | ND | ND | Trb |
| δ-Octalactone | 1,921 | ND | Trb | ND | ND |
| 2-Pentadecanone | 1,953 | ND | ND | 3b | ND |
| Hexadecanal | 2,033 | ND | ND | ND | Trb |
| δ-Decalactone | 2,135 | Trb | 2b | 12b | ND |
| δ-Dodecalactone | 2,363 | ND | 3b | 14b | 8b |
| Miscellaneous compounds | |||||
| α-Farnesene | 1,632 | ND | ND | Trb | ND |
| 3,7,11,15-Tetramethyl-2-hexadecene | 1,926 | ND | ND | ND | Trb |
| Unidentified compounds | |||||
| Unknown | 945 | ND | ND | ND | 2d |
| Unknown | 1,367 | ND | Trd | ND | ND |
| Unknown | 1,661 | ND | ND | ND | Trd |
| Unknown | 1,804 | ND | ND | ND | Tra |
| Unknown | 2,003 | ND | Trd | ND | ND |
| Unknown | >2,400 | ND | ND | ND | 4d |
| Unknown | >2,400 | ND | 6d | 2d | 2d |
Positive identification by mass spectrometry and retention times that agree with those of authentic compounds.
Positive identification by mass spectrometry only.
Tr, compound present at <1 μg/kg cheese (trace).
Detected.
ND, not detected.
Preliminary sensory evaluation.
Feta is a very popular cheese in Greece, and therefore the produced cheeses were compared with commercial feta-type cheese as regards their sensory characteristics. The method of cheese production significantly (P < 0.01) affected the preferences of the tasters. Surface-salted kefir cheeses produced by initial cultures at concentrations of 0.5 and 1.0 g/liter of milk added prior to rennet addition and surface-salted kefir cheese ripened in brine scored the highest values (Table 1) and a cheesy, matured-cheese-like character was predominant compared to rennet cheese. Kefir cheese ripened in brine without previous surface salting scored lower values because of its bad texture, as it lacked cohesion. Amounts of initial freeze-dried kefir coculture greater than 2.0 g/liter of milk resulted in deterioration of cheese texture, and a characteristic alcohol-like flavor dominated. In general, kefir cheeses were approved and accepted by the panel, as they scored values similar to those of commercial feta-type cheese.
Microbiological analysis of unsalted feta-type cheese produced by freeze-dried kefir coculture.
The association of the microbial groups examined during the preservation of unsalted kefir cheese and unsalted rennet cheese preserved at various temperatures is presented in Table 4.
TABLE 4.
Microbiological analysis of unsalted feta-type cheese produced by freeze-dried kefir starter coculture in comparison with unsalted feta-type cheese without starter culture during ripening at various temperatures
| Preservation temp (°C) and time (days) | Total aerobic count (log CFU/g)
|
Coliforms (log CFU/g)
|
Enterobacteria (log CFU/g)
|
Yeasts (log CFU/g)
|
Staphylococci (log CFU/g)
|
Lactococci (log CFU/g)
|
Lactobacilli (log CFU/g)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | |
| 25 | ||||||||||||||
| 0 | 6.71 ± 0.3 | 8.80 ± 0.8 | 5.36 ± 0.2 | 5.90 ± 0.1 | 5.98 ± 0.2 | 6.28 ± 0.2 | 5.91 ± 0.2 | 7.60 ± 0.3 | 3.89 ± 0.1 | 3.65 ± 0.1 | 5.40 ± 0.1 | 8.51 ± 0.5 | 5.52 ± 0.1 | 8.52 ± 0.5 |
| 1 | 7.36 ± 0.3 | 9.23 ± 1.0 | 6.43 ± 0.4 | 6.54 ± 0.5 | 6.48 ± 0.2 | 6.72 ± 0.7 | 6.60 ± 0.2 | 7.93 ± 0.4 | 4.78 ± 0.1 | 4.88 ± 0.1 | 5.85 ± 0.2 | 8.64 ± 0.6 | 5.78 ± 0.2 | 8.79 ± 0.7 |
| 3 | 9.36 ± 0.7 | 9.61 ± 0.6 | 7.96 ± 0.4 | 7.39 ± 0.5 | 8.02 ± 0.3 | 7.63 ± 0.6 | 8.04 ± 0.3 | 8.08 ± 0.4 | 6.67 ± 0.3 | 5.45 ± 0.2 | 8.85 ± 0.8 | 8.97 ± 0.9 | 8.51 ± 0.5 | 8.91 ± 0.9 |
| 5 | 9.98 ± 0.7 | 9.93 ± 0.9 | 8.61 ± 0.6 | 8.60 ± 0.7 | 9.04 ± 0.6 | 8.98 ± 0.9 | 8.28 ± 0.5 | 8.51 ± 0.5 | 7.29 ± 0.3 | 6.01 ± 0.5 | 9.38 ± 0.5 | 9.38 ± 0.3 | 9.06 ± 0.5 | 9.00 ± 0.5 |
| 7 | 10.80 ± 0.2 | 10.30 ± 0.3 | 9.51 ± 0.8 | 9.15 ± 1.0 | 9.58 ± 0.5 | 9.90 ± 0.9 | 8.89 ± 0.8 | 8.60 ± 0.6 | 8.38 ± 0.5 | 6.72 ± 0.3 | 9.93 ± 0.9 | 9.62 ± 0.6 | 9.13 ± 1.0 | 9.45 ± 0.6 |
| 10 | ||||||||||||||
| 0 | 6.71 ± 0.3 | 8.80 ± 0.8 | 5.36 ± 0.2 | 5.90 ± 0.1 | 5.98 ± 0.2 | 6.28 ± 0.2 | 5.91 ± 0.2 | 7.60 ± 0.3 | 3.89 ± 0.1 | 3.65 ± 0.1 | 5.40 ± 0.1 | 8.51 ± 0.5 | 5.52 ± 0.1 | 8.52 ± 0.5 |
| 1 | 7.36 ± 0.3 | 9.23 ± 1.0 | 6.43 ± 0.4 | 6.54 ± 0.5 | 6.48 ± 0.2 | 6.72 ± 0.7 | 6.60 ± 0.2 | 7.93 ± 0.4 | 4.78 ± 0.1 | 4.88 ± 0.1 | 5.85 ± 0.2 | 9.64 ± 0.6 | 5.78 ± 0.2 | 9.79 ± 0.7 |
| 3 | 9.16 ± 1.0 | 9.52 ± 0.7 | 7.79 ± 0.4 | 6.97 ± 0.2 | 7.96 ± 0.5 | 7.42 ± 0.2 | 7.53 ± 0.5 | 8.60 ± 0.2 | 5.55 ± 0.2 | 5.31 ± 0.1 | 7.85 ± 0.3 | 9.78 ± 0.7 | 8.63 ± 0.6 | 9.89 ± 0.6 |
| 5 | 9.45 ± 0.7 | 9.71 ± 0.7 | 8.67 ± 0.6 | 7.67 ± 0.3 | 8.82 ± 0.6 | 8.74 ± 0.4 | 8.21 ± 0.6 | 8.60 ± 0.2 | 6.00 ± 0.3 | 5.98 ± 0.2 | 9.49 ± 0.4 | 9.81 ± 0.8 | 9.09 ± 1.0 | 9.92 ± 0.4 |
| 7 | 9.98 ± 0.9 | 10.00 ± 0.5 | 8.82 ± 0.8 | 8.49 ± 0.4 | 8.92 ± 0.5 | 8.98 ± 0.4 | 8.42 ± 0.6 | 8.78 ± 0.3 | 7.38 ± 0.3 | 5.38 ± 0.2 | 9.60 ± 0.8 | 9.98 ± 0.4 | 9.27 ± 0.2 | 10.01 ± 0.2 |
| 9 | 10.43 ± 0.4 | 10.46 ± 0.4 | 9.13 ± 1.0 | 8.88 ± 0.8 | 9.56 ± 0.5 | 9.07 ± 0.5 | 8.89 ± 0.8 | 8.94 ± 0.5 | 6.37 ± 0.3 | 5.04 ± 0.1 | 9.93 ± 0.9 | 9.40 ± 0.6 | 9.56 ± 0.8 | 10.43 ± 0.4 |
| 5 | ||||||||||||||
| 0 | 6.71 ± 0.3 | 8.80 ± 0.8 | 5.36 ± 0.2 | 5.90 ± 0.1 | 5.98 ± 0.2 | 6.28 ± 0.2 | 5.91 ± 0.2 | 7.60 ± 0.3 | 3.89 ± 0.1 | 3.65 ± 0.1 | 5.40 ± 0.1 | 8.51 ± 0.5 | 5.52 ± 0.1 | 8.52 ± 0.5 |
| 1 | 7.36 ± 0.3 | 9.23 ± 1.0 | 6.43 ± 0.4 | 6.54 ± 0.5 | 6.48 ± 0.2 | 6.72 ± 0.7 | 6.60 ± 0.2 | 7.93 ± 0.4 | 4.78 ± 0.1 | 4.88 ± 0.1 | 5.85 ± 0.2 | 8.64 ± 0.6 | 5.78 ± 0.2 | 8.79 ± 0.7 |
| 4 | 8.36 ± 0.4 | 9.36 ± 0.4 | 7.30 ± 0.3 | 6.93 ± 0.3 | 7.56 ± 0.3 | 7.20 ± 0.2 | 7.13 ± 0.1 | 7.32 ± 0.3 | 5.98 ± 0.1 | 5.79 ± 0.1 | 6.00 ± 0.1 | 8.76 ± 0.3 | 6.00 ± 0.1 | 8.87 ± 0.4 |
| 7 | 8.45 ± 0.4 | 9.66 ± 0.5 | 8.01 ± 0.5 | 7.63 ± 0.3 | 8.02 ± 0.6 | 7.63 ± 0.3 | 7.44 ± 0.4 | 7.65 ± 0.3 | 6.37 ± 0.2 | 6.20 ± 0.2 | 7.77 ± 0.2 | 8.87 ± 0.3 | 7.51 ± 0.2 | 8.98 ± 0.4 |
| 9 | 9.44 ± 0.6 | 9.74 ± 0.6 | 8.68 ± 0.6 | 8.11 ± 0.3 | 8.78 ± 0.4 | 8.30 ± 0.3 | 7.84 ± 0.4 | 8.32 ± 0.4 | 7.05 ± 0.2 | 5.77 ± 0.1 | 7.85 ± 0.2 | 9.03 ± 0.5 | 8.14 ± 0.3 | 9.09 ± 0.5 |
| 12 | 9.73 ± 0.7 | 9.95 ± 0.7 | 9.08 ± 0.4 | 8.26 ± 0.4 | 9.16 ± 0.6 | 8.35 ± 0.3 | 7.95 ± 0.4 | 8.54 ± 0.5 | 6.15 ± 0.1 | 4.89 ± 0.1 | 8.19 ± 0.3 | 9.19 ± 0.5 | 8.41 ± 0.4 | 9.27 ± 0.6 |
| 16 | 10.07 ± 0.7 | 10.28 ± 0.4 | 9.37 ± 0.5 | 8.63 ± 0.4 | 9.51 ± 0.6 | 8.95 ± 0.4 | 8.63 ± 0.6 | 8.65 ± 0.5 | 5.86 ± 0.1 | 4.52 ± 0.1 | 8.51 ± 0.4 | 9.27 ± 0.5 | 8.55 ± 0.4 | 9.34 ± 0.6 |
| 20 | 10.84 ± 0.4 | 8.86 ± 0.4 | 9.00 ± 0.5 | 9.00 ± 0.5 | 4.00 ± 0.2 | 9.48 ± 0.5 | 9.41 ± 0.5 | |||||||
Briefly, spoilage was observed in kefir cheese on the 5th day of preservation at 25°C, on the 9th day at 10°C, and on the 20th day at 5°C. On the other hand, spoilage of rennet cheese at the above temperatures was correspondingly observed on the 5th, 7th, and 16th days of preservation.
In an attempt to evaluate the effects of both kefir coculture and preservation time on the growth of certain microbial taxa at different temperatures, kefir coculture appeared to affect significantly the total aerobic counts at 5°C (P < 0.01); yeast growth at 10 and 5°C (P < 0.01); and staphylococcus, lactococcus, and lactobacillus counts at all of the temperatures tested (P < 0.01). However, preservation time significantly affected (P < 0.05) all microbial counts at all of the temperatures tested (P < 0.05).
All microbial counts, apart from those of staphylococci, increased steadily and reached similar levels in both kefir cheese and rennet cheese. As expected, the increase in counts was slower at low temperatures. The initial lactococcus and lactobacillus counts were significantly (P < 0.01) higher in kefir cheese. In contrast, staphylococcus counts were significantly (P < 0.01) lower in kefir cheese. Salmonella spp. were not detected in any cheese during the whole preservation period. All experiments were duplicated with the corresponding solidified substrates inoculated with the dilution medium itself and incubated in the same way as the original samples in order to control for cross or post contamination. No evidence of contamination was observed.
Physicochemical characteristics of unsalted feta-type cheese produced by freeze-dried kefir coculture.
The physicochemical qualities of unsalted kefir cheese and unsalted rennet cheese preserved at various temperatures are summarized in Table 5.
TABLE 5.
Physicochemical characteristics of unsalted feta-type cheese produced by freeze-dried kefir starter coculture in comparison with unsalted feta-type cheese without starter culture during ripening at various temperatures
| Preservation temp (°C) and time (days) | Lactose (g/100 g cheese)
|
Glucose (g/100 g cheese)
|
Galactose (g/100 g cheese)
|
Lactic acid (g/100 g cheese)
|
pH
|
Ethanol (g/100 g cheese)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | Rennet cheese | Kefir cheese | |
| 25 | ||||||||||||
| 0 | 3.33 ± 0.2 | 0.32 ± 0.02 | Tra | 0.56 ± 0.05 | Tr | 1.01 ± 0.05 | 0.18 ± 0.02 | 0.36 ± 0.02 | 6.71 ± 0.1 | 5.09 ± 0.1 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| 1 | 3.24 ± 0.2 | Tr | Tr | Tr | Tr | Tr | 0.18 ± 0.02 | 1.00 ± 0.05 | 6.05 ± 0.1 | 4.82 ± 0.1 | 0.01 ± 0.001 | 0.04 ± 0.005 |
| 3 | 0.69 ± 0.05 | Tr | Tr | Tr | Tr | Tr | 0.61 ± 0.05 | 0.90 ± 0.05 | 4.82 ± 0.1 | 5.55 ± 0.1 | 0.01 ± 0.001 | 0.03 ± 0.001 |
| 5 | Tr | Tr | Tr | Tr | Tr | Tr | 0.94 ± 0.05 | 0.72 ± 0.05 | 4.80 ± 0.1 | 6.23 ± 0.1 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| 7 | Tr | Tr | Tr | Tr | Tr | Tr | 0.97 ± 0.05 | 0.61 ± 0.05 | 5.13 ± 0.1 | 6.35 ± 0.1 | Tr | 0.01 ± 0.001 |
| 10 | ||||||||||||
| 0 | 3.33 ± 0.2 | 0.32 ± 0.02 | Tr | 0.56 ± 0.05 | Tr | 1.01 ± 0.05 | 0.18 ± 0.02 | 0.36 ± 0.05 | 6.71 ± 0.1 | 5.09 ± 0.1 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| 1 | 3.24 ± 0.2 | Tr | Tr | 0.04 ± 0.01 | Tr | 0.44 ± 0.04 | 0.18 ± 0.02 | 1.00 ± 0.05 | 6.05 ± 0.1 | 4.82 ± 0.1 | 0.01 ± 0.001 | 0.04 ± 0.005 |
| 3 | 2.63 ± 0.2 | Tr | Tr | Tr | Tr | Tr | 0.23 ± 0.03 | 1.08 ± 0.05 | 6.03 ± 0.1 | 4.88 ± 0.1 | 0.01 ± 0.001 | 0.04 ± 0.005 |
| 5 | 0.77 ± 0.05 | Tr | Tr | Tr | Tr | Tr | 0.83 ± 0.05 | 0.94 ± 0.05 | 4.92 ± 0.1 | 5.12 ± 0.1 | 0.02 ± 0.004 | 0.03 ± 0.005 |
| 7 | 0.48 ± 0.02 | Tr | Tr | Tr | Tr | Tr | 0.86 ± 0.05 | 0.86 ± 0.05 | 5.00 ± 0.1 | 5.35 ± 0.1 | 0.02 ± 0.004 | 0.02 ± 0.004 |
| 9 | 0.41 ± 0.02 | Tr | Tr | Tr | Tr | Tr | 0.90 ± 0.05 | 0.65 ± 0.05 | 5.07 ± 0.1 | 5.88 ± 0.1 | 0.05 ± 0.005 | 0.02 ± 0.004 |
| 5 | ||||||||||||
| 0 | 3.20 ± 0.2 | 0.42 ± 0.02 | 0.26 ± 0.02 | 0.47 ± 0.05 | Tr | 1.02 ± 0.05 | 0.18 ± 0.02 | 0.43 ± 0.04 | 6.71 ± 0.1 | 5.09 ± 0.1 | 0.01 ± 0.001 | 0.02 ± 0.001 |
| 1 | 3.15 ± 0.2 | Tr | 0.15 ± 0.02 | Tr | Tr | 0.31 ± 0.02 | 0.18 ± 0.02 | 0.97 ± 0.05 | 6.43 ± 0.1 | 4.91 ± 0.1 | 0.01 ± 0.001 | 0.03 ± 0.005 |
| 4 | 3.00 ± 0.2 | Tr | 0.09 ± 0.01 | Tr | Tr | 0.05 ± 0.01 | 0.25 ± 0.02 | 1.00 ± 0.05 | 6.05 ± 0.1 | 4.79 ± 0.1 | 0.02 ± 0.002 | 0.05 ± 0.005 |
| 7 | 1.41 ± 0.1 | Tr | Tr | Tr | Tr | Tr | 0.72 ± 0.05 | 1.04 ± 0.05 | 5.19 ± 0.1 | 5.01 ± 0.1 | 0.04 ± 0.005 | 0.05 ± 0.005 |
| 9 | 1.11 ± 0.05 | Tr | Tr | Tr | Tr | Tr | 0.76 ± 0.05 | 1.00 ± 0.05 | 5.15 ± 0.1 | 5.06 ± 0.1 | 0.02 ± 0.001 | 0.09 ± 0.005 |
| 12 | 0.72 ± 0.05 | Tr | Tr | Tr | Tr | Tr | 0.72 ± 0.05 | 0.79 ± 0.05 | 5.14 ± 0.1 | 5.32 ± 0.1 | 0.02 ± 0.001 | 0.04 ± 0.005 |
| 16 | 0.67 ± 0.05 | Tr | Tr | Tr | Tr | Tr | 0.68 ± 0.05 | 0.68 ± 0.05 | 5.13 ± 0.1 | 5.77 ± 0.1 | 0.02 ± 0.001 | 0.02 ± 0.001 |
| 20 | Tr | Tr | Tr | 0.61 ± 0.05 | 5.98 ± 0.1 | 0.01 ± 0.001 | ||||||
Tr, compound present at <0.001% (trace).
Addition of the freeze-dried kefir coculture and preservation time significantly (P < 0.01) affected all of the parameters studied at all of the preservation temperatures used, except for glucose concentration at 5°C and pH at 25°C, which were only affected by preservation time (P < 0.01). Moreover, a strong interaction between the two factors (P < 0.01) affecting all of the parameters studied was also observed in all cases.
Quantitative analysis at the end of the production process and before the start of preservation revealed low levels of residual sugar (1.89 to 1.91%) in kefir cheese, in direct contrast to the relatively high levels (3.33 to 3.46%) observed in rennet cheese. During preservation, levels were reduced to traces in kefir cheese at all temperatures, while in rennet cheese such an observation was only obtained during preservation at 25°C. Shifts in lactic acid concentration at all preservation temperatures led to a pH drop, followed by a slight increase that appeared to be sharper in kefir cheese than in rennet cheese. Decreases in sugar and pH and an increase in lactic acid occurred at lower rates at low preservation temperatures.
DISCUSSION
The scope of this study was to determine the suitability of a freeze-dried kefir coculture as a starter in feta-type cheese production. The strategy was aimed at improvement of cheese quality characteristics, acceleration of cheese ripening, and increased shelf life.
Feta-type cheese produced when freeze-dried kefir was added before rennet revealed a better structure, likely because of the action of lactic acid bacteria and the formation of slightly acidic conditions, facilitating the action of rennet (2). This product was also characterized by a rapid decrease in pH and low levels of ethanol which could be attributed to the temperature of curd formation favoring the action of lactic acid bacteria rather than yeasts (4, 5, 43). The increase in pH and ethanol content toward the end of the ripening period, which appeared to be greater when increased amounts of starter culture were used, has previously been reported and associated with the action of yeasts (23). However, this action was limited in surface-salted cheese ripened in brine, as no increase in pH or ethanol content was observed, indicating no development of yeasts derived from the natural kefir microflora (64). An increase in pH due to the action of yeasts is, however, considered important, as it supports the function of non-acid-resistant surface-growing bacteria and their proteolytic and lipolytic activities, which are considered essential for cheese ripening and the development of cheese aroma (13, 67).
The extension of preservation time obtained with kefir cheese compared to rennet cheese by an additional of 25 days before the observation of sparse greenish spots (data not shown) supported our initial hypothesis for extending the shelf life of the final product. Further support was obtained when kefir cheese ripened in brine showed no evidence of mold or yeast growth, even after 70 days of ripening.
Nevertheless, the involvement of ripening processes with brine solutions in dairy factories aims mainly at the formation of a microenvironment capable of prohibiting the development of spoilage and pathogenic microorganisms (64). The use of certain microbial consortia has been shown to create an endogenous shield (22, 45, 54, 55) and could suggest their use as protecting agents. Such a hypothesis for kefir coculture was tested by determining the bacterial diversity of surface-salted kefir and rennet cheeses after a 70-day preservation period. The detection of Staphylococcus and Psychrobacter species, often considered pathogenic and spoilage agents in food technology, in rennet cheese but not in kefir cheese by culture-independent methods could suggest a potential inhibition of species-specific proliferation. This could be attributed to the domination of active members of the genus Lactococcus in the kefir cheese, although bias due to preferential PCR amplification cannot be excluded (18). However, members of the genera Janthinobacterium and Pseudomonas that are considered spoilage bacteria (16) were detected in both cheese types. Further investigations of spiked milk with certain spoilage and pathogenic microorganisms will give more insight into the roles of Lactococcus species and the kefir consortium as endogenous protective agents.
Apart from a product resistant to spoilage, the development of unique aromatic compounds remains an undeniable aim of the cheese industry. Esters, organic acids, alcohols, and carbonyl and sulfur compounds (6, 17, 31, 53) are generally considered the most important compounds usually identified in cheeses by the SPME-GC-MS technique. However, the flavor of cheese appears to depend not on particular key components but rather on a critical balance or a weighted concentration ratio of all of the components present (31).
Most of the esters that were detected in the cheeses analyzed in the present study are characterized by fruity, floral notes. Ethyl acetate was identified in surface-salted kefir cheese ripened in brine and in commercial feta-type cheese, while propyl butanoate was present only in surface-salted kefir cheese. In contrast, ethyl octanoate was detected in both kefir cheese samples. The above esters are known for their fruity aroma contribution (51, 53) and may derive from milk (65). Ethyl butanoate, present in surface-salted kefir cheese ripened in brine, has been identified as one of the most potent odorants in a number of cheeses (14). Phenylethyl acetate, detected in kefir cheese samples and in commercial feta-type cheese, is one of the most important aromatic esters and is known for its floral, rose-like aroma. It was determined to be the major odorant in Camembert cheese (39).
Free fatty acids are important components of cheese flavor and may originate either from milk fat lipolysis or from breakdown of amino acids (66). Hexanoic acid, identified in surface-salted kefir cheese ripened in brine and in commercial feta-type cheese, provides a pungent flavor (51). Octanoic acid, present in all cheeses, and decanoic acid, present in surface-salted kefir cheese ripened in brine and in commercial feta-type cheese, are known for their goaty, rancid flavor (35, 47). Branched-chain fatty acids are characteristic impact compounds of goat and sheep cheeses. Among them, 3-methylbutanoic acid, detected in surface-salted kefir cheese, provides a rancid, cheesy, sweaty, and putrid odor contributing to the very ripened cheese aroma (71). Dodecanoic acid, detected in all cheeses except in rennet cheese, and tetradecanoic acid, present only in surface-salted kefir cheese ripened in brine and in commercial feta-type cheese, play a minor role in cheese flavor, as they have a high perception threshold (47).
Many metabolic pathways are involved in the biosynthesis of alcohols that are encountered in cheese, such as lactose metabolism, methyl ketone reduction, amino acid metabolism, and degradation of linoleic and linolenic acids (47). The alcohols identified included mainly alcohols of the aliphatic series, fusel alcohols, and phenols. Ethanol, identified in all cheeses, provides an alcoholic, mild flavor note; occurs in fresh milk (65); and may derive from lactose metabolism. The presence of branched-chain primary alcohols such as 3-methyl-1-butanol, present in all of the cheese samples analyzed, indicates the reduction of the aldehyde produced by leucine. It was identified as a minor odorant in bovine mozzarella and confers a pleasant aroma of fresh cheese (49). Of the secondary alcohols, which are known to derive from fatty acids (47), 2-heptanol was detected only in rennet cheese. 3-(Methylthio)-1-propanol, detected only in surface-salted kefir cheese, is a reduction product of 3-methylthio-propanal (methional). It plays an important role in the cheese aroma profile, contributing positively to cheese flavor at low concentrations (59). Phenyl ethanol, present in all cheeses, belongs to the most odorous aromatic alcohols, presenting rose flower notes. Phenyl ethanol production from phenylalanine seems to be essentially achieved by yeasts (40). 4-Methylphenol (p-cresol) was detected only in surface-salted kefir cheese ripened in brine. It originates from tyrosine by the action of yeasts and micrococci (33). In general, cresols and phenols may play an important role in cheese produced by sheep milk, providing a distinctive sheep-like or sheepyard-like flavor (35).
The carbonyl compounds identified included mainly aldehydes, ketones, and lactones. Benzaldehyde, identified in kefir cheese samples, is described as having an aromatic note of bitter almond (47) and may originate from α-oxidation of phenylacetaldehyde or from β-oxidation of cinnamic acid (10). Phenylacetaldehyde, detected only in surface-salted kefir cheese, is considered one of the important aromatic aldehydes formed by phenylalanine degradation. It is a major odor-active compound of Gruyère (58, 59) and bovine mozzarella (49). Furfural, detected in surface-salted kefir cheese ripened in brine, is known for its cooked, heated-milk odor and has also been reported as a volatile constituent of cheeses (48). Diacetyl (2,3-butanedione), present only in surface-salted kefir cheese, is considered one of the most important diketones. It is obtained from pyruvate, stemming from lactose and citrate metabolism, and its production is mainly due to the activity of lactic acid bacteria and more specifically Lactococcus lactis (68). This observation is further supported by our molecular analysis, which revealed an active strain of this species only in kefir cheese. Fruity, floral, and musty notes are associated with various methyl ketones, which are formed by a metabolic pathway that is connected to the β-oxidation pathway. 2-Heptanone, identified in all cheese samples except in surface-salted kefir cheese, provides blue cheese notes and is an important flavor compound of Emmental and natural and creamy Gorgonzola cheeses (14). 2-Nonanone, detected only in rennet cheese, is another predominant methyl ketone in natural Gorgonzola and ripened Ragusano cheeses, while 2-undecanone, present in all types of cheese apart from rennet cheese, is considered a key aroma compound of Camembert cheese. Likewise, 2-tridecanone, with a fruity, green flavor note (47), was found only in commercial feta-type cheese. δ-Decalactone, identified in rennet and kefir cheeses, is considered one of the most common and important lactones in cheese. It is a key odorant of Camembert and Emmental cheeses (14). δ-Octalactone, detected only in surface-salted kefir cheese, and δ-dodecalactone, present in all cheeses except in rennet cheese, are known to provide the sensory characteristics of goat cheese (14).
Our results show that a plethora of aroma compounds are formed by the action of a kefir coculture. However, the relationship between the microbial associations and the chemical compounds in cheese is not known and is difficult to interpret because of the great complexity of microbial interactions.
Feta cheese retention time is mainly due to the use of salt as a preservative. Salt addition often constitutes a critical control point in food safety and thus an important agent, although it is not always desirable because of adverse health effects, which may become manifest in the long run among high-risk consumers. The impact of our kefir coculture on preservation time was therefore evaluated in unsalted cheese throughout the maturation process. Both unsalted kefir cheese and unsalted rennet cheese were preserved at room and low temperatures. Although microbial counts of total aerobic bacteria, coliforms, enterobacteria, yeasts, lactococci, and lactobacilli did not result in any substantial differences between the two types of cheese and similar types previously reported (21, 28, 41, 44, 50), staphylococcus counts were significantly lower in unsalted kefir cheese, not only compared to rennet cheese but more importantly compared to similar cheeses which had been salt treated (21, 50).
Given that staphylococci were detected by molecular methods only in salted rennet cheese, it is not unreasonable to suggest the development of a microbial association due to kefir coculture leading to this staphylococcal repression. While pathogenesis or spoilage has not been considered for all staphylococci, such an observation favors the development of technologies aiming at the production of self-preserved foods.
The antimicrobial properties of kefir coculture, as has also previously been suggested for dairy products (26), may be attributed either to its ability to produce lactic acid and therefore cause a drastic reduction in pH or to the production of antimicrobial compounds by certain strains existing in its natural microbial flora (55, 57, 60). Our results show that the sugar consumption, production of lactic acid, and therefore reduction of pH observed in unsalted rennet cheese due to the action of indigenous lactic acid bacteria that survive pasteurization (61) are inadequate to provide the products lacking starter cultures the preservation properties gained by kefir. Besides such properties, starter cultures have the advantage of producing consistent characteristics in the final products, which is an important feature for commerce.
It is important in the food industry to know the likely occurrence of microbial hazards in food and the means (temperature, packaging, preservatives, etc.) needed to obtain a desirable shelf life. The main factors affecting microbial growth are undoubtedly physical parameters such as temperature, pH, and water activity, together with nutrients (oxygen, carbohydrates, protein content, nitrite, etc.). While these factors, in association with food structure, constitute the basis of predictive microbiology (69), the presence of certain microbial populations can result in unexpected associations and therefore in unpredicted microbial compositions. The present study demonstrates the use of a kefir coculture as a potential means to extend the shelf life of feta-type cheese in association with the repression of certain microbial cultures and suggests its use for the production of dairy products for people with high blood pressure. Moreover, a freeze-dried kefir coculture proved to be a suitable starter for feta-type cheese production with respect to quality characteristics, as indicated by the sensory panel. The use of certain consortia may have a major impact in food technology. However, more research in the field is required.
Acknowledgments
We thank the European Social Fund (ESF), Operational Program for Educational and Vocational Training II (EPEAEK II), and particularly the program PYTHAGORAS for funding this work.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anifantakis, E. M. 1993. Enzymes for curd formation, p. 31-33. In E. M. Anifantakis (ed.), Cheese-making. A. Stamoulis, Athens-Pireaus, Greece.
- 3.Aranjo, V. S., V. A. Pagliares, M. L. Queiroz, and A. C. Freitas-Almeido. 2002. Occurrence of Staphylococcus and enteropathogens in soft cheese commercialized in the city of Rio de Janeiro, Brazil. J. Appl. Microbiol. 92:1172-1177. [DOI] [PubMed] [Google Scholar]
- 4.Athanasiadis, I., D. Boskou, M. Kanellaki, V. Kioseoglou, and A. A. Koutinas. 2002. Whey liquid waste of the dairy industry as raw material for potable alcohol production by kefir granules. J. Agric. Food Chem. 50:7231-7234. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiadis, I., D. Boskou, M. Kanellaki, and A. A. Koutinas. 1999. Low-temperature alcoholic fermentation by delignified cellulosic material supported cells of kefir yeast. J. Agric. Food Chem. 47:4474-4477. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri, G., L. Bolzoni, M. Careri, A. Mangia, G. Parolari, S. Spagnoli, and R. Virgill. 1994. Study of the volatile fraction of Parmesan cheese. J. Agric. Food Chem. 42:1170-1176. [Google Scholar]
- 7.Bellesia, F., A. Pinetti, U. M. Pagnoni, R. Rinaldi, C. Zucchi, L. Gaglioti, and G. Palyi. 2003. Volatile components of Grana Parmigiano-Reggiano type hard cheese. Food Chem. 83:53-61. [Google Scholar]
- 8.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, and D. L. Wheeler. 2005. GenBank. Nucleic Acids Res. 33:D34-D38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boylston, T. D., C. G. Vinderola, H. B. Ghoddusi, and J. A. Reinheimer. 2004. Incorporation of bifidobacteria into cheeses: challenges and rewards. Int. Dairy J. 14:375-387. [Google Scholar]
- 10.Casey, J., and R. Dobb. 1992. Microbial routes to aromatic aldehydes. Enzyme Microb. Technol. 14:739-747. [Google Scholar]
- 11.Cevikbas, A., E. Yemni, F. W. Ezzedenn, T. Yardimici, U. Cevikbas, and S. J. Stohs. 1994. Antitumoural, antibacterial and antifungal activities of kefir and kefir grain. Phytother. Res. 8:78-82. [Google Scholar]
- 12.Coia, J. E., Y. Johnson, N. J. Steers, and M. F. Hanson. 2001. A survey of the prevalence of Escherichia coli O157 in raw meats, raw cow's milk and raw-milk cheeses in south-east Scotland. Int. J. Food Microbiol. 66:63-69. [DOI] [PubMed] [Google Scholar]
- 13.Corsetti, A., J. Rossi, and M. Gobbetti. 2001. Interactions between yeasts and bacteria in the smear surface-ripened cheeses. Int. J. Food Microbiol. 69:1-10. [DOI] [PubMed] [Google Scholar]
- 14.Curioni, P. M. G., and J. O. Bosset. 2002. Review: key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 12:959-984. [Google Scholar]
- 15.Elgazzar, F. E., and E. H. Marth. 1992. Salmonellae, salmonellosis, and dairy foods—a review. J. Dairy Sci. 75:2327-2343. [DOI] [PubMed] [Google Scholar]
- 16.Eneroth, A., S. Ahrné, and G. Molin. 2000. Contamination routes of gram-negative spoilage bacteria in the production of pasteurized milk, evaluated by randomly amplified polymorphic DNA (RAPD). Int. Dairy J. 10:325-331. [Google Scholar]
- 17.Engels, W. J. M., R. Dekker, C. de Jong, R. Neeter, and S. Visser. 1997. A comparative study of volatile compounds in the water-soluble fraction of various types of ripened cheese. Int. Dairy J. 7:255-263. [Google Scholar]
- 18.Ercolini, D. 2004. PCR-DGGE fingerprinting: novel strategies for detection of microbes in foods. J. Microbiol. Methods 56:297-314. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa, N., A. Matsuoka, T. Takahasi, and Y. Yamanaka. 1991. Effects of fermented milk on the delayed type hypersensitivity response and survival day in mice bearing Meth-A. Anim. Sci. Technol. 62:579-585. [Google Scholar]
- 20.Garrote, G. L., A. G. Abraham, and G. L. De Antoni. 1997. Preservation of kefir grains, a comparative study. Lebensm.-Wiss. Technol. 30:77-84. [Google Scholar]
- 21.Gerasi, E., E. Litopoulou-Tzanetaki, and N. Tzanetakis. 2003. Microbiological study of Manura, a hard cheese made from raw ovine milk in Greek island Sifnos. Int. J. Dairy Technol. 56(2):117-122. [Google Scholar]
- 22.Ghrairi, T., M. Manai, J. M. Berjeaud, and J. Frère. 2004. Antilisterial activity of lactic acid bacteria isolated from Rigouta, a traditional Tunisian cheese. J. Appl. Microbiol. 97:621-628. [DOI] [PubMed] [Google Scholar]
- 23.Gobbetti, M., S. Lowney, E. Smacchi, B. Battistotti, P. Damiani, and P. F. Fox. 1997. Microbiology and biochemistry of Taleggio cheese during ripening. Int. Dairy J. 7:509-517. [Google Scholar]
- 24.Goncu, A., and Z. Alpkent. 2005. Sensory and chemical properties of white pickled cheese produced using kefir, yoghurt or a commercial cheese culture as a starter. Int. Dairy J. 15:771-776. [Google Scholar]
- 25.Griffiths, R. I., S. A. Whiteley, A. G. O'Donnell, and M. J. Bailey. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulmez, M., and A. Guven. 2003. Survival of Escherichia coli O157:H7, Listeria monocytogenes 4b and Yersinia enterocolitica O3 in different yogurt and kefir combinations as prefermentation contaminant. J. Appl. Microbiol. 95:631-636. [DOI] [PubMed] [Google Scholar]
- 27.Hansen, E. B. 2002. Commercial bacterial starter cultures for fermented foods of the future. Int. J. Food Microbiol. 78:119-131. [DOI] [PubMed] [Google Scholar]
- 28.Hatzikamari, M., E. Litopoulou-Tzanetaki, and N. Tzanetakis. 1999. Microbiological characteristics of Anevato: a traditional Greek cheese. J. Appl. Microbiol. 87(4):595-601. [DOI] [PubMed] [Google Scholar]
- 29.Holzapfel, W. H., R. Geisen, and U. Schillinger. 1995. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int. J. Food Microbiol. 24:342-362. [DOI] [PubMed] [Google Scholar]
- 30.Iconomopoulou, M., A. Becatorou, M. Kanellaki, I. Athanasiadis, V. Kioseoglou, G. Blekas, A. A. Koutinas, and A. Paraskevopoulou. 2001. SCP production using kefir yeast from whey liquid effluent of dairy industry, p. 350-355. In Proceedings of 7th International Conference on Environmental Science and Technology. Global Network for Environmental Science and Technology, Athens, Greece.
- 31.Izco, J. M., and P. Torre. 2000. Characterisation of volatile flavour compounds in Roncal cheese extracted by the purge and trap method and analysed by GC-MS. Food Chem. 70:409-417. [Google Scholar]
- 32.Jay, J. M. 1992. Microbial spoilage of foods, p. 187-248. In J. M. Jay (ed.), Modern food microbiology, 4th ed. Van Nostrand Reinhold, New York, N.Y.
- 33.Jollivet, N., M. C. Bézenger, Y. Vayssier, and J. M. Belin. 1992. Production of volatile compounds in liquid cultures by six strains of coryneform bacteria. Appl. Microbiol. Biotechnol. 36:790-794. [Google Scholar]
- 34.Kieronczyk, A., S. Skeie, T. Langsrud, and M. Yvon. 2003. Cooperation between Lactococcus lactis and nonstarter lactobacilli in the formation of cheese aroma from amino acids. Appl. Environ. Microbiol. 69:734-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Ha, J., and R. C. Lindsay. 1992. Influence of aw on volatile free fatty acids during storage of cheese bases lipolyzed by kid goat pregastric lipase. Int. Dairy J. 2:179-195. [Google Scholar]
- 36.Kirk, R. S., and R. Sawyer. 1991. Pearson's composition and analysis of foods, 9th edition, p. 600-601. Longman Singapore Publishers Ltd., Singapore.
- 37.Kourkoutas, Y., L. Bosnea, S. Taboukos, C. Baras, D. Lambrou, and M. Kanellaki. 2006. Probiotic cheese production using Lactobacillus casei cells immobilized on fruit pieces. J. Dairy Sci. 89:1439-1451. [DOI] [PubMed] [Google Scholar]
- 38.Koutinas, A. A., I. Athanasiadis, A. Bekatorou, M. Iconomopoulou, and G. Blekas. 2005. Kefir yeast technology: scale-up in SCP production using milk whey. Biotechnol. Bioeng. 89:788-796. [DOI] [PubMed] [Google Scholar]
- 39.Kubícková, J., and W. Grosch. 1997. Evaluation of potent odorants of Camembert cheese by dilution and concentration techniques. Int. Dairy J. 7:65-70. [Google Scholar]
- 40.Lee, C. W., and J. Richard. 1984. Catabolism of l-phenylalanine by some microorganisms of cheese origin. J. Dairy Res. 51:461-469. [Google Scholar]
- 41.Litopoulou-Tzanetaki, E., N. Tzanetakis, and A. Vafopoulou-Mastrojiannaki. 1993. Effect of the type of lactic starter on microbiological, chemical and sensory characteristics of feta cheese. Food Microbiol. 10:31-41. [Google Scholar]
- 42.Liu, J. R., and C. Lin. 2000. Production of kefir from soymilk with or without added glucose, lactose or sucrose. J. Food Sci. 65:716-719. [Google Scholar]
- 43.Luis, A., E. Lopez, and C. Lema. 1993. Microflora present in kefir grains of the Galician region. J. Dairy Res. 60:263-267. [DOI] [PubMed] [Google Scholar]
- 44.Manolopoulou, E., P. Sanantinopoulos, E. Zoidou, A. Aktypis, E. Moschopoulou, I. G. Kandarakis, and E. M. Anifantakis. 2003. Evolution of microbial populations during traditional feta cheese manufacture and ripening. Int. J. Food Microbiol. 82:153-161. [DOI] [PubMed] [Google Scholar]
- 45.Maoz, A., R. Mayr, and S. Scherer. 2003. Temporal stability and biodiversity of two complex antilisterial cheese-ripening microbial consortia. Appl. Environ. Microbiol. 69:4012-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michaelidou, A., M. C. Katsiari, E. Kondyli, L. P. Voutsinas, and E. Alichanidis. 2003. Effect of a commercial adjunct culture on proteolysis in low-fat feta-type cheese. Int. Dairy J. 13:179-189. [Google Scholar]
- 47.Moio, L., J. Dekimpe, P. X. Etiévant, and F. Addeo. 1993. Volatile flavor compounds of water buffalo mozzarella cheese. Ital. J. Food Sci. 5:57-68. [Google Scholar]
- 48.Moio, L., D. Langlois, P. X. Etiévant, and F. Addeo. 1993. Powerful odorants in water buffalo and bovine mozzarella cheese by use of extract dilution sniffing analysis. Ital. J. Food Sci. 3:227-237. [Google Scholar]
- 49.Molimard, P., and H. E. Spinnler. 1996. Compounds involved in the flavour of surface mold-ripened cheeses: origins and properties. J. Dairy Sci. 79:169-184. [Google Scholar]
- 50.Nikolaou, E., N. Tzanetakis, E. Litopoulou-Tzanetaki, and R. K. Robinson. 2002. Changes in the microbiological and chemical characteristics of an artisanal, low-fat cheese made from raw bovine milk during ripening. Int. J. Dairy Technol. 55:12-17. [Google Scholar]
- 51.Pérès, C., C. Viallon, and J. L. Berdagué. 2001. Solid-phase microextraction-mass spectrometry: a new approach to the rapid characterization of cheeses. Anal. Chem. 73:1030-1036. [DOI] [PubMed] [Google Scholar]
- 52.Plessas, S., L. Pherson, A. Bekatorou, P. Nigam, and A. A. Koutinas. 2005. Bread making using kefir grains as baker's yeast. Food Chem. 93:585-589. [Google Scholar]
- 53.Qian, M., and G. Reineccius. 2002. Identification of aroma compounds in parmigiano-reggiano cheese by gas chromatography/olfactometry. J. Dairy Sci. 85:1362-1369. [DOI] [PubMed] [Google Scholar]
- 54.Rea, M. C., and T. M. Cogan. 1994. Buttermilk plants: the Irish version of kefir. Ir. Scientist 2:7. [Google Scholar]
- 55.Rodrigues, K. L., L. R. G. Caputo, J. C. T. Carvalho, J. Evangelista, and J. M. Schneedorf. 2005. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 25:404-408. [DOI] [PubMed] [Google Scholar]
- 56.Rudol, M., and S. Scherer. 2001. High incidence of Listeria monocytogenes in European red smear cheese. Int. J. Food Microbiol. 63:91-98. [DOI] [PubMed] [Google Scholar]
- 57.Ryan, M. P., M. C. Rea, C. Hill, and R. P. Ross. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rychlik, M., and J. O. Bosset. 2001. Flavour and off-flavour compounds of Swiss mozzarella cheese. Evaluation of potent odorants. Int. Dairy J. 11:895-901. [Google Scholar]
- 59.Rychlik, M., and J. O. Bosset. 2001. Flavour and off-flavour compounds of Swiss mozzarella cheese. Identification of key odorants by quantitative instrumental and sensory studies. Int. Dairy J. 11:903-910. [Google Scholar]
- 60.Santos, A., M. San Mauro, A. Sanchez, J. M. Torres, and D. Marquina. 2003. The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst. Appl. Microbiol. 26:434-437. [DOI] [PubMed] [Google Scholar]
- 61.Stanton, C., G. Gardiner, P. B. Lynch, J. K. Collins, G. Fitzgerald, and R. P. Ross. 1998. Probiotic cheese. Int. Dairy J. 8:491-496. [Google Scholar]
- 62.Swearingen, P. A., D. J. O. Sullivan, and J. J. Warthesen. 2001. Isolation, characterization, and influence of native, nonstarter lactic acid bacteria on cheddar cheese quality. J. Dairy Sci. 84:50-59. [DOI] [PubMed] [Google Scholar]
- 63.Thierry, A., M.-B. Maillard, and J.-L. Le Quéré. 1999. Dynamic headspace analysis of Emmental aqueous phase as a method to quantify changes in volatile flavour compounds during ripening. Int. Dairy J. 9:453-463. [Google Scholar]
- 64.Tzanetakis, N., and E. Litopoulou-Tzanetaki. 1992. Changes in numbers and kinds of lactic acid bacteria in feta and teleme, two Greek cheeses from ewe's milk. J. Dairy Sci. 75:1389-1393. [Google Scholar]
- 65.Urbach, G. 1995. Contribution of lactic acid bacteria to flavour compound formation in dairy products. Int. Dairy J. 5:877-903. [Google Scholar]
- 66.Urbach, G. 1993. Relations between cheese flavor and chemical composition. Int. Dairy J. 3:389-422. [Google Scholar]
- 67.Viljoen, B. C. 2001. The interaction between yeasts and bacteria in dairy environments. Int. J. Food Microbiol. 69:37-44. [DOI] [PubMed] [Google Scholar]
- 68.Welsh, F. W., W. D. Murry, and R. E. Williams. 1989. Microbiological and enzymatic production of flavour and fragrance chemicals. Crit. Rev. Biotechnol. 9:105-169. [Google Scholar]
- 69.Wilson, P. D. G., T. F. Brocklehurst, S. Arino, D. Thuault, M. Jakobsen, M. Lange, J. Farkas, J. W. T. Wimpenny, and J. F. Van Impe. 2002. Modelling microbial growth in structured foods: towards a unified approach. Int. J. Food Microbiol. 73:275-289. [DOI] [PubMed] [Google Scholar]
- 70.Witthuhn, R. C., T. Schoeman, and T. J. Britz. 2005. Characterisation of the microbial population at different stages of kefir production and kefir grain mass cultivation. Int. Dairy J. 15:383-389. [Google Scholar]
- 71.Yvon, M., and L. Rijnen. 2001. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 11:185-201. [Google Scholar]


