Abstract
A quick and simple method for quantitative detection of Lactobacillus sakei in fermented sausages was successfully developed. It is based on Chelex-100-based DNA purification and real-time PCR enumeration using a TaqMan fluorescence probe. Primers and probes were designed in the L. sakei 16S-23S rRNA intergenic transcribed spacer region, and the assay was evaluated using L. sakei genomic DNA and an artificially inoculated sausage model. The detection limit of this technique was approximately 3 cells per reaction mixture using both purified DNA and the inoculated sausage model. The quantification limit was established at 30 cells per reaction mixture in both models. The assay was then applied to enumerate L. sakei in real samples, and the results were compared to the MRS agar count method followed by confirmation of the percentage of L. sakei colonies. The results obtained by real-time PCR were not statistically significantly different than those obtained by plate count on MRS agar (P > 0.05), showing a satisfactory agreement between both methods. Therefore, the real-time PCR assay developed can be considered a promising rapid alternative method for the quantification of L. sakei and evaluation of the implantation of starter strains of L. sakei in fermented sausages.
Dry fermented sausages are ready-to-eat meat products characterized by a bacterial fermentation process followed by a ripening period. Indigenous microorganisms have been traditionally responsible for fermentation, but starter cultures can also be added to control fermentation and to ensure desired quality (10). Among them, lactic acid bacteria (LAB) play an important role during the fermentation of these products, mainly as a result of competitive growth and the production of inhibitory substances, such as organic acids and bacteriocins (55). The species of LAB most commonly found in meat and meat products, including dry sausages processed with different technologies, are Lactobacillus sakei, Lactobacillus curvatus, and Lactobacillus plantarum (4, 25, 38, 52, 54). LAB have a long safe history of application and consumption in the production of fermented foods and beverages (11, 17, 44). They have been used as a starter culture for the fermentation of meat and meat products to improve microbial safety (21, 26, 27), and L. sakei, a facultative heterofermentative LAB, is the most frequently isolated and one of the best choices for further use as a starter culture (5).
Studies of the composition of LAB communities in meat and fermented sausages have been conducted using conventional methods (25, 48) or several alternatives methods such as species-specific hybridization probes (39, 58), conventional PCR (4, 7, 35), and PCR-denaturing gel electrophoresis (14). Conventional testing methods for the detection of L. sakei in food relying on phenotypical methods have been extensively used (25, 32, 38, 49, 52). They are labor-intensive and time-consuming, in many instances requiring from 8 to 10 days to be completed. One of the current limitations of rapid processing in the laboratory is the requirement to subculture isolates to perform biochemical or other tests needed for bacterial identification: Gram staining, catalase and oxidase testing plus biochemical identification by carbohydrate fermentation profiles, absence of diaminopimelic acid in the cell, production of dl-lactic acid, and hydrolysis of arginine. Moreover, physiological or biochemical criteria are sometimes ambiguous (8, 29). L. sakei is biochemically different from L. curvatus by melibiose consumption and arginine degradation (52), which sometimes may be confusing.
Reliable and fast identification methods are of great importance to control and monitor either endogenous or inoculated starter cultures. Development of a molecular culture-independent enumeration method appears to be particularly valuable in the case of L. sakei, since other available methods are not efficient to enumerate this bacterium in complex microbial communities. Among other molecular enumeration techniques, real-time PCR offers significant advantages for the enumeration of bacteria directly from food samples. This method allows an accurate and unambiguous identification and quantification of nucleic acid sequences (31), and compared to conventional PCR, cross-contamination is reduced while high throughput, wider dynamic range, and automation can be achieved. During the last decade, the genomes of some of the most important LAB have been sequenced (12, 30), which can help in the design of appropriate primers and probes for the molecular identification of LAB species. Real-time PCR has been recently used to enumerate lactic acid bacteria, such as LAB species for milk products (19), several Lactobacillus species in dental caries (9), Lactobacillus thermotolerans in chicken feces (53), Enterococcus in environmental samples (22), or Lactococcus lactis subsp. cremoris in fermented milk (20). Because food samples vary in composition and associated microflora, pre-PCR strategies need to be optimized for each food matrix to produce PCR-compatible samples from which inhibitory substances have been removed. Optimized pre-PCR processing strategies allow accurate DNA amplification without underestimating bacterial loads or giving false-negative results (2, 41, 47).
In this study, we present a rapid and sensitive assay for reliable quantitative identification of L. sakei in meat products based on simple and rapid sample handling and real-time PCR.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All LAB strains were grown in MRS broth (Oxoid, Basingstoke, United Kingdom) at 30°C for 24 h. The other reference strains were grown in tryptic soy broth (catalog no. 211825; Difco Laboratories, Detroit, Mich.) with 0.6% yeast extract (Oxoid) at 37°C for 24 h. A total of 52 L. sakei strains were used for inclusivity tests, and 45 non-L. sakei strains, comprising species closely related to L. sakei or several species that form part of the endogenous microflora of fermented sausages, were used for the exclusivity tests (Table 1).
TABLE 1.
Strains used in this study
| Species | Straina | Source | Real-time PCR resultb |
|---|---|---|---|
| L. sakei | CTC12 | Fermented sausage, Spain | + |
| L. sakei | CTC13 | Fermented sausage, Spain | + |
| L. sakei | CTC14 | Fermented sausage, Spain | + |
| L. sakei | CTC17 | Fermented sausage, Spain | + |
| L. sakei | CTC36 | Fermented sausage, Spain | + |
| L. sakei | CTC38 | Fermented sausage, Spain | + |
| L. sakei | CTC41 | Fermented sausage, Spain | + |
| L. sakei | CTC55 | Fermented sausage, Spain | + |
| L. sakei | CTC55 | Fermented sausage, Spain | + |
| L. sakei | CTC60 | Fermented sausage, Spain | + |
| L. sakei | CTC64 | Fermented sausage, Spain | + |
| L. sakei | CTC65 | Fermented sausage, Spain | + |
| L. sakei | CTC67 | Fermented sausage, Spain | + |
| L. sakei | CTC69 | Fermented sausage, Spain | + |
| L. sakei | CTC90 | Fermented sausage, Spain | + |
| L. sakei | CTC96 | Fermented sausage, Spain | + |
| L. sakei | CTC99 | Fermented sausage, Spain | + |
| L. sakei | CTC100 | Fermented sausage, Spain | + |
| L. sakei | CTC101 | Fermented sausage, Spain | + |
| L. sakei | CTC102 | Fermented sausage, Spain | + |
| L. sakei | CTC103 | Fermented sausage, Spain | + |
| L. sakei | CTC114 | Fermented sausage, Spain | + |
| L. sakei | CTC127 | Fermented sausage, Spain | + |
| L. sakei | CTC130 | Fermented sausage, Spain | + |
| L. sakei | CTC131 | Fermented sausage, Spain | + |
| L. sakei | CTC232 | Fermented sausage, Spain | + |
| L. sakei | CTC494 | Fermented sausage, Spain | + |
| L. sakei | CTC6452 | Fermented sausage, Spain | + |
| L. sakei | CTC6456 | Fermented sausage, Spain | + |
| L. sakei | CTC6464 | Fermented sausage, Spain | + |
| L. sakei | CTC6467 | Fermented sausage, Spain | + |
| L. sakei | CTC6478 | Fermented sausage, Spain | + |
| L. sakei | CTC6490 | Fermented sausage, Spain | + |
| L. sakei | CTC6500 | Fermented sausage, Spain | + |
| L. sakei | CTC6503 | Fermented sausage, Spain | + |
| L. sakei | CTC6505 | Fermented sausage, Spain | + |
| L. sakei | CTC6524 | Fermented sausage, Spain | + |
| L. sakei | CTC6527 | Fermented sausage, Spain | + |
| L. sakei | CTC6535 | Fermented sausage, Spain | + |
| L. sakei | CTC6531 | Fermented sausage, Spain | + |
| L. sakei | CTC6536 | Fermented sausage, Spain | + |
| L. sakei | CTC6559 | Fermented sausage, Spain | + |
| L. sakei | CTC6574 | Fermented sausage, Spain | + |
| L. sakei | CTC6584 | Fermented sausage, Spain | + |
| L. sakei | CTC6607 | Fermented sausage, Spain | + |
| L. sakei | CTC6620 | Fermented sausage, Spain | + |
| L. sakei | CTC6635 | Fermented sausage, Spain | + |
| L. sakei | CTC6644 | Fermented sausage, Spain | + |
| L. sakei | CTC6680 | Fermented sausage, Spain | + |
| L. sakei | CTC6668 | Fermented sausage, Spain | + |
| L. sakei | J23K | Fermented sausage, France | + |
| L. sakei | LTH673 | Meat, Germany | + |
| Other Lactobacillus species | |||
| Lactobacillus curvatus | CTC371 | Fermented sausage, Spain | − |
| L. curvatus | CTC435 | Fermented sausage, Spain | − |
| L. curvatus | CTC6489 | Fermented sausage, Spain | − |
| L. curvatus | CTC6512 | Fermented sausage, Spain | − |
| L. curvatus | LTH1174 | Meat product, Germany | − |
| Lactobacillus plantarum | CTC31 | Fermented sausage, Spain | − |
| L. plantarum | CTC299 | Fermented sausage, Spain | − |
| L. plantarum | CTC300 | Fermented sausage, Spain | − |
| L. plantarum | CTC305 | Fermented sausage, Spain | − |
| L. plantarum | CTC487 | Fermented sausage, Spain | − |
| Lactobacillus agilis | CECT4131 | Sewage, collection | − |
| Lactobacillus alimentarius | CECT570 | Marinated fish product, collection | − |
| Lactobacillus animalis | CECT4060 | Baboon dental plaque, collection | − |
| Lactobacillus brevis | CECT4669 | Green fermenting olive, collection | − |
| Lactobacillus buchneri | CECT4674 | Collection | − |
| Lactobacillus casei | CECT4180 | Dental caries, collection | − |
| Lactobacillus farciminis | CECT571 | Sausage, collection | − |
| Lactobacillus helveticus | CECT800 | Collection | − |
| Lactobacillus hilgardii | CECT4784 | Wine, collection | − |
| Lactobacillus reuteri | CECT925 | Intestine, collection | − |
| Lactobacillus salivarius | CTC2197 | Chicken, Spain | − |
| Non-Lactobacillus species | |||
| Enterococcus faecium | CTC410 | Fermented sausage, Spain | − |
| E. faecium | CTC492 | Fermented sausage, Spain | − |
| E. faecium | CTC496 | Fermented sausage, Spain | − |
| Enterococcus faecalis | CECT481 | Collection | − |
| Leuconostoc mesenteroides | CTC6484 | Fermented sausage, Spain | − |
| Leuconostoc mesenteroides | CTC6571 | Fermented sausage, Spain | − |
| Leuconostoc carnosum | CTC747 | Fermented sausage, Spain | − |
| Weisella halotolerans | CECT573 | Sausage, collection | − |
| Carnobacterium piscicola | CTC779 | Meat | − |
| Lactococcus lactis | CTC776 | Dairy products | − |
| Pediococcus pentosaceus | ATCC 43200 | Fermenting cucumbers, collection | − |
| Staphylococcus xylosus | CTC3050 | Fermented sausage, Spain | − |
| Staphylococcus aureus | CTC1008 | Meat, Spain | − |
| Staphylococcus saprophyticus | CECT235 | Urine, collection | − |
| Staphylococcus carnosus | DSM20501 | Dry sausage, collection | − |
| Staphylococcus epidermidis | CECT231 | Collection | − |
| Staphylococcus warneri | CECT236 | Human skin, collection | − |
| Kocuria varians | CECT230 | Milk, collection | − |
| Listeria monocytogenes | CTC1034 | Fermented sausage, Spain | − |
| Listeria monocytogenes | CTC1011 | Fermented sausage, Spain | − |
| Escherichia coli | CTC1007 | Meat, Spain | − |
| Pseudomonas aeruginosa | CECT108 | Blood, collection | − |
| Salmonella enterica serovar Derby | CTC1022 | Meat, Spain | − |
| Bacillus pumilus | CTC1541 | Fermented sausage, Spain | − |
The strains were obtained from the following sources: CECT, Spanish Type Culture collection; ATCC, American Type Culture Collection; DSM; German Collection of Microorganisms and Cell Cultures; J, FLEC laboratory of INRA, Jouy en Josas, France; LTH, Laboratory of Lebensmittel Technology, Hohemheim, Germany; CTC, IRTA-Meat Technology Center.
+, positive real-time PCR result; −, negative real-time PCR result.
DNA extraction from bacterial strains.
For specificity tests, the genomic DNA of L. sakei and non-L. sakei strains was isolated from overnight culture broth using the DNeasy tissue kit (QIAGEN, Hilden, Germany). The genomic DNA concentration was quantified by the PicoGreen double-stranded DNA quantification kit (Molecular Probes, Inc., Eugene, Oreg.) using a fluorimeter model SFM 25 (Kontron Instruments, Bletchley, United Kingdom). One nanogram of DNA from each reference strain was used to test the specificity of the assay.
Pre-PCR treatment of fermented sausages and meat samples.
Ten grams of fermented sausage or minced meat was diluted 10-fold with 90 ml of buffered peptone water (AES Laboratoires, Combourg, France) and homogenized for 1 min in 125-μm filter stomacher bags (Biochek, Foster City, Calif.). The homogenate (1.5 ml) was centrifuged for 5 min at 14,000 × g and 4°C. The supernatant was carefully discarded, and the pellet was resuspended in 150 μl of 6% Chelex 100 chelating ion-exchange resin (Bio-Rad, Munich, Germany). To achieve microbial cell lysis, the samples were submitted to a microwave treatment at 700 W for 10 min as reported by Cocconcelli et al. (13) and immediately incubated on ice. After a centrifugation step at 14,000 × g for 5 min, 50 μl of the supernatant was transferred to a new tube and stored at −20°C. One microliter was directly used in real-time PCR assays. As an alternative treatment, the commercial Wizard genomic DNA purification kit (Promega, Madison, Wis.) was also evaluated. The same quantity of homogenate (1.5 ml) was purified by following the recommendations of the manufacturer. The isolated DNA was finally rehydrated in 15 μl of distilled water.
Artificially inoculated sausage model.
Ten grams of fermented sausage was diluted (1:10) in 90 ml of buffered peptone water and homogenized for 1 min in stomacher bags, filtered through a 25-μm filter (Miracloth filter; Calbiochem, La Jolla, Calif.), vacuum filtered through a 0.45-μm filter (catalog no. 7141 114; Whatman, Maidstone, United Kingdom) and through a 0.22-μm syringe filter (catalog no. 10462200; Schleicher & Schuell MicroScience, Dassel, Germany). The filter-sterilized extract was artificially inoculated with decreasing amounts of an overnight culture of L. sakei CTC494 to obtain a CFU range equivalent to approximately 6 × 106, 6 × 105, 6 × 104, 6 × 103, 6 × 102, 6 × 101, 32, 16, 8, 4, 2, and 1 cells per amplification reaction mixture. L. sakei counts were determined by plating onto MRS agar. After that, the samples were submitted to the pre-PCR treatment with Chelex 100 and microwaves as described above. The experiment was repeated three independent times with triplicates within each assay.
Primers and probes design.
The sequences of the primers (sakF and sakR) and probes for the target species were designed on the 16S-23S rRNA intergenic transcribed spacer (ITS) region of L. sakei using the software Primer Express v2.0 (Applied Biosystems, Foster City, Calif.), and their sequences are shown in Table 2. Lactobacillus sakei belongs to a group of closely related Lactobacillus species that includes Lactobacillus curvatus and Lactobacillus graminis (7). In this case, rRNA probes cannot be used because of high similarity of rRNA genes (18). Among Lactobacillus species, ITS regions exhibit a higher interspecies variation than rRNA genes without intraspecies variation (7), which can be successfully applied for designing species-specific primers and probes. Primer sakF and primer sakR were selected to amplify an ITS fragment of 78 bp, as shown in Fig. 1. The internal L. sakei probe, sakS, was labeled at the 5′ end with the reporter dye FAM (6-carboxyfluorescein) and designed as a TaqMan minor groove binder (MGB) probe. TaqMan MGB probes form extremely stable complexes when bound to target DNA and, compared to traditional TaqMan probes, have higher melting temperatures (34). The specificity of the sequences was checked against all the available data in the GenBank database. The Internal amplification control (IAC) probe IPCsakei (Table 2) was designed in the inner region of the IAC sequence. This probe was labeled at the 5′ end with the reporter dye VIC and at the 3′ end with the quencher 6-carboxy-tetramethyl-rhodamin (TAMRA). All probes were purchased from Applied Biosystems, and primers were purchased from Roche Molecular Biochemicals (Indianapolis, Ind.).
TABLE 2.
Primers, probes, and IAC designed in this study
| Designation | Target gene/usea | Sequence (5′-3′) | Accession no. | Position |
|---|---|---|---|---|
| SakF | ITS | GATAAGCGTGAGGTCGATGGTT | U97137 | 126-147 |
| SakR | ITS | GAGCTAATCCCCCATAATGAAACTAT | U97137 | 179-204 |
| SakS | ITS | FAM-GCCCATTGTACCAATTT-MGB | U97137 | 161-177 |
| IPCsakei | IAC probe | VIC-AACACCTATTAGACATTCGTTCCATTGGTCGA-TAMRA | X77576 | 9685-9716 |
| IPCsakF | IAC construction | GTGAGGTCGATGGTTGCTGTATAATCGAGC | ||
| IPCsakR | IAC construction | CCCATAATGAAACTATCGGCTCTTCTGCATAG | ||
| IAC sequence | GATAAGCGTGAGGTCGATGGTTGCTGTATAATCGAGCGAGATGAAGGACGAACACCTATTAGACATTCGTTCCATTGGTCGATGGAGAAACAGTACTATGCAGAAGAGCCGATAGTTTCATTATGGGGGATTAGCTC |
The underlined text has identity with the sakF and sakR primers. ITS, 16S-23S rRNA intergenic transcribed spacer region.
FIG. 1.
Alignment of partial 16S-23S rRNA intergenic region sequences of L. sakei (accession number U97137) and other lactic acid bacteria frequently found on meat environment and fermented foods: L. curvatus (accession number U97135), L. plantarum (accession number U97139), P. acidilactici (accession number DQ104396), L. pentosus (accession number U97141), and L. brevis (accession number X74221). Mismatches with the designed L. sakei primers and probe are indicated by gray shading. PCR primers and probe annealing sites are indicated in boldface type. The target for the fluorogenic probe used in real-time quantitative PCR is indicated in italics and boldface type.
Construction of internal control.
A competitive internal control was designed by the composite primer approach in a two-step PCR protocol as reported by Hoorfar et al. (23). The designed IAC consisted of an 89-bp fragment of the acetyl coenzyme A carboxylase gene (BnACCg8, GenBank accession no. X77576) from rapeseed (Brassica napus) flanked by the sakF/R primers. Seeds of Brassica napus were provided by GEVES (Surgeres, France), and DNA was isolated according to the method described by Dellaporta et al. (16). Briefly, 100 ng of rapeseed DNA was PCR amplified using the chimeric primers IPCsakF and IPCsakR (Table 2) in a 20-μl reaction mix containing 2.0 μl of PCR buffer (Invitrogen, Merelbeke, Belgium), 150 μM concentrations of each deoxynucleoside triphosphate (Promega, Madison, Wis.), 3 mM MgCl2, 0.3 U of Taq polymerase (Invitrogen), and 0.3 μM concentrations of each primer. PCRs were run under the following conditions: 1 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C, and a final elongation step of 4 min at 72°C. The amplified DNA was subsequently diluted 1:1,000 and 1:5,000 in 1 mM Tris-HCl (pH 8.0), 0.01 mM EDTA (TE) and used as a template in a second PCR performed with the specific primers sakF/sakR under the same conditions, except the hybridization temperature was raised to 63°C. The amplified IAC was purified using the GeneClean gel extraction kit (Bio 101, La Jolla, Calif.). The eluate was quantified, and the number of copies was adjusted, using TE buffer in the presence of carrier tRNA (5 ng/μl), down to the working concentration.
Real-time PCR conditions.
The TaqMan PCR core reagents kit (Applied Biosystems) was used in a 20-μl reaction volume containing 1× PCR TaqMan buffer A (including 5-carboxy-X-rhodamine as a passive reference dye), 6 mM MgCl2, 200 μM (each) dATP, dCTP, and dGTP, 400 μM dUTP, 50 nM primer sakF, 500 nM primer SakR, 200 nM sakS probe, 200 nM IPCsakei probe, 100 to 200 copies of IAC DNA (137 bp), 1 U of AmpliTaq Gold DNA polymerase, 0.2 units of AmpErase uracil N-glycosylase, and 1 μl of sample.
Reactions were run in an ABI PRISM 7700 sequence detection system device (Applied Biosystems) using the following program: 2 min at 50°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C and 1 min at 63°C. Results were analyzed using the Sequence Detection System software version 1.9.1 (Applied Biosystems). Quantification was performed by interpolation in a standard regression curve of cycle threshold (CT) values generated from samples of known concentrations. Real-time PCR assays with CT values over 40 were considered negative. Nontemplate controls and noninoculated samples were included in all PCR runs and tested negative. The experiment was repeated three independent times with triplicates within each assay.
Standard curves and amplification efficiency.
To determine the sensitivity and amplification efficiency of the real-time PCR assay, two different calibration curves were constructed. One standard curve consisted of purified genomic DNA from L. sakei CTC494, and the other consisted of a sausage model system inoculated with decreasing amounts of L. sakei CTC494. Purified genomic DNA from L. sakei CTC494 was serially diluted with TE buffer (pH 8.0) to obtain a range of DNA concentrations equivalent to approximately 5 × 106, 5 × 105, 5 × 104, 5 × 103, 5 × 102, 250, 125, 62, 31, 15, 8, 4, 2, 1.5, and 1 genome copies per amplification reaction mixture. The number of L. sakei genome equivalents was estimated by considering the genome size of L. sakei 23K (30). Consequently, one L. sakei genome equivalent weighs approximately 2 fg. The sterilized sausage model system, obtained as described above, was inoculated with decreasing levels of L. sakei CTC494 to obtain approximately from 6 × 105 CFU to 1 CFU per amplification reaction mixture. L. sakei counts were determined by plating onto MRS agar. Each experiment was repeated three times, with three replicates within each experiment. By plotting the number of genome equivalents or input cells against the CT value, a linear relationship was formed. The slope of this curve was used to determine the amplification efficiency from the equation E = 10−1/slope − 1 (31).
Quantification of L. sakei in naturally contaminated samples.
Relative accuracy is defined as the closeness of the agreement between the results obtained by an accepted method and the results obtained by an alternative method (3). Eleven samples of fermented sausages and meat batters (comminuted raw meat and fat with the different ingredients included in a sausage formulation) were purchased from local butchers. All samples were submitted to LAB counts by plating on MRS agar (Merck, Darmstadt, Germany) according to AFNOR methodology (1). The incubation was carried at 30°C for 72 h in anaerobiosis (Oxoid jars with Anaerogen; Oxoid). A representative number of colonies, between 20 and 100, of each MRS plate were picked and submitted to L. sakei-specific conventional PCR (4) to determine the percentage of L. sakei in the LAB population of the samples. Each sample was also analyzed in triplicate by the L. sakei real-time PCR method developed. L. sakei estimated counts were calculated by interpolation of the CT values to a standard curve constructed with serial dilutions of a meat model extract.
Statistical analysis.
Statistical analysis of data was carried out using SAS statistical software (SAS System for Windows NT, release 8.1; SAS Institute, Inc., Cary, N.C.). The nonparametric linear statistical method described by Bablok and Passing (6) was used to compare the data between MRS plating and real-time PCR methods.
RESULTS
Design and optimization of L. sakei real-time PCR assay.
The real-time PCR was optimized by varying the concentrations of MgCl2, primers, and probe. It was found that 6 mM MgCl2, 50 nM primer sakF, 500 nM primer sakR, and 200 nM sakS probe (FAM) and IPCsakei probe (VIC) were the optimized concentrations for this real-time PCR assay. All reaction mixtures with amounts of IAC between 100 to 200 molecules showed a positive VIC (reporter dye of the IAC) fluorescence signal without disturbing the FAM (reporter dye of the target) signal.
Specificity of the assay.
The real-time PCR designed was assayed with 1 ng of genomic DNA from each of 52 strains of L. sakei and 45 non-L. sakei strains (Table 1). All L. sakei strains tested were correctly identified, as they showed a positive FAM fluorescence signal. The threshold line of the assay was set to a fluorescence value of 0.02 for FAM, obtaining CT values varying from 17.93 to 21.30 with a CT mean of 18.90 ± 0.49. All 45 non-L. sakei showed CT values over 40, showing that the real-time PCR assay developed was specific for L. sakei.
Sensitivity and quantification limits of the assay.
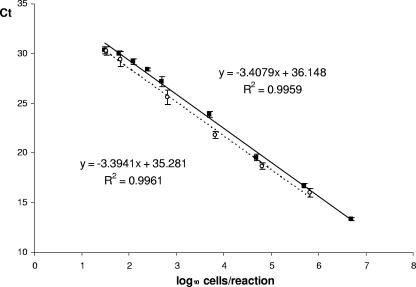
The detection and quantification limits of the PCR assays were determined by (i) 10-fold serially diluted genomic DNA extracted from L. sakei CTC494 overnight cultures and (ii) a sausage model system inoculated with decreasing amounts of L. sakei CTC494 cells. Amplification reactions were performed with a range of concentrations approximately from 5 × 106 to 1 genome equivalent of purified L. sakei DNA. The minimum level of detection was 3 genome equivalents per reaction mixture (positive amplification in all replicates), and it was possible to amplify 1 cell per reaction mixture in 6 of 9 total replicates. The slope of the linear regression curves calculated over a 5-log range from 5 × 106 to 30 genome equivalents was −3.4079 (R2 = 0.996), which indicates an amplification efficiency of 96.5% (Fig. 2).
FIG. 2.
Standard curves for L. sakei real-time PCR. Two different standard curves were constructed, one with serially diluted purified DNA (▪) and the other using a sausage model inoculated with decreasing amounts of L. sakei cells (○). Standard curves were plotted for the estimated log cell number of bacteria versus the CT values. Amplification reactions were performed with a range of concentrations from 5 × 106 to 1 genome equivalent of L. sakei when using purified DNA, and from 6 × 105 to approximately 1 CFU of L. sakei per reaction mixture when using the sausage model. The CT values are the means of three different experiments within triplicates.
The sensitivity of the assay was also investigated by using a sterilized sausage model system, inoculated with an overnight culture of L. sakei CTC494 to obtain different cell inputs, from 6 × 105 to 1 cell per amplification reaction mixture. After the inoculation, the samples were submitted to a pre-PCR treatment with Chelex 100 and microwave lysis and quantified by real-time PCR. With this experiment, the minimum level of detection was 3 cells per reaction equivalent to 3 × 103 CFU/g in the original sample. The linear quantification was determined to be between 32 and 6 × 105 input cells per reaction mixture (R2 = 0.996), with a slope of −3.3941 and an amplification efficiency of 97.1% (Fig. 2).
The estimation of the practical limit of quantification requires consideration of the sampling error, the correlation factor of the calibration curves, and the slope that determines the amplification efficiency. The correlation factor and the amplification efficiency decreased importantly below 30 input cells. Taking into account these results, the quantification limit was established at approximately 30 genome copies and cells per reaction mixture for the DNA and sausage models, respectively.
Enumeration of L. sakei in naturally contaminated samples.
The L. sakei population in food samples was studied to assess the accuracy of the real-time PCR method and the influence of background microorganisms naturally found in meat products. We detected the presence of L. sakei in meat batter and fermented sausages in the range of 3 to 9 log10 CFU/g (Table 3).
TABLE 3.
Real-time PCR-based quantification method of L. sakei compared to traditional plate counta
| Sample no. | Type of sample | MRS count (mean ± SE)b | % of L. sakei ± SEc | L. sakei count (mean ± SE)b | L. sakei count estimated by real-time PCR (mean ± SE)b |
|---|---|---|---|---|---|
| S1 | Fermented sausage | 7.69 ± 0.02 | 100 ± 0.0 | 7.69 ± 0.00 | 7.08 ± 0.05 |
| S2 | Fermented sausage | 7.52 ± 0.06 | 75 ± 8.8 | 6.77 ± 0.61 | 6.24 ± 0.22 |
| S3 | Fermented sausage | 8.79 ± 0.08 | 64 ± 9.6 | 7.99 ± 0.79 | 7.39 ± 0.15 |
| S4 | Fermented sausage | 8.43 ± 0.04 | 100 ± 0.0 | 8.43 ± 0.00 | 8.07 ± 0.03 |
| S5 | Fermented sausage | 8.32 ± 0.06 | 48 ± 9.9 | 8.00 ± 0.83 | 7.85 ± 0.09 |
| S6 | Fermented sausage | 7.52 ± 0.07 | 0 ± 0.0 | ND | ND |
| S7 | Fermented sausage | 9.20 ± 0.02 | 12 ± 3.2 | 7.18 ± 0.26 | 6.84 ± 0.06 |
| M1 | Meat batter | 4.60 ± 0.11 | 0 ± 0.0 | ND | ND |
| M2 | Meat batter | 6.10 ± 0.05 | 68 ± 9.7 | 5.93 ± 0.59 | 5.41 ± 0.01 |
| M3 | Meat batter | 4.87 ± 0.01 | 0 ± 0.0 | ND | ND |
| M4 | Meat batter | 3.62 ± 0.08 | 60 ± 9.2 | 3.40 ± 0.34 | D |
Samples were purified by pre-PCR treatment with Chelex 100. Counts are expressed in log10 CFU/g.
The values are means of triplicates ± standard errors.
Percentages of L. sakei were calculated by the identification of a minimum of 25 colonies from MRS plates, and the standard errors were calculated from a binomial sampling distribution. ND, none detected; D, detected below the quantification limit.
The accuracy of the method was assessed by testing 11 different samples comprising fermented sausages and initial meat batter using the real-time PCR assay developed. The results were compared with MRS counts, followed by determination of the percentage of L. sakei in the samples, checking 25 to 100 colonies per plate by L. sakei-specific PCR (4). The percentage of L. sakei obtained was then multiplied by the total LAB counts in MRS to achieve an estimated L. sakei count for each sample (Table 3). The relative accuracy of the real-time PCR-based method with respect to the plate count method varied from 91.24% to 98.16%. The correlation showed a significant (P < 0.0001) linear relationship between both methods, with an R value of 0.985. Method comparison was also carried out using Passing-Bablok linear regression. The equation obtained was y = 1.0640x − 0.8995 CFU/g, and the 95% confidence interval of the intercept included the value of 0, meaning that there was no constant systematic error between the methods compared. Moreover, the 95% confidence interval of the slope included the value 1, so the measurements between the compared methods were free from proportional systematic error. Therefore, values obtained with the real-time PCR method are not significantly different from those obtained by plate counting (P > 0.05).
L. sakei was not present in three of the samples tested either by plate counts or by real-time PCR. One of the samples presented L. sakei counts near the detection limit and below the limit of quantification.
The Wizard genomic DNA purification kit was also evaluated as a pre-PCR treatment. This method allowed increase of the DNA concentration per sample and raised the sensitivity of the assay by around 1 order of magnitude. Therefore, 10 mg of the purified sample could be added to the real-time PCR instead of 1 mg (using the Chelex 100 procedure) without inhibitory consequences. Using the Wizard genomic DNA purification kit, the detection limit could be established at 3 × 102 CFU/g of sample (results not shown).
DISCUSSION
L. sakei is one of the lactic acid bacteria most frequently described in fermented meat products (5, 15, 25, 40, 42, 43, 48, 50). The study of the implantation of L. sakei during production processes of fermented sausages and its real counts in final products is of great interest for evaluation of the importance of this bacterium in meat fermentation. For this purpose, an effective method for enumeration of L. sakei is required. The real-time PCR method developed for quantitative detection of L. sakei supposes a specific and sensitive technique to enumerate L. sakei in meat and fermented sausages and allows the monitoring of this bacterium in this kind of sample.
Although the 16S-23S rRNA intergenic transcribed spacer region has been used for conventional PCR detection of L. sakei (4, 7), no direct methodology to count L. sakei from samples has been available. Indirect methods are time-consuming, as they required plate counting and confirmation of a number of representative colonies. The real-time PCR method presented here is highly specific. The specificity of the primers and probe was tested both by identity searches in a nucleotide database (GenBank, BLASTN version 2.2.6) and by the screening of 45 representative non-L. sakei and 52 L. sakei strains. The primers and the probe (sakF, sakR, and sakS) designed in this study enabled the specific detection of all of the L. sakei strains isolated from different fermented meat products tested. The mean CT obtained for the L. sakei isolates (18.90 ± 0.49) indicates a low strain-to-strain variation in the CT values. Other Lactobacillus species and the most common species found in meat and meat products were also tested, and no amplification was obtained, suggesting that the primers, the probe, and the real-time PCR conditions applied were specific for L. sakei detection.
The specificity of the probes ensures that no signal is generated by nontarget amplicons. In this system, the IAC has the possibility of detecting underestimation or false-negative results. The competitive design of IAC recommended by Hoorfar et al. (24) avoids the risk of undesired interactions of multiple primers by having both PCRs (the target specific and the IAC specific) working with the same primer set and under identical PCR conditions, as reported. The use of an IAC indicates the presence of DNA polymerase inhibitors or PCR failure caused by any mistake during the process (36). This is of special importance in PCR-based methods performed with complex samples, such as meat products. The IAC used in this assay was a chimerical DNA sequence recognized by a specific probe labeled with VIC. The optimal initial number of IAC copies per PCR mixture was established at 100 to 200 copies. A lower number of IAC copies per reaction mixture caused unstable fluorescence signals even in the presence of low input cells, as described by Malorny et al. (36).
One of the goals of this study was to develop an optimal rapid method for pre-PCR treatment of meat batters and fermented sausages. The Chelex 100-based DNA purification step prior to real-time PCR produced a sensitivity similar to that of pure DNA. The limit of detection using the Chelex 100 purification was established at 3 cells per reaction mixture, similar to the sensitivities of other real-time PCR assays reported (45), and suppose an initial count of 3 × 103 CFU/g of L. sakei in the original sample. The sensitivity of the method could be increased by concentrating the sample. With the Chelex 100 purification procedure, only 1 mg of the purified sample was tested in the PCR vessel, and all attempts to increase this quantity failed as a result of inhibitory processes. As an alternative, we evaluated DNA isolation with the Wizard genomic DNA purification kit (Promega), as suggested by Rodríguez-Lázaro et al. (46), which increased the sensitivity of the method around 1 order of magnitude. This method allowed an increase in the amount of the purified sample added to the real-time PCR to 10 mg without inhibitory consequences. With this procedure, the detection limit could be established at 3 × 102 CFU/g of sample, which would be important in samples containing low counts of L. sakei, such as raw meat or the meat batter used in the elaboration of fermented sausages.
We further evaluated the capacity of the method for quantification of L. sakei. Regression curves correlating the CT values obtained from samples and the corresponding L. sakei inoculums were calculated. Coefficient of correlation (R2) values demonstrated the quantification linearity over a range down to 30 cells per reaction mixture in both DNA and sausage models. Up to this cell input, the R2 has an optimal value of 0.996, and the efficiency of the reaction (33) was determined as 96.5% and 97.1% using the DNA and sausage models, respectively. This efficiency indicated excellent real-time PCR performance when using the pre-PCR treatment with Chelex 100 and microwave lysis, and the similarity to the results obtained when using purified genomic DNA suggests an appropriate adequacy of the pre-PCR treatment of the samples. The estimation of the practical limit of quantification requires the sampling errors to be considered. In view of the efficiency of the reaction, the coefficient of correlation, and the standard deviation on the CT values, we considered that, with CT values higher than 31 (which corresponds to less than 30 cells/reaction mixture), experimental precision would be insufficient. In those cases, quantification is not reliable because the sampling error contributed significantly to the experimental data (51, 57).
A critical parameter in validating an alternative method is the relative accuracy, defined as the closeness of agreement between a test result and the accepted reference value (3). To validate the method, we compared the results obtained with the traditional plating method to enumerate lactic acid bacteria in food (1) followed by species-specific PCR detection. Food samples, and specifically meat and fermented sausages, are highly complex matrices, with high numbers of PCR inhibitors and background microflora (37, 47, 56) that could interfere with the ability of real-time PCR to detect and quantify L. sakei. Therefore, it is important to study the accuracy of the real-time PCR method developed and its suitability for further application in meat products, since the use of model food systems does not always provide an accurate replication of the conditions in food samples (56). With that aim, naturally contaminated fermented sausages and meat batters were analyzed both by a traditional culture method and by real-time PCR. The culture medium used was MRS, and to enumerate L. sakei, a representative number of colonies from plates were identified by L. sakei-specific PCR (4). The relative accuracy obtained indicates a good agreement between methods as reported by other authors (45, 46). Moreover, the statistical analysis performed showed no significant differences between methods. This indicates the reliability of the designed real-time PCR technique to count L. sakei in real samples and also the capacity of the pre-PCR treatment to avoid the interference of the inhibitors present in these kinds of products.
If real-time PCR is intended for quantification, it is a prerequisite to obtain information on both linear range and amplification efficiency to ensure correct quantification (28). In the present study, the linear range obtained allowed us to quantify the range levels of L. sakei currently found in fermented sausages. The detection limit of this technique was also appropriate to enumerate lower numbers of L. sakei, such as in meat batter, but a complete DNA extraction protocol to remove PCR inhibitors and to concentrate the sample could be recommended to quantify L. sakei in fresh meat or meat batter, where counts are expected to be lower than in fermented sausages. We were able to detect L. sakei in a meat batter sample with an estimated count near the detection limit, although the value was below the quantification limit.
In conclusion, this real-time PCR method provides a highly sensitive and specific tool for monitoring L. sakei during meat fermentation and the ripening process both from endogenous microflora or from inoculated starter cultures and to study the distribution and abundance of this bacterium in complex microbial communities.
Acknowledgments
This work was supported by the European Union (TRADISAUSAGE QLK1-CT2002-02240).
We thank Vicenç Oliveras (University of Girona) for technical support. We also thank David Rodríguez (University of Bristol) for helpful suggestions.
REFERENCES
- 1.AFNOR. 1988. Viandes et produits à base de viande-dénombrement des bactéries lactiques (NF V04-503). Association Française de Normalisation, La Plaine Saint-Denis, France.
- 2.Al-Soud, W. A., and P. Rådström. 2000. Effects of amplification facilitators on diagnostic PCR in the presence of blood, feces, and meat. J. Clin. Microbiol. 38:4463-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 1994. Accuracy (trueness and precision) of measurement methods and results. Part 2. Basic method for the determination of repeatability and reproducibility of a standard measurement method (ISO 5725-2:1994). International Organization for Standardization, Geneva, Switzerland.
- 4.Aymerich, M. T., B. Martín, M. Garriga, and M. Hugas. 2003. Microbial quality and direct PCR identification of lactic acid bacteria and nonpathogenic staphylococci from artisanal low-acid sausages. Appl. Environ. Microbiol. 69:4583-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aymerich, M. T., B. Martín, M. Garriga, M. C. Vidal Carou, S. Bover-Cid, and M. Hugas. 2005. Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J. Appl. Microbiol. 100:40-49. [DOI] [PubMed] [Google Scholar]
- 6.Bablok, W., and H. Passing. 1985. Application of statistical procedures in analytical instrument testing. J. Autom. Chem. 7:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthier, F., and S. D. Ehrlich. 1998. Rapid species identification within two groups of closely related lactobacilli using PCR primers that target the 16S/23S rRNA spacer region. FEMS Microbiol. Lett. 161:97-106. [DOI] [PubMed] [Google Scholar]
- 8.Berthier, F., and S. D. Ehrlich. 1999. Genetic diversity within Lactobacillus sakei and Lactobacillus curvatus and design of PCR primers for its detection using randomly amplified polymorphic DNA. Int. J. Syst. Bacteriol. 49:997-1007. [DOI] [PubMed] [Google Scholar]
- 9.Byun, R., M. A. Nadkarni, K. L. Chhour, F. E. Martin, N. A. Jacques, and N. Hunter. 2004. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J. Clin. Microbiol. 42:3128-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell-Platt, G. 1995. Fermented meats—a world perspective, p. 39-52. In G. Campbell-Platt and P. E. Cook (ed.), Fermented meats. Black Academic & Professional, London, United Kingdom.
- 11.Caplice, E., and G. F. Fitzgerald. 1999. Food fermentations: role of microorganisms in food production and preservation. Int. J. Food Microbiol. 50:131-149. [DOI] [PubMed] [Google Scholar]
- 12.Chaillou, S., M. C. Champomier-vergès, M. Cornet, A. M. Crutz Le Coq, A. M. Dudez, V. Martin, S. Beaufils, R. Bossy, E. Darbon-Rongère, V. Loux, and M. Zagorec. 2005. Complete genome sequence of the meat borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 23:1527-1533. [DOI] [PubMed] [Google Scholar]
- 13.Cocconcelli, P. S., D. Porro, S. Galandini, and L. Senini. 1995. Development of RAPD protocol for typing of strains of lactic acid bacteria and enterococci. Lett. Appl. Microbiol. 21:376-379. [DOI] [PubMed] [Google Scholar]
- 14.Cocolin, L., K. Rantsiou, L. Iacumin, R. Urso, C. Cantoni, and G. Comi. 2004. Study of the ecology of fresh sausages and characterization of populations of lactic acid bacteria by molecular methods. Appl. Environ. Microbiol. 70:1883-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppola, R., B. Giagnacovo, M. Iorizzo, and L. Grazia. 1998. Characterization of lactobacilli involved in the ripening of sopressata molisana, a typical southern Italy fermented sausage. Food Microbiol. 15:347-353. [Google Scholar]
- 16.Dellaporta, S., J. Wood, and J. Hicks. 1983. A plant DNA minipreparation version II. Plant Mol. Biol. Rep. 1:19-21. [Google Scholar]
- 17.de Vos, W. M. 1999. Safe and sustainable systems for food-grade fermentations by genetically modified lactic acid bacteria. Int. Dairy J. 9:3-10. [Google Scholar]
- 18.Fox, G. E., J. D. Wisotzkey, and P. Jurtshuk. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 19.Furet, J. P., P. Quenee, and P. Tailliez. 2004. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int. J. Food Microbiol. 97:197-207. [DOI] [PubMed] [Google Scholar]
- 20.Grattepanche, F., C. Lacroix, P. Audet, and G. Lapointe. 2005. Quantification by real-time PCR of Lactococcus lactis subsp. cremoris in milk fermented by a mixed culture. Appl. Microbiol. Biotechnol. 66:414-421. [DOI] [PubMed] [Google Scholar]
- 21.Hammes, W. P., A. Bantleon, and S. Min. 1990. Lactic acid bacteria in meat fermentation. FEMS Microbiol. Rev. 87:165-174. [Google Scholar]
- 22.He, J. W., and S. Jiang. 2005. Quantification of enterococci and human adenoviruses in environmental samples by real-time PCR. Appl. Environ. Microbiol. 71:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoorfar, J., P. Ahrens, and P. Radstrom. 2000. Automated 5′ nuclease PCR assay for identification of Salmonella enterica. J. Clin. Microbiol. 38:3429-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoorfar, J., N. Cook, B. Malorny, M. Wagner, D. De Medici, A. Abdulmawjood, and P. Fach. 2004. Diagnostic PCR: making internal amplification control mandatory. J. Appl. Microbiol. 96:221-222. [DOI] [PubMed] [Google Scholar]
- 25.Hugas, M., M. Garriga, T. Aymerich, and J. M. Monfort. 1993. Biochemical characterization of lactobacilli from dry fermented sausages. Int. J. Food Microbiol. 18:107-113. [DOI] [PubMed] [Google Scholar]
- 26.Hugas, M., M. Garriga, M. T. Aymerich, and J. M. Monfort. 1995. Inhibition of Listeria in dry fermented sausages by the bacteriocinogenic Lactobacillus sake CTC494. J. Appl. Bacteriol. 79:322-330. [Google Scholar]
- 27.Hugas, M., B. Neumeyer, F. Pagés, M. Garriga, and W. P. Hammes. 1997. Comparison of bacteriocin-producing lactobacilli on Listeria growth in fermented sausages. Fleischwirtschaft 76:649-652. [Google Scholar]
- 28.Josefsen, M. H., N. R. Jacobsen, and J. Hoorfar. 2004. Enrichment followed by quantitative PCR both for rapid detection and as a tool for quantitative risk assessment of food-borne thermotolerant campylobacters. Appl. Environ. Microbiol. 70:3588-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandler, O., and N. Weiss. 1986. Regular, nonsporing gram-positive rods, p. 1208-1234. In J. G. Holt and P. H. A. Sneath (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 30.Klaenhammer, T., E. Altermann, F. Arigoni, A. Bolotin, T. F. Breid, J. Broadbent, R. Cano, S. Chaillou, J. Deutscher, M. Gasson, M. van de Guchte, J. Guzzo, A. Hartke, T. Hawkins, P. Hols, R. Hutkins, M. Kleerebezem, J. Kok, O. Kuipers, M. Lubbers, E. Maguin, L. McKay, D. Mills, A. Nauta, R. Overbeek, H. Pel, D. Pridmore, M. Saier, D. van Sinceren, A. Sorokin, J. Steele, D. O'Sullivan, W. de Vos, B. Weimer, M. Zagorec, and R. Siezen. 2002. Discovering lactic acid bacteria by genomics. Antonie Leeuwenhoek 82:29-58. [DOI] [PubMed] [Google Scholar]
- 31.Klein, D. 2002. Quantification using real-time PCR technology: applications and limitations. Trends Mol. Med. 8:257-260. [DOI] [PubMed] [Google Scholar]
- 32.Klein, G., L. M. T. Dicks, A. Pack, B. Hack, K. Zimmermann, F. Dellaglio, and G. Reuter. 1996. Emended description of Lactobacillus sake (Katagiri, Kitahara, and Fukami) and Lactobacillus curvatus (Abo-Elnaga and Kandler): numerical classification revealed by protein fingerprinting and identification based on biochemical patterns and DNA-DNA hybridizations. Int. J. Syst. Bacteriol. 46:367-376. [Google Scholar]
- 33.Knutsson, R., C. Löfström, H. Grage, J. Hoorfar, and P. Radstrom. 2002. Modeling of 5′nuclease real-time responses for optimization of a high-throughput enrichment PCR procedure for Salmonella enterica. J. Clin. Microbiol. 40:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutyavin, I. V., I. A. Afonina, A. Mills, V. V. Gorn, E. A. Lukhtanov, E. S. Belousov, M. J. Singer, D. K. Walburger, S. G. Lokhov, A. A. Gall, R. Dempcy, M. W. Reed, R. B. Meyer, and J. Hedgpeth. 2000. 3′-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 28:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon, H. S., E. H. Yang, S. W. Yeon, B. H. Kang, and T. Y. Kim. 2004. Rapid identification of probiotic Lactobacillus species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol. Lett. 239:267-275. [DOI] [PubMed] [Google Scholar]
- 36.Malorny, B., E. Paccassoni, P. Fack, C. Bunge, A. P. Martin, and R. Helmuth. 2004. Diagnostic real-time PCR for detection of Salmonella in food. Appl. Environ. Microbiol. 70:7046-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martín, B., A. Jofré, M. Garriga, M. Hugas, and M. T. Aymerich. 2004. Quantification of Listeria monocytogenes in fermented sausages by MPN-PCR method. Lett. Appl. Microbiol. 39:290-295. [DOI] [PubMed] [Google Scholar]
- 38.Montel, M. C., R. Talon, J. Fournaud, and M. C. Champomier. 1991. A simplified key for identifying homofermentative Lactobacillus and Carnobacterium spp. from meat. J. Appl. Bacteriol. 70:469-472. [DOI] [PubMed] [Google Scholar]
- 39.Nissen, H., and R. H. Dainty. 1995. Comparison of the use of rRNA probes and conventional methods in identifying strains of Lactobacillus sake and L. curvatus isolated from meat. Int. J. Food Microbiol. 25:311-315. [DOI] [PubMed] [Google Scholar]
- 40.Parente, E., S. Griego, and M. A. Crudele. 2001. Phenotypic diversity of lactic acid bacteria isolated from fermented sausages produced in Basilicata (Southern Italy). J. Appl. Microbiol. 90:943-952. [DOI] [PubMed] [Google Scholar]
- 41.Rådström, P., R. Knutsson, P. Wolffs, M. Lövenklev, and C. Löfström. 2004. Pre-PCR processing: strategies to generate PCR-compatible samples. Mol. Biotechnol. 26:133-146. [DOI] [PubMed] [Google Scholar]
- 42.Rantsiou, K., E. H. Drosinos, M. Gialitaki, R. Urso, J. Krommer, J. Gasparik-Reichardt, S. Tóth, J. Metaxopoulos, G. Comi, L. Cocolin, and C. Cantoni. 2005. Molecular characterization of Lactobacillus species isolated from naturally fermented sausages produced in Greece, Hungary and Italy. Food Microbiol. 22:19-28. [Google Scholar]
- 43.Rantsiou, K., R. Urso, L. Iacumin, C. Cantoni, P. Cattaneo, G. Comi, and L. Cocolin. 2005. Culture-dependent and -independent methods to investigate the microbial ecology of Italian fermented sausages. Appl. Environ. Microbiol. 71:1977-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray, B. 1992. Bacteriocins of starter culture bacteria as food biopreservatives, p. 178-201. In B. Ray and M. Daeschel (ed.), Food biopreservatives of microbial origin. CRC Press, Inc., Boca Raton, Fla.
- 45.Rodríguez-Lázaro, D., A. Jofré, M. T. Aymerich, M. Hugas, and M. Pla. 2004. Rapid quantitative detection of Listeria monocytogenes in meat products by real-time PCR. Appl. Environ. Microbiol. 70:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Lázaro, D., A. Jofré, M. T. Aymerich, M. Garriga, and M. Pla. 2005. Rapid quantitative detection of Listeria monocytogenes in salmon products: evaluation of pre-real-time PCR strategies. J. Food Prot. 68:1467-1471. [DOI] [PubMed] [Google Scholar]
- 47.Rossen, L., P. Norskov, K. Holmstrom, and O. F. Rasmussen. 1992. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int. J. Food Microbiol. 17:37-45. [DOI] [PubMed] [Google Scholar]
- 48.Samelis, J., F. Maurogenakis, and J. Metaxopoulos. 1994. Characterisation of lactic acid bacteria isolated from naturally fermented Greek dry salami. Int. J. Food Microbiol. 23:179-196. [DOI] [PubMed] [Google Scholar]
- 49.Samelis, J., E. Tsakelidou, J. Metaxopoulos, and G. Kalantzopoulos. 1995. Differentiation of Lactobacillus sake and L. curvatus isolated from naturally fermented Greek dry salami by SDS-PAGE of whole-cell proteins. J. Appl. Bacteriol. 78:157-163. [DOI] [PubMed] [Google Scholar]
- 50.Santos, E. M., C. González-Fernández, I. Jaime, and J. Rovira. 1998. Comparative study of lactic acid bacteria house flora isolated in different varieties of “chorizo”. Int. J. Food Microbiol. 39:123-128. [DOI] [PubMed] [Google Scholar]
- 51.Saussoy, P., J.-L. Vaerman, N. Straetmans, V. Deneys, G. Cornu, A. Ferrant, and D. Latinne. 2004. Differentiation of acute myeloid leukemia from B- and T-lineage acute lymphoid leukemias by real-time quantitative reverse transcription-PCR of lineage marker mRNAs. Clin. Chem. 50:1165-1173. [DOI] [PubMed] [Google Scholar]
- 52.Schillinger, U., and F. K. Lücke. 1987. Identification of lactobacilli from meat and meat products. Food Microbiol. 4:199-208. [Google Scholar]
- 53.Selim, A. S. M., P. Boonkumklao, T. Sone, A. Assavanig, M. Wada, and A. Yokota. 2005. Development and assessment of a real-time PCR assay for rapid and sensitive detection of a novel thermotolerant bacterium, Lactobacillus thermotolerans, in chicken feces. Appl. Environ. Microbiol. 71:4214-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw, B. G., and C. D. Harding. 1984. A numerical taxonomic study of lactic acid bacteria from vacuum-packed beef, pork, lamb and bacon. J. Appl. Bacteriol. 56:25-40. [DOI] [PubMed] [Google Scholar]
- 55.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 56.Taylor, T. M., D. Elhanafi, M. Drake, and L. A. Jaykus. 2005. Effect of food matrix and cell growth on PCR-based detection of Escherichia coli O157:H7 in ground beef. J. Food Prot. 68:225-232. [DOI] [PubMed] [Google Scholar]
- 57.Vaerman, J. L., P. Saussoy, and I. Ingargiola. 2004. Evaluation of real-time PCR data. J. Biol. Regul. Homeost. Agents 18:212-214. [PubMed] [Google Scholar]
- 58.Vogel, R., G. Bocker, P. Stolz, M. Ehrmann, D. Fanta, W. Ludwig, B. Pot, K. Kersters, K. Schleifer, and W. Hammes. 1994. Identification of lactobacilli from sourdough and description of Lactobacillus pontis sp. nov. Int. J. Syst. Bacteriol. 44:223-229. [DOI] [PubMed] [Google Scholar]