Abstract
The β-agarase C gene (agaC) of a marine bacterium, Vibrio sp. strain PO-303, consisted of 1,437 bp encoding 478 amino acid residues. β-Agarase C was identified as the first β-agarase that cannot hydrolyze neoagarooctaose and smaller neoagarooligosaccharides and was assigned to a novel glycoside hydrolase family.
Agar is a hydrophilic polysaccharide contained in the cell walls of agarophyte red algae and is composed of agarose and agaropectins (7). β-Agarases (EC 3.2.1.81), which hydrolyze agar, are useful tools for the preparation of neoagarooligosaccharides and the isolation of protoplasts from the algae (4). We isolated a β-agarase-producing bacterium, Vibrio sp. strain PO-303, from a sea environment. Among the β-agarases secreted by the organism, β-agarase C was found to be capable of forming neoagarooligosaccharides longer than neoagarohexaose (DP6) from agar (3). In this paper, we describe the cloning and expression of the β-agarase C gene (agaC) and the characterization of the recombinant enzyme (rAgaC).
Cloning of the agaC gene.
A 759-bp DNA fragment was amplified by PCR using chromosomal DNA of strain PO-303 as a template and the degenerate primers GCNAAYTAYACNGCNWSNAAYGC and CCRTTNGCRAANACNACNGG, composed according to the N-terminal amino acid sequences of β-agarase C and its protease-digested fragment. Sequence analysis showed that the 759-bp fragment derived from the agaC gene. The XbaI-digested chromosomal DNA fragments were purified and self-ligated with a Takara ligation kit, version 2. The upstream and downstream regions of the 759-bp fragments were amplified by inverse PCR with the self-ligated DNA fragments as templates and two outward-facing primers designed according to the sequence given above. The PCR product was cloned into a pT7Blue-2 vector (Novagen) and sequenced. The sequencing results showed that the agaC gene consisted of 1,437 bp encoding 478 amino acid residues with a predicted molecular weight of 50,922. A potential ribosome-binding site (AGGAGA) and the −35 and −10 promoter regions were identified upstream of the coding region (15, 17). A possible transcription terminator was found downstream of the TAG termination codon. Comparison of the deduced amino acid sequence with the entries in databases suggested that β-agarase C might belong to a novel glycoside hydrolase (GH) family, because no known GH was found to have homology with it.
Expression and purification of rAgaC.
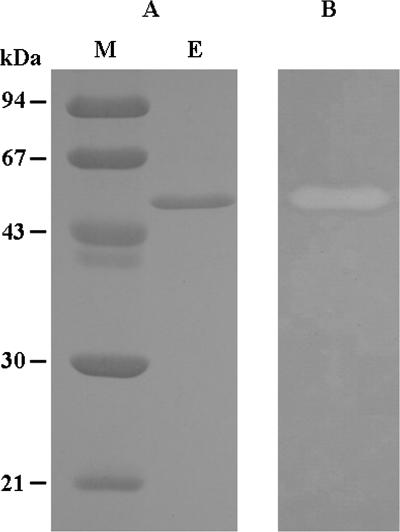
The agaC gene without the signal sequence was amplified by PCR from strain PO-303 chromosomal DNA by using primers GGTGTACATATGGCTAACTATACTGCCAGTAA and GCGGCCCTCGAGCTATTGGCAAGTATAACCTG, which contained artificial NdeI and XhoI sites (italicized). The amplified DNA fragment was digested with NdeI and XhoI and was ligated into a pET22b(+) vector (Novagen) to construct pETAgaC. Escherichia coli BL21(DE3) strains (Novagen) harboring pETAgaC were cultivated for 12 h at 25°C in 1,000 ml of Luria-Bertani medium containing ampicillin and were induced with 1 mM isopropyl-β-d-thiogalactopyranoside. The cells harvested by centrifugation were suspended in sodium acetate buffer (pH 6.0) and disrupted on ice by a sonicator. The supernatant of the cell lysate was collected by centrifugation and purified by chromatography using DEAE-Toyopearl 650M, Ether-Toyopearl 650S, and MonoQ 5/50 GL columns. The activity of the enzyme toward agarose was determined by the Somogyi-Nelson method, which measured the increase in the amount of reduced sugars (18). One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of d-galactose per min. Protein concentrations were measured by the method of Bradford (6). The recombinant AgaC (rAgaC) exhibited an activity of 7,130 U per 1,000 ml of culture and was finally purified 17.9-fold, with a high specific activity of 329 U mg−1 and a final yield of 23.3%. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10) of the purified rAgaC gave a single band with an apparent molecular mass of 47 kDa (Fig. 1A), and activity staining (23) showed that the band had activity against agarose (Fig. 1B). The molecular mass was in good agreement with that deduced from the amino acid sequence (47,240 Da).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (A) and zymogram (B) of purified rAgaC. Lane M, standard markers; lane E, purified rAgaC.
Characterization of rAgaC.
The optimal pH of rAgaC was observed to be around 6.0 when the enzyme activity was assayed at various pHs in Britton-Robinson's universal buffer, and the enzyme was stable between pH 4.0 and 8.0, after being preincubated in the same buffer solutions at various pHs at 25°C for 12 h. The enzyme was optimally active at around 35°C and stable under 37°C.
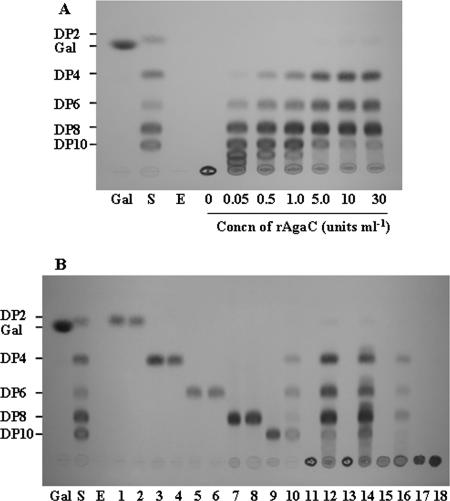
For analysis of the substrate specificity of rAgaC, enzyme reactions were performed at 37°C for 24 h and the hydrolysis products were applied to silica gel 60 thin-layer chromatography plates (solvent, n-butanol-acetic acid-H2O [2:1:1, vol/vol; Merck]). Oligosaccharides were visualized by spraying the plate with diphenylamine/aniline/phosphate reagent (5). The hydrolysis pattern of rAgaC on agarose (Wako Chemicals) was investigated at different enzyme concentrations (0.5 to 30 U ml−1). As shown in Fig. 2A, rAgaC with low activity (0.05 U ml−1) cleaved agarose into longer neoagarooligosaccharides, such as neoagarooctaose (DP8), -decaose (DP10), -dodecaose (DP12), and -tetradecaose (DP14). When the activity was increased to 30 U ml−1, DP8 was produced predominantly in addition to DP6 and DP4. Figure 2B shows the activity patterns of rAgaC (30 U ml−1) against neoagarooligosaccharides (DP2, DP4, DP6, DP8, and DP10), agarose, agar (Ina Food Industry), porphyran, and κ-carrageenan (Wako Chemicals). Porphyran and two neoagarooligosaccharides (DP8 and DP10) were prepared by the methods of Su and Hassid (19) and Aoki et al. (2), respectively. rAgaC did not hydrolyze DP8 or smaller neoagarooligosaccharides but did hydrolyze DP10 into DP4 and DP6 with a small amount of DP8. Therefore, rAgaC was determined to be an enzyme that attacks preferentially the sixth linkage and subsequently the eighth linkage from the nonreducing end of DP10. The hydrolysis products from agar and porphyran were mainly DP8 in addition to DP4 and DP6, results similar to those for agarose. rAgaC did not act on κ-carrageenan.
FIG. 2.
Thin-layer chromatography of hydrolysis products from agarose (A) and from neoagarooligosaccharides and some polysaccharides (B) with rAgaC. Gal, galactose; DP2, DP4, DP6, DP8, and DP10, degrees of polymerization of neoagarooligosaccharides; S, standard neoagarooligosaccharides; E, rAgaC. Lanes 1, 3, 5, 7, and 9, DP2, DP4, DP6, DP8, and DP10, respectively; lanes 2, 4, 6, 8, and 10, hydrolysis products from DP2 to DP10, respectively; lanes 11, 13, 15, and 17, agarose, agar, porphyran, and κ-carrageenan, respectively; lanes 12, 14, 16, and 18, hydrolysis products from agarose, agar, porphyran, and κ-carrageenan, respectively.
Many agarases published to date have been found to act on DP6 to form DP4 and DP2 but not on DP4 (1, 3, 8, 9, 12, 14, 16, 20, 22). Some other agarases have been reported to produce DP2 as the final product (2, 3, 11, 21). Recently, Ohta et al. published a β-agarase that did not act on DP6 (13). To our knowledge, however, no β-agarase unable to degrade not only DP6 but also DP8 has been reported to date. These results have ascertained that β-agarase C is a novel GH family agarase with new substrate specificity.
Nucleotide sequence accession number.
The nucleotide sequence of the agaC gene has been deposited in DDBJ/EMBL/GenBank under accession no. AB218419.
REFERENCES
- 1.Aoki, T., T. Araki, and M. Kitamikado. 1990. Purification and characterization of β-agarase from Vibrio sp. AP-2. Nippon Suisan Gakkaishi 56:825-830. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, T., T. Araki, and M. Kitamikado. 1990. Purification and characterization of a novel β-agarase from Vibrio sp. AP-2. Eur. J. Biochem. 187:461-465. [DOI] [PubMed] [Google Scholar]
- 3.Araki, T., M. Hayakawa, L. Zhang, S. Karita, and T. Morishita. 1998. Purification and characterization of agarases from a marine bacterium, Vibrio sp. PO-303. J. Mar. Biotechnol. 6:260-265. [PubMed] [Google Scholar]
- 4.Araki, T., L. Zhang, and T. Morishita. 1998. Optimization of parameters for isolation of protoplasts from Gracilaria verrucosa (Rhodophyta). J. Mar. Biotechnol. 6:193-197. [PubMed] [Google Scholar]
- 5.Bailey, R. W., and E. J. Bourne. 1960. Colour reactions given by sugars and diphenylamine-aniline spray reagents on paper chromatograms. J. Chromatogr. 4:206-213. [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth, M., and W. Yaphe. 1971. Structure of agar. I. Fractionation of a complex mixture of polysaccharides. Carbohydr. Res. 16:189-197. [Google Scholar]
- 8.Jam, M., D. Flament, J. Allouch, P. Potin, L. Thion, B. Kloareg, M. Czjzek, W. Helbert, G. Michel, and T. Barbeyron. 2005. The endo-β-agarases AgaA and AgaB from the marine bacterium Zobellia galactanivorans: two paralogue enzymes with different molecular organizations and catalytic behaviours. Biochem. J. 385:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirimura, K., N. Masuda, Y. Iwasaki, H. Nakagawa, R. Kobayashi, and S. Usami. 1999. Purification and characterization of a novel β-agarase from an alkalophilic bacterium, Alteromonas sp. E-1. J. Biosci. Bioeng. 87:436-441. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Ohta, Y., Y. Hatada, S. Ito, and K. Horikoshi. 2005. High-level expression of a neoagarobiose-producing β-agarase gene from Agarivorans sp. JAMB-A11 in Bacillus subtilis and enzymatic properties of the recombinant enzyme. Biotechnol. Appl. Biochem. 41:183-191. [DOI] [PubMed] [Google Scholar]
- 12.Ohta, Y., Y. Hatada, Y. Nogi, M. Miyazaki, Z. Li, M. Akita, Y. Hidaka, S. Goda, S. Ito, and K. Horikoshi. 2004. Enzymatic properties and nucleotide and amino acid sequence of a thermostable β-agarase from a novel species of deep-sea Microbulbifer. Appl. Microbiol. Biotechnol. 64:505-514. [DOI] [PubMed] [Google Scholar]
- 13.Ohta, Y., Y. Hatada, Y. Nogi, Z. Li, S. Ito, and K. Horikoshi. 2004. Cloning, expression, and characterization of a glycoside hydrolase family 86 β-agarase from a deep-sea Microbulbifer-like isolate. Appl. Microbiol. Biotechnol. 66:266-275. [DOI] [PubMed] [Google Scholar]
- 14.Ohta, Y., Y. Nogi, M. Miyazaki, Z. Li, Y. Hatada, S. Ito, and K. Horikoshi. 2004. Enzymatic properties and nucleotide and amino acid sequence of a thermostable β-agarase from the novel marine isolate, JAMB-A94. Biosci. Biotechnol. Biochem. 68:1073-1081. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg, M., and D. Court. 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13:319-353. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder, C. D., M. A. Jaffer, and V. E. Coyne. 2003. Investigation of the role of a β(1-4) agarase produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology 149:2919-2929. [DOI] [PubMed] [Google Scholar]
- 17.Shine, J., and L. Dalgarno. 1975. Determinant of cistron specificity in bacterial ribosomes. Nature 254:34-38. [DOI] [PubMed] [Google Scholar]
- 18.Somogyi, M. 1952. Notes on sugar determination. J. Biol. Chem. 195:19-23. [PubMed] [Google Scholar]
- 19.Su, J., and W. Z. Hassid. 1962. Carbohydrates and nucleotides in the red alga Porphyra perforata. I. Isolation and identification of carbohydrates. Biochemistry 1:468-474. [DOI] [PubMed] [Google Scholar]
- 20.Sugano, Y., T. Matsumoto, and M. Noma. 1994. Sequence analysis of the agaB gene encoding a new β-agarase from Vibrio sp. strain JT0107. Biochim. Biophys. Acta 17:105-108. [DOI] [PubMed] [Google Scholar]
- 21.Sugano, Y., T. Matsumoto, H. Kodama, and M. Noma. 1993. Cloning and sequencing of agaA, a unique agarase 0107 gene from a marine bacterium, Vibrio sp. strain JT0107. Appl. Environ. Microbiol. 59:3750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki, H., Y. Sawai, T. Suzuki, and K. Kawai. 2003. Purification and characterization of an extracellular β-agarase from Bacillus sp. MK03. J. Biosci. Bioeng. 95:328-334. [PubMed] [Google Scholar]
- 23.Teather, R. M., and P. J. Wood. 1982. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 43:777-780. [DOI] [PMC free article] [PubMed] [Google Scholar]