Abstract
Salmonella enterica serovar Newport has undergone a rapid epidemic spread in dairy cattle. This provides an efficient mechanism for pathogen amplification and dissemination into the environment through manure spreading on agricultural land. The objective of this study was to determine the survival characteristics of Salmonella serovar Newport in manure and manure-amended soils where the pathogen may be amplified. A multidrug-resistant (MDR) Salmonella serovar Newport strain and a drug-susceptible (DS) strain, both bovine isolates, were inoculated into dairy manure that was incubated under constant temperature and moisture conditions alone or after being mixed with sterilized or nonsterilized soil. Salmonella serovar Newport concentrations increased by up to 400% in the first 1 to 3 days following inoculation, and a trend of steady decline followed. With manure treatment, a sharp decline in cell concentration occurred after day 35, possibly due to microbial antagonism. For all treatments, decreases in Salmonella serovar Newport concentrations over time fit a first-order kinetic model. Log reduction time was 14 to 32 days for 1 log10, 28 to 64 days for 2 log10, and 42 to 96 days for 3 log10 declines in the organisms' populations from initially inoculated concentrations. Most-probable-number monitoring data indicated that the organisms persisted for 184, 332, and 405 days in manure, manure-amended nonsterilized soil, and manure-amended sterilized soil, respectively. The MDR strain and the DS strain had similar survival patterns.
In the past few years, multidrug-resistant (MDR) Salmonella enterica serovar Newport has spread rapidly in animals and humans in the United States (5, 20). This particular Salmonella serotype has recently been named an emerging disease by the American Association of Veterinary Laboratory Diagnosticians (2). Infected dairy herds suffer significant mortality in both adult and young animals, posing a considerable economic loss to producers, and confirmed cases of human salmonellosis ascribed to Salmonella serovar Newport doubled between 1997 and 2001 (5). Although MDR strains of serotype Newport have been known for many years, serotype Newport strains with resistance to cephalosporins have only recently been described (7, 19, 24).
In Pennsylvania, the first bovine case of MDR Salmonella serovar Newport was found in a sample submitted in November 1999 to the Salmonella Reference Center (SRC), University of Pennsylvania. Since then, the SRC has received and tested over 1,000 Salmonella serovar Newport isolates, of which >85% exhibited resistance to four or more antimicrobials and 75% were from bovine sources. Although the true herd prevalence of Salmonella serovar Newport is still unknown, farms that have submitted positive clinical samples represent 3.5% of all dairy herds in the affected counties investigated (S. C. Rankin, unpublished observations). Some individual counties had a prevalence above 10%. A rapid spread of MDR Salmonella serovar Newport on California dairy farms has also occurred since 1999, where strains from the recent outbreaks reflect a phylogeny different from those in an earlier outbreak in the late 1980s (4). Nationally, the organism has been identified in all contiguous states as well as in southern Canada during the same period. At the National Veterinary Services Laboratory, 56% of all Salmonella serovar Newport isolates came from dairy cattle (8). It is apparent that MDR Salmonella serovar Newport has established a reservoir in dairy cattle.
In both clinical and subclinical cases, animals infected with MDR Salmonella serovar Newport shed the bacteria in manure continuously or intermittently for weeks or even months. Concentrations of the organism in manure range from 102 to 107 per gram of fresh feces (H. W. Aceto, personal communication). A mature dairy cow generates about 70 kg (150 lb) of wet manure per day, which would therefore contain millions to billions of the organisms if infected. If the organisms survive or propagate during manure storage, the manure subsequently applied to agricultural fields could be a primary source of Salmonella serovar Newport spread beyond the boundaries of infected farms. If the organisms survive in soils receiving the manure, they may be incorporated into growing plants and transmitted to other animals or humans. However, to date, there have been little data available to address the fate of this organism in the postshed environment.
MDR microorganisms have been present in the environment for many years. There have been growing concerns over their wide spread and the implications for the health of humans and ecosystems. However, research on the persistence of MDR pathogens compared to their counterparts, drug-susceptible (DS) strains, is rare. The primary objective of the present study was to compare the survival characteristics of an MDR with a DS Salmonella serovar Newport strain, both of dairy cow origin, in cattle manure and manure-amended soils. The incubations described in this paper were performed in the laboratory under controlled temperature and moisture conditions. Information obtained from this study will contribute to our understanding of the organism's environmental behavior. Such knowledge is vital for the devising of relevant policies and development of management strategies to prevent this dangerous pathogen from spreading further in the environment.
MATERIALS AND METHODS
Manure.
Fresh manure was obtained from six healthy, lactating dairy cows at a local dairy operation by scooping from the floor immediately after defecation. Sampled cows received no antibiotics in the 2 months prior to sample collection. The total mixed ration formulated and fed to the high-producing cow group from which fecal samples were collected consisted of (dry matter animal−1 day−1) 7.91 kg corn silage, 3.76 kg soybean meal, 3.52 kg ground corn grain, and 2.63 kg haylage. The feed was supplemented with 5.17 kg commercial grain-protein-mineral mix and 0.22 kg Megalac Plus (calcium salts of fatty acids with 7% methionine). The composite fecal sample, after thorough mixing, was frozen at −20°C until laboratory use. Five subsamples of the bulk manure tested negative for presence of Salmonella spp. Physical/chemical analyses of subsamples indicated pH of 6.7, moisture content of 86%, ammonia-N of 0.6 g kg−1, and total N, P2O5, K2O, Ca, and Mg of 4.2, 2.0, 1.2, 1.8, and 0.9 g kg−1 (wet basis), respectively.
Soil.
The soil, mapped as a Conestoga silt loam (14), fine-loamy, mixed, mesic Typic Hapludalf, was obtained from the 0- to 20-cm layer of a local agricultural experiment field. The field plot from which the soil sample was obtained had been planted to silage corn with a barley winter cover crop and had received no manure or fertilizer nitrogen or phosphorus applications for the previous 6 years. The soil had a field water holding capacity of 31.29% and tested negative for Salmonella spp. Other analyses included pH of 6.5, organic matter of 2.2%, cation exchange capacity of 10.6 cmolc kg−1, and available P, K, Ca, and Mg (Mehlich-3 extracts) of 0.068, 0.112, 1.503, and 0.335 g kg−1, respectively.
The bulk soil sample was air-dried, sieved to pass 2.53 mm, and stored in a plastic container at ambient temperature. Upon laboratory use, the bulk soil was divided into two portions; one was autoclaved for 15 min (sterilized soil) and the other was not sterilized. Chemical analyses of the sterilized soil had results similar to those for the nonsterilized soil (above). Prior to inoculation, the soils were weighed into 1,600-g portions and sterilized distilled water was added to bring the soil moisture to 80% of the field water holding capacity, which approximates an optimum moisture condition in agricultural fields.
Bacteria.
Two strains of Salmonella serovar Newport, both isolated from dairy cattle feces, were obtained from the SRC, School of Veterinary Medicine, University of Pennsylvania. Strain 0306-91 was fully susceptible to all drugs tested at the SRC (DS), whereas strain 0007-33 was resistant to ampicillin, chloramphenicol, streptomycin, spectinomycin, sulfamethoxazole, tetracycline, cephalothin, and ceftriaxone (MDR). The two strains were propagated separately in brain heart infusion (BHI) broth (Difco). Briefly, one colony was transferred into 40 ml BHI broth and incubated at 37°C for 24 h; then, 0.5 ml of the actively growing culture was placed into 40 ml BHI broth and incubated again at 37°C for 24 h. Subsequently, the culture was centrifuged (4,000 × g, 20 min, 4°C), washed once in sterile physiological saline solution (PSS) (0.85% NaCl), and resuspended in sterile PSS, resulting in an inoculum of approximately 1010 CFU ml−1 cell concentration.
Inoculation.
The inoculation was performed in two steps. First, 4.5 ml liquid DS or MDR Salmonella serovar Newport inoculum was incorporated into 45 g manure by thorough mixing with a sterilized wooden spatula. The inoculated manure aliquot was then added to 1,600 g manure (manure treatment) or sterilized and nonsterilized soil (manure-plus-soil treatments). The amount of manure in the manure-amended soil resembled a typical dairy manure application rate commonly practiced on farms in the region. After mixing with a sterilized wood spatula, 500 g each of the inoculated manure or manure-amended soil was weighed into three polypropylene containers (2 liters) for incubation at ambient temperature in the laboratory (24.5 ± 1.4°C). The containers were covered with Parafilm to retard moisture loss while allowing air exchange. Throughout the incubation, the moisture content of the samples was maintained by weekly weighing and addition of sterilized distilled water when needed. The weekly moisture loss averaged less than 3% of the total weight. Manure and manure-amended soil samples without inoculation served as controls.
Sampling and enumeration.
Salmonella serovar Newport counts were determined at 0, 1, 3, 6, 9, 14, 20, 27, and 35 days postinoculation and thereafter once every 1 to 4 weeks until the pathogen could no longer be detected. Manure or manure-amended soil samples (2 g each) were aseptically transferred to a sterile 50-ml screw-top centrifuge tube, 18 ml sterile PSS was added, and the mixture was agitated on a reciprocal shaker (200 strokes min−1 for 6 min). The extract was serially diluted (1:10) with sterile PSS, and 0.1-ml aliquots were spread onto XLD (xylose-lysine-deoxycholate) agar plates in triplicate. After incubation at 37°C for 24 h, colonies were counted. Thirty to 300 colonies per plate were considered to be optimal countable numbers.
After pathogen concentrations dropped below the quantitative detection limit (100 CFU g−1) of the direct plating method, the most-probable-number (MPN) technique was performed to monitor the persistence of the organism. The standard MPN procedure for water samples (1) was modified. Briefly, five replicates of 1 g manure or manure-amended soil were diluted in 9 ml of 1× buffered peptone water (BPW); then, 1 ml of the solution was diluted in BPW (1:10), followed by another 1:10 dilution. Subsequently, all three dilutions were incubated at 37°C for 24 h. After incubation, a 0.1-ml aliquot was subcultured into Rappaport-Vassiliadis (RV) enrichment broth and incubated at 41.5°C for 24 h. Finally, 0.1-ml aliquots of the RV enrichment solution were spread onto XLD agar plates and incubated at 37°C for 24 h. Plates with one or more colonies of Salmonella serovar Newport were scored, and the MPN values were calculated (1).
PFGE.
To determine whether isolates of Salmonella serovar Newport obtained over time from the experiment were identical to those introduced, isolates of DS and MDR Salmonella serovar Newport were randomly selected from XLD agar plates on day 35 (all treatments) and on days 42, 107, and 158 from the manure, nonsterilized soil, and sterilized soil treatments, respectively, and subjected to pulsed-field gel electrophoresis (PFGE). The results were compared with the genetic profiles of the two strains used in the experiment. The PFGE procedure followed digestion with the restriction endonuclease XbaI (15, 17). DNA fragments were visualized on a UV transilluminator, photographed with a Kodak EDAS290 digital camera system (Life Technologies, Gaithersburg, MD), and stored as digital image files for further analysis with BioNumerics software (Applied Maths, Inc., Austin, TX).
Data analysis.
The first-order kinetic model (equation 1),
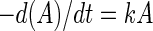 |
(1) |
where t is the survival time (days), A is the cell concentration (CFU g−1) at time t, and k is the deactivation rate, was converted to a linear equation (equation 2):
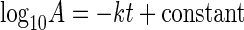 |
(2) |
The experimental data were inserted into equation 2 to obtain the best-fit regression equations for the various treatments, from which the deactivation rate constant was derived. The analysis-of-variance procedure in SAS (21) was performed to determine whether the deactivation rate constant, k, differed for DS and MDR Salmonella serovar Newport (P < 0.01). Analysis of variance with repeated measures across sampling times was conducted to determine whether general differences existed between the DS and MDR Salmonella serovar Newport treatment means (P < 0.01).
RESULTS
The initial DS or MDR Salmonella serovar Newport concentration in inoculated manure was 7.12 log10 CFU g−1, close to the calculated target value of 7 log10 CFU g−1, whereas the cell concentrations in inoculated manure-soil mixtures averaged 6.86 log10 CFU g−1, slightly less than the theoretical target and 45% lower than in inoculated manure. A possible explanation is that the rigorous mechanical stirring during inoculation for the purpose of homogenization resulted in inactivation or death of the organisms.
Survival in manure.
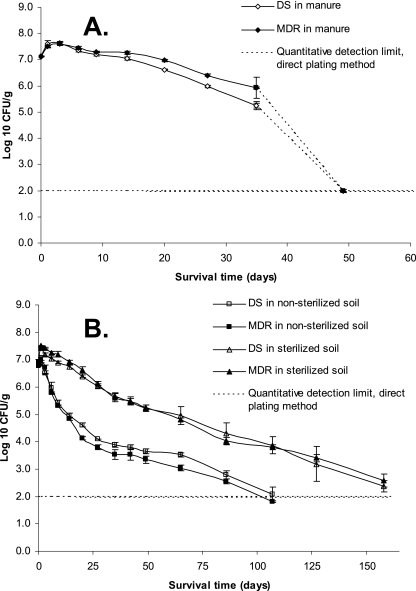
An initial multiplication of the organisms was evidenced by increased cell concentrations, from 7.13 to 7.59 and 7.63 log10 CFU g−1 on days 0, 1, and 3, respectively, in DS Salmonella serovar Newport; the corresponding increases in MDR Salmonella serovar Newport were 7.11 to 7.51 and 7.61 log10 CFU g−1 on days 0, 1, and 3. Increases in cell concentrations between days 0 and 3 calculated on nontransformed data were 317 and 319% for DS and MDR Salmonella serovar Newport, respectively. After the initial increase, the populations of both strains decreased steadily until day 35 (Fig. 1a). Between day 35 and day 49, cell concentrations dropped markedly, from approximately 5 log10 CFU g−1 to below the detection limit of the direct plating method. Nevertheless, the organisms persisted until day 184, as indicated by results from the MPN method (Table 1). Repeated-measures analysis showed that the survival patterns of the MDR and the DS strains did not differ significantly through day 35 of the incubation (P = 0.0293).
FIG. 1.
Salmonella serovar Newport survival in manure (A) and in manure-amended sterilized or nonsterilized soil (B) under constant temperature (24.5 ± 1.4°C) and moisture conditions.
TABLE 1.
Persistence of MDR and DS Salmonella serovar Newport in dairy manure and manure-amended sterilized or nonsterilized soil
| Strain | Treatment | Time to reach detection limit (days)
|
|
|---|---|---|---|
| Direct plating method | MPN method | ||
| DS | Manure | 49 | 184 |
| Manure + nonsterilized soil | 107 | 332 | |
| Manure + sterilized soil | 158 | 405 | |
| MDR | Manure | 49 | 184 |
| Manure + nonsterilized soil | 107 | 332 | |
| Manure + sterilized soil | 158 | 405 | |
Survival in manure-amended soils.
In manure-amended soils, the highest Salmonella serovar Newport concentrations were observed in samples from day 1: 6.88 and 7.02 log10 CFU g−1 for the DS and MDR strains in manure-amended nonsterilized soil (123 and 128% compared to day 0), respectively, and 7.42 and 7.50 log10 CFU g−1 in manure-amended sterilized soil (increases of 310 and 470% compared to day 0), respectively. The initial population increases were followed by steady decreases in all samples until day 107 in the manure-amended nonsterilized soil and day 158 in the manure-amended sterilized soil (Fig. 1b), based on the direct plating method. Results from the MPN method indicated that the organisms persisted until day 332 in the manure-amended nonsterilized soil and day 405 in the manure-amended sterilized soil (Table 1). Survival rates of the DS and MDR Salmonella serovar Newport strains determined by repeated-measures analysis were not different in the manure-amended nonsterilized soil (P = 0.0807) through 107 days of incubation or in the manure-amended sterilized soil (P = 0.4225) through 158 days.
Deactivation model.
Deactivation of Salmonella serovar Newport over time (between the maximum concentration and the last sample that could be quantified by the direct plating method) could be quantitatively described by a first-order kinetic model, with R2 ranging from 0.81 to 0.98. The deactivation rate constants, k (Table 2), did not differ between the susceptible and the resistant strains in the three systems in the study (P values were 0.0579, 0.1298, and 0.9703 for manure, manure-amended nonsterile soil, and manure-amended sterile soil, respectively). From the best-fit linear regression equations (Table 2), log reduction time was calculated to be 14 to 32 days for 1 log10, 28 to 64 days for 2 log10, and 42 to 96 days for 3 log10 reduction from the initially inoculated concentrations. The log reduction time was the longest in manure-amended sterilized soil and shortest in manure, with manure-amended nonsterilized soil intermediate for both strains.
TABLE 2.
Deactivation of MDR and DS Salmonella serovar Newport in dairy manure and manure-amended sterilized or nonsterilized soil
| Strain | Treatment | Regression equationa | R2 | Log reduction time (days)
|
||
|---|---|---|---|---|---|---|
| 1 log10 | 2 log10 | 3 log10 | ||||
| DS | Manure | Log10A = −0.0716t + 7.6732 | 0.98 | 14.0 | 27.9 | 41.9 |
| Manure + nonsterilized soil | Log10A = −0.0394t + 5.8453 | 0.85 | 25.4 | 50.8 | 76.1 | |
| Manure + sterilized soil | Log10A = −0.0311t + 7.0408 | 0.98 | 32.2 | 64.3 | 96.5 | |
| MDR | Manure | Log10A = −0.0515t + 7.6750 | 0.97 | 19.4 | 38.8 | 58.3 |
| Manure + nonsterilized soil | Log10A = −0.0426t + 5.7387 | 0.81 | 23.5 | 47.0 | 70.4 | |
| Manure + sterilized soil | Log10A = −0.0319t + 7.1662 | 0.96 | 31.4 | 62.7 | 94.0 | |
Regression equations for the treatments were obtained by fitting a first-order kinetic model to the experimental data (cell concentrations at different times). Survival data used for model fitting: day 3 to day 35 for manure, day 1 to day 107 for manure-plus-nonsterilized soil, and day 1 to day 158 for manure-plus-sterilized soil. t is survival time (days), and A is Salmonella serovar Newport concentration (CFU g−1) at time t.
PFGE results.
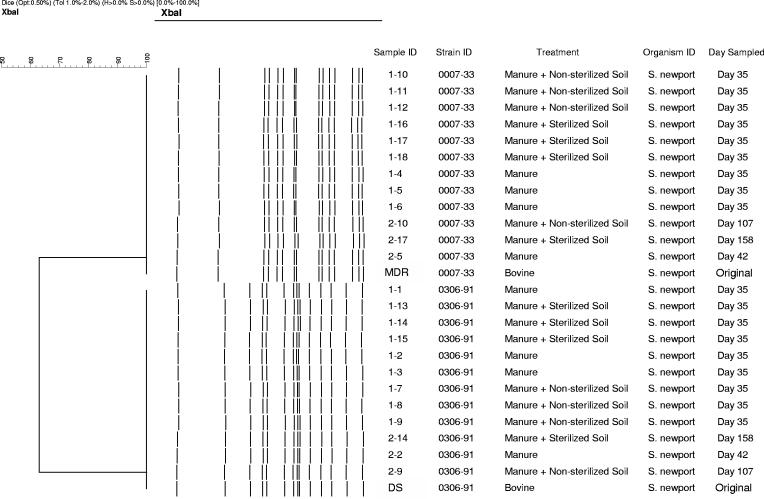
PFGE was performed on 12 DS and 12 MDR Salmonella serovar Newport colonies from the various treatments, and no genetic changes were observed; the PFGE profiles were consistent with the strains that we inoculated (Fig. 2).
FIG. 2.
PFGE typing of selected S. enterica serovar Newport samples with differing treatments and sampling times.
DISCUSSION
MDR Salmonella serovar Newport has undergone a rapid epidemic spread in animals and humans in recent years, with dairy cattle being a major reservoir. This is the first report on the survival characteristics of this pathogen in the postshed environment. The organism was able to amplify in both manure and manure-amended soils after inoculation. The initial propagation of Salmonella serovar Newport in the beginning of the experiment is consistent with previous reports for other pathogenic bacteria. For example, an Escherichia coli O157:H7 population increased by about 2 log10 CFU g−1 in the first 2 days following inoculation into cattle feces prior to a steady decline (23, 25). In inoculated agricultural soils, E. coli O157:H7 and Salmonella enterica serovar Typhimurium populations showed increases in the first 3 days of incubation (10). In the present study, the different growth patterns in manure (population maximum on day 3) and manure-amended soils (maximum on day 1) is perhaps due to higher nutrient availability in manure than in soils.
Microbial antagonism from indigenous organisms against introduced species is a common phenomenon. However, the sharp decline of DS and MDR Salmonella serovar Newport cell concentrations in the manure treatment after day 35, from approximately 5 log10 CFU g−1 to below the detection limit of the direct plating method at day 49, was unexpected. Prior to day 35, there were colonies on XLD agar plates that were morphologically distinct from typical Salmonella colonies in terms of size, color, and shape. After day 35, however, clusters of the unknown organisms overwhelmed the agar plates, making visual identification and enumeration of Salmonella colonies, if present, difficult. Associated with the dominant unidentified organism on the plates was an ammonia-like smell. In addition, we recorded a pH change from 6.70 ± 0.03 at day 0 to 7.62 ± 0.16 at day 37 and 8.00 ± 0.16 at day 72. It has been reported that an increase in pH was associated with declines of E. coli O157:H7 and Salmonella serovar Typhimurium in manure (9, 23). In the current study, the identities of the competing organisms on the XLD agar plates and the physicochemical conditions in the manure associated with the substantial pH change remain to be further investigated.
The effects of microbial antagonism by indigenous soil microbes on the persistence of Salmonella serovar Newport introduced through inoculation were clearly indicated in the results of the manure-amended sterilized soil versus nonsterilized soil treatments. Comparison of the sterilized soil with the nonsterilized soil treatment shows that the initial Salmonella serovar Newport population increase was greater (310% versus 123% for the susceptible strain and 470% versus 127% for the resistant strain), the deactivation was more gradual (Fig. 1b), and the duration of survival was longer (158 days versus 107 days, determined by the direct plating method, and 405 days versus 332 days, by the MPN method [Table 1]). The impacts of native microbes on artificially introduced organisms have been noted for E. coli O157:H7 in a manure-amended soil (12) and for Salmonella in sewage sludge compost (11). It would be interesting to compare the survival behavior of MDR Salmonella serovar Newport introduced through inoculation with that of a “native” MDR Salmonella serovar Newport strain directly shed by infected animals.
For the first time, experimental data allow us to make a direct comparison of postinoculation survival of an MDR strain of Salmonella serovar Newport with a DS strain under the same conditions. Apparently, the DS and MDR Salmonella serovar Newport strains had very similar survival characteristics in manure and manure-amended soils (Fig. 1). There were no significant differences between the two strains in terms of deactivation rate (k) or survival curves. Whether the MDR strain would behave differently from a DS strain under natural conditions with changing environmental factors such as temperature and moisture, particularly in the presence of antimicrobials, remains to be further investigated. Nevertheless, the present study demonstrates that the MDR Salmonella serovar Newport strain survived as well as the DS strain under the experimental conditions.
The fact that the PFGE profiles of the Salmonella serovar Newport isolates collected on days 42, 107, and 158 were the same as those of the isolates used for the inoculation (Fig. 2) has important implications. It has been shown that MDR Salmonella serovar Newport possesses an ampC gene, termed blaCMY-2, which is encoded on a nonconjugative but transferable plasmid responsible for resistance to multiple antimicrobials (19). With its genetic profile consistent over long periods in manure or manure-amended soils, the organism may possess a competitive edge when exposed to natural conditions in an agroecosystem where the presence of antimicrobial residues is common (22). Furthermore, the ampC gene that is responsible for antimicrobial resistance could possibly be transferred to other bacterial species, such as E. coli, with subsequent transfer to animals or humans via the food chain, as suggested elsewhere (18).
Livestock animals often harbor a variety of pathogens, and their excreta are a potential vehicle for transmitting pathogens as well as antibiotic resistance to other animals, food, and the environment. The likelihood of the pathogens spreading into the environment and potentially affecting other species depends, first and foremost, on the ability of the organisms to survive outside of the host. Previous research has demonstrated the survivability and subsequent transmission of some pathogens associated with animal manures. For instance, Baloda et al. (3) found that Salmonella serovar Typhimurium DT104 and DT12 could survive up to 299 days in a terrestrial microcosm study. Jiang et al. (12) were able to isolate E. coli O157:H7 derived from a five-strain inoculum in soil-manure mixtures 200 days after inoculation. Jones (13) reported that Salmonella spp. survived for up to 300 days in soil spread with cattle manure slurry or 259 days in soils amended with animal feces. Natvig et al. (16) grew vegetable crops in controlled-environment chambers in soil mixed with bovine manure inoculated with Salmonella serovar Typhimurium. They found that harvested arugula and radish could be contaminated by Salmonella serovar Typhimurium and native fecal E. coli, although carryover of pathogens to the vegetables could be reduced by the timing of manure application and crop harvest.
The long-term survival of Salmonella serovar Newport in manure or manure-amended soils in the present study indicates the potential risk of environmental spread and subsequent transmission. Typical dairy operations in the region keep manure in storage for weeks to months prior to field application (6). It is therefore possible that in farm settings where MDR Salmonella serovar Newport infection is present, the organism will survive in manure storage and be applied to agricultural fields and increase the potential for dissemination beyond the farm boundaries. Although the transport behaviors of the organism from manure-amended agricultural soils to waters have not been studied in detail, field investigations of dairy farms infected by MDR Salmonella serovar Newport have shown that the organism frequently occurs in samples from locations that receive drainage from animal housing or manure storage areas and has also been regularly recovered from streams and stream edges frequented by cattle (H. W. Aceto, unpublished data). MDR Salmonella serovar Newport represents a clear and present danger to the agricultural community, water resources, and the environment at large. There is an urgent need to study the survival, transport, and functional impact of this organism and to understand how environmental conditions may affect and subsequently be manipulated to alter its fate in the environment.
Acknowledgments
This research was supported by grants from The University of Pennsylvania Research Foundation, Pennsylvania Department of Agriculture, and the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service.
REFERENCES
- 1.American Public Health Association. 1999. Standard methods for the examination of water and wastewater, 20th ed. APHA/AWWA/WEF. American Public Health Association, Washington, D.C.
- 2.Animal Disease Diagnostic Laboratory. 2004. Salmonella Newport—an emerging disease in dairy cattle. [Online.] http://www.addl.purdue.edu/newsletters/2004/summer/salmnewp.htm.
- 3.Baloda, S. B., L. Christensen, and S. Trajcevska. 2001. Persistence of a Salmonella enterica serovar typhimurium DT12 clone in a piggery and in agricultural soil amended with Salmonella-contaminated slurry. Appl. Environ. Microbiol. 67:2859-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berge, A. C. B., J. M. Adaska, and W. M. Sischo. 2004. Use of antibiotic susceptibility patterns and pulsed-field gel electrophoresis to compare historic and contemporary isolates of multi-drug-resistant Salmonella enterica subsp. enterica serovar Newport. Appl. Environ. Microbiol. 70:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January-April, 2002. Morb. Mortal. Wkly. Rep. 51:545-548. [Online.] http://www.cdc.gov/mmwr/PDF/wk/mm5125.pdf. [PubMed] [Google Scholar]
- 6.Dou, Z., D. T. Galligan, C. F. Ramberg, Jr., C. Meadows, and J. D. Ferguson. 2001. A survey of dairy farming in Pennsylvania: nutrient management practices and implications. J. Dairy Sci. 84:966-973. [DOI] [PubMed] [Google Scholar]
- 7.Dunne, E. F., P. D. Fey, P. Kludt, R. Reporter, F. Mostashari, P. Shillam, J. Wicklund, C. Miller, B. Holland, K. Stamey, T. J. Barrett, J. K. Rasheed, F. C. Tenover, E. M. Ribot, and F. J. Angulo. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC β-lactamase. JAMA 284:3151-3156. [DOI] [PubMed] [Google Scholar]
- 8.Ferris, K. E., A. M. Aalsburg, and G. R. Iseminger. 2003. Salmonella serotypes from animals and related sources reported during July 2000-June 2001. U.S. Animal Health Association. [Online.] http://www.usaha.org/meetings/2002/2002_USAHA_Proceedings.pdf.
- 9.Himathongkham, S., and H. Riemann. 1999. Destruction of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes in chicken manure by drying and /or gassing with ammonia. FEMS Microbiol. Lett. 171:179-182. [DOI] [PubMed] [Google Scholar]
- 10.Himathongkham, S., S. Bahari, H. Riemann, and D. O. Cliver. 1999. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251-257. [DOI] [PubMed] [Google Scholar]
- 11.Hussong, D., W. D. Burger, and N. K. Enkiri. 1985. Occurrence, growth, and suppression of Salmonella in composted sewage sludge. Appl. Environ. Microbiol. 50:887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, X. P., J. Morgan, and M. P. Doyle. 2002. Fate of Escherichia coli O157:H7 in manure-amended soils. Appl. Environ. Microbiol. 68:2605-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, P. W. 1986. Sewage sludge as a vector of salmonellosis, p. 21-33. In J. C. Block, A. H. Haielaar, and P. L'Hermite (ed.), Epidemiological studies of risks associated with the agricultural use of sewage sludge. Elsevier, London, England.
- 14.Kunkle, W. M. 1963. Soil survey, Chester and Delaware counties, Pennsylvania. U.S. Department of Agriculture Soil Conservation Service, Washington, D.C.
- 15.Liu, S., and K. E. Sanderson. 1992. A physical map of the Salmonella typhimurium LT2 genome made by using XbaI analysis. J. Bacteriol. 174:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natvig, E. E., S. C. Ingham, B. H. Ingham, L. R. Cooperband, and T. R. Roper. 2002. Salmonella enterica serovar Typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl. Environ. Microbiol. 68:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Old, D. C., S. C. Rankin, and P. B. Crichton. 1999. Assessment of strain relatedness among Salmonella serotypes Salinatis, Duisburg, and Sandiego by biotyping, ribotyping, IS200 fingerprinting, and pulsed-field gel electrophoresis. J. Clin. Microbiol. 37:1687-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramchandani, M., A. R. Manges, C. DebRoy, S. P. Smith, J. R. Johnson, and L. W. Riley. 2005. Possible animal origin of human-associated, multidrug-resistant, uropathogenic Escherichia coli. Clin. Infect. Dis. 40:251-257. [DOI] [PubMed] [Google Scholar]
- 19.Rankin, S. C., H. Aceto, J. Cassidy, J. Holt, S. Young, B. Love, D. Tewari, D. S. Munro, and C. E. Benson. 2002. Molecular characterization of cephalosporin-resistant Salmonella enterica serotype Newport isolates from animals in Pennsylvania. J. Clin. Microbiol. 40:4679-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin, S. C., J. M. Whichard, K. Joyce, L. Stephens, K. O'Shea, H. Aceto, D. S. Munro, and C. E. Benson. 2005. Detection of a blaSHV extended-spectrum β-lactamase in Salmonella enterica serovar Newport MDR-AmpC. J. Clin. Microbiol. 43:5792-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAS Institute. 1999. SAS System for Windows, release 8.00. SAS Institute, Cary, N.C.
- 22.Tolls, J. 2001. Sorption of veterinary pharmaceuticals in soils: a review. Environ. Sci. Technol. 35:3397-3406. [DOI] [PubMed] [Google Scholar]
- 23.Wang, G., T. Zhao, and M. P. Doyle. 1996. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl. Environ. Microbiol. 62:2567-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winokur, P. L., A. Bruggemann, D. L. DeSalvo, L. Hoffman, M. D. Apley, E. K. Uhlenhopp, M. A. Pfaller, and G. V. Doern. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]