Abstract
Phylogenetic analysis was used to compare 16S rRNA sequences from 19 cultured human gut strains of Roseburia and Eubacterium rectale with 356 related sequences derived from clone libraries. The cultured strains were found to represent five of the six phylotypes identified. A new oligonucleotide probe, Rrec584, and the previous group probe Rint623, when used in conjunction with a new helper oligonucleotide, each recognized an average of 7% of bacteria detected by the eubacterial probe Eub338 in feces from 10 healthy volunteers. Most of the diversity within this important group of butyrate-producing gut bacteria can apparently be retrieved through cultivation.
The human colonic microbiota consists of at least 500 bacterial species (9, 18, 31, 35) and plays an important role in maintaining human health by preventing colonization by pathogens, degrading dietary and in situ-produced compounds, producing nutrients, and shaping and maintaining the normal mucosal immunity (12, 21). 16S rRNA-based methods, in particular sequencing of 16S rRNA molecules directly amplified by PCR, have shown that 75% of phylotypes defined by 16S rRNA sequences from the human colon do not correspond to known cultured bacterial species (9, 17, 18, 35); such unidentified phylotypes are particularly common among Firmicute bacteria with low percent G+C contents (9). One possible explanation for this is that a high proportion of human gut bacteria are unculturable by the methods presently employed. On the other hand, there has been considerable success in culturing strict anaerobes from the gut by using anaerobic procedures (2, 11, 31). Recent work, for example, led to the isolation of highly oxygen-sensitive butyrate-producing Firmicute bacteria from the human gut, most of which belong to clostridial clusters IV and XIVa (2, 8, 23). One abundant group, related to Roseburia cecicola or Eubacterium rectale, includes several newly proposed species, including Roseburia intestinalis (2, 5, 7, 8a, 19, 23, 28), while a second abundant group is related to the clostridial cluster IV bacterium Faecalibacterium prausnitzii (6). Butyrate provides the preferred energy source for colonocytes in the human large intestine and has an important impact on gut health (28, 29). The work described here examines further the cultivability and abundance of Roseburia- and E. rectale-related bacteria in human feces, based on analysis of 16S rRNA sequences.
Phylogenetic analysis of Roseburia-related 16S rRNA gene sequences from the human gut.
Seventeen strains related to Roseburia spp. or to E. rectale, isolated by strictly anaerobic procedures (20, 25) and with known 16S rRNA sequences, were available from previous studies (2, 23). The origins of these strains are given in the legend to Fig. 1; culture collection numbers (DSM or ATCC) are indicated for deposited strains in Fig. 1, along with the original strain designations. Two further E. rectale strains (S2Ss2/7 and S2Ss2/2) that were isolated as part of another study (8) were also included; their 16S rRNA sequences were determined in this study.
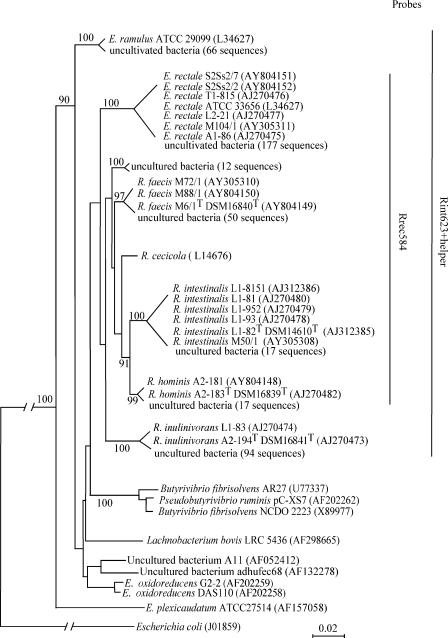
FIG. 1.
Phylogenetic tree, constructed by the neighbor-joining method, based on 16S rRNA sequences of Roseburia- and E. rectale-related clones and species. Isolation of strains T1-815, L2-21, A1-86, L1-8151, L1-81, L1-82T, L1-952, L1-93, A2-181, A2-183T, L1-82, and A2-194T is described in reference 23, and isolation of strains M104/1, M72/1, M88/1, M6/1T, and M50/1 is described in reference 23; for S2Ss2/7 and S2Ss2/2, see the text. 16S rRNA sequence accession numbers are given in parentheses. Numbers above each node are confidence levels (percent) generated from 1,000 bootstrap trials. The Escherichia coli sequence is used as the outgroup to root the tree. The scale bar refers to fixed nucleotide substitutions per sequence position. For the sake of space, the tree is presented in a schematic form that retains the general topology. The branch length of E. coli is not to scale. The probe coverage is shown on the extreme right.
The set of 19 16S rRNA sequences from cultivated Roseburia and E. rectale strains was combined with a subset of 356 related 16S rRNA sequences from available human colonic and fecal 16S rRNA clone libraries (9, 18, 35, 38). To these were added an outgroup set consisting of 68 clone sequences and 9 sequences from cultivated isolates. Phylogenetic analyses were performed using ClustalX alignment and the neighbor-joining method of phylogenetic analysis (30) Statistical validation of tree branching was done by bootstrap analysis (10) involving 1,000 resampled trees.
The 375 sequences related to Roseburia spp. or E. rectale fell into six phylotypes, or operational taxonomic units (OTUs) (Fig. 1). Five out of the six OTUs were interleaved with the cultivated human isolates in the resulting phylogenetic tree. Only one OTU, consisting of 12 clone sequences, had no cultivated counterpart. Seventeen sequences correspond to the known species R. intestinalis (5) and three other OTUs aligned with the newly proposed type strains of Roseburia faecis (50 sequences), Roseburia hominis (17 sequences), and Roseburia inulinivorans (94 sequences) (8a). No sequences clustered together with R. cecicola, which is a murine cecal isolate (33, 34). This species may therefore be absent from or uncommon in the human gut.
The most numerous OTU in the Roseburia and E. rectale cluster, represented by 177 sequences, grouped with the sequences from cultured E. rectale strains (16, 26, 27) (Fig. 1). This group of sequences is represented by six bacterial isolates from five individuals and multiple hits in several clone libraries, thus suggesting the widespread presence of this species in the human gut. The outgroup strain, Eubacterium ramulus (26, 32), fell into an OTU represented by 66 outgroup sequences outside the Roseburia cluster (Fig. 1).
Improved detection of Roseburia and E. rectale strains by fluorescent in situ hybridization (FISH).
Previous work resulted in the design of an oligonucleotide probe, Rint623, specific for the Roseburia and E. rectale group plus E. ramulus (19). Rint623 is, however, targeted to a region of 16S rRNA that is part of a hairpin structure (3). In order to improve the in situ accessibility of this probe to 16S rRNA, in this study a helper oligonucleotide was designed in the region of 16S rRNA directly upstream from the original target site (14). In addition, a new probe, Rrec584, was designed to recognize the majority of the Roseburia and E. rectale group, but it excluded R. inulinivorans and E. ramulus. Both new oligonucleotides were designed using the ARB software package (24) and synthesized by MWG-Biotech (Germany). All probes used in the study, but not the helper oligonucleotide, were labeled with Cy3 dyes, and their sequences are given in Table 1. In validation experiments with human colonic isolates, the specificity of these two probes corresponded to the prediction from in silico analysis (Table 2). No hybridization was obtained with taxonomically distant bacteria in the human gut, such as bacteroides, lactic acid bacteria, proteobacteria, representatives of several other clusters of Clostridium-related bacteria, or neighbors in cluster XIVa such as Clostridium aminovalericum and Clostridium polysaccharolyticum. All Roseburia strains tested, however, were positive with both probes, except that, as predicted, Rrec584 failed to hybridize with R. inulinivorans (Table 2).
TABLE 1.
Nucleotide sequences of oligonucleotide probes
| Probe | Sequence (5′→3′) | Target group | Reference |
|---|---|---|---|
| Eub338 | GCTGCCTCCCGTAGGAGT | Universal eubacterial group | 1 |
| Erec482 | GCTTCTTAGTCAGGTACCG | Subcluster of low-G+C cluster XIVa | 13 |
| Rrec584 | TCAGACTTGCCG(C/T)ACCGC | Roseburia subcluster | 36 |
| Rint623 | TTCCAATGCAGTACCGGG | Roseburia cluster | 19 |
| Rint helper | GTTGAGCCCCGGGCTTT | Rint623 helper | This study |
TABLE 2.
Validation of Roseburia probes
| Control straina | Hybridizationb with:
|
|
|---|---|---|
| Rrec584 | Rint623 + helper | |
| Streptococcus gordonii DL-1 | − | − |
| Streptococcus bovis 26R | − | − |
| Streptococcus mutans DSM 20523 | − | − |
| Enterococcus faecalis JH2-2 | − | − |
| Enterococcus faecium DSM 20477 | − | − |
| Faecalibacterium prausnitzii A2-165 | − | − |
| Faecalibacterium prausnitzii (MMB) | − | − |
| Bifidobacterium adolescentis L2-32 | − | − |
| Bifidobacterium longum NCIMB 8809 | − | − |
| Bifidobacterium infantis DSM 20088 | − | − |
| Bifidobacterium breve MMB 3035 | − | − |
| Eubacterium hallii L2-7 | − | − |
| Coprococcus-like strain L2-50 | − | − |
| Eubacterium ventriosum DSM 3988 | − | − |
| Eubacterium eligens (MMB) | − | − |
| Eubacterium siraeum DSM 3996 | − | − |
| Anaerostipes caccae DSM 14662T | − | − |
| Bacteroides vulgatus DSM 1447T | − | − |
| Bacteroides thetaiotaomicron DSM 2079T | − | − |
| Bacteroides distasonis DSM 20701T | − | − |
| Bacteroides fragilis DSM 2151T | − | − |
| Eubacterium cylindroides T2-87 | − | − |
| Eubacterium cylindroides MMB 3291 | − | − |
| Collinsella aerofaciens DSM 3979 | − | − |
| Lactococcus lactis MG1363 | − | − |
| Lactococcus lactis DSM 20069 | − | − |
| Lactobacillus acidophilus A274 | − | − |
| Megasphaera elsdenii ATCC 25940R | − | − |
| Veillonella parvula DSM 2008 | − | − |
| Acidaminococcus fermentans (MMB) | − | − |
| Escherichia coli JM109 | − | − |
| Escherichia coli ATCC 25922 | − | − |
| Ruminococcus albus SY3R | − | − |
| Ruminococcus flavefaciens 17R | − | − |
| Ruminococcus flavefaciens ATCC 19208R | − | − |
| Ruminococcus bromii L2-63 | − | − |
| Desulfovibrio piger DSM 749 | − | − |
| Clostridium acetobutylicum DSM 792 | − | − |
| Clostridium barkeri MMB 3355 | − | − |
| Clostridium polysaccharolyticum DSM 1801R | − | − |
| Clostridium nexile DSM 1787 | − | − |
| Clostridium aminovalericum DSM 1283S | − | − |
| Clostridium butyricum MMB 3316 | − | − |
| Methanobrevibacter smithii MS1 | − | − |
| Eubacterium rectale A1-86 | + | + |
| Roseburia inulinivorans DSM 16841T | − | + |
| Roseburia hominis DSM 16839T | + | + |
| Roseburia intestinalis DSM 14610T | + | + |
All cultures were of human origin except of those designated R (rumen) and S (sewage). The strains are available from the Deutche Sammlung von Microorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany), American Type Culture Collection (ATCC) (Rockville, Maryland), or Laboratory for Medical Microbiology (MMB) (Groningen, The Netherlands) or otherwise are held in culture at the Rowett Research Institute.
+, positive hybridization; −, no signal.
Abundance of the Roseburia and E. rectale cluster in fecal samples from healthy volunteers.
The validated Rrec584 and Rint623-helper FISH probes were used to estimate the numbers of the Roseburia and E. rectale cells in fecal samples. Freshly voided feces were collected from 10 healthy adult volunteers (5 females and 5 males) between 24 and 60 years old. None of the volunteers had taken antibiotics or other drugs known to influence the fecal microbiota for more than 3 months before the study commenced. Nine of the volunteers consumed typical Western diets, and one volunteer was a vegetarian. Fecal samples were prepared for FISH as described previously (36). Digital images of the slides were viewed with a Leica (Wetzlar, Germany) DMRA2 epifluorescence microscope, and fluorescent cells within 25 to 50 fields of view per well were counted using Quantimet HR600 image analysis software (Leica) (15). For total cell counts 4′,6′-diamidino-2-phenylindole (DAPI) was used by applying 100 μl phosphate-buffered saline plus 1 μl DAPI (500 ng/μl) to the slides and incubating for 5 min at room temperature in a dark room, followed by washing (15 min) in phosphate-buffered saline, rinsing, and drying.
Both probes gave estimates of the numbers for the Roseburia and E. rectale cluster representatives in the healthy gut of between 3 and 15% (average of 7.1% for Rrec584 and 7.4% for Rint623 plus helper) of the total bacterial count obtained with the Eub338 probe (Table 3). Within clostridial cluster XIVa, the proportion of Roseburia- and E. rectale-related bacteria averaged 33% for Rrec584, and 35.4% for Rint623 plus helper (Table 3). Counts for Rrec584 apparently exceeded those for Rint623 plus helper in three cases, which would not be predicted from the specificity of these probes. This may simply reflect more efficient detection by the Rrec584 probe.
TABLE 3.
Enumeration of Roseburia species and E. rectale in fecal samples
| Probe | No. of cells g feces−1a (% Rint623, % Rrec584)b for donor:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| DAPI | 1.23 × 1011 | 7.33 × 1010 | 6.26 × 1010 | 1.20 × 1011 | 1.00 × 1011 | 7.00 × 1010 | 7.83 × 1010 | 9.40 × 1010 | 4.74 × 1010 | 1.11 × 1011 |
| Eub338 | 8.46 × 1010 (6.3, 5.6) | 5.97 × 1010 (4.6, 4.7) | 5.03 × 1010 (5.4, 6.1) | 8.36 × 1010 (6.0, 4.8) | 7.24 × 1010 (8.4, 6.1) | 4.78 × 1010 (11.0, 8.1) | 4.35 × 1010 (12.9, 15.3) | 6.08 × 1010 (3.4, 5.0) | 3.73 × 1010 (12.1, 11.8) | 9.87 × 1010 (3.6, 3.7) |
| Erec482 | 1.72 × 1010 (30.9, 27.7) | 8.90 × 109 (30.9, 31.7) | 1.15 × 1010 (23.7, 26.5) | 1.74 × 1010 (28.7, 23.1) | 1.19 × 1010 (51.0, 37.0) | 1.01 × 1010 (51.9, 38.2) | 1.30 × 1010 (43.1, 51.3) | 8.29 × 109 (25.0, 36.4) | 1.09 × 1010 (50.6, 40.4) | 2.02 × 1010 (17.7, 18.0) |
| Rint623 + helper | 5.31 × 109 | 2.75 × 109 | 2.73 × 109 | 4.99 × 109 | 6.07 × 109 | 5.24 × 109 | 5.60 × 109 | 2.07 × 109 | 5.52 × 109 | 3.57 × 109 |
| Rrec584 | 4.76 × 109 | 2.82 × 109 | 3.05 × 109 | 4.02 × 109 | 4.40 × 109 | 3.86 × 109 | 6.67 × 109 | 3.02 × 109 | 4.40 × 109 | 3.63 × 109 |
Coefficients of variation (based on at least 25 fields per sample) were estimated to be not greater than 0.10. Counts for Rint623 were significantly greater than those for Rrec584 for subjects 1, 4, 5, 6, and 9, while counts for Rint584 were significantly greater than those for Rint623 for subjects 3,7, and 8 (P < 0.001) (but see text).
Rint623 plus helper probe and Rrec584 as proportions of either Eub338 or Erec482.
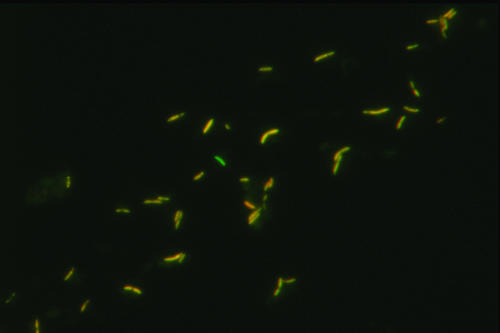
Dual probing was also performed, in which a fecal sample was hybridized with Rint623 labeled with fluorescein isothiocyanate dye and with Rrec584 labeled with Cy3. On the superimposed images, some cells hybridized only with Rint623 plus helper probe, thus confirming that R. inulinivorans and E. ramulus were detectable in low numbers relative to bacteria detected by the Rrec584 probe in the fecal community from donor 9 (Fig. 2). Previous evidence shows that E. ramulus is a significant member of the fecal microbiota of many individuals (9, 32), and R. inulinivorans-related sequences were abundant in amplified 16S rRNA gene libraries from the three individuals in a previous study (9). Use of a FISH probe specific for R. inulinivorans strains in a previous study (19) (there referred to as Eubacterium strains L1-83 and A2-194) detected these organisms at between 108.4 and 1010.1 cells/g feces in 6 of 10 individuals studied, while E. ramulus was detected at between 108 and 109 cells/g feces in 6 of 10 individuals. The extent of interindividual variation in species from the Roseburia and E. rectale group will merit further investigation.
FIG. 2.
Dual probing of a fecal sample from donor 9 (Table 3) with Rint623 plus helper (green label) and Rrec584 (red label) probes for Roseburia species and E. rectale. Orange cells have hybridized with both probes. A bacterium that has hybridized with Rint623 only, indicating that it is either a Roseburia inulinivorans or a Eubacterium ramulus cell, can be seen in the center.
Earlier estimates of Roseburia-related populations in the healthy human gut by using the FISH probe Rint623 gave a mean of 2.3% in relation to total eubacteria (19). This is substantially lower than the estimates obtained here, which gave means of around 7% for both of the group probes employed, including the newly designed probe Rint584, which has a slightly narrower recognition specificity than Rint623. Rint623 recognizes a hairpin structure (3), and the use here of a helper probe was intended to improve access and hybridization (14) of the original Rint623 probe. The lower estimate obtained in the previous study (19) came from a different set of healthy subjects, and it is not possible to ascertain whether the difference is due to the different sample set or whether a higher estimate would have been obtained with the present probes and probe-helper combinations.
Conclusions.
This phylogenetic analysis of the available clone and strain sequences has confirmed the abundance of clostridial cluster XIVa bacteria related to R. intestinalis and E. rectale in the human intestinal microbiota. 16S rRNA sequences from 19 recent isolates of strictly anaerobic bacteria, all butyrate producers obtained from the highest dilutions of human fecal samples, were shown here to cluster with this group. Most significantly, five out of the six OTUs defined by the clone library sequences were shown to incorporate cultivated representatives. Although one OTU is still represented only by clone sequences, this accounted for only 3% of the available clone sequences for the group, and its recovery would presumably require much larger numbers of isolates to be screened by the highest-dilution method. The largest single cluster of cultured strains was found to center on the species E. rectale, which has long been considered one of the most abundant species in the human large intestine (11, 27). E. rectale is more closely related to the Roseburia species than to the Eubacterium type species E. limosum, which belongs to cluster XV (4, 37) and is similar to Roseburia spp. in its phenotypic characteristics, including the production of butyrate and the possession of flagella (5). We can therefore define the Roseburia and E. rectale cluster as those bacteria detected by the Rrec584 probe plus R. inulinivorans. FISH data obtained with the new Rrec584 probe and with the Rint623-helper combination gave similar results, showing that Roseburia-related sequences comprised approximately 7% of total bacterial diversity in fecal samples from the 10 healthy subjects studied. Many sequences from the 16S rRNA gene libraries of pig intestinal microbiota also cluster with the Roseburia and E. rectale group, and they comprise up to 8% of total bacterial diversity in the pig gut (22), which is close to the estimate obtained here for human gut microbiota. This suggests that the Roseburia and E. rectale group may be widespread in the guts of other mammals.
In conclusion, it has been possible to populate five out of six Roseburia and E. rectale-related OTUs defined by molecular diversity analyses of the human gut microbiota with cultured strains isolated under conditions of strict anaerobiosis. If this applies also to other groups of anaerobic bacteria, then it appears that under the right cultivation conditions it may be possible to recover a very high proportion of the total bacterial diversity present in the normal human gut as pure cultures.
Nucleotide sequence accession numbers.
The 16S rRNA sequences of E. rectale strains S2Ss2/7 and S2Ss2/2 have been submitted to GenBank under accession numbers AY804151 and AY804152, respectively.
Acknowledgments
The Rowett Research Institute receives support from the Scottish Executive Environment and Rural Affairs Department (SEERAD). Alan W. Walker was supported by a BBSRC-SEERAD grant.
We are grateful to Grietje Holtrop (BioSS) for statistical analysis.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, and H. J. Flint. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens, S., C. Ruhland, J. Inacio, H. Huber, A. Fonseca, I. Spencer-Martins, B. M. Fuchs, and R. Amann. 2003. In situ accessibility of small-subunit rRNA of members of the domains Bacteria, Archaea, and Eucarya to Cy3-labeled oligonucleotide probes. Appl. Environ. Microbiol. 69:1748-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 5.Duncan, S. H., G. L. Hold, A. Barcenilla, C. S. Stewart, and H. J. Flint. 2002. Roseburia intestinalis sp. nov., a new saccharolytic, butyrate producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 52:1615-1620. [DOI] [PubMed] [Google Scholar]
- 6.Duncan, S. H., G. L. Hold, H. J. M. Harmsen, C. S. Stewart, and H. J. Flint. 2002. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 52:2141-2146. [DOI] [PubMed] [Google Scholar]
- 7.Duncan, S. H., G. Holtrop, G. E. Lobley, G. Calder, C. S. Stewart, and H. J. Flint. 2004. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 91:915-923. [DOI] [PubMed] [Google Scholar]
- 8.Duncan, S. H., P. Louis, and H. J. Flint. 2004. Lactate-utilizing bacteria from human feces that produce butyrate as a major fermentation product. Appl. Environ Microbiol. 70:5810-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Duncan, S. H., R. I. Aminov, K. P. Scott, P. Louis, T. B. Stanton, and H. J. Flint. Proposal of three new species of Roseburia, Roseburia faecis (sp. nov.), Roseburia homins (sp. nov.), Roseburia inulinivorans (sp. nov.), based on isolates from human faeces. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 9.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenstein, J. 1998. PHYLIP—phylogeny interference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 11.Finegold, S. M., V. L. Sutter, and G. E. Mathison. 1983. Normal indigenous flora, p. 3-31. In D. J. Hengtes (ed.), Human intestinal microflora in health and disease. Academic Press, New York, N.Y.
- 12.Flint, H. J. 2006. The significance of prokaryote diversity in the human gastrointestinal tract. SGM Symp. 66:65-90. [Google Scholar]
- 13.Franks, A. H., H. J. M. Harmsen, G. C. Raangs, G. J. Jansen, and G. W. Welling. 1998. Variations of bacterial populations in human feces quantified by fluorescent in situ hybridization with group-specific 16S rRNA-targeted probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs, B. M., F. O. Glockner, J. Wulf, and R. Amann. 2000. Unlabeled helper oligonucleotides increase the in situ accessibility to 16S rRNA of fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 66:3603-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmsen, H. J. M., G. C. Raangs, T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauduroy, P., A. Ehringer, G. Guillot, and J. Magrou. 1937. Dictionnaire des bactéries pathogènes. Masson, Paris, France.
- 17.Hayashi, H., M. Sakamoto, and Y. Benno. 2002. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol. Immunol. 46:535-548. [DOI] [PubMed] [Google Scholar]
- 18.Hold, G. L., S. E. Pryde, V. J. Russell, E. Furrie, and H. J. Flint. 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 39:33-39. [DOI] [PubMed] [Google Scholar]
- 19.Hold, G. L., A. Schwiertz, R. I. Aminov, M. Blaut, and H. J. Flint. 2003. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 69:4320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holdeman, L. V., E. P. Cato, and W. E. C. Moore. 1977. Anaerobe laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg, Va.
- 21.Hooper, L. V. 2004. Bacterial contributions to mammalian gut development. Trends Microbiol. 12:129-134. [DOI] [PubMed] [Google Scholar]
- 22.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Moller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis, P., S. H. Duncan, S. I. McCrae, J. Millar, M. S. Jackson, and H. J. Flint. 2004. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 186:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki, K., J. C. Martin, R. Marinssek-Logar, and H. J. Flint. 1997. Degradation and utilisation of xylans by the rumen anaerobe Prevotella bryantii (formerly P. ruminicola subsp. brevis) B14. Anaerobe 3:373-381. [DOI] [PubMed] [Google Scholar]
- 26.Moore, W. E. C., J. L. Johnson, and L. V. Holdeman. 1976. Emendation of Bacteroidaceae and Butyrivibrio and descriptions of Desulfomonas gen. nov. and 10 new species in genera Desulfomonas, Butyrivibrio, Eubacterium, Clostridium, and Ruminococcus. Int. J. Syst. Bacteriol. 26:238-252. [Google Scholar]
- 27.Moore, W. E. C., and L. V. Holdeman Moore. 1986. Genus Eubacterium Prevot 1938 294AL, p. 1353-1373. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 28.Pryde, S. E., S. H. Duncan, C. S. Stewart, G. L. Hold, and H. J. Flint. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217:133-139. [DOI] [PubMed] [Google Scholar]
- 29.Roediger, W. E. 1980. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet ii:712-715. [DOI] [PubMed] [Google Scholar]
- 30.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 31.Savage, D. C. 1986. Gastrointestinal microflora in mammalian nutrition. Annu. Rev. Nutr. 6:155-178. [DOI] [PubMed] [Google Scholar]
- 32.Simmering, R., B. Kleessen, and M. Blaut. 1999. Quantification of the flavonoid-degrading bacterium Eubacterium ramulus in human fecal samples with a species-specific oligonucleotide hybridization probe. Appl. Environ Microbiol. 65:3705-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton, T. B., and D. C. Savage. 1983. Colonization of gnotobiotic mice by Roseburia cecicola, a motile, obligately anaerobic bacterium from murine ceca. Appl. Environ. Microbiol. 45:1677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanton, T. B., and D. C. Savage. 1983. Motility as a factor in bowel colonization by Roseburia cecicola, an obligately anarobic bacterium from the mouse cecum. J. Gen. Microbiol. 130:173-183. [DOI] [PubMed] [Google Scholar]
- 35.Suau, A., R. Bonnet, M. Sutren, J. J. Gordon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker, A. W., S. H. Duncan, E. C. McWilliam Leitch, M. W. Child, and H. J. Flint. 2005. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71:3692-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willems, A., and M. D. Collins. 1996. Phylogenetic relationships of the genera Acetobacterium and Eubacterium sensu stricto and reclassification of Eubacterium alactlyticum as Pseudoramibacter alactolyticus gen. nov., com. nov. Int. J. Syst. Bacteriol. 46:1083-1087. [DOI] [PubMed] [Google Scholar]
- 38.Zoetendal, E. G., A. D. L. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]