Abstract
The goal of this work was to determine the chemical nature of the red pigment produced by Streptococcus agalactiae, which has been thought to be a carotene. We extracted the pigment with 0.1 M KOH and purified it by column chromatography on Sephadex LH. Data from elemental analysis and mass and nuclear magnetic resonance spectra lead us to propose the structure to be that of a new ornithine rhamno-polyene with 12 conjugated double bonds, to which we have assigned the trivial name granadaene.
Streptococcus agalactiae (group B streptococcus [GBS]) is a hemolytic streptococcus which is the most important bacterium causing life-threatening infections in neonates (7). Production of a red pigment was recognized early in GBS (11), and pigment detection is a popular method for identifying GBS today (5). It was suggested (8) and is accepted that the GBS pigment is a carotene (3, 6), based on its UV-visible spectrum. Surprisingly, no further data support this, and genes coding for carotene biosynthesis are not present in GBS (7). Our data show that the GBS pigment is not a carotene but an ornithine glycopolyene, and we think that this is the first report of a glycopolyene pigment and of a polyene pigment among gram-positive bacteria.
(This work was presented in part at the 7th ASM Conference on Streptococcal Genetics, Saint Malo, France, June 2006.)
S. agalactiae ATCCC 12386 was grown in Erlenmeyer flasks containing Granada medium without agar (9) and incubated at 36°C in a shaker until the broth became deeply red (48 to 72 h). Cells harvested by centrifugation were washed with 0.15 M NaCl, and lipids were extracted with chloroform-methanol (2:1, vol/vol). Afterwards, pigment was extracted with KOH 0.1 M and purified as follows: (i) pigment was precipitated from the extract with methanol-1 M HCl (1:1 vol/vol); (ii) the red precipitate was washed with dimethyl sulfoxide (DMSO) and dissolved in DMSO-0.1% trifluoroacetic acid (TFA); (iii) this solution was kept in an open flask in a desiccator together with a crystallizer containing ammonium hydroxide for several hours, which results in pigment precipitation; (iv) this pigment was redissolved in DMSO-0.1% TFA and chromatographed on a column (950 by 23.5 mm) of Sephadex LH-20 (Amersham Biosciences, Denmark) which was eluted with DMSO-0.1% TFA, and the eluate peak containing the pigment (absorption at 525 nm with minimum absorption at 280 nm) was collected; and (v) pigment was reprecipitated as in step iii. For elemental analysis, pyridine/acetic anhydride acetylation, mass spectrometry (MS), and spectroscopy, the pigment was washed with water and lyophilized. For UV-visible spectroscopy, the pigment was dissolved in DMSO-0.1% TFA. For nuclear magnetic resonance (NMR) (300 MHz for 1H and 75 MHz for 13C) (1H, correlation spectroscopy [COSY], and heteronuclear single quantum correlation [HSQC]), pigment was dissolved in deuterated DMSO-0.1% TFA. For NMR of the acetylated product, it was dissolved in deuterated chloroform.
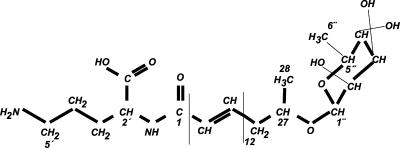
The structure assigned to the GBS pigment is shown in Fig. 1. MS showed an M + H ion at m/z 677.3769, and a nominal mass of 676 is assigned. Elemental analysis showed 3.8% nitrogen and that assigns two N atoms (in accordance with the nitrogen rule). The exact molecular mass and the presence of two N atoms and several hydroxyls (in the MS, fragmentation ions 659, 641, 623, and 605 are detected as result of one, two, three, and four losses of water) match only with the formula C39H53O8N2, which has a double-bond equivalent of 14.5, which corresponds to a chain of 12 double bonds plus two carbonyl and the rhamnose. Peaks at 522, 488, and 460 nm in the UV-visible spectrum (caretonid-like spectrum) together with the integrals of the olefinic protons confirm the presence of the conjugated double bonds.
FIG. 1.
Proposed structure of granadaene.
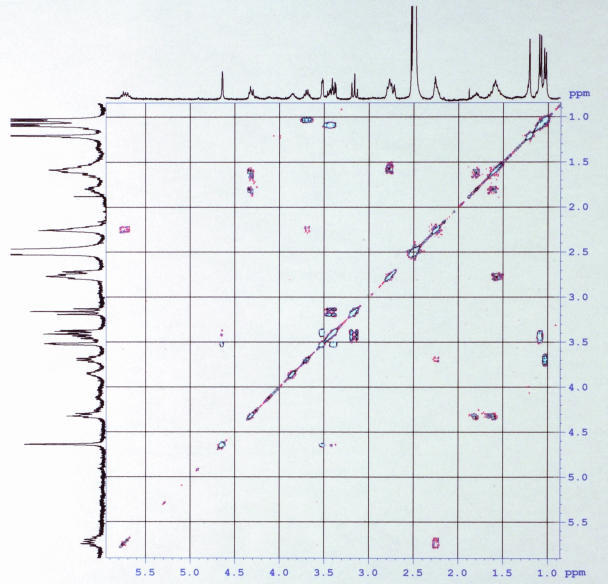
The 1H NMR showed only two methyl signals (at 1.03 and 1.09 ppm) as doublets, whereas the majority of protons resonated between 6.2 and 6.5 ppm (Fig. 2). This pattern is inconsistent with an isoprene, for which a great number of methyl protons should exist (in the edited HSQC, the signals in the region of 1.4 to 3 ppm correspond to methylene groups), and indicates a polyene structure.
FIG. 2.
COSY NMR of granadaene.
The 1H NMR signals at 4.63, 3.52, 3.39, 3.16, 3.43, and 1.09 ppm (and their pattern), which correlated with the 13C signals at 97.42, 70.82, 70.55, 71.79, 68.77, and 17.68 ppm, respectively (Tables 1 and 2), are tentatively assigned to α-l-rhamnose (1). The fact that the sugar is linked at C-27 is demonstrated because (i) in the acetylated product, 1H NMR signals from the 2", 3", and 4" protons shifted to lower fields and the 1"and 27 ones were not affected; (ii) an ion of m/z 531.3232 is detected in the MS fragmentation of the 677 m/z ion, accounting for the loss of the sugar unit from the main structure; and (iii) there were losses of water from the main structure (fragmentation ions mentioned above). The 1H NMR signals at 4.32, 1.64 and 1.80, 1.59, and 2.77 ppm correlated with the 13C signals at 51.15, 27.95, 23.48, and 38.10 ppm, respectively (Tables 1 and 2), and are tentatively assigned to ornithine with an amidic linkage at its amine group at C-2′ (10). In addition, an ion with an m/z of 545.2945 is detected, accounting for the loss of ornithine, and an ion with an m/z of 399.2294 is also detected, accounting for the loss of ornithine and rhamnose. 1H-NMR signals at 7.1 ppm (double doublet, 15.1 and 11 Hz, H-3) and 6.1 ppm (doublet, 15.1 Hz, H-2) indicate the conjugation of the polyene with the carbonyl group (C-1).
TABLE 1.
1H NMR spectral data for granadaene
| Carbon | H1 NMR signal, ppm (DMSO-D6, 0.1% TFA-d) |
1H NMR reference signal, ppm
|
|
|---|---|---|---|
| DMSO-D6 (1) | CD3OD (10) | ||
| 1" | 4.63 (1H; d; 1.5 Hz) | 4.51 (1H; bs) | |
| 2" | 3.52 (1H; dd; 1.5, 3.0 Hz) | 3.63 (1H; bs) | |
| 3" | 3.39 (1H; dd; 3.0, 9.0 Hz) | 3.48 (1H; dd; 3.3, 9.5 Hz) | |
| 4" | 3.16 (1H; t; 9.0 Hz) | 3.26 (1H; t; 9.5 Hz) | |
| 5" | 3.43 (1H; dd; 6.0, 9.0 Hz) | 3.51 (1H; dd; 3.3, 9.5 Hz) | |
| 6 | 1.09 (3H; d; 6.0 Hz) | 1.02 (3H; d; 6.1 Hz) | |
| 2 | 6.1 (1H; d; 15.1 Hz) | 6.07 (d; 15.0 Hz) | |
| 3 | 7.1 (1H; dd; 11, 15.1 Hz) | 7.03 (1h; dd; 11.2, 15.0 Hz) | |
| 5 | 6.66 (1H; dd; 11, 15.1 Hz) | 6.50 (1H; dd; 10.7, 14.8 Hz) | |
| 4, 6-24 | 6.2-6.5 (15H; m) | ||
| 25 | 5.72 (1H; dt; 7.2, 14.5 Hz) | ||
| 26 | 2.25 (1H; m) | ||
| 27 | 3.69 (1H; sextuplet; 6.1 Hz) | ||
| 28 | 1.03 (3H; d; 6.1 Hz) | ||
| 2′ | 4.32 (1H; dd; 5, 8.9 Hz) | 4.36 (1H; dd; 5.3, 7.9 Hz) | |
| 3′ | 1.64 (1H; m), 1.80 (1H; m) | 1.75 (1H; m), 1.89 (1H; m) | |
| 4′ | 1.59 (2H; m) | 1.63 (2H; m) | |
| 5′ | 2.77 (2H; t; 6.7 Hz) | 3.19 (2H; m) | |
TABLE 2.
13C NMR spectral data for granadaene
| Carbon | 13C NMR signal, ppm (DMSO-D6, 0.1% TFA-d) |
13C NMR reference signal, ppm
|
|
|---|---|---|---|
| DMSO-D6 (1) | CD3OD (10) | ||
| 1" | 97.42 | 100.3 | |
| 2" | 70.82 | 70.6 | |
| 3" | 70.55 | 70.8 | |
| 4" | 71.79 | 72.1 | |
| 5" | 68.77 | 68.2 | |
| 6" | 17.68 | 18.1 | |
| 2 | 123.97 | 124.4 | |
| 3 | 138.81 | 141.9 | |
| 5 | 139.55 | 141.2 | |
| 4, 6-24 | 130-136 | ||
| 25 | 131.72 | 129.5 | |
| 26 | 39.9 | ||
| 27 | 70.96 | ||
| 28 | 18.68 | ||
| 2′ | 51.15 | 55.6 | |
| 3′ | 27.95 | 31.2 | |
| 4′ | 23.48 | 26.3 | |
| 5′ | 38.10 | 42.0 | |
These data show that GBS pigment is a 676-Da ornithine rhamno-polyene with a linear chain of 12 conjugated double bonds (Fig. 1). We propose for it the trivial name of granadaene.
Unlike carotenes, granadaene it is not soluble and cannot be extracted from GBS cells with most solvents (water, methanol, ethanol, diethyl ether, acetonitrile, tetrahydrofuran, chloroform, DMSO, or 1 M HCl). Conventional purification by reverse-phase high-pressure liquid chromatography was hindered because of this solubility problem and because granadaene links to hydrophobic ligands.
In DMSO-TFA (and in cell membranes) granadaene shows a carotene-like spectrum (three peaks) (8), but after addition of NaOH the spectrum shifts to one peak (maximum at 420 nm). This change reverses when TFA is added. Granadaene is also produced in the one-peak form when GBS grows in medium containing amylase (or serum). These facts can account for the two GBS pigments previously described (14).
Although polyenic pigments are found in fungi (2), in bacteria they have been reported only for Xanthomonas (4). The GBS pigment is considered a virulence factor based on the assumption that it is a carotene and on the fact that it is always associated with the hemolysin/cytolysin (6, 7). The hemolysin of GBS has not been characterized (7), but the chromosomal locus encoding hemolysin activity has been identified and designated cyl (12), and it includes genes with homology to those that encode enzymes involved in fatty acid (6) and polyene (4) biosynthesis. Understanding that the GBS pigment is a polyene instead of a carotene might help to elucidate its biosynthesis pathway and the role of cyl genes in the universal linkage between pigment and hemolytic activity in GBS (12, 13).
Acknowledgments
We are grateful to Guillermo Gimenez-Gallego and Jesus Jimenez-Barbero from the Spanish Centre of Biological Investigation (CSIC) (Madrid, Spain), to Francisco Santoyo from the Organic Chemistry Department of Granada University, to Fernando Reyes from the Pharmamar Drug Discovery Department, and to Javier Galvan from the Virgen de las Nieves University Hospital for their help and advice.
REFERENCES
- 1.Barrero, A. F., A. Haidour, M. Munoz-Dorado, M. Akssira, A. Sedqui, and I. Mansour. 1998. Polyacetylenes, terpenoids and flavonoids from Bupleurum spinosum. Phytochemistry 48:1237-1240. [Google Scholar]
- 2.Davoli, P., A. Mucci, L. Schenetti, and R. W. Weber. 2005. Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 66:817-823. [DOI] [PubMed] [Google Scholar]
- 3.Doran, K. S., and V. Nizet. 2004. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 54:23-31. [DOI] [PubMed] [Google Scholar]
- 4.Goel, A. K., L. Rajagopal, N. Nagesh, and R. V. Sonti. 2002. Genetic locus encoding functions involved in biosynthesis and outer membrane localization of xanthomonadin in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 184:3539-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta, C., and L. E. Briski. 2004. Comparison of two culture media and three sampling techniques for sensitive and rapid screening of vaginal colonization by group B streptococcus in pregnant women. J. Clin. Microbiol. 42:3975-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, G. Y., and V. Nizet, V. 2004. Extracellular virulence factors for group B streptococci. Front. Biosci. 9:1794-11792. [DOI] [PubMed] [Google Scholar]
- 7.Liu, G. Y., K. S. Doran, T. Lawrence, N. Turkson, M. Puliti, L. Tissi, and V. Nizet. 2004. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defence. Proc. Natl. Acad. Sci. USA 101:14491-14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrit, K., and J. N. Jacobs. 1978. Characterization and incidence of pigment production by human clinical group B streptococci. J. Clin. Microbiol. 8:105-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosa, M., M. Perez, C. Carazo, J. I. Peis, L. Pareja, and L. Hernández. 1992. New Granada medium for detection and identification of group B streptococci. J. Clin. Microbiol. 30:1019-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakemi, S., T. Ichiba, S. Kohmoto, G. Saucy, and T. Higa. 1988. Isolation and structure elucidation of onnadine A, novel bioactive metabolite of a marine sponge; Thaonella sp. J. Am. Chem. Soc. 110:4851-4853. [Google Scholar]
- 11.Sherman, J. M. 1937. The streptococci. Bacteriol. Rev. 1:3-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spellerberg, B., B. Pohl, G. Haase, S. Martin, J. Weber-Heynemann, and R. Lutticken. 1999. Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition. J. Bacteriol. 181:3212-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spellerberg, B., S. Martin, C. Brandt, and R. Lutticken. 2000. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol. Letter. 188:125-128. [DOI] [PubMed] [Google Scholar]
- 14.Tapsall, J. W. 1987. Relationship between pigment production and haemolysin formation by Lancefield group B streptococci. J. Med. Microbiol. 24:83-87. [DOI] [PubMed] [Google Scholar]