Abstract
Enterobacter sakazakii has been associated with life-threatening infections in premature low-birth-weight infants. Contaminated infant milk formula (IMF) has been implicated in cases of E. sakazakii meningitis. Quick and sensitive methods to detect low-level contamination sporadically present in IMF preparations would positively contribute towards risk reduction across the infant formula food chain. Here we report on the development of a simple method, combining charged separation and growth on selective agar, to detect E. sakazakii in IMF. This protocol can reliably detect 1 to 5 CFU of E. sakazakii in 500 g of IMF in less than 24 h.
Enterobacter sakazakii is a gram-negative rod that was formerly known as “yellow-pigmented Enterobacter cloacae” until 1980 (8). This bacterium is an emerging opportunistic pathogen predominantly associated with bacterial meningitis in immunocompromised neonates (2, 9, 14, 20). Other clinical presentations of the infection include bacteremia and necrotizing enterocolitis (14, 18). While it appears that the frequency of E. sakazakii infections is low, progress to understand the epidemiology and institute effective control measures has been poor to date. Reported case-mortality meningitis rates vary from 40 to 80% among infected infants, with the majority of those who survive Enterobacter-associated meningitis (94%) developing an irreversible neurological sequela (20).
The bacterium has been cultured from a variety of food matrices, including cheese, meat, vegetables, grain, bread, herbs, and spices (12, 14). Although the natural habitat of E. sakazakii has yet to be identified, infant milk formula (IMF) has been epidemiologically linked to cases of neonatal meningitis (1, 10, 19).
A protocol previously established by the U.S. Food and Drug Administration is being used to screen IMF for the presence of E. sakazakii (17). No validation studies with this protocol have been reported and, furthermore, no detection limit has been established. Briefly, this method requires at least 5 days to complete and consists of an initial preenrichment step, followed by a second enrichment step and subsequent isolation of pure colonies on violet red bile glucose agar. Several isolated colonies are selected and restreaked onto tryptone soy agar (TSA). Typical yellow-pigmented colonies are detected after an overnight incubation for 48 to 72 h at 25°C. Finally, these presumptive colonies are identified biochemically (17). This approach provides only a generic test for Enterobacteriaceae and lacks the necessary capability to specifically identify E. sakazakii. Recently, a number of selective agars have become available to aid identification. One of these is the chromogenic DFI agar (Druggan-Forsythe-Iversen formulation), which can be used to identify and enumerate E. sakazakii (11, 12).
In this report we describe the development and application of a simple capture and detection protocol for E. sakazakii in dried infant formula. This method requires a short enrichment period, followed by capture of the bacteria and subsequent identification after plating onto DFI agar. The protocol can detect between 1 and 5 CFU in 500 g of powdered formula, following sample pooling, in less than 24 h.
MATERIALS AND METHODS
Pathatrix principle.
Pathatrix (Matrix Microscience Ltd., Newmarket, United Kingdom) is a patented capture system that is based on the use of coated paramagnetic beads. These cationic (positively charged) magnetic beads electrostatically attract the negatively charged lipopolysaccharide on the surface of gram-negative bacteria.
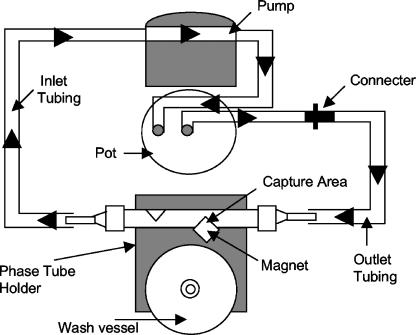
This protocol requires a preprogrammed workstation (Matrix Microscience Ltd.), generic consumables, and (in this study) positively charged (cationic) paramagnetic beads (ZCCB-CAT; Matrix Microscience, Newmarket, United Kingdom) (a general schematic overview of the operation of Pathatrix is shown in Fig. 1). Unlike other particle-based separation techniques, Pathatrix facilitates the sampling and subsequent analysis of a complete homogenate. In the protocol reported here, a weighed IMF sample is homogenized for 1 min and enriched (for 6 h) in buffered peptone water (BPW; Difco Laboratories, Le Pont de Claix, France) at 42°C, in a thermally controlled static incubator. Following transfer of the stomacher bag to the workstation and connection of the circulatory system, the cationic beads are introduced and a 30-minute capture sequence is initiated (Fig. 1). The complete sample homogenate is circulated (flow rate of 450 ml per minute) over the charged cationic beads, to which the bacteria become attached each time they pass over the magnet. Upon completion of this capture phase, bound organisms are washed and eluted to a clean Eppendorf tube. Captured E. sakazakii organisms are plated directly onto DFI agar (Oxoid CM1055; Oxoid, Hampshire, United Kingdom) and incubated at 37°C for 18 h. All experimental analyses were performed in triplicate, and data presented represent the means of those independent evaluations.
FIG. 1.
Schematic diagram showing the Pathatrix system. All relevant features are indicated with arrows. The temperature-controlled pot is indicated.
Bacterial strains and culture conditions.
Enterobacter sakazakii type strain NCTC 11467 was used throughout this study for all optimization and sensitivity experiments. In addition, previously characterized Salmonella enterica serotypes Typhimurium DT104 and Enteritidis (4) were used in competition experiments to determine the selectivity of the cationic beads. Other Enterobacteriaceae known to cause meningitis were also evaluated with the Pathatrix system. Table 1 provides a list of these bacteria.
TABLE 1.
Pathatrix recovery of other Enterobacteriaceae in 100 g of IMF diluted with 900 ml BPWa
| Species | Inoculum (CFU/100 g) | Recovery on DFI agar |
|---|---|---|
| Enterobacter cloacae | 1-5 | +++ |
| Enterobacter cloacae | 6-10 | +++ |
| Citrobacter freundii | 1-5 | + |
| Citrobacter freundii | 6-10 | + |
| Citrobacter diversus | 1-5 | +++ |
| Citrobacter diversus | 6-10 | +++ |
| Klebsiella pneumoniae | 1-5 | + |
| Klebsiella pneumoniae | 6-10 | + |
| Klebsiella oxytoca | 1-5 | + |
| Klebsiella oxytoca | 6-10 | ++ |
A 250-ml volume of the enriched culture was analyzed with the Pathatrix system. Recovery results: +, 1 to 10 CFU; ++, 10 to 100 CFU; +++, >100 CFU.
All bacterial strains were cultured overnight in tryptic soy broth (TSB; Oxoid, Hampshire, United Kingdom) at 37°C in an orbital shaker (Thermo Electron Corp., Ohio). Overnight cultures were subcultured after 18 h into fresh prewarmed TSB, and growth was continued to late log phase (optical density at 610 nm, 0.8). Cell densities were determined by spectrophotometry (Biomate 5; ThermoSpectronic, Cambridge, United Kingdom). To obtain an initial low cell number, 10-fold serial dilutions were performed in phosphate-buffered saline (Oxoid, Hampshire, United Kingdom). Viable cell numbers were determined by direct plating to TSA following incubation at 37°C for 18 to 24 h.
Freeze-drying of bacterial cells.
E. sakazakii NCTC 11467 cells were grown on TSA at 37°C for 24 h and suspended in double-strength skimmed milk. One ml of the suspension was dispensed into a sterile ampoule and connected to a manifold freeze-dryer (EF03; Edwards, Sussex, United Kingdom) for 24 h. The ampoules were sealed using a glass burner and stored at room temperature. Freeze-dried cells were rehydrated in sterile deionized H2O, and the number of CFU were determined by the most-probable-number (MPN) assay (15), using serial dilutions in BPW after aliquoting into 96-well microtiter plates. Based on the growth observed at higher dilutions, the MPN of survivors was calculated using an “eight-tube” technique (3).
IMF.
Eight commercially available dried IMF powders were reconstituted and used in the course of this study. The brands consisted of different formulations and blends, including organic varieties, powders supplemented with prebiotics, and others tailored for specific age groups.
Confirmation by real-time PCR.
A real-time PCR assay (16) targeting the dnaG gene on the macromolecular synthesis operon of E. sakazakii was modified and used to confirm identification of presumptive colonies (6). Briefly, thermal amplification of the target region was performed in a Rotor-Gene RG-300 instrument (Corbett Research, Cambridge, United Kingdom) in a final volume of 20 μl containing 200 μM deoxynucleoside triphosphates, 4.0 mM MgCl2, 2.0 μl of 10× reaction buffer, 500 nM of each primer, 250 nM of probe, 1 U Taq DNA polymerase, and 100 ng of purified template DNA.
A presumptive E. sakazakii colony was taken from each DFI agar plate and suspended in 50 μl sterile water (Sigma, Ayrshire, United Kingdom). Cells were lysed by heating to 80°C for 10 min, and DNA was separated from cellular debris by centrifugation for 2 min at 10,000 × g. Real-time PCR was performed by adding 2 μl (containing approximately 100 ng of DNA template) of this supernatant to 18 μl of PCR master mix (as outlined above).
Defining the limits of detection.
To determine the sensitivity of the method, Pathatrix was operated using three different culture variations. From these data, the limit of detection was defined.
Recovery in BPW without enrichment.
To determine the lower limits of detection using the Pathatrix protocol, mid-log-phase E. sakazakii cells were serially diluted in phosphate-buffered saline to establish a range from 102 through 106 CFU/ml. One-ml volumes were added directly into 249 ml BPW without IMF, prewarmed to 42°C. (No enrichment step was carried out on this occasion.) Following homogenization in a stomacher for 1 min, a 30-min capture over charged paramagnetic beads was performed and E. sakazakii cells were plated directly onto DFI. These plates were incubated for 18 h at 37°C, and the colonies were counted.
Recovery in BPW and IMF without enrichment.
The previous experiment was repeated using the same range of cell concentrations as before, with inocula of 1 ml in 25 g of IMF in 224 ml BPW. No enrichment step was performed.
Recovery in BPW and IMF with enrichment.
Varied cell numbers, from 1 to 15 CFU, were added into 100 g of dry IMF powder, and the mixture was added to 900 ml of prewarmed BPW. Eight IMF formulations were evaluated. Following homogenization, the culture of each IMF was incubated statically in a plastic stomacher bag (Seward Ltd., London, United Kingdom) for 6 h at 42°C. Each IMF sample was individually captured as described previously after 250 ml of the enriched culture was circulated for 30 min. Captured E. sakazakii cells were subsequently eluted and plated directly onto DFI agar.
Competition experiments with Salmonella and other Enterobacteriaceae.
Varied cell numbers of E. sakazakii along with defined numbers of either S. enterica serotype Typhimurium DT104 or serotype Enteritidis were spiked simultaneously into 100 g of IMF in 900 ml BPW. Enrichment and capture steps were performed as described previously. The ability of the Pathatrix protocol to capture the target organism in the presence of low to high (1 to 50 CFU) numbers of Salmonella cells was determined.
Other Enterobacteriaceae known to sporadically contaminate IMF and which have been linked as etiological agents of meningitis in infants were also included for analysis (Table 1). The Pathatrix protocol was applied to these bacteria to assess its ability to capture and detect them (as previously described).
Detection by pooling.
A pooling protocol was developed to facilitate the simultaneous screening of large sample weights of IMF. This strategy was applied postenrichment and used a sampling plan previously approved by the American Organization of Analytical Chemistry Research Institute (AOAC-RI approved for Salmonella spp. [090203B], Listeria spp. [090201B], and Escherichia coli O157 [070502]), based on the analysis of a defined portion of each individual sample.
Detection of E. sakazakii by pooling.
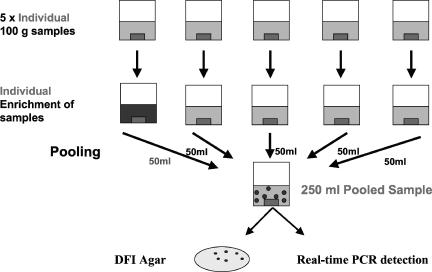
Dry preweighed IMF samples containing various numbers of E. sakazakii cells were enriched as outlined previously. One-hundred-gram IMF samples were placed into a 3.5-liter stomacher bag and mixed with 900 ml BPW. This was replicated an additional four times, equivalent to the analysis of 500 g of powder (Fig. 2). An E. sakazakii inoculum of 1 to 5 CFU was added to one stomacher bag only. All mixes were homogenized for 1 min and incubated statically for 6 h at 42°C. A pooling strategy was employed (Fig. 2) to facilitate the simultaneous screening of all five IMF samples. Each stomacher bag was removed from the incubator, and a 50-ml sample was taken from each and combined to give a final volume of 250 ml. The entire 250-ml sample volume was circulated for 30 min (as before). After capture, the washed cationic-bacteria suspension was concentrated, spread plated directly onto DFI agar, and incubated at 37°C for 18 h. If the 500-g pooling protocol generated a positive result, each 100-g sample was retested to identify the contaminated sample.
FIG. 2.
Pooling protocol approved by the American Organization of Analytical Chemistry for the simultaneous analysis of 500-g quantities of IMF. The dark box in the individual enrichment of samples represents an E. sakazakii-positive sample.
Detection of desiccated E. sakazakii by pooling.
Using the pooling protocol (described above), the performance of Pathatrix to detect dry-stressed E. sakazakii with an extended enrichment time (8 h) was assessed. Desiccated cells were rehydrated in deionized H2O, serially diluted in BPW, and added immediately to the reconstituted IMF. Suspect, contaminated acid casein powders were also evaluated for recovery of stressed E. sakazakii cells. Fifty randomly acquired acid casein dry powdered samples representing part of a microbiological quality assurance program undertaken by a dairy products company supplying ingredients to an IMF manufacturing facility were tested. Levels of E. sakazakii contamination (if any) in these acid casein samples were unknown.
RESULTS
The time required for enrichment of samples was independently determined by evaluating different incubation periods (data not shown). Sufficient cell numbers were generated after 6 h, and these could be successfully captured. The enrichment time can be extended to 8 h when severely stressed E. sakazakii cells are suspected. Similarly, the optimum enrichment temperature was determined (data not shown) by investigating a range of temperatures (including 35, 37, 39, and 42°C). The optimum enrichment temperature was 42°C, although the variation in doubling time was negligible between 39 and 42°C. The upper temperature was chosen for the purpose of conferring a minor selective advantage for the enrichment of E. sakazakii in IMF. All presumptive E. sakazakii colonies were confirmed by real-time PCR.
Defining the limits of detection. (i) Recovery in BPW without enrichment.
To establish the capture efficiency of the cationic beads in the absence of a food matrix, predetermined (low) numbers of E. sakazakii cells were added directly to BPW. On this occasion no enrichment was performed. The limit of detection was determined to be 10 CFU/ml (equivalent to 2.5 × 103 CFU in 250 ml).
(ii) Recovery in BPW and IMF without enrichment.
To investigate possible interference effects of a food matrix, the previous experiment was repeated but with the addition IMF. Again, no enrichment was performed. These data established the limit of detection to be 20 CFU/ml (5 × 103 CFU in 250 ml), indicating a minimal interference effect by the addition of IMF to the system. Epidemiological studies have revealed that initial E. sakazakii cell numbers between 0.36 and 66 CFU/g may be present in contaminated batches of powder (13).
(iii) Recovery in BPW and IMF with enrichment.
Following the confirmation of the test's ability to capture E. sakazakii in IMF, low numbers of E. sakazakii were spiked into IMF and enriched for 6 h at 42°C. Table 2 summarizes the findings of these experiments. This method reliably detected between 1 and 5 CFU E. sakazakii in 100 g IMF, with higher inocula producing a higher recovery of E. sakazakii (Table 2). Minor variations in recovery were observed between some IMF blends, which was possibly due to individual compositions enhancing the growth of E. sakazakii or through background interference by other bacteria.
TABLE 2.
Pathatrix detection of E. sakazakii in 100 g of IMF diluted with 900 ml BPWa
| IMF sample no. | Inoculum (CFU/100 g) | Recovery on DFI agar |
|---|---|---|
| 1 | 1-5 | ++ |
| 2 | 1-5 | ++ |
| 3 | 1-5 | ++ |
| 4 | 1-5 | +++ |
| 5 | 1-5 | +++ |
| 6 | 1-5 | ++ |
| 7 | 1-5 | ++ |
| 8 | 1-5 | ++ |
| 8 | 6-10 | ++ |
| 8 | 11-15 | +++ |
A 250-ml volume of the enriched culture was analyzed with the Pathatrix system. Recovery results: +, 1 to 10 CFU; ++, 10 to 100 CFU; +++, >100 CFU.
Competition experiments with Salmonella and capture of other Enterobacteriaceae.
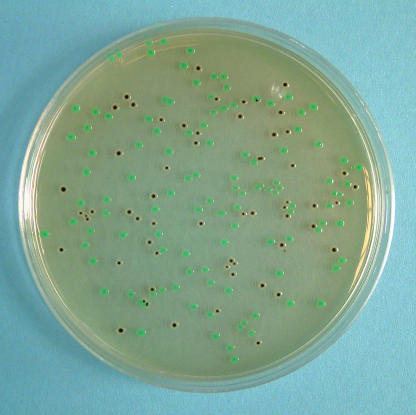
IMF is not a sterile product and may contain other related organisms (12, 13). These other bacteria could potentially interfere with the detection of E. sakazakii. To test this hypothesis, competition studies were undertaken with two Salmonella enterica serotypes (Typhimurium DT104 and Enteritidis). Table 3 provides a summary of these data. S. enterica serotypes were easily discriminated on DFI agar from the blue-green colonies of E. sakazakii, as the former appeared as a distinct black colony (Fig. 3). Although the competing Salmonella organisms reduced the recovery of E. sakazakii, complete exclusion was not observed and no false negatives were recorded.
TABLE 3.
Interference study with two S. enterica serotypes and 100 g IMF diluted with 900 ml BPWa
| S. enterica serotype and IMF sample no. | Inoculum (CFU/100 g)
|
Recovery on DFI agar
|
||
|---|---|---|---|---|
| S. enterica | E. sakazakii | S. enterica | E. sakazakii | |
| Typhimurium | ||||
| 1 | 10 | 1-5 | +++ | ++ |
| 2 | 20 | 1-5 | ++ | +++ |
| 3 | 50 | 1-5 | ++ | ++ |
| Enteritidis | ||||
| 1 | 1-5 | 1-5 | +++ | ++ |
| 2 | 1-5 | 1-5 | +++ | +++ |
| 2 | 10-15 | 1-5 | +++ | +++ |
| 3 | 1-5 | 1-5 | +++ | +++ |
| 3 | 10-15 | 1-5 | +++ | ++ |
A 250-ml volume of the enriched culture was analyzed with the Pathatrix system. For each IMF sample tested, a 100-g aliquot was used. Recovery results: +, 1 to 10 CFU; ++, 10 to 100 CFU; +++, >100 CFU.
FIG. 3.
DFI agar plate showing mixed colony types containing E. sakazakii and S. enterica serotype Typhimurium DT104 (black).
Individual 25-gram samples of IMF containing a range of other Enterobacteriaceae that are known etiological agents of neonatal meningitis (5) were also evaluated for capture and detection by this method. Many of these have been previously isolated from IMF preparations (12, 13). Table 1 shows that all of these bacteria could be recovered and detected in IMF.
Detection by pooling. (i) Detection of E. sakazakii by pooling.
A pooling protocol was employed to facilitate the testing of 500 g of IMF (Fig. 2). Pooling of enriched IMF preparations permits the simultaneous screening of multiple preweighed IMF samples. The technique detected low numbers of E. sakazakii in 500 g of IMF, ranging from 1 to 5 CFU/ml, and was successfully applied using all IMF blends (Table 4).
TABLE 4.
Pathatrix E. sakazakii detection with poolinga
| IMF sample no. | Inoculum (CFU/500 g) | Recovery on DFI agar |
|---|---|---|
| 1 | 1-5 | +++ |
| 2 | 1-5 | ++ |
| 3 | 1-5 | +++ |
| 4 | 1-5 | ++ |
| 5 | 1-5 | +++ |
| 6 | 1-5 | ++ |
| 7 | 1-5 | ++ |
| 8 | 1-5 | ++ |
Five 100-g samples of IMF were each enriched in 900 ml BPW. One was inoculated. Following enrichment, a 50-ml aliquot was taken from each and the pooled 250 ml of homogenates was analyzed with the Pathatrix system. Recovery results: +, 1 to 10 CFU; ++, 10 to 100 CFU; +++, >100 CFU.
(ii) Detection of desiccated E. sakazakii by pooling.
The Pathatrix capture method and the pooling protocol above were further evaluated for the ability to recover dry-stressed cells. An extended enrichment time was employed on this occasion to provide an adequate period for the bacteria to be resuscitated. Again, the protocol was applied at low inoculum levels. Composite values from separate MPN assays established that a desiccated inoculum equivalent to 2.2 cells was detectable in 500 g of IMF in 24 h. Furthermore, acid- and dry-stressed E. sakazakii cells were also successfully recovered directly from powdered acid casein samples.
DISCUSSION
In this study we described the development and application of a user-friendly capture and detection protocol for E. sakazakii. Currently, the U.S. Food and Drug Administration method is applied to detect E. sakazakii and can take at least 5 days for complete identification. This protocol is limited by its broad selectivity for Enterobacteriaceae. Development of an effective testing protocol is an essential component of any control program. Implementation of a method that could reliably capture and identify E. sakazakii would be important in reducing the risk of product contamination and in protecting the health of vulnerable groups. Furthermore, it would facilitate the implementation of a positive release strategy as part of a quality assurance program for powder manufacturers.
The protocol described in this report can be completed in less than 24 h and is not affected by background bacterial contamination. In addition, the technique is amenable to alteration for the analysis of a smaller quantity of IMF in a greater volume of diluent and for the screening of IMF precursors, other dried foodstuffs, and environmental dust or scrapings for the presence of E. sakazakii, thereby permitting the environmental monitoring of a manufacturing facility. In this study the type strain E. sakazakii NCTC 11467 was used along with various IMF formulations to validate the protocol described. The capture and detection of other E. sakazakii strains from environmental sources, cultured as part of a manufacturing facility surveillance program, were also evaluated. All of these isolates when present could be recovered and successfully identified.
Enterobacter sakazakii cells, if present in dried powder, are in a stressed desiccated state in routine analysis of IMF. We simulated these conditions by freeze-drying cells and spiking reconstituted formula prior to the enrichment step. An extended enrichment time of 8 h was sufficient to resuscitate the bacteria and to increase the cell numbers to a detectable level, thereby facilitating capture. We also report the successful recovery and detection of stressed E. sakazakii from acid casein powders that were submitted to us for independent testing. Acid casein may become sporadically contaminated with E. sakazakii, which could result in the contamination of IMF. The capture and detection protocol outlined previously was applied to all individual samples. Our method could reliably detect stressed (due to low pH and desiccation) E. sakazakii cells from the dried powder using the protocols previously established. The identity of any recovered organisms was confirmed by biotyping and their corresponding color reaction on DFI agar. All presumptive E. sakazakii samples produced a positive signal by real-time PCR (data not shown).
The presence of Enterobacteriaceae is often used as an indicator of product quality and hygienic manufacturing practice. This simple enrichment, capture, and detection method is useful for microbiological screening of IMF and its constituent ingredients, as it can identify the presence of human pathogens, including Salmonella spp., E. sakazakii, and other known etiological agents of bacterial meningitis. Implementation of this approach would positively contribute towards an effective quality control program in the IMF manufacturing industry, thereby limiting the risk of transmission of such pathogens to infants fed powdered formula.
Effective user-friendly detection methods are more likely to gain acceptance by the IMF industry as a means of ensuring product safety, brand protection, and guaranteeing consumer confidence in their products. Recently, the European Food Safety Authority (7) recommended the introduction of a performance objective for powered infant formula and follow-on formula. Implementation of this performance objective is designed to eliminate Salmonella and E. sakazakii from manufactured infant milk powder. Verification of compliance can be confirmed by testing for Enterobacteriaceae in both the product and the manufacturing environment. The protocol described here will support the IMF industry's objective of meeting these requirements.
Acknowledgments
We acknowledge the financial support provided through the Irish Government's Food Institutional Research Measure grant no. 05/R&D/D/363 and by the Newman Scholarship Programme, University College Dublin (D.D. is the Diageo Newman Scholar in Food Safety).
We also thank our colleague John Flynn for freeze-drying bacterial cells.
REFERENCES
- 1.Bar-Oz, B., A. Preminger, O. Peleg, C. Block, and I. Arad. 2001. Enterobacter sakazakii infection in the newborn. Acta Paediatr. 90:356-358. [PubMed] [Google Scholar]
- 2.Biering, G., S. Karlsson, N. C. Clark, K. E. Jonsdottir, P. Ludvigsson, and O. Steingrimsson. 1989. Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J. Clin. Microbiol. 27:2054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blodget, R. 2001. Biological analytical manual, 8th ed. [Online.] http://www.cfsan.fda.gov/∼ebam/bam-a2.html. Accessed 20 March 2006.
- 4.Daly, M., J. Buckley, E. Power, and S. Fanning. 2004. Evidence for a chromosomally located third integron in Salmonella enterica serovar Typhimurium DT104b. Antimicrob. Agents Chemother. 48:1350-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desinor, O. Y., J. L. Silva, and M. J. Menos. 2004. Neonatal sepsis and meningitis in Haiti. J. Trop. Pediatr. 50:48-50. [DOI] [PubMed] [Google Scholar]
- 6.Drudy, D., M. O'Rourke, M. Murphy, N. Mullane, R. O'Mahony, L. Kelly, M. Fischer, S. Sanjaq, P. Shannon, P. Wall, M. O' Mahony, P. Whyte, and S. Fanning. 2006. Characterization of a collection of Enterobacter sakazakii isolates from environmental and food sources. Int. J. Food Microbiol. 110:127-134. [DOI] [PubMed] [Google Scholar]
- 7.European Food Safety Authority. 2004. Microbiological risks in infant formulae and follow-on formulae. EFSA J. 113:1-34. [Google Scholar]
- 8.Farmer, J. J., III, M. A. Asbury, F. W. Hickmann, and D. J. Brenner. 1980. Enterobacter sakazakii: a new species of “Enterobacteriaceae” isolated from clinical specimens. Int. J. Syst. Bacteriol. 30:569-584. [Google Scholar]
- 9.Gallagher, P. G., and W. S. Ball. 1991. Cerebral infarctions due to CNS infection with Enterobacter sakazakii. Pediatr. Radiol. 21:135-136. [DOI] [PubMed] [Google Scholar]
- 10.Himelright, I., E. Harris, V. Lorch, and M. Anderson. 2002. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee. JAMA 287:2204-2205. [PubMed] [Google Scholar]
- 11.Iversen, C., P. Druggan, and S. J. Forsythe. 2004. A selective differential medium for Enterobacter sakazakii. Int. J. Food Microbiol. 96:133-139. [DOI] [PubMed] [Google Scholar]
- 12.Iversen, C., and S. J. Forsythe. 2004. Isolation of Enterobacter sakazakii and other Enterobacteriaceae from powdered infant formula and related products. Food Microbiol. 21:771-776. [Google Scholar]
- 13.Muytjens, H. L., H. Roelofs-Willemse, and G. H. J. Jaspar. 1988. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J. Clin. Microbiol. 26:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazarowec-White, M., and J. M. Farber. 1997. Enterobacter sakazakii: a review. Int. J. Food Microbiol. 34:103-113. [DOI] [PubMed] [Google Scholar]
- 15.Russek, E., and R. R. Colwell. 1983. Computation of the most probable numbers. Appl. Environ. Microbiol. 45:1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo, K. H., and R. E. Brackett. 2005. Rapid, specific detection of Enterobacter sakazakii in infant formula using a real-time PCR assay. J. Food Prot. 68:59-63. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. 2002. Isolation and enumeration of Enterobacter sakazakii from dehydrated infant formula. [Online.] http://www.cfsan.fda.gov/∼comm/mmesakaz.html. Accessed 20 March 2006.
- 18.van Acher, J., F. de Smet, G. Muyldermans, A. Bougatef, A. Naessens, and S. Lauwers. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powered milk formula. J. Clin. Microbiol. 39:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir, E. 2002. Powdered infant formula and fatal infection with Enterobacter sakazakii. CMAJ 166:1570. [PMC free article] [PubMed] [Google Scholar]
- 20.Willis, J., and J. E. Robinson. 1988. Enterobacter sakazakii meningitis in neonates. Pediatr. Infect. Dis. J. 7:196-199. [DOI] [PubMed] [Google Scholar]