Abstract
There is evidence that drinking water may be a source of infections with pathogenic nontuberculous mycobacteria (NTM) in humans. One method by which NTM are believed to enter drinking water distribution systems is by their intracellular colonization of protozoa. Our goal was to determine whether we could detect a reduction in the prevalence of NTM recovered from an unfiltered surface drinking water system after the addition of ozonation and filtration treatment and to characterize NTM isolates by using molecular methods. We sampled water from two initially unfiltered surface drinking water treatment plants over a 29-month period. One plant received the addition of filtration and ozonation after 6 months of sampling. Sample sites included those at treatment plant effluents, distributed water, and cold water taps (point-of-use [POU] sites) in public or commercial buildings located within each distribution system. NTM were recovered from 27% of the sites. POU sites yielded the majority of NTM, with >50% recovery despite the addition of ozonation and filtration. Closely related electrophoretic groups of Mycobacterium avium were found to persist at POU sites for up to 26 months. Water collected from POU cold water outlets was persistently colonized with NTM despite the addition of ozonation and filtration to a drinking water system. This suggests that cold water POU outlets need to be considered as a potential source of chronic human exposure to NTM.
Nontuberculous mycobacteria (NTM) are acid-fast bacilli commonly found in soil, dust, vegetation, and surface waters (2, 11, 14). Several species of NTM, such as Mycobacterium avium, are frequently detected in clinical specimens and are considered human pathogens. Although exposure to pathogenic NTM is believed to commonly occur, the young, the elderly, the chronically ill, and the immunocompromised are most likely to develop clinical illness (4, 13, 15, 18, 25, 30, 31). There is evidence that drinking water may be an important environmental source of NTM infections in both community and health care settings (3, 7, 20, 35), although the proportion of disease associated with exposure to drinking water is unknown.
NTM have been reported in treated and distributed drinking water (8, 9, 12, 17, 32). M. avium has been reported to be resistant to chlorine disinfection (29). In addition, NTM may survive the process of drinking water disinfection by intracellular colonization in free-living amoebae (6, 28). Because of their size, amoebae are most likely to seed the distribution systems of unfiltered drinking water or systems in which there has been a water treatment failure. A previous study suggests that filtration may reduce NTM in distribution systems by removing mycobacteria associated with the particles that contribute to turbidity in the source water (10).
Our goal was to determine whether we could detect a reduction in the prevalence of NTM recovered from an unfiltered surface drinking water system after the addition of ozonation and filtration treatment and to characterize NTM isolates by using molecular methods.
MATERIALS AND METHODS
Study site selection.
We selected an unfiltered municipal drinking water system that received surface water from protected watersheds. The system serves a major metropolitan area in the western United States. The system is comprised of two sources of surface water, each with its own treatment plant. Plant A did not change its treatment during the study period. Plant B was due to undergo a treatment change that included the addition of ozonation and filtration. Treatment at plant A consisted of fluoridation, primary disinfection with chlorine gas, and pH adjustment using lime. Initially, water treatment at plant B consisted of fluoridation, primary disinfection with chlorine gas, and pH adjustment with lime and soda ash. During the winter of 2000-2001, six dual-bay, deep-bed, flow-controlled, gravity-type anthracite medium filters were added to plant B. Inline particle counting was initiated, with the goal of producing finished water with a turbidity of less than 0.1 nephelometric turbidity unit. Ozone was added for primary disinfection, with CTs (ozone concentration multiplied by contact time) averaging from 4.2 to 13.3 min-mg/liter as measured daily at peak hourly flow. Chlorine gas was used for secondary disinfection. Fluoridation treatment methods were unchanged. Corrosion control changed from lime and soda ash to lime and carbon dioxide; however, pH and alkalinity targets remained the same.
Watersheds for plants A and B were protected and closed to public access and recreational activities. Historically, the water quality has been very good, and the municipality has been able to meet the filtration avoidance criteria of the 1989 Surface Water Treatment Rule (34). Because of the variability that may occur in water quality over time, areas served by plant A acted as a comparable distribution system, subject to similar temperatures and seasonal effects as the plant B system, without the addition of a treatment change.
We initially screened 16 sites at cold water taps (point of use [POU]) in public or commercial buildings for the presence of NTM, 4 sites from the plant A distribution system, and 12 sites from the plant B distribution system. Of 16 samples, 3 (19%) yielded evidence of NTM by multiplex PCR (16). Final POU study sites included the three sites that yielded evidence of NTM: one site from the plant A system and two sites from the plant B system. We then selected an additional POU site from the plant B system that did not yield evidence of NTM during initial screening. Distribution system sample sites were selected “upstream” of POU sample sites.
We systematically sampled 12 total sites on each sampling day: two plant influent (raw water) sites, two plant effluent (finished water) sites, four distributed water sites, and four POU sites. Sampling was conducted over 15 days, either monthly or bimonthly during the spring through autumn over a 29-month period, from June 2000 through November 2002.
Collection of water samples.
Water samples were collected and analyzed for NTM, heterotrophic plate count (HPC) bacteria, total coliforms, temperature, and free chlorine concentrations. Bacillus spores were enumerated as indicators of water treatment efficacy. Escherichia coli organisms were cultured as microbial indicators of fecal pollution. To evaluate the overall water quality, the numbers of Bacillus spores, heterotrophic plate count bacteria, total coliforms, and E. coli were assessed at plant influent and effluent sites, and free chlorine was measured at plant effluent sites. Water temperature and free-chlorine concentrations were measured at distributed sites. “First-pull” water samples were collected for NTM analyses at all sites.
Water samples for NTM, HPC, and Bacillus spore analyses were collected in sterile sample bottles containing sodium thiosulfate (100 mg/liter). Samples were shipped overnight on blue ice in an insulated, double-walled container to the laboratory for analysis and primary isolation.
Laboratory procedures.
HPCs were performed in duplicate by using the R2A spread plate method (1). Water samples were analyzed for Bacillus spores as previously described (22, 26). Total coliforms, E. coli, water temperature, and free chlorine concentrations were analyzed by standard methods (1). All field equipment was calibrated against primary standards according to a laboratory protocol that included daily checks against secondary standards.
NTM samples were analyzed by the method reported by Covert et al. (8). Briefly, a 30-min sample exposure to 0.04% cetylpyridinium chloride (Sigma Chemical Company, St. Louis, MO) was used to reduce background organisms. Sample volumes (500 ml) from each sample were filtered through 0.45-μm-pore-size black grid HABG 47-mm membrane filters (Millipore Corp., Bedford, MA) after exposure to cetylpyridinium chloride. After filtration, the filters were rinsed with buffer (1) and transferred to Middlebrook 7H10 agar (Difco Laboratories, Detroit, MI) plates containing 500 mg of cycloheximide (Sigma) per liter. The plates were incubated at 37°C with 10% CO2. The filters were examined at biweekly intervals for up to 8 weeks with a stereoscopic microscope. NTM colony types were acid-fast stained, enumerated, and transferred to Middlebrook 7H10 agar slants for further identification. The selection of NTM colonies was based on colonial morphologies previously described (12).
Isolates were identified to the species level when possible by high-performance liquid chromatography analysis of mycolic acids (5). Multilocus enzyme electrophoresis (MEE) was used for typing of M. avium isolates (36). M. avium isolates which shared an electrophoretic type (ET) were then compared by pulsed-field gel electrophoresis (PFGE) to determine genetic relatedness (21).
Statistical procedures.
All data were analyzed by using SAS version 8.2 (SAS Institute, Inc., Cary, NC). Basic descriptive summaries were used to describe ranges, frequencies, and measures of the central tendency of water characteristics and microbial concentrations. For the purpose of data analysis, when NTM were >500 CFU/500 ml or too numerous to count, the data were entered as the number “501”; all microbial values of <1 were entered as zero. The frequency of NTM recovery was calculated for water samples. A post hoc power calculation was performed after NTM recovery from water samples was determined (27). The Wilcoxon rank sum test using the exact statement was performed to test the means of water characteristics before and after water treatment. Tests were two-sided; significance was set at an α of 0.05.
Ambient temperature and chlorine concentrations were analyzed as potential predictor variables since they may be associated with NTM growth or recovery. Spearman's correlation coefficients were calculated for the numbers of CFU of NTM isolated from the POU and the free chlorine concentration in the distribution system and for the numbers of CFU of NTM isolated from the POU and the water temperature in the distribution system.
Water sample sites were repeatedly measured over time; the samples therefore could not be considered independent samples for analytic purposes. To evaluate the effect of repeated sampling at each site, the generalized estimating equation approach was used (19). Specifically, the genmod procedure was used with a repeated-measures statement for the sample site variable. A negative binomial distribution was specified as the CFU data were overdispersed. Two models were evaluated by using the number of CFU of NTM as the outcome variable; either distribution system chlorine or distribution system temperature was specified as the predictor variable.
RESULTS
Prevalence of NTM recovered from water samples.
A total of 167 water samples were analyzed; of these, 28 were collected from treatment plant influent sites. NTM were not detected in any of the influent water samples due to the overgrowth of background organisms. Therefore, 139 samples were included in the analysis of NTM occurrence.
NTM were isolated from 27% (38 of 139) of all remaining samples. Representative isolates of every NTM colony type found in each of the 38 samples were identified. Of these, 30 isolates were identified as M. avium, 5 were identified as M. kansasii, 2 were identified as M. fortuitum complex, and 1 isolate was classified as an unidentified member of the M. avium complex. CFU were enumerated in 36 of 38 samples from which NTM were recovered. Among these 36 samples, NTM ranged from 1 to >500 CFU/500 ml, with a median of 161 CFU/500 ml.
M. fortuitum complex was isolated from two finished water samples, one from each treatment plant. The unidentified member of the M. avium complex was isolated from a single plant A distribution system sample. Water samples collected at POU sites yielded the majority of NTM; 30 POU samples yielded M. avium, and 5 yielded M. kansasii. NTM were isolated from water collected at all four POU sites.
Water quality characteristics.
HPCs and the numbers of Bacillus spores and total coliform bacteria were reduced significantly during water treatment at both plants (Table 1). No E. coli were detected in any plant effluent samples.
TABLE 1.
Water treatment efficacy
| Count type and parameter | Plant A
|
Plant B
|
||||
|---|---|---|---|---|---|---|
| Influent | Effluent | P > Z | Influent | Effluent | P > Z | |
| HPC (CFU/ml) | ||||||
| No. of samples | 12 | 11 | 12 | 12 | ||
| Mean ± SD | 5,934 ± 5,190 | 285 ± 625 | 3,032 ± 5,542 | 17 ± 34 | ||
| Median | 4,850 | 10 | 0.0007 | 270 | <1 | 0.0005 |
| Range | 310-20,700 | <1-1,820 | 20-17,600 | <1-110 | ||
| Total no. of coliforms (CFU/100 ml) | ||||||
| No. of samples | 13 | 12 | 13 | 13 | ||
| Mean ± SD | 701 ± 771 | <1 ± 0.4 | 186 ± 292 | <1 ± 3 | ||
| Median | 411 | <1 | 0.0002 | 22 | <1 | 0.0002 |
| Range | 46-2,419 | <1-1 | 2-921 | <1-10 | ||
| Bacillus spore count/liter | ||||||
| No. of samples | 13 | 12 | 13 | 13 | ||
| Mean ± SD | 238 ± 301 | 25 ± 24 | 73 ± 79 | 14 ± 13 | ||
| Median | 150 | 20 | 0.0001 | 40 | 10 | 0.0016 |
| Range | 60-1,200 | <1-60 | 5-300 | <1-40 | ||
Free chlorine concentrations were assessed on site at all four distribution system sites on sampling days during the study period. The distribution system samples all contained detectable residual chlorine concentrations. The mean free chlorine concentration of the 12 plant A distribution system samples was 0.69 mg/liter, with a range of 0.23 to 1.14 mg/liter; the mean free chlorine concentration of the 35 plant B distribution system samples was 0.58 mg/liter, with a range of 0.10 to 1.12 mg/liter.
Temperatures were assessed at all four distribution system sites on sampling days during the study period. The mean temperature of the 12 plant A samples was 16.1°C, with a range of 11.2 to 21.5°C; the mean temperature of the 34 plant B samples was 14.5°C, with a range of 9.9 to 20.5°C.
There was no statistically significant correlation between the CFU of NTM and the chlorine concentration in the distribution system or between the CFU of NTM and the temperature in the distribution system. Correlations were evaluated overall and by each specific portion of the distribution system above each POU site. Correlations were evaluated before and after the treatment change at plant B.
There was no statistically significant association between the numbers of CFU of NTM at the POU sites and the distribution system water characteristics during the repeated-measures analysis. The generalized estimating equation model yielded a z-statistic of 0.29 (P = 0.77) for the effect of the distribution system chlorine on the numbers of CFU of NTM, and a z-statistic of −0.26 (P = 0.79) for the effect of distribution system temperature on CFU of NTM.
Effect of ozonation and filtration.
We evaluated the microbial indicators of water treatment in the effluent of plant B before and after the addition of filtration and ozonation. We found no statistically significant differences between samples collected during these two time periods (Table 2).
TABLE 2.
Effect of the addition of filtration and ozonation to plant B effluent quality
| Count typea and parameter | Before addition | After addition | P > Z |
|---|---|---|---|
| HPC (CFU/ml) | |||
| No. of samples | 4 | 8 | |
| Mean ± SD | 24 ± 26 | 14 ± 39 | |
| Median | 18 | <1 | 0.06 |
| Range | <1-60 | <1-110 | |
| Total no. of coliforms (CFU/100 ml) | |||
| No. of samples | 5 | 8 | |
| Mean ± SD | <1 ± 0 | 1 ± 4 | |
| Median | <1 | <1 | 1.0 |
| Range | <1-<1 | <1-10 | |
| Bacillus spore count/liter | |||
| No. of samples | 5 | 8 | |
| Mean ± SD | 14 ± 5 | 15 ± 17 | |
| Median | 10 | 8 | 0.60 |
| Range | 10-20 | <1-40 |
That is, in plant B effluents before and after the addition of filtration and ozonation.
Before the treatment change, 51 water samples were collected from plant effluents, distribution systems, and POU sites; NTM were recovered from 14 (27%) of these. NTM were recovered from 5 of 16 (31%) of the samples from the plant A system and 9 of 35 (26%) of the samples from the plant B system. The majority of samples yielding NTM were collected at POU sites. We found that 50% of the POU samples collected from the plant A system and 53% of POU samples collected from the plant B system yielded NTM during primary isolation (Table 3).
TABLE 3.
Recovery of NTM from water samples by site before the addition of filtration to plant Ba
| Species or group | No. of positive samples (%)
|
|||||
|---|---|---|---|---|---|---|
| Plant A
|
Plant B
|
|||||
| Effluent (n = 5) | Distributed (n = 5) | POU (n = 6) | Effluent (n = 5) | Distributed (n = 15) | POU (n = 15) | |
| M. avium | 0 | 0 | 0 | 0 | 0 | 8 (53) |
| M. kansasii | 0 | 0 | 3 (50) | 0 | 0 | 0 |
| M. fortuitum complex | 1 (20) | 0 | 0 | 1 (20) | 0 | 0 |
| M. avium complex | 0 | 1 (20) | 0 | 0 | 0 | 0 |
Samples were collected from June to November 2000. n, total number of samples tested. POU, point-of-use public buildings.
After the addition of ozonation and filtration to plant B, a total of 88 samples were collected from plant A and B effluents, distribution systems, and POU sites. NTM were recovered from 24 (27%) of these samples. Specifically, NTM were recovered from 8 of 26 (31%) total samples from the plant A system and 16 of 62 (26%) total samples from the plant B system. The majority of the samples yielding NTM were collected at POU sites. We found that 89% of the POU samples collected from the plant A system and 62% of POU samples collected from the plant B system yielded NTM during primary isolation (Table 4).
TABLE 4.
Recovery of NTM from water samples by site after the addition of filtration to plant Ba
| Species or group | No. of positive samples (%)
|
|||||
|---|---|---|---|---|---|---|
| Plant A
|
Plant B
|
|||||
| Effluent (n = 8) | Distributed (n = 9) | POU (n = 9) | Effluent (n = 9) | Distributed (n = 27) | POU (n = 26) | |
| M. avium | 0 | 0 | 6 (67) | 0 | 0 | 16 (62) |
| M. kansasii | 0 | 0 | 2 (22) | 0 | 0 | 0 |
| M. fortuitum complex | 0 | 0 | 0 | 0 | 0 | 0 |
Samples were collected from May 2001 to November 2002. n, total number of samples tested. POU, point-of-use public buildings.
When recovered, NTM ranged from 1 to >500 CFU/500 ml at the POU sites. No significant reductions in NTM CFU enumeration were found after the treatment change at plant B (Table 5).
TABLE 5.
NTM counts before and after the addition of filtration and ozonationa
| Site and plant category | No. of samples | Mean no. of NTM ± SD (CFU/500 ml), median (range)
|
||
|---|---|---|---|---|
| M. avium | M. kansasii | M. fortuitum complex | ||
| Site before addition | ||||
| Plant A | ||||
| Effluent | 1 | − | − | 1 |
| Distributed | 1 | * | − | − |
| POU | 3 | − | 78 ± 75, 83 (1-151) | − |
| Plant B | ||||
| Effluent | 1 | − | − | * |
| Distributed | 0 | − | − | − |
| POU | 8 | 225 ± 184, 184 (4->500) | − | − |
| Site after addition | ||||
| Plant A | ||||
| Effluent | 0 | − | − | − |
| Distributed | 0 | − | − | − |
| POU | 8 | 362 ± 215, n = 6, >500 (60->500) | 302 ± 282, n = 2, 302 (102->500) | − |
| Plant B | ||||
| Effluent | 0 | − | − | − |
| Distributed | 0 | − | − | − |
| POU | 16 | 205 ± 191, 161 (2->500) | − | − |
Note that for samples of >500 CFU or CFU too numerous to count, the number 501 was used for data analysis. *, CFU not enumerated for this sample. -, not determined.
We were unable to determine whether the true prevalence of NTM changed due to the treatment change at plant B due to the limited power of our study. We performed a post hoc power calculation based on NTM recovery in 53% of POU water samples before filtration and recovery in 62% of POU samples after filtration at plant B. Given this observed prevalence, we would have required a total of 475 samples collected and analyzed before the treatment change and 475 samples collected and analyzed after the change to achieve 80% power with a two-sided α of 0.05.
Molecular analysis of M. avium.
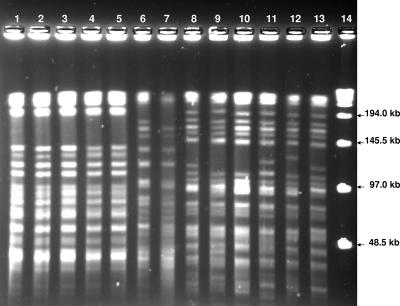
Of the 30 M. avium isolates, 27 were characterized by MEE analysis. These 27 isolates were separated by MEE into eight ETs. Four major ETs, ET 1 to ET 4, accounted for 23 of the isolates. Eight isolates belonged to ET 1, nine isolates belonged to ET 2, four isolates belonged to ET 3, and two isolates belonged to ET 4. Further analysis by PFGE revealed that the isolates within each ET shared identical or closely related restriction patterns and were designated PFGE groups 1 through 4. PFGE group 1 was found only at POU 2; it was detected during a total of 22 months of sampling. PFGE group 2 was found at POU 3 during a total of 26 months of sampling; group 2 was also found at POU 4 in a single water sample. PFGE group 3 was found only at POU 1; it was detected during a total of 18 months of sampling. Group 4 was detected in two samples: one sample from POU 1 and one sample from POU 2. We found persistence of PFGE groups 1 to 3 at three POU sites despite the change in water treatment (Fig. 1).
FIG. 1.
Pulsed-field electrophoresis gel depicting electrophoretic bands of group 3 M. avium isolates derived from samples collected at POU 1 sample sites over an 18-month period (lanes 1 to 5), group 1 M. avium isolates derived from samples collected at POU 2 sample sites over a 22-month period (lanes 6 to 7), and group 2 M. avium isolates derived from samples collected at POU 3 sample sites over a 26-month period (lanes 8 to 13). A 48.5-kb ladder is shown in lane 14.
DISCUSSION
We detected persistence of M. avium electrophoretic groups for up to 26 months at POU sampling sites located within small to medium-sized public and commercial buildings. Although M. avium has been previously reported to persist as long as 41 months in a hospital's recirculating hot water system (35), to our knowledge, ours is the first report of persistence of electrophoretic groups of M. avium in cold-water POU outlets. This suggests that drinking water systems, once colonized, may represent a chronic source of M. avium exposure.
We had hypothesized that the addition of filtration at plant B could reduce the numbers of mycobacteria entering the system (10). We found that despite the addition of filtration and ozonation to plant B, treatment efficacy, as measured by Bacillus spore removal, was not significantly improved. We could not detect a reduction in NTM due to the change in treatment; the prevalences of M. avium at plant B POU sites were similar before and after the addition of filtration and ozonation. However, with the low recovery of NTM, the power of the present study was limited to formally test a hypothesis of a reduction in NTM with the change in treatment.
Factors previously hypothesized to facilitate the persistence of NTM in buildings' plumbing systems include the complexity of the buildings' plumbing systems, hot-water systems, and low residual disinfectant concentrations (8, 9, 35). The buildings in our study were small to medium-sized public or commercial buildings; the complexities of the plumbing systems were not characterized. We sampled only cold-water outlets. Disinfection residuals were detectable at all “upstream” distribution system sample sites but were not determined at the POU sites.
NTM have been reported to be particularly persistent in areas where biofilms may enhance their survival (10, 24). Copper pipes, widely used in buildings' plumbing systems during the 20th century, appear to provide favorable conditions for M. avium colonization and survival during periods when disinfection concentrations are sufficient to eliminate the heterotrophic bacterial population (23). Tsintzou et al. reported a reduction in mycobacteria after the replacement of portions of the distribution system; however, replacement of the distribution system alone will not prevent future colonization (33). Norton et al. reported the effect of variables such as distribution pipe composition, organic carbon concentrations in water, and disinfectant residuals on the survival of M. avium in a model distribution system biofilm (23). Our finding of NTM persistence at POU sites is consistent with M. avium colonization of biofilms that form within drinking water distribution systems.
Generally, primary isolation of NTM in environmental samples uses techniques, selective media, and decontamination procedures developed for the analysis of clinical samples. The most common approaches include decontamination of samples with NaOH, oxalic acid, or cetylpyridinum chloride to help reduce background organisms, followed by methods to concentrate the cells. Despite measures to help control background organisms, overgrowth of background organisms such as Bacillus spp. and fungi remain a problem. In the present study, all of the influent samples were overgrown by Bacillus spp., and no NTM were isolated. The current “harsh” decontamination techniques cause a loss of at least 50 to 70% of the target NTM (T. Covert, unpublished data). This leads to underestimations of the numbers of samples that contain NTM, the numbers of NTM per sample and, potentially, the loss of NTM strains that are most sensitive to the effects of decontamination agents.
We did not detect a significant difference in effluent water quality parameters after the introduction of filtration and ozonation at plant B. This system's water quality met all federal drinking water quality standards at all sample times. Indicator organisms such as total coliforms and E. coli are used to indicate the potential presence of enteric pathogens and to monitor treatment efficacy. However, these indicators are not useful to indicate the presence of M. avium since NTM are not typically associated with intestinal flora and are more chlorine resistant than these indicator organisms.
We found persistence of M. avium groups at drinking water POU sites, despite the addition of ozonation and filtration treatment to plant B. Our findings support previous studies that indicate that treated, distributed drinking water is a source of human exposure to NTM (3, 7, 20).
Future studies are needed to determine the proportion of human NTM infections associated with exposure to drinking water versus other recognized routes of exposure. The genetic heterogeneity of M. avium enables specific associations to be made between isolates derived from infected persons and drinking water. Comparison of M. avium groups that are persistent in the environment to those groups of M. avium isolated from human clinical samples by using molecular methods will provide more information about sources of human exposure and infection.
Acknowledgments
We gratefully acknowledge the technical assistance and expertise of Sandy Dunkel, Brian Hoyt, and Moya Joubert from the participating utility; Cliff Johnson of the U.S. Environmental Protection Agency, Office of Research and Development, National Risk Management Research Laboratory, Cincinnati, Ohio; and Cara Carty of the University of Washington, Seattle.
The views expressed in this report are those of the individual authors and do not necessarily reflect the views and policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Inc., Washington, D.C.
- 2.Argueta, C., S. Yoder, A. E. Holtzman, T. W. Aronson, N. Glover, O. G. Berlin, G. N. Stelma, Jr., S. Froman, and P. Tomasek. 2000. Isolation and identification of nontuberculous mycobacteria from foods as possible exposure sources. J. Food Prot. 63:930-933. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, T., A. Holtzman, N. Glover, M. Boian, S. Froman, O. G. Berlin, H. Hill, and G. Stelma, Jr. 1999. Comparison of large restriction fragments of Mycobacterium avium isolates recovered from AIDS and non-AIDS patients with those of isolates from potable water. J. Clin. Microbiol. 37:1008-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonomo, R. A., J. M. Briggs, W. Gross, M. Hassan, R. C. Graham, W. R. Butler, and R. A. Salata. 1998. Mycobacterium celatum infection in a patient with AIDS. Clin. Infect. Dis. 26:243-244. [DOI] [PubMed] [Google Scholar]
- 5.Butler, W. R., L. Thibert, and J. O. Kilburn. 1992. Identification of Mycobacterium avium complex strains and some similar species by high-performance liquid chromatography. J. Clin. Microbiol. 30:2698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirillo, J. D., S. Falkow, L. S. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conger, N. G., R. J. O'Connell, V. L. Laurel, K. N. Olivier, E. A. Graviss, N. Williams-Bouyer, Y. Zhang, B. A. Brown-Elliott, and R. J. Wallace, Jr. 2004. Mycobacterium simiae outbreak associated with a hospital water supply. Infect. Control Hosp. Epidemiol. 12:1050-1055. [DOI] [PubMed] [Google Scholar]
- 8.Covert, T. C., M. R. Rodgers, A. L. Reyes, and G. N. Stelma, Jr. 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol. 65:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.du Moulin, G. C., K. D. Stottmeier, P. A. Pelletier, A. Y. Tsang, and J. Hedley-Whyte. 1988. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 260:1599-1601. [DOI] [PubMed] [Google Scholar]
- 10.Falkinham, J. O., C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falkinham, J. O., III. 2002. Nontuberculous mycobacteria in the environment. Clin. Chest Med. 23:529-551. [DOI] [PubMed] [Google Scholar]
- 12.Glover, N., A. Holtzman, T. Aronson, S. Froman, O. G. Berlin, P. Dominguez, K. A. Kunkel, G. Overturf, G. N. Stelma, C. Smith, and M. Yakrus. 1994. The isolation and identification of Mycobacterium avium complex (MAC) recovered from Los Angeles potable water, a possible source of infection in AIDS patients. Int. J. Environ. Health Res. 4:63-72. [Google Scholar]
- 13.Horsburgh, C. R., D. L. Cohn, R. B. Roberts, H. Masur, R. A. Miller, A. Y. Tsang, and M. D. Iseman. 1986. Mycobacterium avium-M. intracellulare isolates from patients with or without acquired immunodeficiency syndrome. Antimicrob. Agent Chemother. 30:955-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichiyama, S., K. Shimokata, and M. Tsukamura. 1988. The isolation of Mycobacterium avium complex from soil, water, and dusts. Microbiol. Immunol. 32:733-739. [DOI] [PubMed] [Google Scholar]
- 15.Koek, L., T. Debord, M. Fabre, V. Vincent, J. D. Cavallo, and R. L. Vagueresse. 1996. Disseminated Mycobacterium simiae infection in a patient with AIDS. Clin. Infect. Dis. 23:832-833. [DOI] [PubMed] [Google Scholar]
- 16.Kulski, J. K., C. Khinsoe, T. Pryce, and K. Christiansen. 1995. Use of multiplex PCR to detect and identify Mycobacterium avium and Mycobacterium intracellulare in blood culture fluids of AIDS patients. J. Clin. Microbiol. 33:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Dantec, C., J. P. Duguet, A. Montiel, N. Dumoutier, S. Dubrou, and V. Vincent. 2002. Occurrence of mycobacteria in water treatment lines and in water distribution systems. Appl. Environ. Microbiol. 68:5318-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy-Frebault, V., B. Pangon, A. Bure, C. Katlama, C. Marche, and H. L. David. 1987. Mycobacterium simiae and Mycobacterium avium-M. intracellulare mixed infection in acquired immune deficiency syndrome. J. Clin. Microbiol. 25:154-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang, K.-Y., and S. L. Zeger. 1986. Longitudinal data analysis using generalized linear models. Biometrika 4:695-702. [Google Scholar]
- 20.Marras, T. K., R. J. Wallace, Jr., L. L. Koth, M. S. Stulbarg, C. T. Cowl, and C. L. Daley. 2005. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest 2:664-671. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek, G. H., S. Hartman, Y.-S. Zhang, B. A. Brown, J. S. R. Hector, D. Murphy, and R. J. Wallace, Jr. 1993. Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J. Clin. Microbiol. 31:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieminski, E. C., W. D. Bellamy, and L. R. Moss. 2000. Using surrogates to improve plant performance. J. Am. Water Works Assoc. 92:67-78. [Google Scholar]
- 23.Norton, C. D., M. W. LeChevallier, and J. O. Falkinham III. 2004. Survival of Mycobacterium avium in a model distribution system. Water Res. 38:1457-1466. [DOI] [PubMed] [Google Scholar]
- 24.Peters, M., C. Muller, S. Rusch-Gerdes, C. Seidel, U. Gobel, H. D. Pohle, and B. Ruf. 1995. Isolation of atypical mycobacteria from tap water in hospitals and homes: is this a possible source of disseminated MAC infection in AIDS patients? J. Infect. 31:39-44. [DOI] [PubMed] [Google Scholar]
- 25.Pransky, S. M., B. K. Reisman, D. B. Kearns, A. B. Seid, D. L. Collins, and H. F. Krous. 1990. Cervical facial mycobacterial adenitis in children: endemic to San Diego? Laryngoscope 100:920-925. [DOI] [PubMed] [Google Scholar]
- 26.Rice, E. W., R. K. Fox, R. J. Miltner, D. A. Lytle, and C. J. Johnson. 1996. Evaluating plant performance with endospores. J. Am. Water Works Assoc. 88:122-130. [Google Scholar]
- 27.Selvin, S. 1996. Statistical power and sample-size calculations, p. 91-92. In J. L. Kelsey, M. G. Marmot, P. D. Stolley, and M. P. Vessey (ed.), Statistical analysis of epidemiological data: monographs in epidemiology and biostatistics, vol. 25, 2nd ed. Oxford University Press, New York, N.Y. [Google Scholar]
- 28.Steinert, M., K. Birkness, E. White, B. Fields, and F. Quinn. 1998. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl. Environ. Microbiol. 64:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor, R. H., J. O. Falkinham III, C. D. Norton, and M. W. LeChevallier. 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66:1702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen, V. O., U. B. Dragsted, J. Bauer, K. Fuursted, and J. Lundgren. 1999. Disseminated infection with Mycobacterium genavense: a challenge to physicians and mycobacteriologists. J. Clin. Microbiol. 37:3901-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres, R. A., J. Nord, R. Feldman, V. LaBombardi, and M. Barr. 1991. Disseminated mixed Mycobacterium simiae-Mycobacterium avium complex infection in acquired immunodeficiency syndrome. J. Infect. Dis. 164:432-433. [DOI] [PubMed] [Google Scholar]
- 32.Torvinen, E., S. Suomalainen, M. J. Lehtola, I. T. Miettinen, O. Zacheus, L. Paulin, M. L. Katila, and P. J. Martikainen. 2004. Mycobacteria in water and loose deposits of drinking water distribution systems in Finland. Appl. Environ. Microbiol. 70:1973-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsintzou, A., A. Vantarakis, O. Pagonopoulou, A. Athanassiadou, and M. Papapetropoulou. 2000. Environmental mycobacteria in drinking water before and after replacement of the water distribution network. Water Air Soil Pollut. 120:273-282. [Google Scholar]
- 34.U.S. Environmental Protection Agency. 1989. 40 CFR parts 141 and 142: drinking water; national primary drinking water regulations; filtration, disinfection; turbidity, Giardia lamblia, viruses, Legionella, and heterotrophic bacteria; final rule. Fed. Regist. 54:274486-274541. [Google Scholar]
- 35.Von Reyn, C. F., J. N. Maslow, T. W. Barber, J. O. Falkinham III, and R. D. Arbeit. 1994. Persistent colonization of potable water as a source of Mycobacterium avium infection in AIDS. Lancet 343:1137-1141. [DOI] [PubMed] [Google Scholar]
- 36.Yakrus, M. A., M. W. Reeves, and S. B. Hunter. 1992. Characterization of isolates of Mycobacterium avium serotypes 4 and 8 from patients with AIDS by multilocus enzyme electrophoresis. J. Clin. Microbiol. 30:1474-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]