Abstract
The antibacterial activity of photocatalytic titanium dioxide (TiO2) substrates is induced primarily by UV light irradiation. Recently, nitrogen- and carbon-doped TiO2 substrates were shown to exhibit photocatalytic activities under visible-light illumination. Their antibacterial activity, however, remains to be quantified. In this study, we demonstrated that nitrogen-doped TiO2 substrates have superior visible-light-induced bactericidal activity against Escherichia coli compared to pure TiO2 and carbon-doped TiO2 substrates. We also found that protein- and light-absorbing contaminants partially reduce the bactericidal activity of nitrogen-doped TiO2 substrates due to their light-shielding effects. In the pathogen-killing experiment, a significantly higher proportion of all tested pathogens, including Shigella flexneri, Listeria monocytogenes, Vibrio parahaemolyticus, Staphylococcus aureus, Streptococcus pyogenes, and Acinetobacter baumannii, were killed by visible-light-illuminated nitrogen-doped TiO2 substrates than by pure TiO2 substrates. These findings suggest that nitrogen-doped TiO2 has potential application in the development of alternative disinfectants for environmental and medical usages.
Disinfectants are antimicrobial agents that are used extensively in hospitals and other health care settings for a variety of topical and hard-surface applications. They are essential for infection control and aid in the prevention of nosocomial infections (18). Compared to antibiotics, which provide comparatively selective activity against microorganisms, disinfectants typically have a broader biocidic spectrum (28) and are usually used with inanimate objects (33). A wide variety of active chemical agents exhibit bactericidal activities. Some of the most widely used, including alcohols, iodine, and chlorine, have been employed for a long time in disinfection and preservation (28). Compared to these widely used disinfectants, applications of photocatalyst-based antimicrobial disinfectant technologies are still in the developmental stage. Photocatalytic titanium dioxide (TiO2) substrates have been shown to eliminate organic compounds and to function as disinfectants (26). Upon UV light excitation, the photon energy excites valance electrons and generates pairs of electrons and holes (electron vacancy in valence band) that diffuse and become trapped on or near the TiO2 surface. These excited electrons and holes have strong reducing and oxidizing activities and react with atmospheric water and oxygen to yield active oxygen species, such as hydroxyl radicals ( · OH) and superoxide anions (O2−) (12). Electron holes, · OH, and O2− are extremely reactive upon contact with organic compounds. Complete oxidation of organic compounds and Escherichia coli cells to carbon dioxide could be achieved (16, 23). Reactive oxygen species (ROS), such as · OH, O2−, and hydrogen peroxide (H2O2) generated on irradiated TiO2 surfaces, have been shown to operate in concert to attack polyunsaturated phospholipids in bacteria (26). In addition, it has been shown that photoirradiated TiO2 catalyzed site-specific DNA damage via generation of H2O2 (14). These findings suggested that TiO2 might exert antimicrobial effects similar to those of the peroxygen disinfectant H2O2 (28). The oxidation of bacterial cell components, such as lipids and DNA, might therefore result in subsequent cell death (26).
Due to the widespread use of antibiotics and the emergence of more-resistant and -virulent strains of microorganisms (1, 32, 33), there is an urgent need to develop alternative sterilization technologies. The TiO2 photocatalytic process is a conceptually feasible technology. The TiO2 photocatalyst, however, is effective only upon irradiation by UV light at levels that would induce serious damage to human cells. This greatly restricts the potential applications of TiO2 substrates for use in our living environments. Recently, the anion-doped anatase TiO2-based photocatalysts were identified, which work by irradiation with visible light (3, 15), offering the potential to overcome this problem. We previously developed several vapor deposition methods to prepare visible-light photocatalysts, such as films of nitrogen-doped TiO2 [TiO2 (N)] and carbon-doped TiO2 [TiO2 (C)], on various substrates, including silicon, glass, and quartz coupons (44, 45). The TiO2 films absorbed only UV light (wavelength < 380 nm), while the TiO2−xNx and TiO2−xCx (where x represents the dopant [N or C] concentration in molar fraction in the host crystal [TiO2]) films showed visible-light absorption with the absorption edges red shifted by approximately 565 and 425 nm, respectively. The prepared nanosized carbon- and nitrogen-doped thin films showed an enhancement in the photodegradation efficiency of methylene blue under visible-light (≥400 nm) irradiation compared to pure TiO2 thin film. The crystallinities and compositions of photocatalysts are correlated to their hydrophilic properties and photocatalytic activities during methylene blue degradation (44, 45). However, the antibacterial activity of these anion-doped TiO2 films has not been clearly demonstrated.
The aim of this study was to investigate the antibacterial activity of the visible-light-irradiated nitrogen- and carbon-doped TiO2. We tested several human pathogens, including Shigella flexneri, Listeria monocytogenes, Vibrio parahaemolyticus, Streptococcus pyogenes, Staphylococcus aureus, and Acinetobacter baumannii. Among these microorganisms, S. flexneri, L. monocytogenes and V. parahaemolyticus were usually found in contaminating water, plants, and sewage (24, 27, 34, 42) and frequently lead to outbreaks in regions with poor sanitary conditions (9, 24). S. pyogenes and S. aureus are exotoxin-producing pathogens which can cause diseases such as soft tissue infections, food-borne disease, and toxic shock syndrome in humans (34). The emergence and rapid spread of multidrug-resistant A. baumannii isolates causing nosocomial infections are of great concern worldwide (30). Although the optimal antimicrobial conditions remain to be fully established, we found that the TiO2 (N)-coated substrates developed for this study possessed bactericidal activities that could reduce the bacterial population of all tested pathogens when illuminated by visible light. Our data suggest that TiO2 (N) is an effective antibacterial photocatalyst which is user friendly compared to traditional UV-driven TiO2 photocatalysts.
MATERIALS AND METHODS
Preparation of TiO2-, TiO2 (C)-, and TiO2 (N)-coated substrates.
The TiO2, TiO2−xCx, and TiO2−xNx films were prepared in an ion-assisted electron beam evaporation system (Branchy Vacuum Technology Co., Ltd., Taoyuan, Taiwan). The distance between the rotating substrate holder and the electron beam evaporation source was 550 mm. The chamber was evacuated by a mechanical pump (ALCATEL-2033SD) and a cryopump (CTI-Cryo-Torr8) to a base pressure below 2.7 × 10−4 Pa. The substrates used were polished Si(100), quartz, and glass coupons, which were sputter etched with argon ions (Ar+) for 5 min prior to the deposition to remove any residual pollutants on the surface. The substrate temperature was maintained at 300°C by a quartz lamp. The TiO2 films were deposited in an oxygen atmosphere (6.7 ×10−3 Pa) using rutile TiO2 (99.99%) as the source material. The nitrogen flow for TiO2−xNx films was 15 standard cm3 min−1 through the ion gun at a constant pumping speed, and the chamber pressure was at 4.4 ×10−2 Pa. The carbon dioxide gas flow for TiO2−xCx films was 7 standard cm3 min−1, and the chamber pressure was 2.6 × 10−2 Pa. The ion gun beam current of 10 mA and voltage of −1,000 V were maintained by a Commonwealth Scientific ion beam power supply controller. Sufficient energy and current of the ion beam are critical to incorporate significant dopant concentration in the film. Without ion bombardment, it is difficult for the dopant to compete with the oxygen for incorporation into anatase titania. The deposition rate was adjusted to 0.2 nm s−1, using a quartz crystal monitor for all films deposited at a thickness of 1.2 μm. The three films were prepared under the optimized conditions for their categories of anatase crystallinity and dopant concentration (44, 45).
Bacterial strains and culture.
E. coli (strain OP50) (31) was maintained and cultured in Luria-Bertani (LB) broth or LB agar (MDBio, Inc., Taipei, Taiwan) at 37°C using a standard laboratory E. coli culture method (2, 35). L. monocytogenes (laboratory strain 10430S) was provided by Eric Pamer (Sloan-Kettering Cancer Center) (46). A clinical isolated strain of S. flexneri was collected from central Taiwan in 1996 (9). Pandrug-resistant A. baumannii (strain M36788), S. pyogenes (strain M29588) (39), and S. aureus (strain SA02) were clinical isolates from Buddhist Tzu-Chi General Hospital in Hualien, Taiwan. All clinical isolates were initially differentiated into gram-positive and gram-negative strains, based on the results of preliminary identification. Both gram-positive and -negative strains were directly cultured in tryptic soy broth supplemented with 0.5% yeast extract (TSBY) and LB at 37°C for 16 h and then identified by biochemical methods according to routine clinical laboratory procedures (29). E. coli, S. flexneri, and A. baumannii were maintained and grown in LB medium or LB agar at 37°C. S. pyogenes and S. aureus were grown in TSBY broth or TSBY broth agar (MDBio, Inc., Taipei, Taiwan) at 37°C. V. parahaemolyticus (strain 15427, serovar O3:K6) was a clinical isolate obtained from the Center for Disease Control in Taiwan (8). The strains were maintained and grown at 37°C in tryptic soy broth (Difco) supplemented with 3% NaCl. All bacteria were stored in 50% medium and 50% glycerol solution in freezers at −80°C before use. To reactivate bacteria from frozen stocks, 25 μl bacterial stock solution was transferred to a test tube containing 5 ml of freshly prepared culture medium and then incubated at 37°C under agitation overnight (16 to 18 h).
Photocatalytic reaction and detection of viable bacteria.
In this study, bacterial concentrations were either determined by the standard plating method or inferred from optical density readings at 600 nm (OD600). For each bacterium, a factor for converting the OD600 values of the bacterial culture to concentration values (CFU/ml) was calculated as follows. A fresh bacterial culture was diluted by factors of 10−1 to 10−7, and an OD600 of these dilutions was measured. Bacterial concentrations of these dilutions were determined by the standard plating method. The OD600 values were plotted against the bacterial concentration log values, and the conversion factors for particular bacteria were calculated. The conversion factor for E. coli OP50, for example, was calculated to be 6 × 108 CFU/ml per OD600 by this method.
In order to determine the bactericidal effects of the TiO2-related substrates, 200 μl of bacterial overnight culture was transferred into 5 ml of culture medium and incubated at 37°C until an OD600 of 0.3 to 0.6 (log phase) was reached. The bacterial concentrations were calculated using the conversion factor for the bacteria, and the cultures were diluted to 5 × 107 CFU/ml with culture medium. Fifty microliters (2.5 × 106 CFU) was then applied to an area of approximately 1 cm2 of the TiO2-related substrates by using a plastic yellow tip. The bacterium-containing substrates were placed under an incandescent lamp (Classictone incandescent lamp, 60W, Philips; Taiwan) for photocatalytic reaction, and a light meter (model LX-102; Lutron Electronic Enterprises, Taiwan) was used to record the illumination density. In the dose dependence experiments, illuminations were carried out for 5 min at distances of 5, 10, and 15 cm from the lamp, corresponding to illumination densities of 3 × 104, 1.2 × 103, and 3 × 102 lux (lumen/m2), respectively. In the kinetic analysis experiments, illuminations were carried out for 1, 5, 10, 15, and 25 min at a distance of 5 cm, corresponding to an illumination density of 3 × 104 lux. Unless specified, illuminations were carried out in a 4°C cold room. After illumination, the bacterial solutions were recovered from the TiO2-related substrates, and an aliquot of fresh culture medium was used to collect the residual bacteria on the substrates. The two bacterial solutions were pooled to make a total of 100 μl. The bacterial concentration was determined by the standard plating method immediately after the bacterial collection, and the percentage of surviving bacteria was calculated.
In the experiments for determining the mitigation effect of protein and dye in photocatalyst-mediated killing, a log-phase E. coli culture (OD600 of 0.3 to 0.6) was diluted to 5 × 107 CFU/ml as described above, and 50 μl was mixed with an equal volume of normal saline solutions containing either 5%, 1%, and 0.2% bovine serum albumin (BSA) (wt/vol), or 4, 2, and 0.4 OD600 of bromophenol blue. The mixtures were then applied to an approximately 1-cm2 area of the TiO2-related substrates, and illumination was carried out under the incandescent lamp for 5 min at 4°C at a distance of 5 cm, corresponding to an illumination density of 3 × 104 lux. Recovery of the surviving bacteria was performed as described above, with the exception of a total of 150 μl solution being obtained. The bacterial concentrations were determined by the standard dilution and plating methods, and the percentage of surviving bacteria was calculated. In the experiments of photocatalyst-mediated killing of E. coli of different concentrations, a log-phase E. coli culture (OD600 of 0.3 to 0.6) was adjusted to 0.5, 1, and 2 OD600 either by dilution with culture medium or by centrifugation and resuspension of the cell pellets into culture medium. Aliquots of 2.5 × 107 CFU bacteria were applied to the TiO2-related substrates. Illumination was carried out under the incandescent lamp for 5 min at 4°C at a distance of 5 cm, corresponding to an illumination density of 3 × 104 lux. Recovery of the surviving bacteria and calculation of the percentage of surviving bacteria were carried out as in the dose dependence and kinetic analysis experiments.
Septic shock mouse model.
Six- to 8-week-old C57BL/6J mice were purchased from the National Experimental Animal Center (Taipei, Taiwan) (20). A log-phase E. coli OP50 culture (OD600 of 0.3 to 0.6) was adjusted to 2 × 1010 CFU/ml saline by centrifugation, followed by resuspension of the cell pellet in sterile normal saline solution, using the conversion factor of E. coli OP50 (6 × 108 CFU/ml per OD600 unit) for the bacterial concentration calculation. The E. coli solution was divided into aliquots of 250 μl (5 × 109 CFU). Each mouse in the control groups received an intravenous injection of 5 × 109 CFU E. coli, a lethal dose for mice. In the experimental groups, mice were injected with the same batch of bacterial aliquots, but the E. coli solution was pretreated with visible-light illuminations on TiO2 or TiO2 (N) substrates for 5 min at 4°C. The distance between the lamp and the bacterium-containing substrates was 5 cm, corresponding to an illumination density of 3 × 104 lux. The mortality of mice in this septic shock model, which was affected by the viability of treated bacteria, was then recorded. The Animal Care and Use Committee of Tzu-Chi University approved the protocol of the mouse experiments.
Statistical analysis.
All results were calculated from the data of three independent experiments. A t test was used to assess the statistical significance of differences in results of antimicrobial effects. A P value of less than 0.05 was considered significant. The statistical tests were carried out and output to graphs using Microsoft Excel (Microsoft Taiwan, Taipei, Taiwan) and SigmaPlot (Systat Software, Point Richmond, CA) softwares.
RESULTS
Bactericidal activities of TiO2 versus nitrogen- and carbon-doped TiO2.
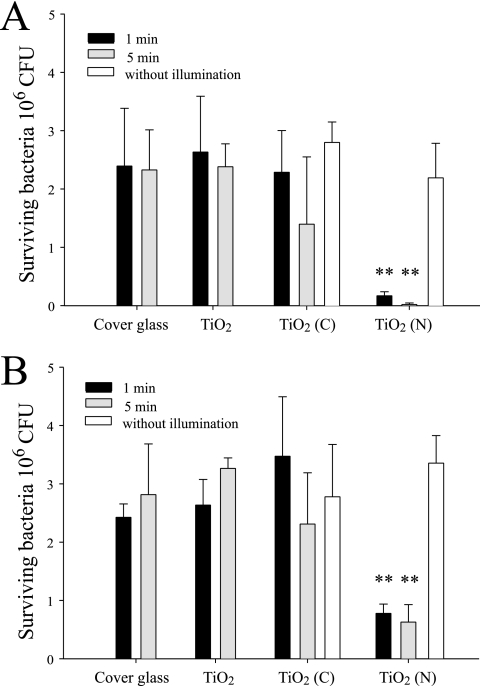
To determine the bactericidal activities of TiO2 (N) and TiO2 (C), we first placed 2.5 × 106 CFU E. coli on various substrates, including cover glass (silica, without TiO2 coating) and silica substrates coated with thin films of TiO2, TiO2 (N), and TiO2 (C). These preparations were then irradiated with visible light, and the levels of surviving bacteria were quantified. The antibacterial activities of TiO2 (N) and TiO2 (C) were more pronounced when experiments were conducted at room temperature than at 4°C (Fig. 1A and B). The irradiation produced heat after absorption by the photocatalyst, and this greatly influenced bacterial survival. To avoid the effects of heat, we performed the same experiments in a 4°C cold room but maintained the temperature of TiO2 substrate surfaces at 4°C during irradiation. Although the antibacterial activity of TiO2 substrates was reduced under these conditions, TiO2 (N) still exhibited a significantly greater ability to reduce the number of E. coli than TiO2 and TiO2 (C) (P < 0.01) (Fig. 1B). To control for the effects of heat and determine the pure “ROS-mediated killing” effect, all subsequent bacterium-killing experiments were performed in a 4°C cold room.
FIG. 1.
Bactericidal activity analysis. Bactericidal activities of the TiO2-related substrates after visible-light illumination at 25°C (A) or 4°C (B) were analyzed. Illumination was carried out at a light density of 3 × 104 lux for either 1 or 5 min. “Without illumination” indicates experiments conducted in a dark room without illumination. **, P < 0.01 compared to either the respective cover glass groups or the TiO2 (N) groups without visible-light.
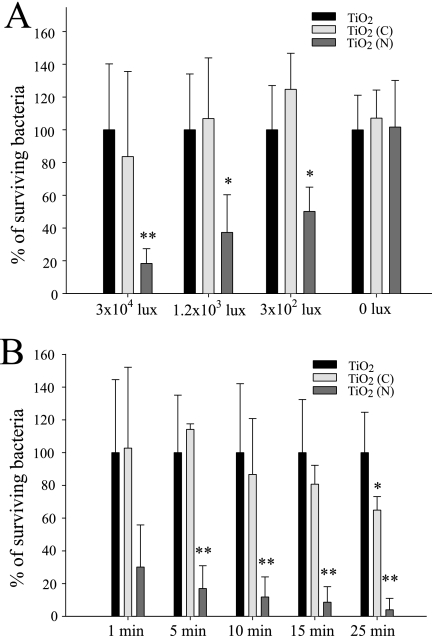
To obtain dose-dependent and kinetic data for E. coli on TiO2 substrates, we further analyzed the effects of illumination by visible light at various time points or at various distances (5 cm, 10 cm, and 20 cm and with respective illumination intensities of 3 × 104, 1.2 × 103, and 3 × 102 lux) (Fig. 2). The results showed that TiO2 (N) substrates could kill E. coli in minutes when exposed to various degrees of illumination by visible light (Fig. 2A). The bacterium-killing efficiency in the TiO2 (N) groups was significantly greater than that of the respective TiO2 groups (Fig. 2A). On the other hand, the TiO2 (C) substrates had less bactericidal effectiveness. Although prolonged illuminations seemed to increase the bacterium killing of TiO2 (C) substrates (25 min) (Fig. 2B), the killing efficiency still did not match that of the TiO2 (N) substrates (bacterial survival rate of 4% versus 70%) (Fig. 2B).
FIG. 2.
Dose dependency and kinetics. Dose dependency (A) and kinetic analysis (B) of the bactericidal activity of the TiO2-related substrates after visible-light illumination are shown. Illumination was carried out either at different light densities for 5 min (A) or at a light density of 3 × 104 lux for different times (B). Under each illumination condition, the percentages of the surviving bacteria on the TiO2 (C) and TiO2 (N) substrates were normalized to the percentage of the surviving bacteria on the TiO2 substrates (100%). *, P < 0.05; **, P < 0.01 (compared to the respective TiO2 groups).
Bactericidal activity of TiO2 (N) in solutions contaminated by protein- or light-absorbing substances.
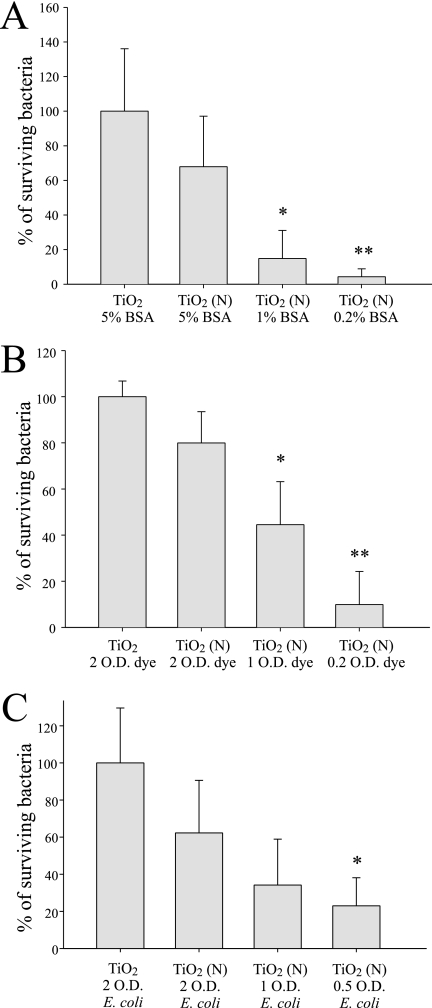
In order to investigate the potential for use of TiO2 (N) in environmental or medical materials which may become contaminated with protein- or light-absorbing substances, we introduced various concentrations of BSA or the dye bromophenol blue into E. coli incubation medium and then measured the bactericidal activity of TiO2 (N). The results showed that the TiO2 (N) substrates became less effective only when the contaminants reached a high level (Fig. 3A and B). When the protein concentration was 1% or the dye contaminant was present at 1 OD, the TiO2 (N) substrate exhibited significant antibacterial ability (bacterial inhibition of 82% or 58%, respectively) (Fig. 3).
FIG. 3.
Protein- and light-absorbing substances. The effects of BSA (A), bromophenol blue dye (B), and bacterial concentrations (C) on the bactericidal activity of the TiO2-related substrates after visible-light illumination are shown. The percentages of surviving bacteria were normalized to the percentage of the surviving bacteria on the TiO2 substrate plus 5% BSA (A), to that on the TiO2 substrate plus 2 OD of dye (B), or to that from 2 OD of E. coli on the TiO2 substrate (C). *, P < 0.05 compared to the group with TiO2 plus 5% BSA (A), the group with TiO2 plus 2 OD of dye (B), or the group with 2 OD of E. coli on TiO2 (C); **, P < 0.01 compared to the group with TiO2 plus 5% BSA (A) or the group with TiO2 plus 2 OD of dye (B).
To control for the OD and light-shielding effects of bacterial concentration, the TiO2 (N)-mediated killing experiments were further performed using different concentrations (OD) of E. coli cells in bacterial solution with equal amounts (2.5 × 107 CFU) of E. coli. We found that the bactericidal activity was not significant when the concentration of E. coli solution was adjusted to 1 OD or greater (Fig. 3C).
Bactericidal activities of TiO2 (N) to eliminate pathogens.
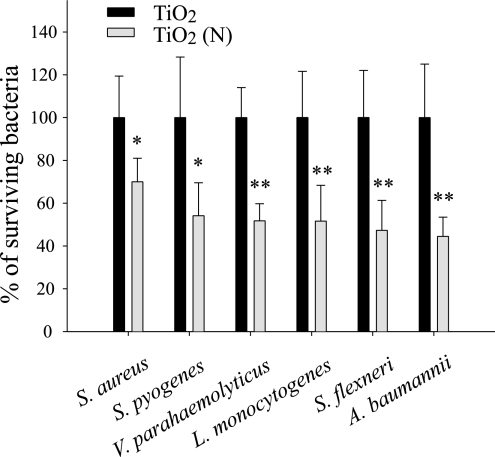
In pathogen experiments, TiO2 (N) was significantly more effective at killing all of the tested pathogens, including S. flexneri, L. monocytogenes, V. parahaemolyticus, S. pyogenes, S. aureus, and A. baumannii, than TiO2 substrates. This effectiveness was not influenced by whether the target was gram-positive or gram-negative bacteria (Fig. 4).
FIG. 4.
Pathogen analysis. For each pathogen, the percentage of surviving bacteria on the TiO2 (N) substrate was normalized to that on the TiO2 substrate. *, P < 0.05; **, P < 0.01 (compared to the TiO2 group).
Experimental sepsis mouse model.
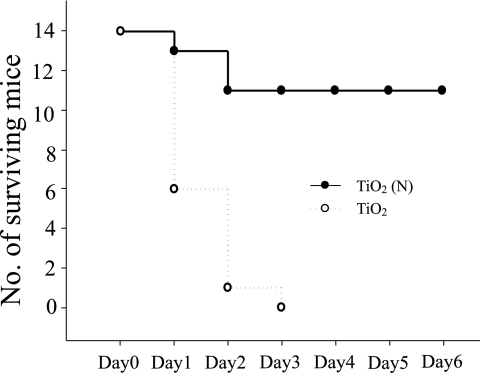
Septic shock experiments in a mouse model were used to investigate whether previous exposure of bacteria to TiO2 photocatalyst killing would result in less severe symptoms after host inoculation. The results showed that killing by TiO2 (N) substrates reduced the number of viable cells of E. coli and significantly reduced their ability to cause host mortality on inoculation (Fig. 5).
FIG. 5.
Septic shock mouse model. Mortality of the C57BL/6J mice after intravenous injection of a lethal dose of E. coli (5 × 109 CFU) preexposed to TiO2 or TiO2 (N) substrates and visible-light illumination (n = 14).
DISCUSSION
Pure TiO2 photocatalyst is effective against bacteria upon UV irradiation. Exposure of humans to UV light at the necessary levels, however, would cause great damage to the skin and eyes (13, 37), thus limiting the potential for the use of TiO2 substrates in environments where humans would be exposed. Public environments are ideal places for the transmission of pathogens (7, 41). The visible-light-induced antibacterial activity of TiO2 (N) offers the potential for use as a disinfectant in public areas, specifically those indoor environments without adequate air circulation, such as public toilets, schools, hospitals, stations, airports, hotels, and public transportation. The surfaces of objects such as door handles and push buttons are constantly contacted by people, and a method which provides a constant disinfection process may be able to limit pathogen spread (6, 38). Since these objects would also be exposed to natural and/or artificial light sources, a TiO2 (N)-coated surface offers the potential for developing such a solution. Many techniques have been developed to coat surfaces with photocatalysts, including wet methods, such as sol-gel and spraying, to achieve the fixation of powder as a film as well as dry processes, such as evaporation, ion-assisted deposition, sputtering, and metal-organic chemical vapor deposition (19).
In this study, we investigated the antimicrobial properties of visible-light photocatalyst TiO2 (N) against human pathogens. Human pathogens were more resistant to TiO2 (N)-mediated killing than the laboratory E. coli strain OP50, with a killing efficiency of approximately 50% versus 80 to 95%. Several pathogens were shown to evolve resistance mechanisms against ROS. For example, specific enzyme systems for the elimination of ROS were found in S. aureus, S. flexneri, and S. pyogenes (11, 17, 25). Because ROS production by phagocytes is part of the innate immune system of hosts (5, 36), these anti-ROS mechanisms are often associated with pathogen virulence (11, 17, 25). The greater resistance against TiO2 (N)-mediated killing of these pathogens than that of E. coli might be attributable to the presence of these enzyme systems, although this possibility remains to be investigated. Even though exposure of inoculates to visible-light photocatalysts significantly reduced the mortality in our septic shock mouse model, this bactericidal efficiency was not comparable to that of commonly used disinfectants, which can almost completely eliminate the target microorganisms (28). The TiO2-based photocatalysts, however, have several advantages compared to other disinfectants. First, because TiO2 is a chemically stable and inert material, it could continuously exert antimicrobial action when illuminated by light. Second, because it is inert, a previous study showed that it is not harmful when ingested by animals (4). Third, the bactericidal activity can be switched on and off or modulated by controlling the light intensity. These advantages might be complementary to existing disinfectants and provide the potential for developing a variety of alternative antimicrobial applications. In addition, several recent technical advancements, such as metal (silver dopant), the addition of the electric field, and the creation of mixed-phase crystals of TiO2, could enhance the photocatalysis activity of TiO2-based photocatalysts (10, 40; Chou et al., unpublished), furthering their potential for use in the design of disinfection technology.
Due to urbanization, population growth, and heavy traveling, infectious diseases can quickly spread worldwide from one local area; the epidemic of severe acute respiratory syndrome during 2003 is an example (21, 22, 43). Visible-light photocatalysts have the potential for use in a variety of settings to reduce the transmission of pathogens in public environments. The emergence of increasingly virulent and antibiotic-resistant pathogens in hospital settings (1, 32) provides another motivation for the development of alternative disinfection approaches using visible-light photocatalysts.
This study demonstrated that TiO2 (N) has better visible-light photocatalytic bactericidal activity against human pathogens than TiO2 or TiO2 (C). Our results showed that the number of microorganisms was greatly reduced after treatment with a visible-light photocatalyst. These results suggest that TiO2 (N) has the potential for use in the development of applications for environmental and medical decontamination.
Acknowledgments
We would like to thank Tony Jer-Fu Lee, Dean of the College of Life Sciences, Tzu-Chi University, for his scientific suggestions and assistance in preparing the manuscript.
We also appreciate the financial support of the National Science Council of Taiwan, R.O.C., under grant no. NSC 93-2216-E-259-002 and NSC 93-2120-M-259-001.
REFERENCES
- 1.Aiello, A. E., and E. Larson. 2003. Antibacterial cleaning and hygiene products as an emerging risk factor for antibiotic resistance in the community. Lancet Infect. Dis. 3:501-506. [DOI] [PubMed] [Google Scholar]
- 2.Alterthum, F., and L. O. Ingram. 1989. Efficient ethanol production from glucose, lactose, and xylose by recombinant Escherichia coli. Appl. Environ. Microbiol. 55:1943-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahi, R., T. Morikawa, T. Ohwaki, K. Aoki, and Y. Taga. 2001. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269-271. [DOI] [PubMed] [Google Scholar]
- 4.Bernard, B. K., M. R. Osheroff, A. Hofmann, and J. H. Mennear. 1990. Toxicology and carcinogenesis studies of dietary titanium dioxide-coated mica in male and female Fischer 344 rats. J. Toxicol. Environ. Health 29:417-429. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 6.Booth, T. F., B. Kournikakis, N. Bastien, J. Ho, D. Kobasa, L. Stadnyk, Y. Li, M. Spence, S. Paton, B. Henry, B. Mederski, D. White, D. E. Low, A. McGeer, A. Simor, M. Vearncombe, J. Downey, F. B. Jamieson, P. Tang, and F. Plummer. 2005. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 191:1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, K. T., P. Y. Chen, R. B. Tang, Y. F. Huang, P. I. Lee, J. Y. Yang, H. Y. Chen, J. Bresee, E. Hummelman, and R. Glass. 2005. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001-2003. J. Infect. Dis. 192(Suppl. 1):S44-S48. [DOI] [PubMed] [Google Scholar]
- 8.Chiou, C.-S., S.-Y. Hsu, S.-I. Chiu, T.-K. Wang, and C.-S. Chao. 2000. Vibrio parahaemolyticus serovar O3:K6 as cause of unusually high incidence of food-borne disease outbreaks in Taiwan from 1996 to 1999. J. Clin. Microbiol. 38:4621-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou, C.-S., W.-B. Hsu, H.-L. Wei, and J.-H. Chen. 2001. Molecular epidemiology of a Shigella flexneri outbreak in a mountainous township in Taiwan, Republic of China. J. Clin. Microbiol. 39:1048-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egerton, T. A., S. A. Kosa, and P. A. Christensen. 2006. Photoelectrocatalytic disinfection of E. coli suspensions by iron doped TiO2. Phys. Chem. Chem. Phys. 8:398-406. [DOI] [PubMed] [Google Scholar]
- 11.Franzon, V. L., J. Arondel, and P. J. Sansonetti. 1990. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect. Immun. 58:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujishima, A., and K. Honda. 1972. Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37-38. [DOI] [PubMed] [Google Scholar]
- 13.Hearing, V. J. 2005. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J. Dermatol. Sci. 37:3-14. [DOI] [PubMed] [Google Scholar]
- 14.Hirakawa, K., M. Mori, M. Yoshida, S. Oikawa, and S. Kawanishi. 2004. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radic. Res. 38:439-447. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki, M., M. Hara, H. Kawada, H. Tada, and S. Ito. 2000. Cobalt ion-doped TiO(2) photocatalyst response to visible light. J. Colloid Interface Sci. 224:202-204. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby, W. A., P. C. Maness, E. J. Wolfrum, D. M. Blake, and J. A. Fennel. 1998. Mineralization of bacterial cell mass on a photocatalytic surface in air. Environ. Sci. Technol. 32:2650-2653. [Google Scholar]
- 17.Janulczyk, R., S. Ricci, and L. Björck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 71:2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampf, G., and A. Kramer. 2004. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 17:863-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneko, M., and I. Okura (ed.). 2002. Photocatalysis. Springer-Verlag, New York, N.Y.
- 20.Kau, J. H., D. S. Sun, W. J. Tsai, H. F. Shyu, H. H. Huang, H. C. Lin, and H. H. Chang. 2005. Antiplatelet activities of anthrax lethal toxin are associated with suppressed p42/44 and p38 mitogen-activated protein kinase pathways in the platelets. J. Infect. Dis. 192:1465-1474. [DOI] [PubMed] [Google Scholar]
- 21.La Montagne, J. R., L. Simonsen, R. J. Taylor, and J. Turnbull. 2004. Severe acute respiratory syndrome: developing a research response. J. Infect. Dis. 189:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan, Y. C., T. T. Liu, J. Y. Yang, C. M. Lee, Y. J. Chen, Y. J. Chan, J. J. Lu, H. F. Liu, C. A. Hsiung, M. S. Ho, K. J. Hsiao, H. Y. Chen, and Y. M. Chen. 2005. Molecular epidemiology of severe acute respiratory syndrome-associated coronavirus infections in Taiwan. J. Infect. Dis. 191:1478-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legrini, O., E. Oliveros, and A. M. Braun. 1993. Photochemical processes for water treatment. Chem. Rev. 93:671-698. [Google Scholar]
- 24.Lima, A. A. 2001. Tropical diarrhoea: new developments in traveller's diarrhoea. Curr. Opin. Infect. Dis. 14:547-552. [DOI] [PubMed] [Google Scholar]
- 25.Mandell, G. L. 1975. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal-leukocyte interaction. J. Clin. Investig. 55:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maness, P.-C., S. Smolinski, D. M. Blake, Z. Huang, E. J. Wolfrum, and W. A. Jacoby. 1999. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 65:4094-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martino, M. C., G. Rossi, I. Martini, I. Tattoli, D. Chiavolini, A. Phalipon, P. J. Sansonetti, and M. L. Bernardini. 2005. Mucosal lymphoid infiltrate dominates colonic pathological changes in murine experimental shigellosis. J. Infect. Dis. 192:136-148. [DOI] [PubMed] [Google Scholar]
- 28.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken. 2003. Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 30.Navon-Venezia, S., R. Ben-Ami, and Y. Carmeli. 2005. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr. Opin. Infect. Dis. 18:306-313. [DOI] [PubMed] [Google Scholar]
- 31.Rodger, S., B. S. Griffiths, J. W. McNicol, R. W. Wheatley, and I. M. Young. 2004. The impact of bacterial diet on the migration and navigation of Caenorhabditis elegans. Microb. Ecol. 48:358-365. [DOI] [PubMed] [Google Scholar]
- 32.Russell, A. D. 2004. Bacterial adaptation and resistance to antiseptics, disinfectants and preservatives is not a new phenomenon. J. Hosp. Infect. 57:97-104. [DOI] [PubMed] [Google Scholar]
- 33.Russell, A. D. 2003. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 3:794-803. [DOI] [PubMed] [Google Scholar]
- 34.Salyers, A. A., and D. D. Whitt. 1994. Bacterial pathogenesis: a molecular approach. ASM Press, Washington, D.C.
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Segal, A. W. 2005. How neutrophils kill microbes. Annu. Rev. Immunol. 23:197-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominski, A., and J. Pawelek. 1998. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin. Dermatol. 16:503-515. [DOI] [PubMed] [Google Scholar]
- 38.Tong, T. R. 2005. Airborne severe acute respiratory syndrome coronavirus and its implications. J. Infect. Dis. 191:1401-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai, P. J., Y. H. Chen, C. H. Hsueh, C. H. Hsieh, Y. H. Liu, J. J. Wu, and C. C. Tsou. 2006. Streptococcus pyogenes induces epithelial inflammatory responses through NF-kappaB/MAPK signaling pathways. Microbes Infect. 8:1440-1449. [DOI] [PubMed] [Google Scholar]
- 40.Vohra, A., D. Y. Goswami, D. A. Deshpande, and S. S. Block. 2005. Enhanced photocatalytic inactivation of bacterial spores on surfaces in air. J. Ind. Microbiol. Biotechnol. 32:364-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walther, B. A., and P. W. Ewald. 2004. Pathogen survival in the external environment and the evolution of virulence. Biol. Rev. Camb. Philos. Soc. 79:849-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong, H.-C., S.-H. Liu, T.-K. Wang, C.-L. Lee, C.-S. Chiou, D.-P. Liu, M. Nishibuchi, and B.-K. Lee. 2000. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 66:3981-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, H. S., Y. C. Hsieh, I. J. Su, T. H. Lin, S. C. Chiu, Y. F. Hsu, J. H. Lin, M. C. Wang, J. Y. Chen, P. W. Hsiao, G. D. Chang, A. H. Wang, H. W. Ting, C. M. Chou, and C. J. Huang. 2004. Early detection of antibodies against various structural proteins of the SARS-associated coronavirus in SARS patients. J. Biomed. Sci. 11:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, M. C., T. S. Yang, and M. S. Wong. 2004. Nitrogen-doped titanium oxide films as visible light photocatalyst by vapor deposition. Thin Solid Films 469-470:1-5. [Google Scholar]
- 45.Yang, T. S., C. B. Shiu, and M. S. Wong. 2004. Structure and hydrophilicity of titanium oxide films prepared by electron beam evaporation. Surface Sci. 548:75-82. [Google Scholar]
- 46.Zhong, M. X., W. A. Kuziel, E. G. Pamer, and N. V. Serbina. 2004. Chemokine receptor 5 is dispensable for innate and adaptive immune responses to Listeria monocytogenes infection. Infect. Immun. 72:1057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]