Abstract
Campylobacter jejuni is a major cause of bacterial food-borne infection in the industrial world. There is evidence that C. jejuni is present in eggs and hatchery fluff, opening the possibility for vertical transmission from hens to progeny. Poultry operations in Iceland provide an excellent opportunity to study this possibility, since breeding flocks are established solely from eggs imported from grandparent flocks in Sweden. This leaves limited opportunity for grandparents and their progeny to share isolates through horizontal transmission. While Campylobacter was not detected in all grandparent flocks, 13 of the 16 egg import lots consisted of eggs gathered from one or more Campylobacter-positive grandparent flocks. No evidence of Campylobacter was found by PCR in any of the 10 relevant quarantine hatchery fluff samples examined, and no Campylobacter was isolated from the parent birds through 8 weeks, while they were still in quarantine rearing facilities. After the birds were moved to less biosecure rearing facilities, Campylobacter was isolated, and 29 alleles were observed among the 224 isolates studied. While three alleles were found in both Sweden and Iceland, in no case was the same allele found both in a particular grandparent flock and in its progeny. We could find no evidence for vertical transmission of Campylobacter to the approximately 60,000 progeny parent breeders that were hatched from eggs coming from Campylobacter-positive grandparent flocks. If vertical transmission is occurring, it is not a significant source for the contamination of chicken flocks with Campylobacter spp.
Campylobacter jejuni is a serious human pathogen and is widely acknowledged as the most common cause of bacterial food poisoning in the developed world (6). Since most cases of campylobacteriosis are associated with raw or undercooked poultry meat (6), interventions that reduce the prevalence of C. jejuni within poultry flocks may have a major impact on human health.
One possible path for introduction of C. jejuni into broiler flocks is vertical transmission, from the hen through the egg to the chick (12). Cox et al. (3) found identical ribotypes and flaA short-variable-region (SVR) alleles in a commercial broiler breeder flock and its progeny broiler flock. Other studies have shown the presence of amplifiable C. jejuni DNA in hatchery fluff and eggshell samples (7). Further, C. jejuni has been found within the reproductive tracts of breeder hens (1, 8) and in the semen of roosters (4). On the other hand, Sahin et al. (15) could not detect C. jejuni in 1,500 eggs collected from commercial broiler breeding operations.
The poultry industry in Iceland provides an excellent opportunity to assess vertical transmission in a commercial system. Iceland imports no fresh poultry food products and no hatched live poultry, and the poultry industry is small enough that almost every flock associated with commercial broiler production could be sampled. Iceland produces broiler chicken flocks and commercial egg-laying flocks from separate breeding flocks; these breeding flocks are populated from eggs imported from grandparent flocks in Sweden. By sampling the grandparent flock and the progeny broiler breeder flocks associated with a particular egg import lot, we have studied a system in which there was limited chance for horizontal transmission between the grandparents in Sweden and their progeny in Iceland. This has increased the likelihood that genetically identical isolates shared between Sweden and Iceland flocks could be identified as the result of vertical transmission.
MATERIALS AND METHODS
Sample collection.
From each of 10 Swedish grandparent flocks, 10 pooled samples of 5 fresh feces from the pen floor were taken from 1 to 12 weeks after egg collection, for a total of 24 grandparent flock samples. These samples were express shipped on ice in insulated containers to Iceland for laboratory enumeration. From each of 25 highly biosecure quarantine rearing flocks, the Chief Veterinary Office of Iceland provided either 20 or 30 cecal samples taken from birds dissected at an age of 6 to 8 weeks and combined into 4 (for 21 flocks) or 6 (for 4 flocks) pooled samples of 5 ceca each. Likewise, 10 pooled samples of 5 fresh feces were collected from the nonquarantine rearing barns housing each of 43 parent flocks in Iceland when the progeny were 18 weeks old. Pooled cecal samples and fecal samples were directly plated onto Campy-Cefex agar (17). Colonies characteristic of Campylobacter were confirmed using a latex agglutination kit recognizing C. jejuni, Campylobacter coli, and Campylobacter lari (Panbio Diagnostics). One confirmed Campylobacter colony per positive sample was restreaked, placed in Wang's transport medium (20), and shipped to the USDA labs in Athens, Ga., for molecular typing. Upon arrival in Georgia, isolates were streaked out onto Campy-Cefex agar and stored in glycerol at −80°C. Prior to August 2002, isolates that arrived unrecoverable in Georgia were discarded; after that date, the transport tubes of unrecoverable isolates were stored at 4°C for later molecular analysis.
Detection of Campylobacter in hatchery fluff.
Fluff samples were collected at hatching from each of 13 egg import lots. One hundred grams of fluff was aseptically gathered into sterile plastic bags from the hatching trays; these samples were shipped to Georgia for PCR detection of Campylobacter jejuni. For five of the first fluff samples, we used the method of Hiett et al. (7) to extract DNA and amplify Campylobacter jejuni flaA SVR. For eight subsequent samples, we extracted 200 mg of fluff using the QIAmp DNA stool minikit (QIAGEN, Inc.). The final elution volume was 200 μl, and bovine serum albumin was added to a final concentration of 0.1 μg μl−1. This DNA was then used for two PCRs. For the first, two eubacterial 16S primers, 8FPL (5′-AGT TTG ATC CTG GCT CAG-3′) and 806R (5′-GGA CTA CCA GGG TAT CTA AT-3′) (14), were used as a control to verify the presence of amplifiable bacterial DNA. For this reaction, 5 μl of fluff DNA was used as a template in a 50-μl reaction volume, with 3.0 mM MgCl2, 0.125 μM each primer, 0.8 mM each deoxynucleoside triphosphate (dNTP), and 1.25 U AmpliTaq DNA polymerase (Applied Biosystems). Before the DNA was added, the tubes containing the PCR mix were irradiated for 1 min on a UV transilluminator by following the recommendations of Relman (14), and every reaction was performed with a negative control to check for contamination. The tubes were subjected to 40 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 1 min, followed by a 5-min extension at 72°C. Ten microliters of PCR product was electrophoresed and visualized on a 1% agarose gel.
After the presence of bacterial DNA was confirmed, we used 5 μl DNA as a template for a 50-μl flaA SVR amplification using the degenerate primers FlaA242FU (5′-CTA TGG ATG AGC AAT TWA AAA T-3′) and FlaA625RU (5′-CAA GWC CTG TTC CWA CTG AAG-3′) (10), with concentrations of magnesium, primer, dNTPs, and polymerase identical to those for the 16S amplification described above. The tubes were subjected to 35 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 1 min, followed by a 5-min extension at 72°C. Ten microliters of PCR product was visualized on a 1% agarose gel.
Molecular typing.
DNA was prepared from frozen isolates by placing 10 μl of glycerol stock in 50 μl sterile distilled water. Cells were lysed at 100°C for 5 min. Microcentrifuge tubes containing DNA samples were spun briefly to precipitate cellular debris (8). DNA was prepared for unrecoverable isolates by pipetting 40 μl of Wang's transport medium into 200 μl sterile distilled water, boiling these samples at 100°C for 5 min, and spinning to precipitate cellular debris (2). In both cases, the flaA SVR (a hypervariable part of the flagellin gene) was then amplified using primers Fla4F (5′-GGA TTT CGT ATT AAC ACA AAT GGT GC-3′) (11) and FlaA625RU with a reaction mixture containing 2 mM MgCl2, 0.125 μM each primer, 0.8 mM each dNTP, and 2.5 U AmpliTaq in a 100-μl volume. Tubes were subjected to 35 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 1 min, followed by a 5-min extension at 72°C. Sequencing was performed using degenerate primers Fla106F (5′-GAY GAT GCT TCW GGK ATG-3′) and FlaA625RU by BigDye Terminator, version 3.1, chemistry (Applied Biosystems). Sequence data were obtained using a 3730 DNA analyzer (Applied Biosystems). The 359-nucleotide region between primers FlaA242FU and FlaA625RU was used for allelic comparisons. In all, 44 isolates were sequenced from 10 grandparent flocks, and 180 isolates were sequenced from 27 parent rearing flocks.
Phylogenetic analysis.
Phylogenetic analysis was performed using the PHYLIP 3.65 suite of programs (5), following alignment of unique alleles by CLUSTAL W (19). SEQBOOT was used to generate a data set of 500 bootstrap replicates from the alignment of unique alleles, and DNAML was used to generate trees from these data replicates. CONSENSE was used to create a consensus tree and calculate the bootstrap support for each branch. This tree was then reinput into DNAML as a user tree with the original sequence data in order to assign maximum-likelihood lengths to the branches previously determined through bootstrapping the tree. Branches with less than 50% bootstrap support or with branch lengths that were not significantly different from zero were collapsed.
Nucleotide sequence accession numbers.
The sequences of unique alleles in Campylobacter strains isolated in this study can be found in GenBank under accession numbers DQ335538 to DQ335565.
RESULTS
As can be seen in Table 1, Campylobacter was never detected in 4 of the 10 grandparent flocks. However, this should be viewed as a conservative look at colonization in the grandparents, since fecal samples varied in freshness, and samples were subjected to enumeration on selective media rather than enrichment, which would have provided the greatest chance for recovering ailing bacteria. Likewise, prevalence cannot be determined from these data, since the samples were pooled. An indication that some of the samples might not have been fresh is that one flock with only one positive sample (S54 on 7 April 2003) had a higher log CFU count per gram of feces than the mean log count of a flock (S52 on 8 July 2002) with eight positive samples. One would expect a flock with few Campylobacter-colonized birds to have a lower CFU count than a flock with almost all birds colonized (18).
TABLE 1.
Campylobacter colonization in the Swedish grandparent flocks
| Grandparent flock | Sample datea | No. of positive pooled samples | Import lot | Date of egg import |
|---|---|---|---|---|
| S47 | 12 June 2001 | 0 | R49 | 27 Mar. 2001 |
| S48 | 12 June 2001 | 5 | R49 | 27 Mar. 2001 |
| R50 | 2 May 2001 | |||
| 30 Oct. 2001 | 6 | R52 | 9 Aug. 2001 | |
| S49 | 30 Oct. 2001 | 1 | R52 | 9 Aug. 2001 |
| R53a | 9 Oct. 2001 | |||
| R53b | 24 Oct. 2001 | |||
| Not sampled | R55 | 6 Feb. 2002 | ||
| S50 | 30 Oct. 2001 | 0 | R53a | 09 Oct. 2001 |
| R53b | 24 Oct. 2001 | |||
| 5 Mar. 2002 | 0 | R55 | 6 Feb. 2002 | |
| 15 Apr. 2002 | 0 | R56 | 20 Mar. 2002 | |
| S51 | 17 Dec. 2001 | 0 | R54 | 5 Dec. 2001 |
| 15 Apr. 2002 | 0 | R56 | 20 Mar. 2002 | |
| 8 July 2002 | 0 | R57 | 26 June 2002 | |
| S52 | 15 Apr. 2002 | 3 | R56 | 20 Mar. 2002 |
| 8 July 2002 | 8 | R57 | 26 June 2002 | |
| 9 Sept. 2002 | 10 | R59 | 8 Aug. 2002 | |
| 7 Oct. 2002 | 8 | R60 | 18 Sept. 2002 | |
| S53 | 8 July 2002 | 1 | R57 | 26 June 2002 |
| 9 Sept. 2002 | 0 | R59 | 8 Aug. 2002 | |
| 7 Oct. 2002 | 0 | R60 | 18 Sept. 2002 | |
| S54 | 9 Sept. 2002 | 0 | R59 | 8 Aug. 2002 |
| 7 Oct. 2002 | 0 | R60 | 18 Sept. 2002 | |
| 20 Jan. 2003 | 3 | R61a | 13 Nov. 2002 | |
| R61b | 27 Nov. 2002 | |||
| R62 | 8 Jan. 2003 | |||
| 7 Apr. 2003 | 1 | R63 | 11 Mar. 2003 | |
| S55 | 20 Jan. 2003 | 6 | R61b | 27 Nov. 2002 |
| 7 Apr. 2003 | 5 | R63 | 11 Mar. 2003 | |
| S57 | 10 June 2003 | 0 | R64 | 30 Apr. 2003 |
Date of fecal sample collection from the grandparent flocks.
Because eggs derived from noncolonized grandparents cannot be used to test for vertical transmission, we have attempted to consider only those parent flock birds hatched from eggs from colonized grandparents. The eggs for each import lot were collected from 1 to 3 grandparent flocks (Table 2), and for 3 import lots (R54, R55, and R64), none of the grandparent flocks tested positive. These lots were excluded from further analysis. For the remaining egg import lots, the proportion of eggs collected from colonized grandparent flocks was multiplied by the number of parent flock birds placed from that lot to estimate the number of parent flock birds that might have demonstrated vertical transmission.
TABLE 2.
Description of egg import lots
| Import lot | No. of eggs shipped | Fluff resulta | No. of contributing GPb flocks | No. of positive GP flocks | Proportion of eggs from positive GP flocks | No. of placed parent birds | No. of placed parent birds possibly associated with vertical transmissionc |
|---|---|---|---|---|---|---|---|
| R49 | 33,480 | N/A | 2 | 1 | 0.247 | 11,508 | 2,846 |
| R50 | 12,600 | − | 1 | 1 | 1.000 | 3,857 | 3,857 |
| R52 | 29,160 | N/A | 2 | 2 | 1.000 | 6,408 | 6,408 |
| R53a | 19,800 | − | 2 | 1 | 0.327 | 6,528 | 2,136 |
| R53b | 19,800 | − | 2 | 1 | 0.364 | 7,566 | 2,751 |
| R54 | 28,800 | − | 1 | 0 | 0.000 | 9,315 | 0 |
| R55 | 20,880 | − | 2 | 0 | 0.000 | 7,283 | 0 |
| R56 | 38,520 | − | 3 | 1 | 0.645 | 12,301 | 7,932 |
| R57 | 38,520 | − | 3 | 2 | 0.561 | 9,531 | 5,344 |
| R59 | 21,600 | − | 3 | 1 | 0.617 | 5,297 | 3,266 |
| R60 | 38,520 | − | 3 | 1 | 0.262 | 8,434 | 2,207 |
| R61a | 19,440 | N/A | 1 | 1 | 1.000 | 5,255 | 5,255 |
| R61b | 20,000 | − | 2 | 2 | 1.000 | 6,608 | 6,608 |
| R62 | 28,800 | − | 1 | 1 | 1.000 | 7,579 | 7,579 |
| R63 | 24,480 | − | 2 | 2 | 1.000 | 4,160 | 4,160 |
| R64 | 28,440 | − | 1 | 0 | 0.000 | 10,296 | 0 |
Presence or absence of a PCR amplicon of flaA SVR in the hatchery fluff sample from the indicated import lot. N/A, no fluff sample was available for testing. A minus sign indicates that the sample was PCR negative for Campylobacter flaA SVR.
GP, grandparent.
The proportion of eggs contributed by positive grandparent flocks was multiplied by the number of birds placed to calculate the number of placed birds that might have been colonized through vertical transmission.
As can be seen in Table 3, no fluff samples from the quarantine hatchery were positive for Campylobacter spp. by PCR. In addition, none of the quarantine rearing cecal samples was positive for Campylobacter by culture. Not until the birds were transferred to the less biosecure rearing facilities were any of the flocks positive. As a result, alleles can be compared only between the grandparent flocks that supplied the eggs for a particular import lot and the nonquarantine parent rearing flocks that were hatched from those eggs.
TABLE 3.
Prevalence of Campylobacter spp. through the parent rearing stage in Icelanda
| Facility (time in wks) | Flock % positive (no. positive/no. tested) | No. (type) of specimens tested | Total no. of parent flock birds placed | Total no. of parent flock birds possibly associated with vertical transmission |
|---|---|---|---|---|
| Quarantine hatchery | 0 (0/13) | 13 (fluff) | ||
| Quarantine rearing (8) | 0 (0/20) | 86 (cecal contents) | 121,926 | 60,351 |
| Rearing (18) | 75 (27/36) | 366 (fresh feces) |
Sampling was conducted from 1 May 2001 through 15 March 2004.
Of the 29 alleles found by sequencing, 4 were found only in the Swedish grandparent flocks and 22 were found only in Iceland. The remaining three alleles were found in both Sweden and Iceland. Of the 22 alleles found only in Iceland, 6 alleles were seen only in birds hatched from egg import lots R54, R55, and R64, which were hatched from eggs from noncolonized grandparent flocks. Of the remaining 16 alleles, 2 alleles appeared as single isolates and another 8 were restricted to a single flock in this data set. Of the four alleles found only in Sweden, one allele was found in a single isolate. Another two alleles were found within single flocks at two consecutive sampling times.
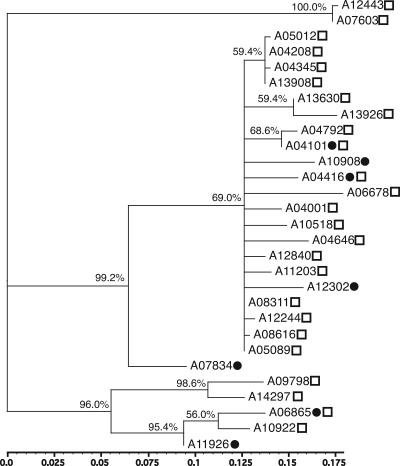
The flaA SVR sequences are not very tree-like, as can be seen in Fig. 1. While there are three strongly supported clades, the largest clade (in terms of the number of unique alleles) has little supported structure. However, Swedish and Icelandic alleles are found in two of the strongly supported clades, and there appears to be no segregation of Swedish alleles into any particular clade.
FIG. 1.
Unrooted maximum-likelihood tree of Campylobacter flaA SVR alleles. Filled circles indicate alleles present in Swedish grandparent isolates. Open squares indicate alleles present in Icelandic parent rearing samples. The percentage of bootstrap support (out of 500 bootstrap replicates) for each node is given above the branch. Scale bar, substitutions per site.
A closer examination of the individual egg import lots from Sweden to Iceland (Table 4) shows that at no point is an allele seen in both the grandparent flock from which the eggs were taken and the parent rearing flocks hatched from those eggs. Excluded from this table are three egg import lots for which the grandparent flocks were not colonized with Campylobacter and two early lots for which the grandparent isolates arrived in Georgia noncultivable. The fourth column of Table 4 shows the minimum number of mutations required to transform an allele found in a grandparent flock to its closest relative among the alleles found in the parent flocks hatched from that egg import lot. The smallest number of mutations for any of the import lots is 9.
TABLE 4.
Unique alleles found in grandparent and parent rearing flocks for each egg import lot
| Egg import lot | Grandparent flock allele (flock, no. of isolates with each allele)a | Parent flock allele (no. of isolates with each allele)a | Minimum no. of mutations among allelesb |
|---|---|---|---|
| R52 | A04416 (S48, n = 3) | A04101 (n = 1) | 11 |
| A06865 (S48, n = 1; S49, n = 1) | A04345 (n = 1) | ||
| R53 | A06865 (S49, n = 1) | A04101 (n = 9) | 62 |
| A04646 (n = 1) | |||
| A05012 (n = 2) | |||
| A07603 (n = 3) | |||
| A12443 (n = 1) | |||
| R56 | A07834 (S52, n = 3) | A08616 (n = 6) | 33 |
| A10922 (n = 9) | |||
| R57 | A07834 (S52, n = 6) | A04101 (n = 4) | 25 |
| A04208 (n = 1) | |||
| A04416 (n = 5) | |||
| A05089 (n = 2) | |||
| A08616 (n = 11) | |||
| A09798 (n = 4) | |||
| A11203 (n = 3) | |||
| R59 | A04101 (S52, n = 8) | A06865 (n = 18) | 63 |
| A10908 (S52, n = 1) | |||
| R60 | A04101 (S52, n = 7) | A04416 (n = 8) | 9 |
| A10908 (S52, n = 1) | A08616 (n = 4) | ||
| A09798 (n = 1) | |||
| A12244 (n = 2) | |||
| R61 | A10908 (S54, n = 1; S55, n = 7) | A10518 (n = 12) | 13 |
| A11926 (S54, n = 2) | |||
| R62 | A10908 (S54, n = 1) | A04101 (n = 4) | 12 |
| A11926 (S54, n = 2) | A04345 (n = 13) | ||
| A04416 (n = 5) | |||
| A12244 (n = 1) | |||
| R63 | A10908 (S55, n = 2) | A04001 (n = 2) | 9 |
| A11926 (S54, n = 1) | A13630 (n = 6) | ||
| A12302 (S55, n = 1) |
Alleles seen in both Sweden and Iceland are boldfaced.
The minimum number of mutations required to change an allele from the grandparent flock into one from the parent flock.
DISCUSSION
Because of the large geographic distance and intervening ocean between the grandparent flocks in Sweden and the parent flocks in Iceland, this data set presents a unique opportunity to study egg-borne transmission of Campylobacter from parent to offspring with minimal chances of confounding horizontal or common environmental source transmission. While the hatched breeder chicks were reared within a high-containment facility that maintained a “shower-in/shower-out,” restricted-access protocol with careful pest proofing to enforce the biosecurity of the animals, there were 6 to 8 weeks for any isolate transmitted vertically to spread throughout the flock without other possible sources of Campylobacter colonization to muddy the interpretation. Stern et al. (18) showed that with broilers 1 to 6 weeks old, 70 to 100% of birds within an experimental flock began shedding Campylobacter within 5 days after the introduction of a “seeder” bird, and 95 to 100% of the birds were colonized with Campylobacter by the seventh day. Were vertical transmission to occur, most or all of the birds within a flock would be colonized in less than 6 weeks.
The fact that the alleles from grandparent flocks were not phylogenetically distinct from the alleles in Iceland might argue for vertical transmission. However, if vertical transmission were occurring in our samples, we would expect the alleles seen in the grandparent flocks to be seen in the offspring parent flocks. This was not observed during our study. Even if one allows the possibility that mutation might obscure the identity by descent of a vertically transmitted allele in the parent rearing samples, the smallest number of mutations required is 9. This is an unlikely scenario to explain the lack of any genetic matches. The lack of a phylogenetic separation between alleles of Campylobacter isolates from Sweden and those from Iceland may indicate that there is some genetic exchange occurring between these nations; such exchange could be explained not only by rare vertical transmission events but by rare horizontal transmission events through long-range migrants, such as birds or humans.
The possibility for vertical transmission was previously investigated by Jacobs-Reitsma (9), who wished to characterize this potential. By comparing serotypes of Campylobacter from hens and progeny chicks, no matches were made, and no evidence was observed to support such a hypothesis. More recently, Smith et al. (16) examined the relationship of transmission from breeder hens to young turkeys in a longitudinal study of Campylobacter colonization. In their study, two sibling pairs of turkey flocks were sampled. None of the restriction fragment length polymorphism flagellar types of Campylobacter isolated from the breeders matched those from the progeny stock. Although no amount of evidence can ever be provided to indicate that the phenomenon of vertical transmission never occurs, Smith et al. concluded that there was a lack of evidence to support such transmission of Campylobacter between flock generations.
Taking a contrary position, Pearson et al. (13) suggested that the isolation rate and type of Campylobacter isolates in broiler chickens were associated with the hatchery supplying chicks for broiler production. Further, they suggested that Campylobacter was introduced by vertical transmission. Cox et al. (3) subsequently supported the position that breeder hens can serve as a source for Campylobacter contamination in broiler flocks. Their evidence consisted of culturing isolates from broiler breeder flocks and from corresponding progeny broiler flocks, 60 miles apart. The isolates were characterized by both ribotyping and flaA SVR sequencing, which suggested that the isolates were of clonal origin. However, the potential for widely distributed Campylobacter clones as an alternative explanation was not considered in that study.
In this study, we sampled parent flocks representing more than 60,000 offspring birds that might have demonstrated vertical transmission. We found no evidence of Campylobacter colonization in any of the birds while they remained in quarantine conditions. Even if we ignore the cultural evidence and look only at the genetic data, the flaA SVR alleles seen in the less biosecure parent rearing flocks did not match those found in the grandparent flocks. While this does not disprove the possible existence of vertical transmission, it suggests that egg-borne transmission, if occurring, is not a common event. While 60,000 birds is not a huge number relative to the millions of broilers hatched each year, it is large relative to the number of birds in any particular broiler house: vertical transmission occurring at a rate of 1 in 60,000 birds is not a large risk for a house containing 20,000 birds, let alone a flock of 10,000 or fewer birds. In the face of much higher frequencies of horizontal and environmental source transmission, eliminating Campylobacter in breeder flocks will not be an effective means of controlling Campylobacter in broilers, where it presents a risk to human health.
Acknowledgments
This work was supported by National Research Initiative grant 2002-35212-12369 from the Epidemiological Approaches to Food Safety program of the USDA Cooperative State Research, Education, and Extension Service (awarded to N.J.S., R.L., and K.L.H.) and through the USDA Agricultural Research Service (CRIS 6612-32000-034-00).
This work was performed as part of the Campy-on-Ice Consortium, which consists of Haraldur Briem and Gudrún Sigmundsdóttir (Directorate of Health, Reykjavik, Iceland), Hjördís Harðardóttir and Karl Kristinsson (Landspitali National University Hospital, Reykjavik, Iceland), Vala Friðriksdóttir and Eggert Gunnarsson (Institute of Experimental Pathology, Reykjavik, Iceland), Franklín Georgsson (Food Laboratory, The Environmental and Food Agency of Iceland, Reykjavik, Iceland), Jarle Reiersen (Icelandic Veterinary Services, Reykjavik, Iceland), Eva Berndtson (Swe-Chick, Kristianstad, Sweden), Jean-Robert Bisaillon and Ruff Lowman (Canadian Food Inspection Agency, Ottawa, Ontario, Canada), Aamir Fazil and Pascal Michel (Public Health Agency of Canada Laboratory for Food-Borne Zoonoses, Guelph, Ontario, and St. Hyacinth, Quebec, Canada), Greg Paoli (Decisionalysis Risk Consultants, Inc., Ottawa, Ontario, Canada), and Kenneth Callicott, Kelli Hiett, and Norman Stern (USDA Agricultural Research Service, Poultry Microbiological Safety Research Unit, Athens, Ga.).
We thank Susan Brooks, Tabitha Mashburn, and Latoya Wiggins of the USDA Agricultural Research Service for technical assistance.
The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
REFERENCES
- 1.Buhr, R. J., N. A. Cox, N. J. Stern, M. T. Musgrove, J. L. Wilson, and K. L. Hiett. 2002. Recovery of Campylobacter from segments of the reproductive tract of broiler breeder hens. Avian Dis. 46:919-924. [DOI] [PubMed] [Google Scholar]
- 2.Callicott, K. A., N. J. Stern, K. L. Hiett, and the Campy on Ice Consortium. 2005. Isolation of DNA for PCR assays from noncultivable Campylobacter jejuni isolates. Poult. Sci. 84:1530-1532. [DOI] [PubMed] [Google Scholar]
- 3.Cox, N. A., N. J. Stern, K. L. Hiett, and M. E. Berrang. 2002. Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens. Avian Dis. 46:535-541. [DOI] [PubMed] [Google Scholar]
- 4.Cox, N. A., N. J. Stern, J. L. Wilson, M. T. Musgrove, R. J. Buhr, and K. L. Hiett. 2002. Isolation of Campylobacter spp. from semen samples of commercial broiler breeder roosters. Avian Dis. 46:717-720. [DOI] [PubMed] [Google Scholar]
- 5.Felsenstein, J. 2004. PHYLIP (phylogeny inference package), version 3.6. Department of Genome Sciences, University of Washington, Seattle. [Online.] http://evolution.genetics.washington.edu/phylip.html.
- 6.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of C. jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 7.Hiett, K. L., N. A. Cox, and N. J. Stern. 2002. Direct polymerase chain reaction detection of Campylobacter spp. in poultry hatchery samples. Avian Dis. 46:219-223. [DOI] [PubMed] [Google Scholar]
- 8.Hiett, K. L., N. A. Cox, R. J. Buhr, and N. J. Stern. 2002. Genotype analysis of Campylobacter isolated from distinct segments of the reproductive tracts of broiler breeder hens. Curr. Microbiol. 45:400-404. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs-Reitsma, W. F. 1995. Campylobacter bacteria in breeder flocks. Avian Dis. 39:355-359. [PubMed] [Google Scholar]
- 10.Meinersmann, R. J., L. O. Helsel, P. I. Fields, and K. L. Hiett. 1997. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J. Clin. Microbiol. 35:2810-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachamkin, I., K. Bohachick, and C. M. Patton. 1993. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 31:1531-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson, A. D., M. H. Greenwood, R. K. A. Feltham, T. D. Healing, J. Donaldson, D. M. Jones, and R. R. Colwell. 1996. Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: intermittent common source, vertical transmission, and amplification by flock propagation. Appl. Environ. Microbiol. 62:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relman, D. A. 1993. Universal bacterial 16S rDNA amplification and sequencing, p. 489-495. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 15.Sahin, O., P. Kobalka, and Q. Zhang. 2003. Detection and survival of Campylobacter in chicken eggs. J. Appl. Microbiol. 95:1070-1079. [DOI] [PubMed] [Google Scholar]
- 16.Smith, K., N. Reimers, H. J. Barnes, B. C. Lee, R. Siletzky, and S. Kathariou. 2004. Campylobacter colonization of sibling turkey flocks reared under different management conditions. J. Food Prot. 67:1463-1468. [DOI] [PubMed] [Google Scholar]
- 17.Stern, N. J., B. Wojton, and K. Kwiatek. 1992. A differential-selective medium and dry-ice generated atmosphere for recovery of Campylobacter jejuni. J. Food Prot. 55:514-517. [DOI] [PubMed] [Google Scholar]
- 18.Stern, N. J., N. A. Cox, and M. T. Musgrove. 2001. Incidence and levels of Campylobacter in broilers after exposure to an inoculated seeder bird. J. Appl. Poult. Res. 10:315-318. [Google Scholar]
- 19.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, W.-L., N. W. Luechtefeld, L. B. Reller, and M. J. Blaser. 1980. Enriched brucella medium for storage and transport of cultures of Campylobacter fetus subsp. jejuni. J. Clin. Microbiol. 12:479-480. [DOI] [PMC free article] [PubMed] [Google Scholar]