Abstract
Potent activation of human T-cell leukemia virus type 1 (HTLV-1) gene expression is mediated by the virus-encoded transactivator protein Tax and three imperfect 21-bp repeats in the viral long terminal repeats. Each 21-bp repeat contains a cAMP-responsive-element core flanked by 5′ G-rich and 3′ C-rich sequences. Tax alone does not bind DNA. Rather, it interacts with basic domain-leucine zipper transcription factors CREB and ATF-1 to form ternary complexes with the 21-bp repeats. In the context of the ternary complexes, Tax contacts the G/C-rich sequences and recruits transcriptional coactivators CREB-binding protein (CBP)/p300 to effect potent transcriptional activation. Using an easily transduced and chromosomally integrated reporter system derived from a self-inactivating lentivirus vector, we showed in a BRG1- and BRM1-deficient adrenal carcinoma cell line, SW-13, that Tax- and 21-bp repeat-mediated transactivation does not require BRG1 or BRM1 and is not enhanced by BRG1. With a similar reporter system, we further demonstrated that Tax- and tumor necrosis factor alpha-induced NF-κB activation occurs readily in SW-13 cells in the absence of BRG1 and BRM1. These results suggest that the assembly of stable multiprotein complexes containing Tax, CREB/ATF-1, and CBP/p300 on the 21-bp repeats is the principal mechanism employed by Tax to preclude nucleosome formation at the HTLV-1 enhancer/promoter. This most likely bypasses the need for BRG1-containing chromatin-remodeling complexes. Likewise, recruitment of CBP/p300 by NF-κB may be sufficient to disrupt histone-DNA interaction for the initiation of transcription.
Human T-cell leukemia virus type 1 (HTLV-1), a deltaretrovirus, is the etiologic agent of adult T-cell leukemia and a neurological disorder known as HTLV-1-associated myelopathy/tropical spastic paraparesis. The viral regulatory protein Tax, encoded by the env-pX region of HTLV-1, plays a critical role in HTLV-1 replication and pathogenesis. Tax performs two major functions during the viral life cycle: first, it mediates potent activation of viral transcription, and second, it usurps regulatory mechanisms critical for cell growth and division to facilitate viral replication. The effects Tax exerts on cells are pleiotropic and include potent NF-κB activation, cell cycle perturbation, and cell transformation.
Tax does not bind DNA directly but activates transcription by recruiting or modifying the activities of cellular transcription factors, including cyclic AMP-responsive-element-binding protein (CREB), serum-responsive factor (SRF), and NF-κB. The mechanism by which Tax activates viral transcription has been studied extensively. The viral transcriptional enhancer consists of three imperfect 21-bp repeats, each containing a cAMP-responsive element (CRE) flanked by 5′ G-rich and 3′ C-rich sequences. The CREs in the 21-bp repeats are bound by the cellular basic domain-leucine zipper (bZip) transcription factors CREB and ATF-1. Tax, CREB/ATF-1, and the 21-bp repeats assemble stable ternary complexes (2, 44, 49, 58, 61) in which Tax binds the basic domains of CREB/ATF-1 (1, 3, 38, 60) and makes contacts with the minor groove of the G/C-rich sequences that flank the CRE, thus achieving the exquisite DNA sequence specificity of Tax-mediated long terminal repeat (LTR) transactivation (7, 26, 27, 32, 33, 37, 45, 59). In the context of the ternary Tax-CREB/ATF-1-21-bp-repeat complex, Tax further recruits transcriptional coactivators CREB-binding protein (CBP)/p300 and, possibly, p300-CBP associated factor for potent gene activation (5, 16, 17, 22, 29, 32). In the presence of Tax, gene expression driven by multiple copies of the 21-bp repeat element can increase 100-fold or more.
Tax also activates the classical and the alternative NF-κB pathways. Activation of the classical NF-κB pathway by Tax is due in part to a direct interaction between Tax and the regulatory subunit of the IκB kinase (IKK), NF-κB essential modulator (NEMO)/IKKγ (6, 23, 43, 55, 57). Recent data have indicated that via a tripartite interaction, Tax, protein phosphatase 2A (PP2A), and NEMO/IKKγ form a stable ternary complex. In this multiprotein complex, the activity of PP2A to inactivate phosphorylated/activated IKK is inhibited or diminished (12). These results suggest that PP2A is a negative regulator of activated phospho-IKK, and PP2A inhibition by NEMO/IKKγ-bound Tax maintains IKK in a phosphorylated and active state, causing constitutive phosphorylation and degradation of IκB, nuclear translocation of NF-κB/Rel, and potent activation of genes under the control of RelA/NF-κB1. The alternative NF-κB pathway is mediated by the RelB/NF-κB2 complex. This pathway is important for B-cell proliferation and lymphoid organogenesis and is activated in response to a subset of NF-κB inducers, such as lymphotoxin β and B-cell activating factor (18). Normally, the alternative pathway is regulated through NF-κB-inducing kinase and IKKα and is independent of IKKγ (41, 54). Activated NF-κB-inducing kinase-IKKα complex phosphorylates the precursor of NF-κB2, p100, and targets it for ubiquitination and processing (47, 52). Tax-mediated p100 processing, however, requires both IKKα and IKKγ (47, 51, 53). Recent data suggest that Tax binds p100 directly and activates IKKγ/IKKα to facilitate p100 phosphorylation and processing (47, 51, 53). NF-κB activation by Tax up-regulates many cellular genes, including those encoding interleukin-2 (IL-2) receptor α chain, costimulatory surface receptors OX40/OX40L, IL-13, IL-15, ICAM1, and antiapoptotic proteins such as IAP1 (8, 15) that are important for proinflammatory responses and lymphocyte survival. They are likely to contribute to the development of HTLV-1-associated myelopathy/tropical spastic paraparesis and adult T-cell leukemia.
To date, most studies of the transactivation functions of Tax have relied on transient transfection of reporter plasmids and in vitro reconstitution of protein-protein and protein-DNA interactions. Chromosomally integrated HTLV-1 proviral DNA represents a surrogate of cellular transcriptional unit and assembles in nucleosome configuration with the full complement of histones and possibly nonhistone proteins. In this sense, it is probably not surprising that Tax is involved in the recruitment of histone acetylases (HATs) CBP/p300 to the viral transcriptional enhancer to promote mRNA transcription. What remains unclear is whether the assembly of CBP/p300-containing complexes on the 21-bp repeats is both necessary and sufficient for Tax-mediated transactivation or, alternatively, the ATP-dependent chromatin-remodeling complexes such as SWI/SNF may also be involved. Here we report the construction of two enhanced green fluorescence protein (EGFP) reporter systems for monitoring the transactivation functions of Tax: one reporter is driven by 18 copies of the 21-bp repeats inserted upstream of a minimal HTLV-1 promoter (18x21), and the other is based on the E-selectin transcriptional regulatory region. Using a self-inactivating (SIN) lentivirus vector, we generated high-titer vector particles that easily integrate these two reporters in the chromosomes of a wide variety of cultured cell lines. The chromosomally integrated reporters are highly Tax-inducible and produce minimal background signals. Using these two reporters, we show that potent Tax/21-bp repeat-mediated transactivation and both Tax- and tumor necrosis factor alpha (TNF-α)-induced NF-κB activation occur readily in SW-13, a cell line deficient in BRG1 and BRM1, the ATPase subunits of the SWI/SNF chromatin-remodeling complexes.
MATERIALS AND METHODS
Cell lines.
HEK293T (human embryonic kidney cell line 293 expressing simian vacuolating virus 40 T antigen), HeLa, PX1 (a fibroblast cell line derived from an LTR tax transgenic mouse), and SW-13 (a human adrenocortical carcinoma cell line deficient in BRG1 and BRM1; a generous gift of Kejie Zhao, NIH) and TSU-Pr1 (a bladder transitional cell carcinoma cell line that lacks BRG1 and BRM1; generously provided by Seong-jin Kim, NIH) cells were maintained in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 units/ml penicillin-streptomycin, and 2 mM l-glutamine. T-cell lines MT4 (an HTLV-1-transformed human T-cell line) and Jurkat (an HTLV-unrelated human T-lymphoblast-like cell line) were maintained in RPMI 1640 with the same supplements.
Plasmid construction.
The cytomegalovirus (CMV) immediate early enhancer/promoter was deleted from the SIN lentivirus vector SMPU-CMV-EGFP following EagI and BamHI restriction endonuclease digestion. The vector DNA fragment was blunt ended by the Klenow fragment enzyme, treated with calf intestinal alkaline phosphatase to remove the 5′ phosphate, and ligated with a blunt-ended 1.1-kb SmaI-SalI fragment containing 18 copies of the HTLV-1 21-bp repeats and a minimal HTLV-1 promoter. For construction of the NF-κB reporter SMPU-E-sel-EGFP, a DNA fragment containing the E-selectin promoter (positions −730 to +52) (40) was derived by PCR using the E-selectin-Luc plasmid as the template and the primer pair 5′ AGTCGGCCGCCAAAGTGGTGGGATTA 3′ and 5′ TCAGGATCCGTCTCAGGTCAGTATA 3′ (40). These two primers also incorporate EagI and BamHI restriction sites into the 5′ and 3′ ends of the PCR product, respectively. The PCR product was then digested with EagI and BamHI and used to replace the CMV immediate early promoter in SMPU-CMV-EGFP.
Production and transduction of recombinant lentivirus vector.
To generate lentiviral vector particles, 3 × 106 HEK293T cells were plated in a T75 flask the day before transfection. On the second day, cells in each T75 flask were transiently transfected with 10 μg pCMVΔR8.2, 2 μg pCMV-VSV-G, and 10 μg vector plasmid (SMPU-18x21-EGFP, SMPU-E-sel-EGFP, or LV-Tax) by using the calcium phosphate method. Culture medium was removed the next day, and the cells washed with phosphate-buffered saline (PBS) and then grown in fresh medium. Forty-eight hours after transfection, culture supernatants were collected and centrifuged at 2,250 rpm for 10 min in a low-speed centrifuge to remove debris. The titer of the reporter virus was determined by counting the number of EGFP-positive cells after infection of MT4 cells. To obtain single colonies transduced with the SMPU-18x21-EGFP reporter, HeLa cells were trypsinized 1 day after transduction and seeded on 10-cm dishes at a density of approximately 100 cells/dish. Individual colonies were picked and seeded in 24-well culture plates in duplicate. Positive clones were identified by positive EGFP expression after secondary transduction with a lentivirus vector carrying the tax gene.
Western blotting.
Whole cell extracts were prepared under denaturing conditions. Protein samples from total cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membrane using a semidry transfer apparatus. After a blocking for 1 h at room temperature with 5% milk in PBS containing 1% Tween (PBST), the membrane was incubated with rabbit polyclonal antibody against BRG1 (Santa Cruz) at a 1:2,000 dilution or a mouse hybridoma antibody (4C5) against Tax (1:100) for 1 h at room temperature. Thereupon, the membrane was washed three times with PBST for 10 min, each at room temperature, and incubated with horseradish peroxidase-conjugated second antibodies for 1 h at room temperature. The membrane was then washed three times with PBST and visualized with enhanced chemiluminescence reagents (Amersham-Pharmacia).
Transfection and luciferase assay.
Approximately 3 × 105 SW-13 cells/well in a 12-well plate were cotransfected using Lipofectamine 2000 (Invitrogen Corp.) with the E-selectin-Luc plasmid (0.5 μg) or the HTLV-1 LTR-luciferase plasmid (0.5 μg) together with pCMV Tax (100 ng) in the presence or absence of 1.0 μg pREP7-BRG1 expression plasmid. As a positive control, 0.5 μg K8P (220-bp K-bZip promoter), together with 100 ng Rta with or without 1.0 μg pREP7-BRG1, was transiently transfected into SW-13 cells. The total amount of transfected DNA in each well was kept constant by the addition of empty vector. Cell lysates were prepared 48 h after transfection by dissolving the DNA-transfected cells from each well in 250 μl of the reporter lysis buffer (Promega Corp.). Twenty microliters of the lysate was then placed in each well of a 96-well plate. After injection of 100 μl of luciferase substrate buffer, the luciferase activity was measured immediately in an MLX microtiter plate luminometer (Dynex Technologies). Transfections and luciferase assays for each experiment were performed in triplicate with the averages and standard deviations of luciferase activities shown.
Flow cytometry.
Reporter-transduced SW-13 cells were trypsinized, rinsed twice with ice-cold PBS, and fixed in 1% freshly prepared, cold paraformaldehyde (Sigma-Aldrich, St. Louis, Mo.). FACS analysis was then performed using EPICS XL-MCL (Beckman coulter). A minimum of 10,000 events were routinely counted. Data analyses were carried out using the EXP032 ADC software. EGFP expression was quantified by summing up the fluorescence signals registered by the flow cytometer and subtracting the background fluorescence in untreated cells.
Immunofluorescence.
Approximately 105 SW-13 cells were seeded in a six-well plate and transduced with SMPU-18x21-EGFP and LV-Tax together in the next day. Forty-eight hours later, cells were fixed with freshly made formaldehyde (2% final concentration) for 10 min at room temperature followed by incubation with 3% bovine serum albumin (BSA) for 1 h at 4°C. After washing the cells in PBS for 3 times, a mouse antivimentin antibody (1:100 dilution in phosphate-buffered saline containing 3% BSA) was added to the well and incubated with the cells at 4°C overnight. After three washes with PBS (2 ml each time), the rhodamine-conjugated anti-mouse immunoglobulin G (1:200; Sigma) was applied at room temperature for 1 h. Cells were washed again with 2 ml of phosphate-buffered saline five times and observed with an Olympus IX81 fluorescence microscope.
RESULTS
Construction of 18x21-EGFP and E-sel-EGFP reporters using the SIN lentivirus vector SMPU.
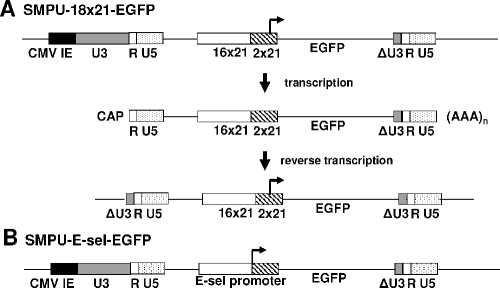
Using the HTLV-1 long terminal repeat as the backbone, we have previously derived a minimal HTLV-1 promoter that contains the HTLV-1 TATA element, the complete R region, and a part of the U5 region (13). Oligonucleotides representing the two promoter-proximal Tax-responsive 21-bp repeats were then synthesized and inserted upstream of the minimal HTLV-1 promoter (13). In this configuration, the promoter element has very low basal activity and is highly Tax responsive (13). To increase the Tax responsiveness of the promoter, eight additional duplications of the two synthetically derived 21-bp repeats were made and inserted upstream of the previously cloned 21-bp repeats to give rise to the 18x21 promoter. The 18x21 promoter cassette was then used to replace the CMV promoter upstream of the EGFP coding sequence in a SIN lentivirus vector, SMPU-CMV-EGFP, to produce SMPU-18x21-EGFP (Fig. 1A). Because most of the U3 region in the 3′ LTR of SMPU has been deleted, after reverse transcription and integration of the SMPU-18x21-EGFP vector, the only functional enhancer/promoter element in the integrated DNA is the Tax-responsive 18x21 promoter, which alone drives the expression of the EGFP reporter (Fig. 1A). In this configuration, the SMPU-18x21-EGFP vector is ideally suited for rapid detection of Tax expression and HTLV-1 infection. The ease of production and the high titer of the SIN vector-based reporter also greatly facilitate derivation of stable reporter cell lines for those purposes (see below).
FIG. 1.
Schematic diagrams of lentivirus vectors SMPU-18x21-EGFP and SMPU-E-sel-EGFP. In the SMPU vectors, the majority of the human immunodeficiency virus gag coding region and the complete coding region for pol, env, and accessory genes have been removed. The 5′ U3 region is replaced by the CMV enhancer/promoter, and a deletion is made in the 3′ U3 region (ΔU3 R). The reporter gene to be transduced, the EGFP gene in this case, is driven by the 18x21 (A) or E-selectin (E-sel) promoter cassette (B). Upon introduction of the reporter vector into a target cell, the U5 region in the 5′ LTR and the ΔU3 region in the 3′ LTR of the vector mRNA are duplicated through reverse transcription. The only functional enhancer/promoter element in the integrated DNA is the Tax-responsive 18x21 promoter or the E-sel promoter.
Similarly, to generate an NF-κB reporter, we amplified by PCR the 5′-proximal promoter region (positions −730 to +52) of the E-selectin gene and inserted it upstream of EGFP to yield SMPU-E-sel-EGFP (Fig. 1B) The E-selectin promoter contains three NF-κB binding sites and is highly responsive to NF-κB (40). As expected and as indicated below, this reporter is highly inducible by Tax and TNF-α.
The 18x21-EGFP and E-sel-EGFP reporter cassettes can be readily transduced by the SMPU vector and are highly Tax responsive.
To derive lentivirus vector particles for the transcriptional reporter cassettes, 293T cells were transiently transfected with the SMPU-18x21-EGFP or the SMPU-E-sel-EGFP construct together with two packaging plasmids, pCMVΔR8.2 and pCMV-VSV-G. pCMVΔR8.2 has env and vpr deleted, and as such, encodes all human immunodeficiency virus structural and accessory proteins except Env and Vpr. The plasmid pCMV-VSV-G allows lentiviral vector particles pseudotyped with the pantropic vesicular stomatitis virus G envelope to be produced. We next transduced the 18x21-EGFP reporter cassette into two Tax-expressing cell lines (MT4, an HTLV-1 transformed human T-cell line, and PX1, a mouse fibroblast cell line derived from a mouse transgenic for Tax) and two human cell lines not related to HTLV-1 (HeLa and Jurkat) by infection with the SMPU-18x21-EGFP vector. Forty-eight hours after gene transduction, the expression of EGFP in these cell lines was monitored under a fluorescence microscope. Approximately 90% of the MT4 cells and 20% of the PX1 cells became EGFP positive (Fig. 2A and B). As expected, no significant EGFP expression could be detected in HeLa or Jurkat cells (Fig. 2C and E). By contrast, when HeLa and Jurkat cells were transduced concurrently with both the reporter vector and a Tax lentivirus vector, LV-Tax, strong EGFP-expression was readily seen (Fig. 2D and F). These results indicate that EGFP expression as directed by the 18x21 enhancer/promoter cassette is exquisitely Tax dependent. In the absence of Tax, little or no basal transcription of EGFP is detectable. In the presence of Tax, potent transactivation occurred. The SMPU-18x21-EGFP virus titer of any given vector preparation is routinely estimated by counting the number of EGFP-positive cells 48 h after transduction of MT4 cells. Typically, a titer greater than or equal to 5 × 106/ml culture supernatant can be achieved. In aggregate, these results indicate that the 18x21-EGFP vector and 18x21-EGFP-transduced cells are uniquely suitable for detecting Tax expression and HTLV-1 infection.
FIG. 2.
The 18x21-EGFP reporter cassettes can be readily transduced by the SMPU vector and are highly Tax responsive. Four cell lines, MT4, PX1, HeLa, and Jurkat, were selected for transduction by SMPU-18x21-EGFP. MT4 is an HTLV-1-transformed human T-cell line that expresses Tax constitutively. PX1 is a fibroblast cell line derived from an LTR-tax transgenic mouse. PX1 also constitutively produces Tax. HeLa and Jurkat are cell lines unrelated to HTLV-1. Twenty-four hours before transduction, cells were seeded in six-well plates. The same amount of the reporter vector was then added to each cell line individually. EGFP expression was observed 48 h after transduction using an Olympus IX81 inverted fluorescence microscope. Panels on the bottom show fluorescence images of the transduced cells. Panels on the top are corresponding bright-field images. Cell lines used for panels A to F are as labeled. All were transduced with the SMPU-18x21-EGFP reporter. Cell lines in panels D and F were additionally transduced with LV-Tax, a lentivirus vector for HTLV-1 Tax.
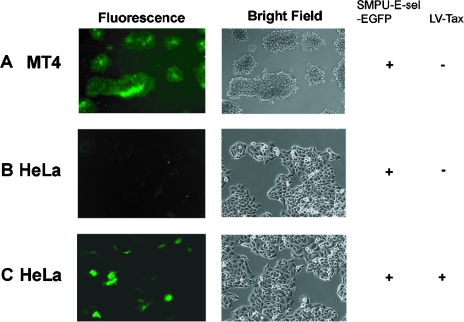
The SMPU-E-sel-EGFP reporter virus was similarly prepared and introduced into both MT4 and HeLa cells. As expected, the E-sel-EGFP reporter was specifically activated by Tax (Fig. 3A and C), but not in its absence (Fig. 3B), albeit the level of transcriptional activation by Tax is lower than that of the 18x21 reporter, as indicated by the intensity of EGFP signal (Fig. 3A and C). This result is consistent with the quantitative luciferase reporter assays, wherein the Tax-activated transcriptional activity of the HTLV-1 LTR was about 10-fold higher than that of the E-selectin promoter (see Fig. 4A and B).
FIG. 3.
Characterizations of the NF-κB reporter SMPU-E-sel-EGFP. SMPU-E-sel-EGFP vector particles were produced as described in Materials and Methods and analyzed similarly to the method described in the legend to Fig 2. Fluorescence images are shown on the left and the corresponding bright-field images on the right.
FIG. 4.

Transactivation of viral LTR and E-selectin promoter by Tax does not require BRG1. DNA transfections of SW-13 cells were carried out as described in Materials and Methods. The amounts of plasmids used in each transfection are as indicated. DNA transfection was done in triplicates. The averages of the luciferase reporter activities and standard deviations were calculated and plotted. The reporter and transactivators used for panels A, B, and C were Tax/LTR-Luc, Tax/E-sel-Luc, and Rta/K8-Luc, respectively.
Tax-mediated transactivation of chromosomally integrated 21-bp repeats does not require chromatin-remodeling factors, BRG1 and BRM1, and is not enhanced by BRG1.
The mechanism by which Tax activates viral transcription has been studied extensively. The viral transcriptional enhancer consists of three imperfect 21-bp repeats, each containing a CRE core flanked by 5′ G-rich and 3′ C-rich sequences. Works from many laboratories have shown that the CREs in the 21-bp repeats interact with the cellular bZip transcription factors CREB and ATF-1 and possibly other bZIP proteins. Tax, CREB/ATF-1, and the 21-bp repeats form stable ternary complexes (2, 44, 49, 58, 61) in which Tax binds the bZIP domains of CREB/ATF-1, stabilizes CREB/ATF1 dimers (1, 3, 38, 60), and makes contacts with the minor groove of the G/C-rich sequences that flank the CRE (9-11, 24, 28, 39, 40). Tax-DNA interaction in the ternary complex confers exquisite DNA sequence specificity to Tax-mediated LTR transactivation (7, 26, 27, 32, 33, 37, 45, 59). In the context of the ternary Tax-CREB/ATF-1-21-bp repeat complex, Tax further recruits transcriptional coactivators CREB-binding protein (CBP)/p300 and possibly other transcriptional coactivators for potent gene activation (5, 16, 17, 22, 29, 32).
Activation of eukaryotic transcription requires the establishment—around the transcriptional promoter—of a localized chromatin structure permissive for access by basal transcription factors and RNA polymerase II. Both HATs and chromatin-remodeling complexes have been shown to be critical for this process (9-11, 28, 39). It is thought that HATs such as CBP/p300, by adding acetyl groups to the N-terminal tails of histones, reduce the positive charges on histone tails, thereby releasing DNA from the repressive interaction of histones (4, 19, 20, 46, 48). Chromatin-remodeling complexes such as SWI/SNF, by contrast, can hydrolyze ATP and facilitate nucleosome sliding and histone octamer transfer to alter nucleosome structure for transactivation or repression (34, 35, 42). While the recruitment of CBP and/or p300 to the 21-bp repeats by Tax plays a critical role in transactivation, it is not clear whether ATP-dependent chromatin-remodeling factors such as BRG1 also participate in Tax-mediated transactivation. Since HTLV-1 provirus has been shown to integrate into the transcriptional control regions of cellular genes, it is conceivable that chromatin-remodeling may play a role in regulating viral gene expression.
To test the role of BRG1 in Tax-mediated transactivation, we transiently cotransfected reporter plasmids pLTR-Luc (containing the HTLV-1 LTR) and pE-selectin-Luc (an NF-κB reporter) with pCMV-Tax into the SW-13 cell line, a cell line deficient in chromatin-remodeling factors BRG1 and BRM1 (30). As indicated in Fig. 4A and B, Tax dramatically activated LTR and NF-κB reporters in SW-13 cells. The inclusion of BRG1 (pREP7-BRG1) in DNA transfection had no effect on Tax activity. Similar results were also obtained using another BRG1-null cell line, TSU-Pr1 (data not shown). It has been shown previously that the Kaposi's sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) immediate early transactivator Rta requires BRG1 for transactivation (14). By using this system as a positive control, we confirmed that in SW-13 cells, Rta only minimally affected expression of a luciferase reporter construct containing the Rta-response element derived from the HHV-8 K8 promoter. In contrast to LTR and NF-κB activation by Tax, the inclusion of pREP7-BRG1 expression plasmid significantly augmented Rta transactivation in SW-13 cells (Fig. 4C).
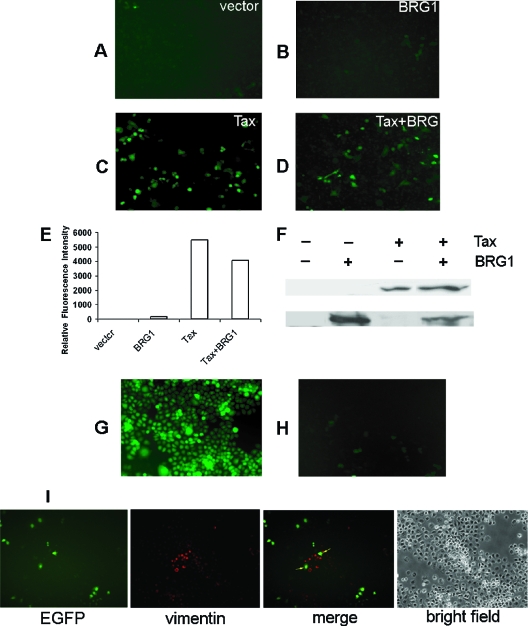
Because the DNA templates in transient transfection are not necessarily in chromatin configuration, we sought to test the transactivating activities of Tax on chromatin templates. To this end, SW-13 cells were transduced with the SMPU-18x21-EGFP reporter. Forty-eight hours later, cells were trypsinized and seeded in six-well plates at a density of 4 × 105 per well and secondarily transfected with pCMV-Tax and pREP7-BRG1 individually or in combination. As indicated in Fig. 5A and B, few EGFP-positive cells could be detected in the cell population transduced with the reporter alone or among the reporter-transduced cells that had been further transfected by BRG1. While transfection of Tax dramatically increased the number of EGFP-positive cells, no significant difference could be observed with (Fig. 5D) or without (Fig. 5C) the inclusion of pREP7-BRG1. To quantify Tax-induced EGFP expression as a function of BRG1, the fluorescence signals of individual cells detected by flow cytometry (Fig. 5C and 5D) were summed up directly and the background signal (from cells transduced with reporter only) subtracted. As shown in Fig. 5E, BRG1 had no detectable effect on Tax transactivation of the 18x21-EGFP reporter. It should also be pointed out that transfection of BRG1 did not increase the number of EGFP-positive cells or their individual fluorescence signals. The expression of Tax and BRG1 in transfected SW-13 cells was verified by immunoblotting (Fig. 5F).
FIG. 5.
Tax-mediated transactivation of chromosomally integrated 21-bp repeats does not require chromatin-remodeling factor BRG1 or BRM1. A total of 2.5 million SW-13 cells was seeded on a 10-cm plate to 30% confluence. Approximately 5 × 106 infectious units of SMPU-18x21-EGFP was added to the culture medium together with 8 μg/ml polybrene. After 48 h, transduced SW-13 cells were trypsinzed and reseeded in a six-well plate at a density of approximately 4 × 105 cells per well. Cells in each well were then transfected with pCMV-Tax (4 μg) and pREP7-BRG1 (4 μg) individually or in combination. Forty-eight hours later, cells were examined under a fluorescence microscope. Panels A to D represent SW-13 cells transduced with SMPU-18x21-EGFP vector only (labeled as “vector”) (A), cells similar to those in panel A but further transfected with pREP7-BRG1 (BRG1) (B), cells transfected with pCMV-Tax construct (Tax) (C), and cells cotransfected with both pCMV-Tax and pREP7-BRG1 (Tax+BRG1) (D). (E) The extent of EGFP expression of cells in panels A to D was determined by subjecting the cells to flow cytometry, followed by integrating the fluorescence signals (in arbitrary units) of all cells that have been sorted. The fluorescence signal corresponding to panel A (deemed background) was subtracted from the signal of each set in panels A to D, and the results were plotted. (F) Immunoblot analyses of cells in panels A to D. A rabbit polyclonal antibody against BRG1 (Santa Cruz Biotechnology, Inc.) and a mouse hybridoma Tax antibody 4C5 were used. Panels G and H show fluorescence images of an SW-13/18x21-EGFP stable cell line transduced with LV-Tax (G) and SMPU-SV40-Puro (H), respectively. (I) Cells were cotransduced with SMPU-18x21-EGFP and LV-Tax. Vimentin expression in SW-13 cells was detected by indirect immunofluorescence 48 h later. Cells expressing both vimentin and EGFP are marked with arrows.
It has been reported previously that commonly used SW-13 cells are not homogeneous with respect to BRG1 expression (56). Most SW-13 cells lack both BRG1 and BRM1 and, as a result, do not produce vimentin, whose expression is BRG1 dependent. A small fraction, however, express a low level of BRG1 and are vimentin positive as a result (56). To rule out the possibility that the EGFP expression detected was due solely to BRG-1-positive (and vimentin-positive) SW-13 cells, we first transduced SW-13 cells with SMPU-18x21-EGFP and LV-Tax and then detected vimentin expression by immunofluorescence as a surrogate for the presence of BRG1 in these cells. In agreement with published results, the fraction of vimentin-positive cells in the SW-13 cell culture is small. Importantly and as expected, strong EGFP expression was detected in vimentin-negative cells (Fig. 5I). Furthermore, the EGFP signals of those vimentin-positive (hence BRG1-positive) cells that became EGFP positive as a result of SMPU-18x21-EGFP and LV-Tax transduction (Fig. 5I) were not significantly different from those of their vimentin-negative counterparts, again suggesting that BRG1 is not important for Tax-mediated transactivation.
Finally, we established a stable SW-13 cell line containing the 18x21-EGFP reporter by transducing SW-13 cells with the SMPU-18x21-EGFP vector. One hundred or less reporter-transduced SW-13 cells were then seeded on a 10-cm plate and allowed to form colonies. Single clones were then picked and grown individually in 24-well plates. Because the titer of the SMPU-18x21-EGFP vector was sufficiently high, stable SW-13 clones containing the 18x21-EGFP cassette could be readily isolated. When such an SW-13/18x21-EGFP cell line—which, incidentally, was vimentin negative (not shown)—was transduced with a control retroviral vector SMPU-SV-Puro, little or no expression of EGFP could be detected (Fig. 5H). By contrast, when the cell line was transduced with LV-Tax, a strong induction of EGFP expression was observed (Fig. 5G), indicating that Tax-mediated transactivation of nucleosomal 18x21 promoter occurred readily in the absence of the BRG1-containing chromatin-remodeling complexes.
NF-κB transactivation does not require chromatin-remodeling factor BRG1 or BRM1.
Published data indicate that BRG1 is not required for NF-κB function (25). Because Tax activates NF-κB by causing constitutive phosphorylation and degradation of I-κB, it is expected that Tax-mediated activation of NF-κB-regulated promoters will be BRG1 independent. To demonstrate this, SW-13 cells were transduced with the SMPU-E-sel-EGFP lentivirus vector at a multiplicity of infection of approximately 2. The transduced cells expressed low but detectable levels of EGFP in the absence of any stimuli (Fig. 6A). Consistent with the notion that NF-κB transactivation is independent of chromatin-remodeling complexes, EGFP expression in the reporter-transduced SW-13 cells became dramatically increased when cotransduced with LV-Tax (Fig. 6B), and it was significantly induced after TNF-α treatment (Fig. 6C), as visualized by fluorescence microscopy (Fig. 6B and C) and quantified by flow cytometry (Fig. 6D and E).
FIG. 6.
Tax and NF-κB transactivates chromosomally integrated E-selectin promoter in the absence of BRG1 and BRM1. SW-13 cells were seeded in a six-well plate and transduced at a multiplicity of infection of 2 with SMPU-E-sel-EGFP reporter vector alone (untreated) (A). Forty-eight hours after transduction with the reporter vector, cells were concurrently cotransduced with LV-Tax vector (B) or treated with 20 ng/ml TNF-α (panel C). Both fluorescence and corresponding bright-field images of the cells are shown. (D) Flow cytometry of cells from panels A to C. Flow cytometry was carried out as detailed in Materials and Methods. (E) The relative levels of EGFP expression were determined as described in the legend to Fig. 5E, except the background fluorescence signal of untransfected and reporterless cells was subtracted.
DISCUSSION
In this study, we have derived two HTLV-1 reporters, SMPU-18x21-GFP and SMPU-E-sel-EGFP, based on the self-inactivating lentivirus vector SMPU. Cells transduced by these two reporter vectors produce minimal background EGFP signals, and their EGFP expression is highly Tax dependent. Both reporter vectors have been used to generate stable cell lines in a wide variety of backgrounds that allow easy detection of the transactivation activities of Tax (Fig. 2 and 3), NF-κB activation (Fig. 6), and HTLV infection (not shown). Although several mammalian cell lines containing HTLV-1 LTR-driven reporters (luciferase or EGFP) have been created before (21, 36), these earlier studies produced reporter cell lines by plasmid transfection followed by antibiotic selection. This approach is limited by the transfectability of the cell types of interest. To date, T-lymphocyte cell lines containing such reporters are lacking, and rapid detection of HTLV-1 infection of primary cells remains technically challenging. By contrast, the ease of use of the SMPU-18x21-EGFP and SMPU-E-sel-EGFP vectors is likely to facilitate detection of HTLV-1 infection and Tax functions in many settings. Furthermore, the SMPU-E-sel-EGFP vector can also be used to measure NF-κB activation by various extracellular or intracellular stimuli. Finally, reporter cassettes for specific promoters or for detecting infection by other viruses that express unique transcriptional activators can be easily derived using a similar strategy.
By itself, Tax does not bind DNA. For LTR transactivation, Tax acts by interacting with CREB/ATF-1 and by recruiting CBP/p300 to the Tax-responsive viral 21-bp repeats. Much of what we have learned about LTR transactivation by Tax relies on transient cotransfection of Tax and reporter plasmids. Because transiently transfected plasmids do not readily assume the nucleosome structure seen in chromosomally integrated genes, current understandings of Tax-mediated transactivation based on transient transfections may not completely reflect the regulation of stably integrated HTLV LTRs. More recently, Okada and Jeang (36) have constructed cell lines which contained an integrated HTLV LTR luciferase gene and found that there are different requirements for transactivation of transiently transfected versus stably integrated HTLV-1 LTR. By generating stable SW-13 cell lines carrying 18x21-EGFP and E-sel-EGFP, we now show that potent LTR and NF-κB activation by Tax occurs independently of chromatin-remodeling factors BRG1 and possibly BRM1. In agreement with this conclusion, similar results were obtained for TSU-Pr1, a bladder carcinoma cell line with a homozygous BRG1 deletion (data not shown). These results contrast with a previously published report which suggested that BRG1 interacts with Tax physically, and functionally assisted Tax-mediated LTR transactivation using C33A cells (50).
Although our results cannot rule out the possibility that other chromatin-remodeling factors may be involved in Tax-mediated transactivation, we think the assembly of a stable multiprotein complex containing Tax, CREB/ATF-1, and CBP/p300 on the 21-bp repeats is likely to be the principal mechanism utilized by Tax to preclude nucleosome formation at the HTLV-1 enhancer/promoter, and the recruitment of CBP/p300 and possibly other HATs and basal transcription factors by Tax to the HTLV-1 LTR appears to be responsible for achieving potent transcriptional activation. The HAT activity of CBP/p300, in turn, is expected to modify the nucleosome structure around the HTLV-1 enhancer/promoter such that ATP-dependent chromatin-remodeling complexes are not needed for initiating viral mRNA synthesis. A recent report showing that the assembly of Tax-CREB-p300 on the HTLV-1 LTR excluded nucleosome formation supports this conclusion (31). Our data also confirm the data from previous studies showing that mRNA transcription driven by NF-κB is independent of BRG1 chromatin-remodeling complexes (25), but KSHV Rta requires BRG1 for its transactivating function (14). Together, these results indicate that the transactivation mechanisms employed by Tax are fundamentally different from that adopted by other viral activators such as the KSHV/HHV-8 Rta. We noticed that the reporter activity of fully activated HTLV-1 LTR is 5 to 10 times that of the fully activated E-selectin promoter and 20 to 30 times that of the Rta-activated KSHV/HHV-8 K8 promoter. Whether the transcriptional strengths of the respective viral or cellular promoters determine the choice of CBP/p300-like HATs versus chromatin-remodeling complexes for transactivation is a question that remains to be answered.
Acknowledgments
We thank K. Zhao of the National Institutes of Health for the SW-13 cell line and the pREP7-BRG1 plasmid, S. J. Kim, also of the National Institutes of Health, for the TSU-Pr1 cell line, and Chunhua Chen for technical assistance.
This work was supported by grants RO1CA48709 and RO1CA/GM75688 from the National Institutes of Health.
REFERENCES
- 1.Adya, N., L. J. Zhao, W. Huang, I. Boros, and C. Z. Giam. 1994. Expansion of CREB's DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282-284 near the conserved DNA-binding domain of CREB. Proc. Natl. Acad. Sci. USA 91:5642-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, A. P., A. A. Franklin, M. N. Uittenbogaard, H. A. Giebler, and J. K. Nyborg. 1993. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc. Natl. Acad. Sci. USA 90:7303-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baranger, A. M., C. R. Palmer, M. K. Hamm, H. A. Giebler, A. Brauweiler, J. K. Nyborg, and A. Schepartz. 1995. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature 376:606-608. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, W. R., J. J. Hayes, J. H. White, and A. P. Wolffe. 1994. Nucleosome structural changes due to acetylation. J. Mol. Biol. 236:685-690. [DOI] [PubMed] [Google Scholar]
- 5.Bex, F., M. J. Yin, A. Burny, and R. B. Gaynor. 1998. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol. Cell. Biol. 18:2392-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu, Z. L., J. A. Di Donato, J. Hawiger, and D. W. Ballard. 1998. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J. Biol. Chem. 273:15891-15894. [DOI] [PubMed] [Google Scholar]
- 7.Datta, S., N. H. Kothari, and H. Fan. 2000. In vivo genomic footprinting of the human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat enhancer sequences in HTLV-1-infected human T-cell lines with different levels of Tax I activity. J. Virol. 74:8277-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Fuente, C., L. Deng, F. Santiago, L. Arce, L. Wang, and F. Kashanchi. 2000. Gene expression array of HTLV type 1-infected T cells: Up-regulation of transcription factors and cell cycle genes. AIDS Res. Hum. Retrovir. 16:1695-1700. [DOI] [PubMed] [Google Scholar]
- 9.Dilworth, F. J., and P. Chambon. 2001. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene 20:3047-3054. [DOI] [PubMed] [Google Scholar]
- 10.Falbo, K. B., and X. Shen. 2006. Chromatin remodeling in DNA replication. J. Cell Biochem. 97:684-689. [DOI] [PubMed] [Google Scholar]
- 11.Fry, C. J., and C. L. Peterson. 2001. Chromatin remodeling enzymes: who's on first? Curr. Biol. 11:R185-R197. [DOI] [PubMed] [Google Scholar]
- 12.Fu, D. X., Y. L. Kuo, B. Y. Liu, K. T. Jeang, and C. Z. Giam. 2003. Human T-lymphotropic virus type I tax activates I-kappa B kinase by inhibiting I-kappa B kinase-associated serine/threonine protein phosphatase 2A. J. Biol. Chem. 278:1487-1493. [DOI] [PubMed] [Google Scholar]
- 13.Giam, C. Z., and Y. L. Xu. 1989. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J. Biol. Chem. 264:15236-15241. [PubMed] [Google Scholar]
- 14.Gwack, Y., H. J. Baek, H. Nakamura, S. H. Lee, M. Meisterernst, R. G. Roeder, and J. U. Jung. 2003. Principal role of TRAP/mediator and SWI/SNF complexes in Kaposi's sarcoma-associated herpesvirus RTA-mediated lytic reactivation. Mol. Cell. Biol. 23:2055-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harhaj, E. W., L. Good, G. Xiao, and S. C. Sun. 1999. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene 18:1341-1349. [DOI] [PubMed] [Google Scholar]
- 16.Harrod, R., Y. L. Kuo, Y. Tang, Y. Yao, A. Vassilev, Y. Nakatani, and C. Z. Giam. 2000. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J. Biol. Chem. 275:11852-11857. [DOI] [PubMed] [Google Scholar]
- 17.Harrod, R., Y. Tang, C. Nicot, H. S. Lu, A. Vassilev, Y. Nakatani, and C. Z. Giam. 1998. An exposed kid-like domain in human t-cell lymphotropic virus type 1 tax is responsible for the recruitment of coactivators cbp/p300. Mol. Cell. Biol. 18:5052-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda, K., D. J. Steger, A. Eberharter, and J. L. Workman. 1999. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol. Cell. Biol. 19:855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, T., T. Ikehara, T. Nakagawa, W. L. Kraus, and M. Muramatsu. 2000. p300-mediated acetylation facilitates the transfer of histone H2A-H2B dimers from nucleosomes to a histone chaperone. Genes Dev. 14:1899-1907. [PMC free article] [PubMed] [Google Scholar]
- 21.Jewell, N. A., and L. M. Mansky. 2005. Construction and characterization of deltaretrovirus indicator cell lines. J. Virol. Methods 123:17-24. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, H., H. Lu, R. L. Schiltz, C. A. Pise-Masison, V. V. Ogryzko, Y. Nakatani, and J. N. Brady. 1999. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J. Biol. Chem. 274:17402-17405. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, C. N., N. L. Adkins, and P. Georgel. 2005. Chromatin remodeling complexes: ATP-dependent machines in action. Biochem. Cell Biol. 83:405-417. [DOI] [PubMed] [Google Scholar]
- 25.Kadam, S., G. S. McAlpine, M. L. Phelan, R. E. Kingston, K. A. Jones, and B. M. Emerson. 2000. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev. 14:2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimzey, A. L., and W. S. Dynan. 1999. Identification of a human T-cell leukemia virus type I tax peptide in contact with DNA. J. Biol. Chem. 274:34226-34232. [DOI] [PubMed] [Google Scholar]
- 27.Kimzey, A. L., and W. S. Dynan. 1998. Specific regions of contact between human T-cell leukemia virus type I Tax protein and DNA identified by photocross-linking. J. Biol. Chem. 273:13768-13775. [DOI] [PubMed] [Google Scholar]
- 28.Krebs, J. E., C. J. Fry, M. L. Samuels, and C. L. Peterson. 2000. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102:587-598. [DOI] [PubMed] [Google Scholar]
- 29.Kwok, R. P., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman. 1996. Control of camp-regulated enhancers by the viral transactivator tax through creb and the co-activator cbp. Nature 380:642-646. [DOI] [PubMed] [Google Scholar]
- 30.Leibovitz, A., W. M. McCombs III, D. Johnston, C. E. McCoy, and J. C. Stinson. 1973. New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J. Natl. Cancer Inst. 51:691-697. [PubMed] [Google Scholar]
- 31.Lemasson, I., N. J. Polakowski, P. J. Laybourn, and J. K. Nyborg. 2006. Tax-dependent displacement of nucleosomes during transcriptional activation of human T-cell leukemia virus, type 1. J. Biol. Chem. 281:13075-13082. [DOI] [PubMed] [Google Scholar]
- 32.Lenzmeier, B. A., H. A. Giebler, and J. K. Nyborg. 1998. Human T-cell leukemia virus type I Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol. Cell. Biol. 18:721-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundblad, J. R., R. P. Kwok, M. E. Laurance, M. S. Huang, J. P. Richards, R. G. Brennan, and R. H. Goodman. 1998. The human T-cell leukemia virus-1 transcriptional activator Tax enhances cAMP-responsive element-binding protein (CREB) binding activity through interactions with the DNA minor groove. J. Biol. Chem. 273:19251-19259. [DOI] [PubMed] [Google Scholar]
- 34.Muchardt, C., and M. Yaniv. 1993. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 12:4279-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muchardt, C., and M. Yaniv. 1999. ATP-dependent chromatin remodelling: SWI/SNF and Co. are on the job. J. Mol. Biol. 293:187-198. [DOI] [PubMed] [Google Scholar]
- 36.Okada, M., and K. T. Jeang. 2002. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 76:12564-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paca Uccaralertkun, S., L. J. Zhao, N. Adya, J. V. Cross, B. R. Cullen, I. M. Boros, and C. Z. Giam. 1994. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator, Tax. Mol. Cell. Biol. 14:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perini, G., S. Wagner, and M. R. Green. 1995. Recognition of bZIP proteins by the human T-cell leukaemia virus transactivator Tax. Nature 376:602-605. [DOI] [PubMed] [Google Scholar]
- 39.Pollard, K. J., and C. L. Peterson. 1998. Chromatin remodeling: a marriage between two families? Bioessays 20:771-780. [DOI] [PubMed] [Google Scholar]
- 40.Schindler, U., and V. R. Baichwal. 1994. Three NF-kappa B binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol. Cell. Biol. 14:5820-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 42.Shen, X., G. Mizuguchi, A. Hamiche, and C. Wu. 2000. A chromatin remodelling complex involved in transcription and DNA processing. Nature 406:541-544. [DOI] [PubMed] [Google Scholar]
- 43.Sun, S. C., and D. W. Ballard. 1999. Persistent activation of NF-kappaB by the tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene 18:6948-6958. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, T., J. I. Fujisawa, M. Toita, and M. Yoshida. 1993. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc. Natl. Acad. Sci. USA 90:610-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang, Y., F. Tie, I. Boros, R. Harrod, M. Glover, and C. Z. Giam. 1998. An extended alpha-helix and specific amino acid residues opposite the DNA-binding surface of the camp response element binding protein basic domain are important for human T cell lymphotropic retrovirus type I tax binding. J. Biol. Chem. 273:27339-27346. [DOI] [PubMed] [Google Scholar]
- 46.Tse, C., T. Sera, A. P. Wolffe, and J. C. Hansen. 1998. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol. Cell. Biol. 18:4629-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uhlik, M., L. Good, G. Xiao, E. W. Harhaj, E. Zandi, M. Karin, and S. C. Sun. 1998. NF-kappaB-inducing kinase and IkappaB kinase participate in human T-cell leukemia virus I Tax-mediated NF-kappaB activation. J. Biol. Chem. 273:21132-21136. [DOI] [PubMed] [Google Scholar]
- 48.Utley, R. T., K. Ikeda, P. A. Grant, J. Cote, D. J. Steger, A. Eberharter, S. John, and J. L. Workman. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394:498-502. [DOI] [PubMed] [Google Scholar]
- 49.Wagner, S., and M. R. Green. 1993. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science 262:395-399. [DOI] [PubMed] [Google Scholar]
- 50.Wu, K., M. E. Bottazzi, C. de la Fuente, L. Deng, S. D. Gitlin, A. Maddukuri, S. Dadgar, H. Li, A. Vertes, A. Pumfery, and F. Kashanchi. 2004. Protein profile of tax-associated complexes. J. Biol. Chem. 279:495-508. [DOI] [PubMed] [Google Scholar]
- 51.Xiao, G., M. E. Cvijic, A. Fong, E. W. Harhaj, M. T. Uhlik, M. Waterfield, and S. C. Sun. 2001. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 20:6805-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao, G., A. Fong, and S. C. Sun. 2004. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 279:30099-30105. [DOI] [PubMed] [Google Scholar]
- 53.Xiao, G., E. W. Harhaj, and S. C. Sun. 2000. Domain-specific interaction with the I kappa B kinase (IKK)regulatory subunit IKK gamma is an essential step in tax-mediated activation of IKK. J. Biol. Chem. 275:34060-34067. [DOI] [PubMed] [Google Scholar]
- 54.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 55.Xiao, G., and S. C. Sun. 2000. Activation of IKKalpha and IKKbeta through their fusion with HTLV-I tax protein. Oncogene 19:5198-5203. [DOI] [PubMed] [Google Scholar]
- 56.Yamamichi-Nishina, M., T. Ito, T. Mizutani, N. Yamamichi, H. Watanabe, and H. Iba. 2003. SW13 cells can transition between two distinct subtypes by switching expression of BRG1 and Brm genes at the post-transcriptional level. J. Biol. Chem. 278:7422-7430. [DOI] [PubMed] [Google Scholar]
- 57.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 58.Yin, M. J., and R. B. Gaynor. 1996. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol. Cell. Biol. 16:3156-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin, M. J., and R. B. Gaynor. 1996. Htlv-1 21 bp repeat sequences facilitate stable association between tax and creb to increase creb binding affinity. J. Mol. Biol. 264:20-31. [DOI] [PubMed] [Google Scholar]
- 60.Yin, M. J., E. Paulssen, J. Seeler, and R. B. Gaynor. 1995. Chimeric proteins composed of Jun and CREB define domains required for interaction with the human T-cell leukemia virus type 1 Tax protein. J. Virol. 69:6209-6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao, L. J., and C. Z. Giam. 1991. Interaction of the human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc. Natl. Acad. Sci. USA 88:11445-11449. [DOI] [PMC free article] [PubMed] [Google Scholar]