Abstract
Murine primary cells are poorly permissive to human immunodeficiency virus type 1 (HIV-1) vector infection. Retroviral infectivity is influenced by dominant inhibitors such as TRIM5α. Sensitivity to TRIM5α is altered by interactions between cyclophilin A and the HIV-1 capsid. Here we demonstrate that competitive inhibitors of cyclophilins, cyclosporine or the related Debio-025, stimulate HIV-1 vector transduction of primary murine cells, including bone marrow and macrophages, up to 20-fold. Unexpectedly, the infectivity of an HIV-1 mutant or a simian lentivirus that does not recruit cyclophilin A is also stimulated by these drugs. We propose that cyclosporine and related compounds will be useful tools for experimental infection of murine primary cells. It is possible that HIV-1 infection of murine cells is inhibited by dominant factors related to immunophilins.
Experiments with animals are often necessary for preclinical gene therapy studies, as well as for other vector applications. It would therefore be advantageous if lentiviral vectors could transduce nonhuman cells as efficiently as they can human cells, particularly those from monkeys and mice. However, human immunodeficiency virus type 1 (HIV-1), macaque simian immunodeficiency virus (SIVmac), and equine infectious anemia virus vectors clearly exhibit distinct species specificity, even if entry blocks are overcome by pseudotyping (10, 11). Importantly, HIV-1 vectors poorly transduce simian and murine cells (10, 11).
A significant factor controlling HIV-1 permissivity in nonhuman primate cells is the antiviral restriction factor TRIM5α (24). TRIM5α blocks viral infection early after entry, usually before significant reverse transcription (24). The viral determinants of sensitivity reside in the capsid protein (4, 6, 7, 9, 14, 16, 20). The mechanism of restriction remains unclear, but the simplest model is a direct interaction between incoming capsid and TRIM5α that leads to perturbation of the rearrangement of the viral core. HIV-1 permissivity is influenced by drugs, including cyclosporine (CSA), and capsid mutations that prevent interactions between the host protein cyclophilin A (CypA) and the viral capsid (25). Preventing such interactions tends to increase HIV-1 infectivity in nonhuman primate cells but reduces infectivity in human cells. A possible explanation for these observations is that CypA binding alters the availability of the capsid for restriction factor binding (2, 5, 13, 15). HIV-1 vector transduction of rodent cells is also inhibited at a postentry, preintegration step (1, 8). Here we investigate the effect of CSA on HIV-1 vector transduction of mouse primary cells.
Maturation-dependent block of HIV-1 in murine primary cells.
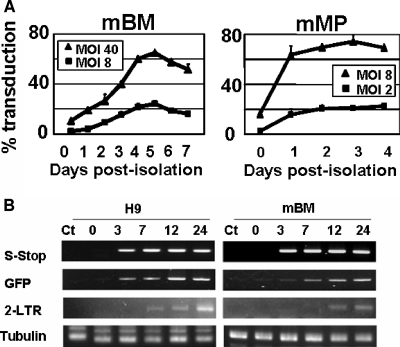
Recently, we showed that HIV-1 vectors can be used to transduce mouse bone marrow (BM)-derived premature dendritic cells (DC) and that the lentivirally transduced DC can elicit potent antigen-specific immune responses (21). During the study, we noticed that freshly isolated mouse BM cells were highly resistant to HIV-1 vector transduction. We thus first examined the block of HIV-1 vector transduction in mouse BM cells. Mouse BM was collected from the tibias and femurs of female BALB/c and C57BL/6 mice (2 to 4 months old) and cultured as described previously (21). BALB/c BM cells were allowed to differentiate in culture with granulocyte-macrophage colony-stimulating factor. Vesicular stomatitis virus G (VSV-G)-pseudotyped HIV-1 vector, which encoded a green fluorescent protein (GFP) gene driven by a spleen focus-forming virus promoter, was prepared as previously described (4) and concentrated by ultracentrifugation (23). On various days postisolation, cells were exposed to the HIV-1 vector at doses equivalent to multiplicities of infection (MOI) of 8 and 40 on 293T cells, referred to as 293T MOI. As shown in Fig. 1A, freshly isolated BM cells were refractory to transduction by wild-type HIV-1 GFP. When the vectors were used at a 293T MOI of 8 and 40, less than 2 and 15%, respectively, of the cells became transduced. Increasing the dose to a 293T MOI of >120 resulted in a small nonlinear increase in transduction to 22% (data not shown). However, after 4 days of culture, the BM-DC became progressively more susceptible to infection. Infection at doses of 293T MOI of 8 and 40 resulted in transduction of above 20 and 60% of cells, respectively (Fig. 1A). Similar patterns of maturation-dependent block were observed in BM cells derived from a different mouse strain C57BL/6 (data not shown).
FIG. 1.
Maturation-dependent block of HIV-1 in murine primary cells. (A) The block of HIV-1 vector transduction in mouse primary cells was examined. BM-derived DC and peritoneal macrophages were exposed to VSV-G-pseudotyped HIV-1 GFP-encoding vector at 293T MOI of 8 and 40 at various time points. The GFP-positive cell populations were analyzed by FACSA 7 days after infection. (B) Semiquantitative PCR of DNA synthesis in mouse BM cells. HIV GFP vector was pretreated with DNase for 40 min at 37°C in the presence of 1 mM Mg2+. Freshly isolated mouse BM cells, as well as human H9 cells, were infected with HIV GFP vector at a 293T MOI of 2. At the indicated time points, total DNA was extracted, and 100 ng of total DNA was subjected to semiquantitative PCR. The primer pairs used were as follows: 5′-GGGTCTCTCTGGTTAGACCAGATCT-3′ and 5′-TGCTGCTAGAGATTTTCCACACTGA-3′ for strong stop DNA, 5′-GCAAGCTGCCCGTGCCCTGGCCC-3′ and 5′-ATGTTGTGGCGGATCTTGAAGTT-3′ for GFP, 5′-AGATCTGGTCTAACCAGAGAGACCC-3′ and 5′-GAGCCTGGGAGCTCTCTGGCTAACT-3′ for 2LTR circle, and 5′-AAGAAGTCCAAGCTGGAGTTC-3′ and 5′-GTTGGTCTGGAATTCTGTCAG-3′ for alpha-tubulin.
Next, we examined a second primary cell type, peritoneal macrophages. Peritoneal macrophages were harvested from the peritoneal cavity, and adherent macrophages were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum. When freshly isolated cells were infected at a 293T MOI of 8 and 40, ca. 10 and 40% of cells, respectively, became transduced (Fig. 1A). Thus, the HIV-1 vector could transduce freshly isolated mouse peritoneal macrophages more efficiently than freshly isolated mouse BM cells. However, similar to BM-DC transduction, the macrophages became more susceptible to infection after 1 day of culture. When the cells were infected at 1 day after isolation, more than 90% of the cells were transduced at a 293T MOI of 8 (Fig. 1A).
Next, we analyzed whether reverse transcription of vector genomes could occur in freshly isolated BM cells. Freshly isolated BM cells were infected with the GFP-carrying vector pseudotyped with VSV-G at a 293T MOI of 2. We used a human lymphoid cell line, H9, as a permissive cell control. At 0, 3, 7, 12, and 24 h after exposure to vector, cells were harvested. Semiquantitative PCR was performed to detect a reverse transcription intermediate as described previously (18). PCR products of GFP sequence increased over time in both H9 and mouse BM cells (Fig. 1B). However, appearance of 2-LTR circles was delayed in BM cells. These data suggest that the block to transduction in mouse BM cells is at a level between the completion of reverse transcription and nuclear entry.
Block of HIV-1 infection in murine BM cells is eliminated by treatment with CSA.
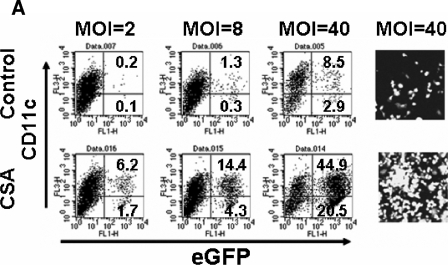
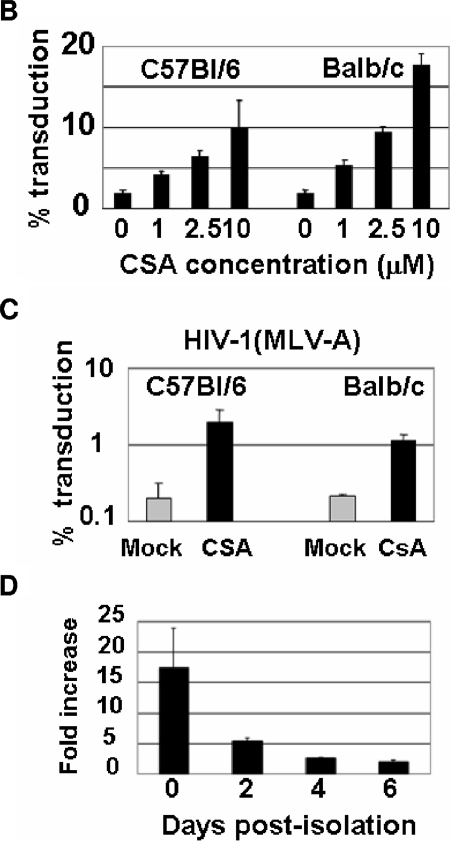
We assumed that the resistance to HIV-1 infection in freshly isolated mouse primary cells might occur by similar mechanisms to Lv1 restriction in primate cells. We therefore examined the effect of CSA treatment on the vector transduction efficiency of mouse BM cells. CSA was dissolved in dimethyl sulfoxide to prepare a 50 mM stock solution. Freshly isolated BM cells were incubated with CSA at a final concentration of 10 μM during an overnight infection. Equivalent amounts of dimethyl sulfoxide were added as a no-CSA control. The cells were further maintained for 7 days in growth medium. Ten days after infection, GFP-positive cells were counted by fluorescence-activated cell sorting (FACSA). CSA treatment increased the transduction efficiency by more than 20 times (Fig. 2A). There was no significant difference in levels of CD11c expression between CSA-treated and nontreated cells at 10 days after infection (Fig. 2A). Moreover, we did not see any difference in morphology between CSA-treated and nontreated cells at 12 days after infection, suggesting that CSA treatment did not affect normal DC maturation. The effects of CSA were dose dependent, and similar results were observed with BM cells from BALB/c and C57BL/6 mice (Fig. 2B). CSA also enhanced the transduction of BM cells by HIV-1 vectors pseudotyped with the MLV-A envelope, which, unlike the pH-dependent VSV-G, fuses at the cell surface (Fig. 2C). This observation indicated that the effect of CSA was not affected by route of entry.
FIG. 2.
Block of HIV-1 infection in murine BM cells is eliminated by treatment with CSA. (A) Freshly isolated mouse BM cells were infected with HIV-1 vector at 293T MOI of 2, 8, and 40 in the presence or absence of CSA (10 μM). Ten days after infection, CD11c- and GFP-positive DC populations were analyzed by FACSA with Cy5-conjugated monoclonal antibody to mouse CD11c. GFP-positive cells were also analyzed by fluorescence microscopy. (B) BM cells from BALB/c and C57BL/6 mice were infected with HIV-1 vector at 293T MOI of 8 in the presence of various concentrations of CSA. Since prolonged treatment with CSA at a concentration of 10 μM showed some toxicity, the CSA-containing media were replaced with complete media after overnight infection. (C) Freshly isolated BM cells were infected with a HIV-1 vector pseudotyped with MLV amphotropic Env [HIV-1(MLV-A)] in the presence or absence of CSA (2.5 μM). HIV-1(MLV-A) vector was prepared from stable producer line, 293T-GPRT1+R1-Ampho-HV#2 (12). (D) Freshly isolated BM and BM-derived DC were infected with the HIV-1 vector at MOI of 2 in the presence of 10 μM CSA at the indicated time points. The GFP-positive cell populations were analyzed 7 days after infection.
Next, we examined the effects of CSA on HIV-1 vector infection of freshly isolated peritoneal macrophages. The cells were infected by HIV-1 GFP in the presence or absence of CSA. The GFP-positive populations were counted by FACSA analysis 6 days after infection. CSA treatment improved HIV-1 infectivity of the macrophage culture by up to 15-fold. We also tested the effect of CSA treatment on the transduction of primary kidney cells; CSA treatment had no significant effect on the transduction efficiency (data not shown), suggesting that the effects of CSA are tissue specific. We then sought to determine whether CSA enhanced the transduction efficiency of HIV-1 vectors in BM-derived cells after prolonged culture in vitro. Enhancement of the vector transduction efficiency by CSA was maturation dependent in that as the cells remained in culture they became more permissive to HIV-1 infection and less sensitive to the enhancing effects of CSA (Fig. 2D).
Previously, we have reported a similar maturation-dependent block of HIV-1 in human monocytes (18). We therefore examined whether CSA could remove the block of HIV-1 vector transduction in human monocytes, although CSA generally reduces HIV-1 transduction in human cells (25). Freshly isolated human monocytes were cultured in the RPMI growth medium supplemented with autologous human serum. The cells were incubated with CSA at final concentrations of 0, 0.6, 1.2, 2.5, and 5.0 μM during an overnight infection at a 293T MOI of 10. The cells were further maintained for 7 days in the growth medium with 10% human serum. Seven days after infection, GFP-positive cells were analyzed. As reported previously, freshly isolated monocytes were refractory to HIV-1-based vector transduction, and only 2.5 to 3.5% of the cells became GFP positive in the absence of CSA. CSA treatment at 0.6 μM further reduced the vector transduction by >20-fold, whereas no GFP-positive cells were detected in the cultures treated with CSA at concentrations higher than 1.2 μM (data not shown). Clearly, CSA could not remove the maturation-dependent postentry block to HIV-1 in human monocytes.
The block of HIV-1 in murine BM cells is not saturable with virus-like particles.
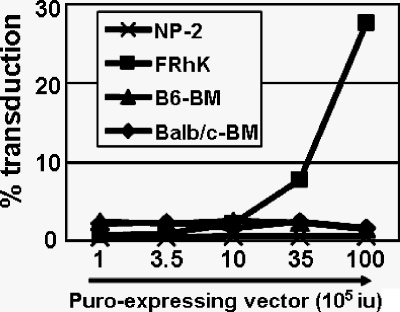
We examined whether the resistance in mouse BM cells could be saturated by high levels of virus particles. Freshly isolated mouse BM cells from BALB/c and C57BL/6 mice, rhesus monkey FRhK4 cells, and human NP2 cells were coinfected with fixed amount of GFP-expressing HIV-1 vector and increasing amounts of HIV-1 vector expressing puromycin resistance gene (puro). Although coinfection with high levels of the puro-expressing vector resulted in a 30-fold increase in GFP vector infectivity in monkey FRhK4 cells, no increase in infectivity was observed in mouse BM cells by such coinfection (Fig. 3).
FIG. 3.
The block of HIV-1 in murine BM cells is not saturable with virus-like particles. Human NP2, rhesus macaque FRhK-4, and mouse BM cells were coinfected with GFP- and puro-expressing HIV-1 vectors. The percentage of infection was measured by analysis of GFP expression 72 h later (cell lines) and 7 days later (BM cells) by FACSA.
Abrogation of block by CSA is independent of capsid CypA interactions and calcineurin inhibition.
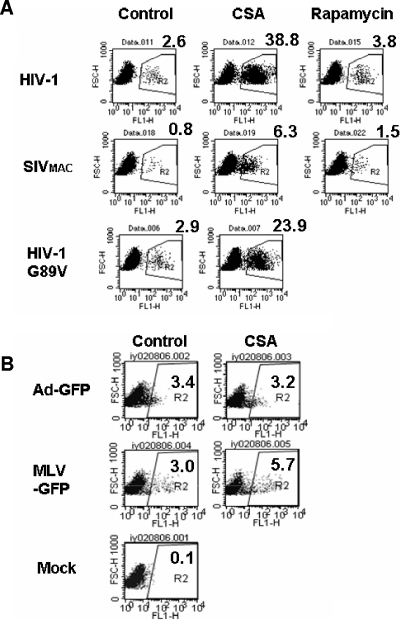
Next, we examined the effects of CSA on the SIVmac-based vector, which does not incorporate CypA into viral particles. SIVmac251-derived vector plasmids, pSIV3+ and pSIV-GFP, were kindly provided by F.-L. Cosset, ENS de Lyon (17). CSA treatment enhanced the transduction of BM cells by SIVmac vectors by ∼6-fold (Fig. 4A). This suggested that the stimulation of infectivity is independent of capsid-CypA interactions. We therefore tested whether the prevention of capsid-CypA interactions in HIV-1 is responsible for the transduction enhancement by CSA treatment in mouse BM cells. We used the HIV-1 vector with G89V capsid mutation, which prevents CypA incorporation into HIV-1 capsid (27). Intriguingly, the G89V mutant was still sensitive to CSA treatment, indicating that direct binding of CypA to the viral capsid does not strongly affect the block in mouse BM cells (Fig. 4A). In contrast, CSA did not affect the transduction of mouse BM cells by a GFP-expressing adenoviral vector and only marginally (up to 2.5-fold) enhanced the transduction by a MLV-based vector (Fig. 4B).
FIG. 4.
Abrogation of the block by CSA is independent of capsid- CypA interactions and calcineurin inhibition. (A) Effects of CSA on lentiviral infection in mouse BM cells. Freshly isolated BM cells were infected with HIV-1, SIVmac, and HIV-1 G89V capsid mutant vectors in the presence of CSA (5 μM). Rapamycin (2.5 nM) was used as a control in infection with HIV and SIVmac vectors. GFP-positive populations were analyzed by FACSA 12 days after infection. (B) Freshly isolated BM cells were infected with adenoviral (Ad-GFP) and retroviral (MLV-GFP) vectors in the presence or absence of CSA (5 μM). GFP-positive populations were analyzed by FACSA 5 days after infection. (C) Effects of a nonimmunosuppressive CSA-related drug, Debio-025, on HIV-1 vector infection in mouse BM and spleen-derived T cells. Freshly isolated BM cells and concanavalin A-stimulated spleen-derived T cells from BALB/c mice were infected with HIV-1 vectors in the presence of the indicated concentrations of Debio-025 or CSA. GFP-positive populations were analyzed by FACSA 6 days after infection.
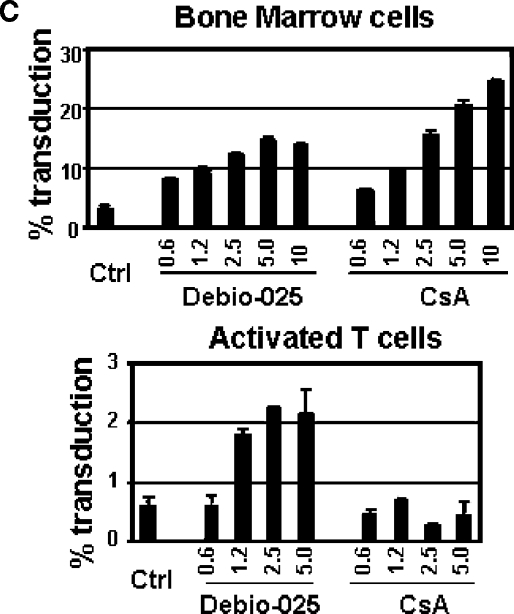
CSA not only prevents CypA-capsid interactions but also binds to calcineurin in a complex with CypA and inhibits its ability to activate NFAT. In order to dissociate these two effects of CSA, we used a CSA-related drug, Debio-025. Debio-025 prevents CypA-capsid interaction (5) but does not cause immunosuppression. Similar to CSA treatment, the Debio-025 treatment enhanced HIV-1 vector infectivity in mouse BM cells, suggesting that the effects of CSA on HIV-1 vector infectivity in mouse cells is independent of its inhibition of calcineurin. We also examined the effects of CSA and Debio-025 treatment on HIV-1 transduction of primary murine T cells. Although CSA did not enhance HIV-1 vector transduction in the cells, possibly due to its strong cytotoxicity in T cells, Debio-025 increased HIV-1 infectivity up to fourfold (Fig. 4C).
The infectivity of retroviruses, and of retroviral vectors derived from them, is restricted by the expression of antiviral host factors. It is likely that a greater understanding of the block and viral sensitivity to the block will allow improvement of vectors designed to deliver therapeutic genes. TRIM5α, also known as Lv1, is an important restriction factor in primates, active against HIV-1. Sensitivity to TRIM5α is affected by drugs that block interactions between the HIV-1 capsid and the cellular prolyl isomerase, CypA (3, 13). Here we report that HIV-1 vector transduction of murine cells, including freshly isolated primary murine cells, can be dramatically increased by CSA treatment. The resistance to HIV-1 vectors in primary cells decreased after culture in vitro and differential CSA sensitivity was observed among cells from different organs from the same mouse. These results suggest the presence of tissue-specific restriction factors active against HIV-1, which are regulated during differentiation. We assume that the resistance of murine cells to HIV-1 is due to restriction factor(s), rather than to a lack of essential positive factors, because it is unlikely that CSA and Debio-025 can substitute for essential factors normally lacking in murine cells (Fig. 4C).
Treatment of owl monkey cells with CSA increases HIV-1 titer 100 to 1,000 times, as does mutation of the CypA binding site in the HIV-1 capsid with the G89V mutation (25). In owl monkeys there is a fusion between the primate restriction factor TRIM5 and CypA (19, 22) called TRIM-Cyp. It appears that the restriction activity of the TRIM domain of TRIM-Cyp is recruited to the incoming HIV-1 capsid by the CypA domain (26). Drugs or mutations that block CypA interacting with the capsid, such as capsid G89V, also block TRIM-Cyp interactions and therefore rescue the infectivity of HIV-1 in owl monkey cells. Here we show that in murine cells CSA can also increase HIV-1 infectivity. However, in this case the infectivity of the HIV-1 G89V mutant is also increased by drug treatment. This result suggests that the effect of CSA on HIV-1 infectivity in mouse BM cells is independent of interactions between the HIV-1 capsid and CypA, at least at the site that recruits CypA into HIV-1 particles. Intriguingly CSA also enhanced the transduction efficiency of SIVmac-based vectors, which, like HIV-1 G89V, do not incorporate CypA into viral particles. These observations suggest a rescue mechanism that is independent of capsid-CypA interactions and possibly of CypA itself. The drug CSA is known to bind a range of immunophilins, and it may be that a murine immunophilin, related to CypA, which can bind to the wild-type HIV-1 capsid, the G89V mutant, and SIVmac, is involved in inhibiting infection. How CSA rescues HIV-1 infectivity in murine cells remains unclear. It may be related to previously described capsid-dependent restriction of SIVmac in murine cells (8).
Experiments with animals are essential for preclinical gene therapy studies, as well as other lentiviral vector applications. Efficient transduction of nonhuman cells, especially those from monkeys and mice, would be advantageous. The addition of CSA at the time of infection may represent an easy addition to protocols aiming to efficiently transduce simian or murine cells. CSA-related, nonimmunosuppressive drugs may be particularly useful for increasing infectivity of systemically administered lentiviral vectors.
Acknowledgments
We thank Zuzana Keckesova and Yasuhiro Takeuchi, University College London for reagents, helpful discussion, and encouragement.
Cancer Research UK, the Wellcome Trust, the Medical Research Council UK, the Biotechnology and Biological Sciences Research Council, and the Mayo Foundation supported this study.
REFERENCES
- 1.Baumann, J. G., D. Unutmaz, M. D. Miller, S. K. Breun, S. M. Grill, J. Mirro, D. R. Littman, A. Rein, and V. N. KewalRamani. 2004. Murine T cells potently restrict human immunodeficiency virus infection. J. Virol. 78:12537-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2005. Cyclophilin A is required for TRIM5α-mediated resistance to HIV-1 in Old World monkey cells. Proc. Natl. Acad. Sci. USA 102:14849-14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2004. Lv1 inhibition of human immunodeficiency virus type 1 is counteracted by factors that stimulate synthesis or nuclear translocation of viral cDNA. J. Virol. 78:11739-11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterji, U., M. D. Bobardt, R. Stanfield, R. G. Ptak, L. A. Pallansch, P. A. Ward, M. J. Jones, C. A. Stoddart, P. Scalfaro, J. M. Dumont, K. Besseghir, B. Rosenwirth, and P. A. Gallay. 2005. Naturally occurring capsid substitutions render HIV-1 cyclophilin a independent in human cells and trim-cyclophilin resistant in owl monkey cells. J. Biol. Chem. 280:40293-40300. [DOI] [PubMed] [Google Scholar]
- 6.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorfman, T., and H. G. Gottlinger. 1996. The human immunodeficiency virus type 1 capsid p2 domain confers sensitivity to the cyclophilin-binding drug SDZ NIM 811. J. Virol. 70:5751-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatziioannou, T., S. Cowan, and P. D. Bieniasz. 2004. Capsid-dependent and -independent postentry restriction of primate lentivirus tropism in rodent cells. J. Virol. 78:1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda, Y., M. K. Collins, P. A. Radcliffe, K. A. Mitrophanous, and Y. Takeuchi. 2002. Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther. 9:932-938. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda, Y., Y. Takeuchi, F. Martin, F. L. Cosset, K. Mitrophanous, and M. Collins. 2003. Continuous high-titer HIV-1 vector production. Nat. Biotechnol. 21:569-572. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, Y., L. M. Ylinen, M. Kahar-Bador, and G. J. Towers. 2004. Influence of gag on human immunodeficiency virus type 1 species-specific tropism. J. Virol. 78:11816-11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kootstra, N. A., C. Munk, N. Tonnu, N. R. Landau, and I. M. Verma. 2003. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. USA 100:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mische, C. C., H. Javanbakht, B. Song, F. Diaz-Griffero, M. Stremlau, B. Strack, Z. Si, and J. Sodroski. 2005. Retroviral restriction factor TRIM5α is a trimer. J. Virol. 79:14446-14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negre, D., P. E. Mangeot, G. Duisit, S. Blanchard, P. O. Vidalain, P. Leissner, A. J. Winter, C. Rabourdin-Combe, M. Mehtali, P. Moullier, J. L. Darlix, and F. L. Cosset. 2000. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther. 7:1613-1623. [DOI] [PubMed] [Google Scholar]
- 18.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 75:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nisole, S., C. Lynch, J. P. Stoye, and M. W. Yap. 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. USA 101:13324-13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens, C. M., P. C. Yang, H. Gottlinger, and J. Sodroski. 2003. Human and simian immunodeficiency virus capsid proteins are major viral determinants of early, postentry replication blocks in simian cells. J. Virol. 77:726-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmowski, M. J., L. Lopes, Y. Ikeda, M. Salio, V. Cerundolo, and M. K. Collins. 2004. Intravenous injection of a lentiviral vector encoding N.Y.-ESO-1 induces an effective CTL response. J. Immunol. 172:1582-1587. [DOI] [PubMed] [Google Scholar]
- 22.Sayah, D. M., E. Sokolskaja, L. Berthoux, and J. Luban. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569-573. [DOI] [PubMed] [Google Scholar]
- 23.Strang, B. L., Y. Takeuchi, T. Relander, J. Richter, R. Bailey, D. A. Sanders, M. K. Collins, and Y. Ikeda. 2005. Human immunodeficiency virus type 1 vectors with alphavirus envelope glycoproteins produced from stable packaging cells. J. Virol. 79:1765-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 25.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9:1138-1143. [DOI] [PubMed] [Google Scholar]
- 26.Yap, M. W., S. Nisole, and J. P. Stoye. 2005. A single amino acid change in the SPRY domain of human TRIM5α leads to HIV-1 restriction. Curr. Biol. 15:73-78. [DOI] [PubMed] [Google Scholar]
- 27.Yoo, S., D. G. Myszka, C. Yeh, M. McMurray, C. P. Hill, and W. I. Sundquist. 1997. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. J. Mol. Biol. 269:780-795. [DOI] [PubMed] [Google Scholar]