Abstract
Viruses have to adjust to the host cell to guarantee their life cycle and survival. This aspect of the virus-host cell interaction is probably performed by viral proteins, such as serine-threonine kinases, that are present early during infection. Vaccinia virus has an early Ser-Thr kinase, B1R, which, although required for successful viral infection, is poorly characterized regarding its effects on cellular proteins, and thus, its potential contribution to pathogenesis is not known. Signaling by mitogen-activated protein kinase (MAPK) is mediated by the assembly of complexes between these kinases and the JIP scaffold proteins. To understand how vaccinia virus B1R can affect the host, its roles in the cellular signaling by MAPK complexes and c-Jun activation have been studied. Independently of its kinase activity, B1R can interact with the central region of the JIP1 scaffold protein. The B1R-JIP1 complex increases the amount of MAPK bound to JIP1; thus, MKK7 and TAK1 either bind with higher affinity or bind more stably to JIP1, while there is an increase in the phosphorylation state of JNK bound to JIP1. The functional consequence of these more stable interactions is an increase in the activity of transcription factors, such as c-Jun, that respond to these complexes. Furthermore, B1R is also able to directly phosphorylate c-Jun in residues different from those targeted by JNK and, thus, B1R can also cooperate by an independent route in c-Jun activation. Vaccinia virus B1R can thus modulate the signaling of pathways that respond to cellular stress.
Viral infection represents a major cellular stress, and therefore it is likely that stress pathways are activated or modulated whenever an infection occurs. The activation of stress responses aims to get rid of the infection, but as a consequence of evolution, an adaptation between virus and host cells has been selected to allow the survival of the virus. To achieve this survival, interactions between viral and cellular proteins with roles in the signaling pathway must occur and are most likely mediated by early viral proteins; otherwise, the cell might get rid of the virus before infection progression. Thus, the interaction of an early viral protein with host signals might be an important component of strategies for achieving viral survival. The vaccinia virus has a large genome of 200 kb and is the prototype virus of the poxvirus family, which includes the smallpox, variola, and ectromelia viruses (26). Among its early viral proteins is B1R, a serine-threonine kinase that is present in infecting virions and required for viral DNA synthesis, as shown by the phenotype of two temperature-sensitive mutants that each express a very labile B1R protein without kinase activity (2, 23). However, B1R must be involved in other viral processes since, in permissive conditions, B1R mutants show 60% of the viral DNA replication of normal virus but only 15% of virus production (2). Also, it was impossible to generate a virus lacking the B1R open reading frame, which shows that it is an essential gene even without kinase activity (2), probably because of its ability to modulate other proteins by a direct interaction. So far, the identification of B1R substrates, either viral or cellular, is very limited; among its viral protein substrates is H5R (3), and among the cellular substrates are ribosomal proteins (1, 4), BAF (30), and p53, which is hyperphosphorylated, triggering a downregulation of apoptotic signals (37) that could contribute to the survival of infected cells by transiently interfering with the cellular stress response and thus allowing the course of the infection to progress.
Poxvirus can modulate the cell response to inflammatory cytokines (22, 27, 38) and apoptosis (12, 44). Recently, the F1L protein emerged as a key protein in the inhibition of host apoptosis due to its ability to interact with Bak and prevention of the mitochondrion-dependent activation of apoptosis (13, 32, 50) even in absence of other vaccinia antiapoptotic proteins such as SPI-2 (51). However, F1L expression is detected at high levels only 4 h after infection (32). Therefore, the virus should have a way of preventing host apoptosis in the early steps of the infection. B1R is a good candidate to perform this role since it is present in virions that enter the cells and is expressed 1 h after infection. In addition, B1R is a kinase and can therefore mediate fast responses by phosphorylating cellular proteins.
Cellular responses to different types of stress are mediated by a variety of signaling pathways where the mitogen-activated protein kinases (MAPKs) are major components. MAPK pathways are formed by a group of three consecutive kinases, and for each step, there are several kinases, thus permitting large flexibility in the modulation and types of effects mediated by the MAPK routes (8). In some systems, the three kinases are assembled in a complex by a scaffold protein of the JIP family (21, 53). Many of these signals converge on the transcription factor c-Jun, a major component of the AP1 complex (41, 52). In different types of infections, there is a functional interaction between viral proteins and the MAPK signaling pathways. The LMP1 protein of the Epstein-Barr virus activates the TAK1-MKK4/7-JNK route (11, 49). The protein X of hepatitis B virus is able to activate c-Jun-dependent transcription and protects cells from apoptosis by activating the JNK pathway (10). Also, in cells infected with varicella-zoster virus (34, 35), herpesvirus (25), and cytomegalovirus (7), there is an activation of MAPK. Therefore the activation of MAPK seems to be a common target for different viruses in order to guarantee their life cycles.
In this report, we have examined the effect of B1R on the formation of the signaling complex assembled by JIP1 and the activation of c-Jun-dependent transcription. B1R interacts stably with JIP1 and increases the interaction of this protein with a MAPKKK and a MAPKK, resulting in the activation of JNK by phosphorylation and the consequent activation of the c-Jun transcriptional response at a level consistent with survival signals.
MATERIALS AND METHODS
Kinase assay.
The in vitro kinase activity was determined by assaying protein phosphorylation in a mixture containing 20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 0.5 mM dithiothreitol, 150 mM KCl, and 5 μM (5 μCi) [γ-32P]ATP with 5 μg of glutathione S-transferase (GST)-JIP1 or GST-c-Jun as a substrate and immunoprecipitated hemagglutinin (HA)-B1R expressed in Cos1 or 1 μg of GST-B1R expressed in bacteria as a kinase. The reaction was performed for 1 h at 30°C (24, 28, 29).
Tryptic phosphopeptide map analysis.
GST-c-Jun and GST-c-Jun S63/73A proteins were phosphorylated in vitro by B1R (bacterially expressed and then separated from GST by thrombin digestion), followed by transfer to an Immobilon-P membrane (Millipore, Bedford, MA). The band with the phosphorylated protein was excised and incubated with 0.5% polyvinyl-pyrrolidone in 100 mM acetic acid for 30 min at 37°C, followed by extensive washes with water and fresh 50 mM NH4HCO3. The tryptic map was determined as previously reported (24, 39, 40).
Plasmids.
The B1R constructs pGEX-4T-B1R, pSG-HA-B1R, and its kinase-dead form, pSG-HA-B1R(K149Q), have been reported previously (37). The pTRE-HA-B1R construct was obtained by cloning B1R in the HindIII and NotI sites of pTRE-HA (BD Biosciences Clontech, Palo Alto, CA). JNK was obtained from the plasmid pHA-JNK (S. Gutkind, Bethesda, MD) by digestion with BamHI and HindIII and cloned in pCMVT-Flag (Furou-Cho, Nagoya, Japan) to obtain pFlag-JNK. The prokaryotic pGST-c-Jun and pGST-c-Jun(Ser63/73Ala) were from M. Karin (San Diego, CA). The plasmids for JIP1 mammalian expression construct fused to GST, pGST-JIP1 (full length), pGST-JIP1(ΔJBD), pGST-JIP1 (residues 1 to 127), pGST-JIP1 (residues 127 to 282), pGST-JIP1 (residues 283 to 660), and pGST-JIP1 (residues 471 to 660) were from R. J. Davies (University of Massachusetts). The human c-Jun tagged with HA epitope, pMT107-c-Jun-HA, was from D. Bohmann (EMBL, Germany). For luciferase reporter assays, the N-terminal domain of c-Jun fused to GAL4, p-c-Jun-GAL4, and p-c-Jun(S63/73A)-GAL4 and p5xGAL4-Luc were from M. Karin (University of California, San Diego). The −517/+63 collagenase (MMP1) promoter cloned in the pGL3-basic vector to form the pColl-luciferase plasmid was a gift of G. Thiel (University of Saarland, Saarbrücken, Germany) (45). The plasmids used to express MAPK were pHA-TAK1 and pFlag-TAB1 from Furou-Cho (Nagoya, Japan) and pFlag-MKK7 from A. Whitmarsh (Patterson Institute, Manchester, United Kingdom). Plasmid pMEKK1 expressing a constitutively active kinase and the pAP1-Luc reporter were from Stratagene (San Diego, CA). Plasmid pRL-tk from Promega Biotech (Madison, WI) was used as an internal control in luciferase assays.
Cell culture, infection, and transfection.
BSC-1 cells were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 5% fetal bovine serum (FBS). Vaccinia virus Western Reserve strain infections were carried out in media containing 2% FBS and incubated at 37°C in a 5% CO2 atmosphere (15). HeLa-HA-B1R cells stably transfected with a pTRE-HA-B1R plasmid were obtained with the “Tet-off gene expression system” from Clontech following the manufacturer's instructions and were grown in DMEM supplemented with Tet system-approved FBS (BD Biosciences Clontech). Cos1 cells were grown in DMEM with 10% fetal calf serum and supplemented with antibiotics in a humidified atmosphere with 5% CO2. Totals of 300,000 and 500,000 cells were plated in a P60 and a P100 dish, respectively, and used for transfection 24 h later. Cells were transfected with the JetPEI reagent from Polytransfection (Illkirch, France) (39, 40, 47). After 48 h, the cells were lysed in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 plus protease inhibitors) or GST pull-down buffer (20 mM Tris-HCl, pH 7.4, 137 mM NaCl, 2 mM EDTA, 25 mM β-glycerophosphate, 2 mM pyrophosphate, 10% [vol/vol] glycerol and 1% Triton X-100 plus protease inhibitors). GST pull-down assays were performed, incubating 1 mg of total cell extract with glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) for 12 h at 4°C. Sepharose beads were washed three times with lysis buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Antibodies.
The following antibodies were used: HA epitope monoclonal antibody from Covance (Berkeley, CA), Flag epitope polyclonal antibody and β-actin monoclonal AC-15 antibody from (Sigma, St. Louis, MO), β-actin monoclonal AC-15 antibody from Sigma, and GST monoclonal antibody (sc-138) from Santa Cruz Biotechnology (Santa Cruz, CA). JIP1 was detected with monoclonal B7 antibody (sc-25267) or rabbit polyclonal antibody (sc-15353) from Santa Cruz. Anti-JNK was from BD Pharmingen; phospho-(Thr183/Tyr185)-JNK with monoclonal G7 antibody (sc-6254) was from Santa Cruz Biotechnology; c-Jun was detected with a rabbit polyclonal antibody from Cell Signaling (Beverly, MA). Goat anti-mouse horseradish peroxidase and goat anti-rabbit horseradish peroxidase (Amersham Pharmacia Biotech) were used as secondary antibodies. For analysis, 50 μg of whole-cell extracts were fractionated in sodium dodecyl sulfate-polyacrylamide gels and transferred to Immobilon-P membranes (Millipore), and the immunoblots were developed with the ECL detection reagents (Amersham Biotech).
Transcriptional assays.
Reporter gene assays were performed with the dual-luciferase reporter assay system (Promega, Madison, WI) in cell extracts prepared at 36 to 48 h after transfection. The assay is based on the use of pSG-424-c-Jun or pSG-424-c-Jun S63/73A constructs with a GAL4 DNA binding domain and a p5xGAL4-Luc reporter that were cotransfected in combination with pRL-tk Renilla luciferase as an internal control for transfection efficiency as previously reported (24, 39, 40). Other luciferase reporter constructs used were pAP1-luc and pColl-luciferase. The amount of DNA within the experiments was kept constant by adding the respective empty vector plasmid DNA to the transfection mixtures. Luciferase activity was determined with 20 μl of total cell extract using the dual luciferase reporter assay kit from Promega (Madison, WI). The generated light was detected with a MiniLumat LB9506 luminometer (Egg-Berthold, Bad Wildbad, Germany) as previously described (5). The results were obtained in at least three different experiments. All results were analyzed by comparing with the control (unpaired Student's t test), and the means and standard deviations (SD) of independent triplicate cultures are shown in the figures.
Cell viability.
A total of 500,000 HeLa-HA-B1R cells (stably transfected with the inducible pTRE-HA-B1R plasmid) were seeded in a P60 plate, and the expression of HA-B1R was controlled (inducible) with doxycycline. Cell death was induced using 7.5 μg/ml etoposide C (Sigma), and cell viability was measured at several time points by counting the percentage of cells that excluded or adsorbed the trypan blue dye (Sigma).
RESULTS
Accumulation of c-Jun during vaccinia virus infection.
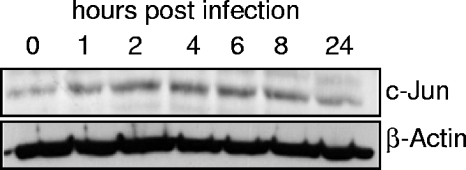
Any viral infection represents a cellular stress; therefore, it is likely that stress response pathways are activated in the infected cell as a cellular attempt to abort the infection. To test this possible stress response, the levels of c-Jun protein were determined in the course of vaccinia infection. BSC-1 cells were infected with vaccinia virus, and the level of c-Jun was determined by Western blot analysis at different time points. Following vaccinia infection, there was a transient stabilization of the endogenous c-Jun protein that reached a peak between 2 to 8 h (Fig. 1).
FIG. 1.
Accumulation of endogenous c-Jun protein during vaccinia virus infection. Extracts were prepared at the indicated times after infection and analyzed by Western blotting with an anti c-Jun antibody.
B1R stabilizes and phosphorylates c-Jun.
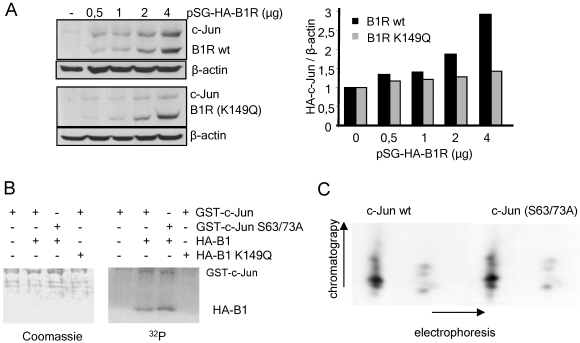
The phosphorylation of c-Jun by JNK results in its stabilization and accumulation (14). The accumulation of c-Jun may also be due to phosphorylation as a consequence of an infection by an early viral kinase. The only early vaccinia kinase (B1R) has been shown to hyperphosphorylate and destabilize p53, another stress response protein (37). Therefore, whether B1R could have an effect on c-Jun stability was determined. For this aim, Cos1 cells were transfected with a fixed amount of pMT107-c-Jun-HA and increasing amounts of pSG-HA-B1R (Fig. 2A, top) or pSG-HA-B1R(K149Q) that expresses a kinase-dead B1R protein (Fig. 1B, bottom). As the amount of B1R protein increased in the cells, so did the amount of c-Jun protein detected in Western blots (Fig. 2A). This c-Jun accumulation required the kinase activity since it did not occur with the kinase-dead B1R, as better reflected in their quantification (Fig. 2A, graph). However, no stable interaction could be detected between c-Jun and B1R proteins in reciprocal immunoprecipitation experiments (not shown), suggesting that their interaction is transient, as is expected for a phosphotransfer reaction.
FIG. 2.
(A) Stabilization of c-Jun by vaccinia virus B1R. Cos1 cells were transfected with pMT107-c-Jun-HA (100 ng) and the indicated amounts of pSG-HA-B1R wild type (wt) or kinase dead (K149Q). β-Actin was used as a loading control. The c-Jun and B1R proteins were detected with an anti-HA antibody. The blots were quantified by densitometry in the linear response range using β-actin as a normalization control and are shown in the bar diagram. −, without. (B) Phosphorylation of c-Jun by B1R. An in vitro kinase assay was performed using GST-c-Jun or GST-c-Jun(Ser63/73Ala) as the substrates of the wild-type or inactive B1R proteins expressed in Cos1 cells from plasmid pSG-HA-B1R or pSG-HA-B1R(K149Q), respectively, and immunoprecipitated with anti-HA antibody. −, without; +, with. (C) Phosphopeptide analysis of wild-type and mutant c-Jun with Ser63- and Ser73-to-Ala substitutions. Both c-Jun proteins, wild type and mutant, were phosphorylated in vitro with a bacterially expressed GST-B1R fusion protein and separated from the GST moiety by thrombin digestion. The two-dimensional (electrophoresis and chromatography) phosphopeptide analysis is described in Materials and Methods.
The previous experiment suggested that c-Jun might be phosphorylated by B1R. To test the possible phosphorylation of c-Jun by B1R, Cos1 cells were transfected with the plasmid pSG-HA-B1R or the inactive pSG-HA-B1R(K149Q) and the kinase proteins were immunoprecipitated with an anti-HA antibody and used for an in vitro kinase assay to phosphorylate either GST-c-Jun or GST-c-Jun(Ser63/73Ala), in which the residues targeted by JNK have been mutated and cannot be phosphorylated. In this assay, B1R, but not the inactive B1R(K164Q), phosphorylated both forms of c-Jun (Fig. 2B), suggesting that phosphorylation occurs in residues different from those targeted by JNK. Similar observations were obtained using a bacterially purified GST-B1R protein, but its size was very close to that of GST-c-Jun substrate and interfered with the isolation of the phosphorylated band for further analysis. To confirm this observation, c-Jun proteins were phosphorylated by B1R (expressed in bacteria as GST-B1R and then separated from GST by digestion with trypsin) and their phosphopeptide maps were determined. The two forms of c-Jun proteins, wild-type and mutant, displayed similar maps consisting of several spots (Fig. 2C), some of which were resolved only in the chromatography and barely separated in the electrophoresis, indicating that B1R phosphorylated c-Jun in several residues independently of Ser63 and Ser73, the targets of JNK and VRK1. This map is different from that of c-Jun phosphorylation in Ser63 and Ser73 (39).
B1R directly activates c-Jun-dependent transcription.
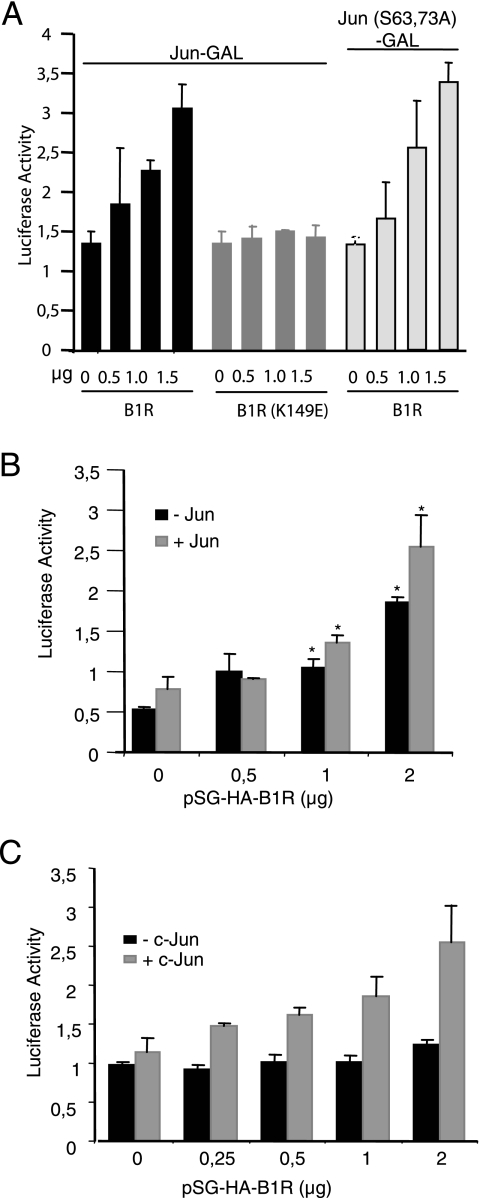
The phosphorylation of c-Jun by B1R in residues different from those targeted by JNK raises the question of whether B1R can activate c-Jun-dependent transcription, as it is carried out when it is phosphorylated in Ser63/Ser73 by either JNK (14) or VRK1 (39) since c-Jun phosphorylation favors its dimerization (14). To test the effect on c-Jun-dependent transcription, Cos1 cells were transfected with different combinations of B1R (active or inactive kinase) and c-Jun substrates (wild type or mutant), using a reporter system based on the fusion of the N terminus of c-Jun with a GAL4 DNA binding domain (p-c-Jun-GAL4) and a luciferase reporter vector, p5xGAL4-Luc, that is activated when it is recognized by dimers of the DNA binding domain, which are formed only when the c-Jun moiety of the fusion protein is phosphorylated (14). The active B1R increased c-Jun-dependent transcription in a dose-dependent manner but not that of the kinase-dead B1R (Fig. 3A). This activation was similarly induced when c-Jun(Ser63/73Ala) was used (Fig. 3A), confirming its independence from these two residues. These results indicated that c-Jun phosphorylation by vaccinia virus B1R can activate its transcriptional role in a manner independent of JNK activation and by phosphorylation of residues other than those phosphorylated by JNK.
FIG. 3.
(A) Activation of c-Jun-dependent transcription. Cos1 cells were transfected with different pSG-HA-B1R (active) and pSG-HA-B1R-K149Q (kinase dead) (indicated at the bottom), as well as two types of reporter plasmid with a wild-type c-Jun-GAL4 and a mutant c-Jun(S63/73A)-GAL4 that cannot respond to JNK-mediated signals. The means and SD of three independent experiments determined in triplicate are shown. (B) Assay of c-Jun-dependent transcription by B1R using the plasmid pAP1-Luc as a reporter. This experiment was performed with the endogenous c-Jun (−) or by cotransfection with exogenous pMT107-c-Jun-HA (+). In the transfection, pAP1-Luc (1 μg) as a reporter, pRL-tk (20 ng) for control and normalization, and the indicated amounts of pSG-HA-B1R and pMT107-c-Jun-HA (0.5 μg) were used. The values were normalized with respect to the internal control. The means and SD of six experiments are shown. *, P was <0.005. (C) Activation of the collagenase promoter in a c-Jun-dependent manner. This experiment was performed with the endogenous c-Jun (−) or by cotransfection with exogenous pMT107-c-Jun-HA (+). In the transfection, MMP1 (collagenase) promoter (0.5 μg of pColl-luciferase) as a reporter, pRL-tk (50 ng) for control and normalization, and the indicated amounts of pSG-HA-B1R with or without pMT107-c-Jun-HA (1 μg) were used. The means and SD of three independent experiments determined in triplicate are shown. Error bars indicate standard deviations.
The activation of c-Jun-dependent transcription was also determined based on a different type of assay with a reporter plasmid (pAP1-Luc that depends on the endogenous wild-type c-Jun protein) containing several AP1 response elements. In this case, the transcriptional activity was also activated in a dose-dependent manner by B1R without significant changes when we overexpressed c-Jun (Fig. 3B). Next, the effect was tested using the promoter of a gene that responded to AP1 activation and, for this aim, we used the human collagenase (MMP1) gene promoter coupled with luciferase, plasmid pColl-luciferase. These experiments were performed in the presence of exogenous c-Jun protein to avoid the endogenous protein becoming limiting. B1R activated c-Jun-dependent transcription via the AP1 sites present in a human MMP1 gene promoter (Fig. 3C).
B1R interacts with and phosphorylates JIP1.
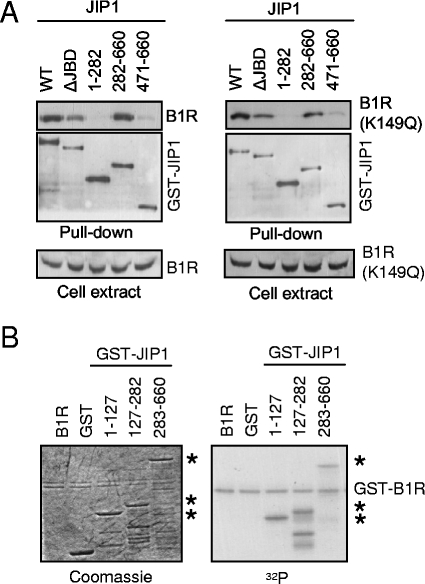
The c-Jun protein is activated by phosphorylation by JNK, which forms a signaling complex held together by the JIP scaffold proteins and is implicated in the response to different types of stimulation (53, 55). The fact that the B1R protein is essential for the virus, even lacking kinase activity, suggests the importance of the interaction with other proteins for this role (2). Therefore, it was decided to test whether B1R could also stably interact with the human JIP1 scaffold protein. This potential interaction was studied by analyzing its formation as a complex in Cos1 cells transfected with plasmids expressing active (pSG-HA-B1R) or kinase-dead (pSG-HA-B1R-K149Q) B1R proteins in combination with a eukaryotic vector (pGST-JIP1) expressing different regions of the JIP1 protein: the full length, residues 1 to 282, 282 to 660, and 471 to 660, and the JBD domain (ΔJBD) (lacking the binding domain to JNK, residues 127 to 282). The fusion proteins were used for glutathione-Sepharose pull-down experiments of whole-cell extracts. Both active and inactive forms of B1R interacted stably with GST-JIP1 fusion proteins (Fig. 4A). The JIP1 protein constructs that lack the region comprised between residues 283 to 471 did not interact with B1R (Fig. 3A). The loss of the JBD domain (ΔJBD) did not affect the interaction with JIP1-B1R, thus indicating that B1R interacted with JIP1 by a region different from that required by JNK.
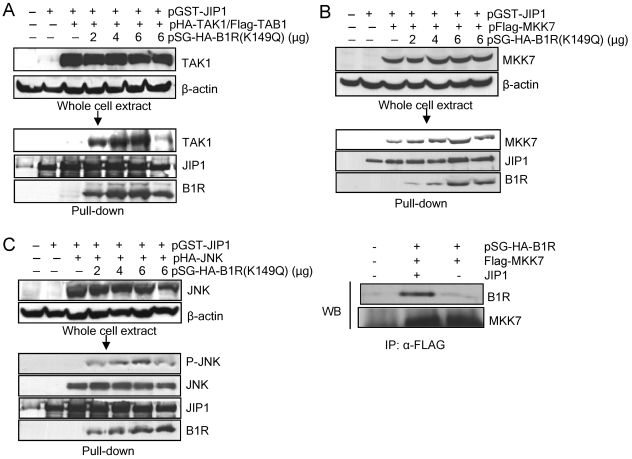
FIG. 4.
(A) Interaction between JIP1 and B1R. Cos1 cells were cotransfected with active B1R (pSG-HA-B1R), or kinase-dead B1R (pSG-HA-B1R-K149Q), with different mammalian expression constructs containing different regions of the JIP1 protein fused to GST as indicated in the figure. The extracts were subjected to a pull down with glutathione-Sepharose, and the proteins were determined with either an anti-HA antibody to detect B1R or anti-GST to confirm that GST-JIP1 was indeed brought down in the pull down. In the lower panel, the correct expression of B1R in the extracts is shown. The ΔJBD construct lacks the JNK binding region (residues 183 to 282). WT, wild type. (B) Phosphorylation of JIP1 by B1R. Different fragments of the GST-JIP1 protein expressed in Escherichia coli were used as substrates for an in vitro kinase assay of GST-B1R protein. The proteins used were detected by Coomassie blue (left) and incorporated radioactivity (right). The asterisks indicate the GST-JIP1 fusion proteins.
Next, whether B1R can phosphorylate JIP1 was determined. An in vitro kinase assay was performed using a GST-B1R kinase expressed from plasmid pGEX4T-B1R (37) and, as a substrate, different GST-JIP1 proteins spanning regions including residues 1 to 127, 127 to 282, and 283 to 660. All three of these JIP1 constructs, but not the GST moiety (Fig. 4B), were phosphorylated, suggesting that the phosphorylation was very complex since it occurred in multiple sites throughout the JIP1 molecule. This situation was already observed with p53, which is also hyperphosphorylated by B1R (37). The autophosphorylation of B1R was also detected in the assay.
B1R promotes JIP1 interaction with TAK1, MKK7, and JNK.
The interaction of B1R with JIP1 suggests that it might be affecting the formation of signaling complexes of JIP1 (53, 55). Therefore, it was decided to test whether the B1R-JIP1 complex has any effect on JIP1 interaction with different MAPKs. Three consecutive MAPKs, TAK1/TAB1, MKK7, and JNK, which are known to be in the same pathway, were tested sequentially. For this purpose, Cos1 cells were transfected with plasmid pSG-HA-B1R or kinase-dead pSG-HA-B1R-K149Q), pGST-JIP, and the kinase corresponding to each step of the signaling cascade. In all cases, their expression was checked in whole-cell extract before proceeding to a pull-down experiment with glutathione-Sepharose of the GST-JIP1 protein and its associated proteins that were detected in Western blot analysis. The first interaction studied was that of JIP1 with TAK1/TAB1. TAK1 was cotransfected with TAB1 since TAK1 binds JIP1 only when it is activated by forming a complex with TAB1 (18, 31). The presence of the different proteins in the whole-cell extract was checked to determine their correct expressions. TAK1 was equally expressed in all of the corresponding points (Fig. 5A, top). In the pull-down experiment for GST-JIP1, it was observed that there was a very weak interaction between JIP1 and TAK1, and in the presence of B1R, the amount of bound TAK1 increased significantly in a dose-dependent manner (Fig. 5A).
FIG. 5.
(A) Effect of B1R on JIP1 interaction with TAK1/TAB1. Cos1 cells were transfected with pHA-TAK1 and pFlag-TAB1, different amounts of pSG-HA-B1R or pSG-HA-B1R(K149Q), and pGST-JIP1 as indicated. The correct expression of the proteins in the whole-cell extract is shown in the top gel. These extracts were used for a pull down with glutathione-Sepharose (arrow), and the proteins detected in a Western blot are shown in the middle panel. (B) Effect of B1R on the JIP1 interaction with MKK7. Cos1 cells were transfected with pFlag-MKK7, different amounts of pSG-HA-B1R or pSG-HA-B1R(K149Q), and pGST-JIP1 as indicated. The proteins in the whole-cell extract were used for a pull down with glutathione-Sepharose and Western blot analysis (middle blot). The interaction was confirmed by an alternative method using immunoprecipitation (lower right panel). For the detection of the interaction of B1R and MKK7 through JIP1, Cos1 cells were transfected with the plasmids pSG-HA-B1R and pFlag-MKK7 (1 μg) with and without pGST-JIP1. The extract was immunoprecipitated with anti-Flag (brings down MKK7 and its associated proteins), and the immunoprecipitated proteins were analyzed with an anti-HA antibody that detects B1R and anti-Flag to detect MKK7. (C) Effect of B1R on the JIP1 interaction with JNK. Cos1 cells were transfected with plasmids pFlag-JNK, different amounts of pSG-HA-B1R or pSG-HA-B1R(K149Q) and pGST-JIP1 as indicated. The expression of the proteins in the whole-cell extract is shown in the top gel, and the extract was used for a pull-down with glutathione-Sepharose and the proteins were detected in a Western blot (WB). −, without; +, with.
Next, whether the B1R binding to JIP1 might affect MKK7-JIP1 interaction was analyzed. For this aim, Cos1 cells were transfected with different combinations of pSG-HA-B1R or the inactive pSG-HA-B1R (K149Q), and pFlag-MKK7 with or without pGST-JIP1. The proteins were correctly expressed in the whole-cell extract (Fig. 5B, top). Next, a pull-down assay of GST-JIP1-associated proteins was performed. B1R was detected only when JIP1 was included, suggesting that B1R and MKK7 did not interact directly, but both formed a stable complex with JIP1 (Fig. 5B, lanes 2 and 3). MKK7 binds to GST-JIP1, and as the amount of B1R was increased, so did the amount of bound MKK7. To confirm this indirect interaction in a different way, an immunoprecipitation experiment was performed. Cos1 cells were transfected with pSG-HA-B1R and pFlag-MKK7 with and without JIP1. The MKK7 extract was immunoprecipitated with an anti-Flag antibody, and the presence of B1R in the immunoprecipitate was determined with an anti-HA antibody. B1R was detected in only the MKK7 immunoprecipitate when JIP1 was present (Fig. 5B), supporting the observation that MKK7 and B1R do not interact directly, but both bind to the larger central region of JIP1 between residues 280 to 471.
A similar pull-down experiment was performed with the third component of the complex, JNK. JNK was bound to JIP1 in the absence of B1R. In the presence of increasing amounts of B1R, there appeared to be no increase in this binding (Fig. 5C), but B1R appeared to facilitate an increase in the activity of the bound JNK in the complex since an increase in JNK-specific phosphorylation in Thr183/Tyr185 residues was clearly detected with a specific antibody that is dependent on the amount of B1R present (Fig. 5C, lower panel).
When using the kinase-dead B1R (K149Q), the level of interaction with any of the three MAPKs was lower than that with an active B1R, suggesting that phosphorylation might contribute to the stability of the complex and permit the phosphorylation of the last kinase. From a functional point of view, only phosphorylated JNK can increase c-Jun-dependent transcription (9, 17). The inactive B1R(K149Q), although it binds to the JIP1 complex, does not facilitate the increase in phosphorylation of JNK (Fig. 5C, right lane).
Cooperation of B1R and other MAPK in activation of transcription.
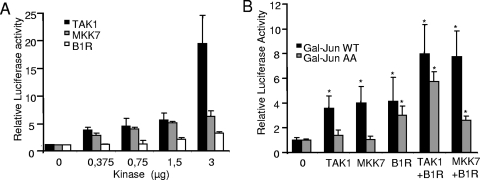
Since B1R phosphorylates c-Jun in residues different from those targeted by JNK, it is very likely that the signal from both kinases, at suboptimal doses, might have an additive effect on c-Jun-dependent transcription. To test this possibility, the previously described c-Jun-GAL reporter system was used. Before testing a potential cooperation, a dose-response curve of activation was determined for each kinase in order to select a suboptimal dose for each of them. There is a dose-dependent activation of transcription by the three kinases, but the effect of the active first kinase, TAK1/TAB1, is stronger than that of the middle kinase, MKK7, and B1R by themselves (Fig. 6A).
FIG. 6.
Cooperation of B1R with other MAP kinases. (A) Effect of different amounts of B1R, TAK1/TAB1, or MKK7 on the activation of transcription. In these experiments, 200,000 Cos1 cells were transfected with p-c-Jun-GAL4 (0.3 μg), p5xGAL4-Luc (0.3 μg), pRL-tk (20 ng), and the amount of kinase indicated in the figure. The means and SD of three experiments are shown. (B) Cooperation in activation of transcription. Cos1 cells were transfected with p-c-Jun-GAL4 (WT) (0.3 μg) or p-c-Jun(Ser63/73Ala)-GAL4 (AA) (0.3 μg), p5xGal4-Luc (0.3 μg), and one of the kinases B1R (1.5 μg), TAK1/TAB1(0.375 μg each), or MKK7 (0.375 μg) by themselves or in combination. pRL-tk (20 ng) was used for control and normalization. The means of seven experiments and SD are shown. *, P was <0.001. Error bars indicate standard deviations.
Next, the response to a unique suboptimal dose of kinase (0.375 μg for TAK1 and MKK7 and 1.5 μg for B1R) was studied. Combinations of TAK1/TAB and MKK7, with and without B1R, were tested using the same reporter system. In the case of wild-type c-Jun, there was an additive effect in the activation of transcription, while in the case of the mutant c-Jun(Ser63/73Ala), only the B1R response was detected (Fig. 6B). These data suggested that B1R can cooperate in the activation of the JNK pathway in two different ways.
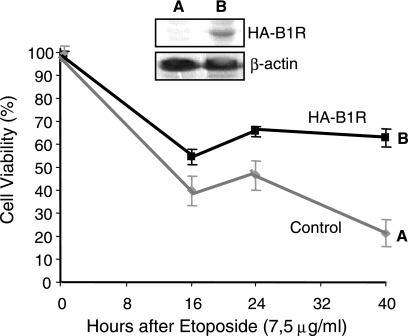
Increase of cell viability induced by B1R.
The activation of the c-Jun and JNK pathway can inhibit or activate apoptosis, depending on the type and context of stimulation. However, in cells lacking p53 activity, it usually leads to the inhibition of apoptosis (54) and vaccinia-infected cells show a decrease in p53 protein levels (48), which is probably induced by B1R (37). A functional consequence of the expression of B1R in the cell and its interaction with the JNK and p53 response pathways (37) is that B1R might make the cell less sensitive to cell death. To test this possibility, it was decided to determine whether B1R could increase the viability of cells in which apoptosis had been induced by etoposide. Etoposide is a chemotherapeutic drug that activates stress response pathways (16, 43) and activates p53 (46). HeLa cells with an inducible B1R were grown in the presence or absence of doxycycline to shut off or on the expression of B1R, respectively. The expression of B1R was determined by Western blotting (Fig. 7, insert). Etoposide (7.5 μg/ml) was added to the culture, and cell viability was measured at several time points using a trypan blue assay. We observed that the expression of B1R protected cells from apoptosis induced by etoposide (Fig. 7), and the response appeared to be biphasic, probably due to the fact that the cell cultures were not synchronized and sensitivity to DNA-damaging agents depends on the cell cycle. These data suggest that B1R can contribute to a transient inhibition of host stress response and apoptosis during infection.
FIG. 7.
Effect of B1R on cell viability in response to an etoposide DNA-damaging drug. HeLa cells stably transfected with a B1R induced by doxycycline were treated with etoposide to induce DNA damage. The expression and repression of B1R were determined by Western blotting, as shown in the insert. The viability of the cell population in uninduced and induced cells was determined at different time points following the addition of the genotoxic agent. The result is the mean of three experiments with the standard deviation. In the insert, the level of B1R protein in uninduced (A) and induced (B) cultures is shown.
DISCUSSION
Vaccinia is a cytolytic virus, and thus, the effect of any viral protein on host proteins should be very short and aim to permit successful viral replication. There is no need to develop fine tuning to maintain a stable long-term interaction as would be required for cytopathic viruses. Therefore, cytolytic viruses require only a delay in the stress response of the host cell after infection to gain enough time to complete its life cycle, which in the case of vaccinia, is approximately 24 h and results in cell death to release the viral particles.
The catalytic domain of B1R is related to the mammalian vaccinia-related kinase (VRK) family (24, 28, 29), and the cellular proteins targeted by B1R are similar to those so far identified for human VRK1, such as p53 (24, 47) or c-Jun (39). However, while the human VRK1 targets the phosphorylations of specific residues, B1R has a much more relaxed specificity, resulting in the multiphosphorylation of its targets. Probably as a consequence of this, human or murine VRK can only partially rescue the B1R-deficient phenotype (6). This apparent lower specificity or multiphosphorylation by the vaccinia kinase B1R of host proteins is probably enough to induce the required effect that will promote viral survival, and therefore, there is no additional selective pressure for fine tuning. Alternatively, it could also be a consequence of the low number of kinases (two) in the viral genome that perhaps have to play several and different roles during the viral life cycle.
Many cellular stress responses either converge on p53 or are modulated by the JNK pathway and, in the end, affect proliferation and survival (42). These pathways are better known in the context of responses to genotoxic stress than to viral lytic infections or persistent infections. Regarding p53, there has been identified a reduction in p53 levels during vaccinia virus infection (48) and B1R has been shown to downregulate p53 and the expression of the proapoptotic protein Bax (37), which indicate it could delay apoptosis and thus permit the formation of viral particles that are released at the time of cell death. The interaction of viral proteins with MAPK pathways is partially known in nonlytic viruses (11, 49), but nothing is known regarding cytolytic viruses such as vaccinia.
Signaling routes mediated by c-Jun control cell survival and apoptosis depending on the magnitude of the stimulation (42). The effect of B1R on this pathway that we describe here results in the transcriptional activation of c-Jun by two different mechanisms, as can be seen by the additive effect of B1R and MAPK in the activation of c-Jun-dependent transcription.
Regarding the first effect, B1R can directly phosphorylate c-Jun in residues different from those targeted by JNK and, interestingly, by its human homologue, VRK1 (39). However, both B1R and VRK1 induce c-Jun accumulation (in keeping with the effect of vaccinia infection that we describe) and, probably as a consequence of its dimerization, prevent c-Jun degradation by the proteasome and promote its binding to DNA and transcriptional activity (14). In that way, B1R permits bypassing its activation in response to stress signals that use MAPK signaling complexes but allows c-Jun to still be regulated by the canonical JNK pathway. The phosphorylation of c-Jun in residues different from those, Ser63/Ser73, targeted by JNK may alter the type of active dimers that are formed as well as the transcriptional specificity of these active dimers. Depending on the activated target, the response may be different. For example, c-Jun can activate the expression of FasL that induces apoptosis (42), but it can also inhibit the expression of Fas (20) and induce the activation of the apoptosis inhibitor bcl3 (36). Thus, the specificity, magnitude, and duration of the c-Jun response can determine its biological effect.
Regarding the second effect, it is a consequence of the direct interaction between B1R and the JIP1 scaffold protein that assembles MAPK signaling complexes. Thus, JIP1 interactions through its central domain can contribute to switching or altering the balance of signals that pass through the MAPK signaling complex. The interaction between B1R and JIP1 is independent of its kinase activity. The B1R-JIP1 complex appears to promote or stabilize the association of JIP1 with two kinases of the MAPK cascade tested in this work (TAK1 and MKK7), increasing the phosphorylation state and thus resulting in the activation of JNK bound to JIP1 without increasing the JNK-JIP1 interaction. The consequence of this is an activation of JNK activity as detected by its phosphorylation in specific tyrosine residues, probably as a consequence of increased formation of the upstream complexes since B1R does not phosphorylate tyrosine residues and by the transcriptional activity of c-Jun, AP-1, and the collagenase promoter. This mechanism is similar to that found in hepatitis C virus; its X protein can interact with the MAPK scaffold 14-3-3, forming a complex together with MAPK (10).
JNK negatively regulates p53 in the absence of stress, inhibiting apoptosis, and both JNK and c-Jun inhibit apoptosis in cells lacking p53 (33, 54). Considering that B1R downregulates p53 and inhibits Bax expression (37), the effect on c-Jun activation is consistent with an inhibition or delay of apoptosis. This, in the context of vaccinia lytic cycle, makes biological sense since what is needed is a delay in the apoptotic response for the time needed to complete viral assembly, just before the cell is lysed to release the viral particles. Also, it is consistent with our observation that B1R increases cell viability in the presence of a DNA damage-inducing drug that can cause cell death. B1R could be involved in an early-phase delay of apoptosis by a rapid mechanism, such as phosphorylation, since the F1L protein, which appears to be of critical importance due to its ability to interact with the proapoptotic protein Bak and the inhibition of the mitochondrion-dependent activation of apoptosis (13, 32, 50, 51) is expressed at only 4 h after infection.
The possible role of B1R in the early prevention apoptosis depends on two different mechanisms: the phosphorylation of transcription factors like p53 and c-Jun and the activation of the JNK pathway by interaction with JIP1. The latter could explain why a vaccinia virus mutant that lacks functional B1R kinase activity has a significantly reduced viral production (2) and why it was not feasible to isolate virus deficient in B1R (2) since these viruses probably induce apoptosis of infected cells before viral production occurs.
In summary, the vaccinia virus B1R kinase, the only viral kinase present early during infection, is able to modulate the cellular responses to c-Jun by two different mechanisms, direct phosphorylation in novel residues and modulation of JIP1-MAPK complexes. Thus, the activation of c-Jun transcription can be achieved by two cooperating pathways. It is known that the activation of JNK leads to the protection of apoptosis (42). B1R is able to both activate JNK and downregulate p53 (19), thus contributing to the survival of the infected cell long enough to permit the completion of its lytic cycle.
Acknowledgments
The help of Rafael Blasco with vaccinia virus infection experiments is greatly appreciated.
C.R.S., S.B., and A.S. have predoctoral fellowships from Fundação para a Ciência e a Tecnologia (Portugal), Ministerio de Educación y Ciencia (Spain), and Consejo Superior de Investigaciones Científicas, respectively. This work was funded by grants from Fondo de Investigación Sanitaria (FIS02/0585), Ministerio de Educación y Ciencia (SAF2004-02900), Junta de Castilla y León (CSI05A05), and Fundación de Investigación Médica MM to P.A.L.
REFERENCES
- 1.Banham, A. H., D. P. Leader, and G. L. Smith. 1993. Phosphorylation of ribosomal proteins by the vaccinia virus B1R protein kinase. FEBS Lett. 321:27-31. [DOI] [PubMed] [Google Scholar]
- 2.Banham, A. H., and G. L. Smith. 1992. Vaccinia virus B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology 191:803-812. [DOI] [PubMed] [Google Scholar]
- 3.Beaud, G., R. Beaud, and D. P. Leader. 1995. Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate virus B1R protein kinase. J. Virol. 69:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaud, G., A. Sharif, A. Topa-Masse, and D. P. Leader. 1994. Ribosomal protein S2/Sa kinase purified from HeLa cells infected with vaccinia virus corresponds to B1R protein kinase and phosphorylates in vitro the viral ssDNA-binding protein. J. Gen. Virol. 75:283-293. [DOI] [PubMed] [Google Scholar]
- 5.Blanco, S., L. Klimcakova, F. M. Vega, and P. A. Lazo. 2006. The subcellular localization of vaccinia-related kinase 2 (VRK2) isoforms determines their different effect on p53 stability in tumour cell lines. FEBS J. 273:2487-2504. [DOI] [PubMed] [Google Scholar]
- 6.Boyle, K. A., and P. Traktman. 2004. Members of a novel family of mammalian protein kinases complement the DNA-negative phenotype of a vaccinia virus ts mutant defective in the B1 kinase. J. Virol. 78:1992-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruening, W., B. Giasson, W. Mushynski, and H. D. Durham. 1998. Activation of stress-activated MAP protein kinases up-regulates expression of transgenes driven by the cytomegalovirus immediate/early promoter. Nucleic Acids Res. 26:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 9.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 10.Diao, J., A. A. Khine, F. Sarangi, E. Hsu, C. Iorio, L. A. Tibbles, J. R. Woodgett, J. Penninger, and C. D. Richardson. 2001. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J. Biol. Chem. 276:8328-8340. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., and L. S. Young. 1998. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene 16:1731-1742. [DOI] [PubMed] [Google Scholar]
- 12.Everett, H., and G. McFadden. 2002. Poxviruses and apoptosis: a time to die. Curr. Opin. Microbiol. 5:395-402. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, S. F., H. Ludwig, J. Holzapfel, M. Kvansakul, L. Chen, D. C. Huang, G. Sutter, M. Knese, and G. Hacker. 2006. Modified vaccinia virus Ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3L to block virus-associated apoptosis. Cell Death Differ. 13:109-118. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, S. Y., B. Xie, V. Adler, V. A. Fried, R. J. Davis, and Z. Ronai. 1997. c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J. Biol. Chem. 272:32163-32168. [DOI] [PubMed] [Google Scholar]
- 15.Geada, M. M., I. Galindo, M. M. Lorenzo, B. Perdiguero, and R. Blasco. 2001. Movements of vaccinia virus intracellular enveloped virions with GFP tagged to the F13L envelope protein. J. Gen. Virol. 82:2747-2760. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa, J., C. Depatie, M. Ohmichi, and D. Mercola. 2003. The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J. Biol. Chem. 278:20582-20592. [DOI] [PubMed] [Google Scholar]
- 17.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7:2135-2148. [DOI] [PubMed] [Google Scholar]
- 18.Holtmann, H., J. Enninga, S. Kalble, A. Thiefes, A. Dorrie, M. Broemer, R. Winzen, A. Wilhelm, J. Ninomiya-Tsuji, K. Matsumoto, K. Resch, and M. Kracht. 2001. The MAPK kinase kinase TAK1 plays a central role in coupling the interleukin-1 receptor to both transcriptional and RNA-targeted mechanisms of gene regulation. J. Biol. Chem. 276:3508-3516. [DOI] [PubMed] [Google Scholar]
- 19.Huang, S., L. Shu, M. B. Dilling, J. Easton, F. C. Harwood, H. Ichijo, and P. J. Houghton. 2003. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1). Mol. Cell 11:1491-1501. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov, V. N., A. Bhoumik, M. Krasilnikov, R. Raz, L. B. Owen-Schaub, D. Levy, C. M. Horvath, and Z. Ronai. 2001. Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol. Cell 7:517-528. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke, A., M. P. Czech, and R. J. Davis. 2004. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 18:1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston, J. B., and G. McFadden. 2003. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 77:6093-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, S., W. Chen, and S. S. Broyles. 1992. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J. Virol. 66:2717-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Borges, S., and P. A. Lazo. 2000. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene 19:3656-3664. [DOI] [PubMed] [Google Scholar]
- 25.Marsters, S. A., T. M. Ayres, M. Skubatch, C. L. Gray, M. Rothe, and A. Ashkenazi. 1997. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J. Biol. Chem. 272:14029-14032. [DOI] [PubMed] [Google Scholar]
- 26.Moss, B. 1996. Poxviridae: the viruses and their replication, p. 2637-2671. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 27.Moss, B., and J. L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59-66. [DOI] [PubMed] [Google Scholar]
- 28.Nezu, J., A. Oku, M. H. Jones, and M. Shimane. 1997. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics 45:327-331. [DOI] [PubMed] [Google Scholar]
- 29.Nichols, R. J., and P. Traktman. 2004. Characterization of three paralogous members of the mammalian vaccinia related kinase family. J. Biol. Chem. 279:7934-7946. [DOI] [PubMed] [Google Scholar]
- 30.Nichols, R. J., M. S. Wiebe, and P. Traktman. 2006. The vaccinia-related kinases phosphorylate the N′ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol. Biol. Cell 17:2451-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, J. M., H. Brady, M. G. Ruocco, H. Sun, D. Williams, S. J. Lee, T. Kato, Jr., N. Richards, K. Chan, F. Mercurio, M. Karin, and S. A. Wasserman. 2004. Targeting of TAK1 by the NF-κB protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 18:584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postigo, A., J. R. Cross, J. Downward, and M. Way. 27. Jan 2006, posting date. Interaction of F1L with the BH3 domain of Bak is responsible for inhibiting vaccinia-induced apoptosis. Cell Death Differ. [Online.] doi: 10.1038/sj.cdd.4401853. [DOI] [PubMed]
- 33.Potapova, O., M. Gorospe, R. H. Dougherty, N. M. Dean, W. A. Gaarde, and N. J. Holbrook. 2000. Inhibition of c-Jun N-terminal kinase 2 expression suppresses growth and induces apoptosis of human tumor cells in a p53-dependent manner. Mol. Cell. Biol. 20:1713-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahaus, M., N. Desloges, and M. H. Wolff. 2004. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol. 85:3529-3540. [DOI] [PubMed] [Google Scholar]
- 35.Rahaus, M., and M. H. Wolff. 2003. Reciprocal effects of varicella-zoster virus (VZV) and AP1: activation of jun, fos and ATF-2 after VZV infection and their importance for the regulation of viral genes. Virus Res. 92:9-21. [DOI] [PubMed] [Google Scholar]
- 36.Rebollo, A., L. Dumoutier, J. C. Renauld, A. Zaballos, V. Ayllon, and A. C. Martinez. 2000. Bcl-3 expression promotes cell survival following interleukin-4 deprivation and is controlled by AP1 and AP1-like transcription factors. Mol. Cell. Biol. 20:3407-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos, C. R., F. M. Vega, S. Blanco, R. Barcia, and P. A. Lazo. 2004. The vaccinia virus B1R kinase induces p53 downregulation by an Mdm2-dependent mechanism. Virology 328:254-265. [DOI] [PubMed] [Google Scholar]
- 38.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 39.Sevilla, A., C. R. Santos, R. Barcia, F. M. Vega, and P. A. Lazo. 2004. c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK). Oncogene 23:8950-8958. [DOI] [PubMed] [Google Scholar]
- 40.Sevilla, A., C. R. Santos, F. M. Vega, and P. A. Lazo. 2004. Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J. Biol. Chem. 279:27458-27465. [DOI] [PubMed] [Google Scholar]
- 41.Shaulian, E., and M. Karin. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131-E136. [DOI] [PubMed] [Google Scholar]
- 42.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-2400. [DOI] [PubMed] [Google Scholar]
- 43.Shimada, K., M. Nakamura, E. Ishida, M. Kishi, S. Yonehara, and N. Konishi. 2003. c-Jun NH2-terminal kinase-dependent fas activation contributes to etoposide-induced apoptosis in p53-mutated prostate cancer cells. Prostate 55:265-280. [DOI] [PubMed] [Google Scholar]
- 44.Shisler, J. L., and B. Moss. 2001. Immunology 102 at poxvirus U: avoiding apoptosis. Semin. Immunol. 13:67-72. [DOI] [PubMed] [Google Scholar]
- 45.Steinmuller, L., G. Cibelli, J. R. Moll, C. Vinson, and G. Thiel. 2001. Regulation and composition of activator protein 1 (AP-1) transcription factors controlling collagenase and c-Jun promoter activities. Biochem. J. 360:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson, T., C. Tovar, H. Yang, D. Carvajal, B. T. Vu, Q. Xu, G. M. Wahl, D. C. Heimbrook, and L. T. Vassilev. 2004. Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J. Biol. Chem. 279:53015-53022. [DOI] [PubMed] [Google Scholar]
- 47.Vega, F. M., A. Sevilla, and P. A. Lazo. 2004. p53 stabilization and accumulation induced by human vaccinia-related kinase 1. Mol. Cell. Biol. 24:10366-10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wali, A., and D. S. Strayer. 1999. Infection with vaccinia virus alters regulation of cell cycle progression. DNA Cell Biol. 18:837-843. [DOI] [PubMed] [Google Scholar]
- 49.Wan, J., L. Sun, J. W. Mendoza, Y. L. Chui, D. P. Huang, Z. J. Chen, N. Suzuki, S. Suzuki, W. C. Yeh, S. Akira, K. Matsumoto, Z. G. Liu, and Z. Wu. 2004. Elucidation of the c-Jun N-terminal kinase pathway mediated by Epstein-Barr virus-encoded latent membrane protein 1. Mol. Cell. Biol. 24:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasilenko, S. T., L. Banadyga, D. Bond, and M. Barry. 2005. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J. Virol. 79:14031-14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasilenko, S. T., A. F. Meyers, K. Vander Helm, and M. Barry. 2001. Vaccinia virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore. J. Virol. 75:11437-11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weston, C. R., and R. J. Davis. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12:14-21. [DOI] [PubMed] [Google Scholar]
- 53.Whitmarsh, A. J., C. Y. Kuan, N. J. Kennedy, N. Kelkar, T. F. Haydar, J. P. Mordes, M. Appel, A. A. Rossini, S. N. Jones, R. A. Flavell, P. Rakic, and R. J. Davis. 2001. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 15:2421-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisdom, R., R. S. Johnson, and C. Moore. 1999. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 18:188-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasuda, J., A. J. Whitmarsh, J. Cavanagh, M. Sharma, and R. J. Davis. 1999. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19:7245-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]