Abstract
Feline calicivirus is a major causative agent of respiratory disease in cats. It is also one of the few cultivatable members of Caliciviridae. We have examined the entry process of feline calicivirus (FCV). An earlier study demonstrated that acidification in endosomes may be required. We have confirmed this observation and expanded upon it, demonstrating, using drugs to inhibit the various endocytic pathways and dominant-negative mutants, that FCV infects cells via clathrin-mediated endocytosis. We have also observed that FCV permeabilizes cell membranes early during infection to allow the coentry of toxins such as α-sarcin. Inhibitors of endosome acidification such as chloroquine and bafilomycin A1 blocked this permeabilization event, demonstrating that acidification is required for uncoating of the genome and access to the cytoplasm.
The initial stages in virus infection involve attachment of virions to cell surface receptors followed by penetration of the cell membranes to release the capsids or their contents into the cytoplasm. Many viruses, both enveloped and nonenveloped, require endocytosis to enter cells, and they penetrate the cytoplasm from endocytic vesicles (reviewed in references 42 and 47). A number of different routes of endocytosis have been characterized: clathrin-mediated endocytosis, endocytosis via caveolae/lipid rafts, macropinocytosis, and phagocytosis. Using drugs or dominant-negative mutants of key proteins, it is possible to distinguish between these different routes and so discover the routes of entry for different viruses.
Many nonenveloped viruses have been shown to use the clathrin-mediated endocytic route to infect cells; examples include canine parvovirus (41, 59), adenovirus (60), and picornaviruses including rhinovirus (15, 22), foot-and-mouth disease virus (7, 39), and coxsackievirus B3 (11). Recently, two viruses, simian virus 40 and parechovirus 1, have been shown to use caveolae in order to infect cells (3, 27), while human echovirus 11 and coxsackievirus B4 have been shown to enter cells in a cholesterol-dependent manner suggesting entry via lipid rafts (51, 54).
A phenomenon observed during entry of both enveloped and nonenveloped viruses (21, 32, 34, 35, 40, 41, 62) is the permeabilization of cellular membranes to toxins such as α-sarcin and hygromycin, both of which inhibit protein synthesis. Normally, these toxins are unable to cross the plasma membrane and their passage into cells (indicated by the inhibition of protein synthesis) can be used as a marker for penetration of either intact capsids or viral nucleic acid into the cytoplasm.
Little is known about the attachment and entry processes of members of the Caliciviridae, as many of the group cannot be propagated in vitro. Feline calicivirus (FCV), however, grows to high titers in vitro and has been used as a model for calicivirus infection. FCV is a member of the Vesivirus genus of the Caliciviridae family. Infection with FCV is widespread in cats, and the virus is a major causative agent of upper respiratory tract disease. FCV is a nonenveloped virus with a 7.7-kb positive-strand RNA genome (10) covalently linked to a viral protein, VPg (9, 25). The genome encodes three open reading frames (ORFs). ORF1 encodes the nonstructural proteins including a 2C picornaviruslike helicase, a 3C-like protease, and a 3D-like polymerase (38, 49). ORFs 2 and 3 are encoded by a VPg-linked subgenomic RNA species. ORF2 encodes the capsid protein, and ORF3 encodes a small protein recently shown to be a minor virion component possibly involved in RNA encapsidation (50).
Early studies demonstrated that the entry of FCV is sensitive to the effects of chloroquine, suggesting that acidification is necessary for efficient infection by this virus (31). We have examined the entry process of FCV in more detail using drugs and dominant-negative mutants to examine specific endocytosis routes. We have shown that FCV entry is dependent upon clathrin-mediated endocytosis and acidification. Entry of FCV permeabilizes cells to allow coentry of α-sarcin and hygromycin B, and this step can be inhibited by bafilomycin A1 and chloroquine, demonstrating that acidification of the virions in endosomes is required for uncoating of the genome and access to the cytoplasm.
MATERIALS AND METHODS
Reagents and antibodies.
The following chemicals were purchased from Sigma: chlorpromazine, chloroquine, bafilomycin A, nystatin, brefeldin A, cytochalasin D, amiloride, nocodazole, and α-sarcin. The concentrations used are shown in Table 1. Antibodies used were as follows: anti-FCV capsid (Chemicon), rat anti-alpha tubulin (Serotec), Alexa Fluor 488 goat anti-mouse (Molecular Probes), Alexa Fluor 488 goat anti-rat (Molecular Probes), and Alexa Fluor 594 phalloidin (Molecular Probes). Anti-FCV and anti-alpha tubulin were used at 1/1,000, phalloidin was used at 1/200, and all secondary antibodies were used at 1/1,000.
TABLE 1.
Drugs, effects, and concentrations useda
| Drug | Effect | Optimum concentration | Concentration resulting in side effects |
|---|---|---|---|
| Chlorpromazine | Prevents assembly and disassembly of clathrin lattices at cell surface and on endosomes and inhibits clathrin-mediated endocytosis | 14 μM (in water) | 28 μM |
| Nystatin | Sequesters cholesterol, inhibits endocytosis via lipid rafts/caveolae | 25 μM (in DMSO) | 50 μM |
| Chloroquine | Inhibits acidification of endosomes | 100 μM (in water) | 200 μM |
| Bafilomycin A1 | Inhibits vacuolar-type H+-ATPase, inhibits acidification of endosomes | 200 nM (in DMSO) | 500 nM |
| Brefeldin A | Inhibits vesicle transport, disrupts Golgi apparatus, inhibits picornavirus RNA replication | 18 μM (in DMSO) | Not tested |
| Cytochalasin D | Prevents actin polymerization, disrupts actin cytoskeleton | 4 μM (in DMSO) | 10 μM |
| Nocodazole | Disrupts microtubules, inhibits trafficking of endosomes | 20 μM (in DMSO) | >50 μM |
| Amiloride | Inhibits Na+/H+exchanger, inhibits macropinocytosis | 100 μM (in DMSO) | 250 μM |
| EIPA | Inhibits Na+/H+exchanger, inhibits macropinocytosis | 25 μM (in DMSO) | 100 μM |
| Benzamil | Inhibits Na+/H+exchanger, inhibits macropinocytosis | 50 μM (in DMSO) | 200 μM |
| Verapamil | Inhibits Ca2+ ion channels | 50 μM (in DMSO) | Not tested |
| PP2 | Inhibits Src family of tyrosine kinases | 10 μM (in DMSO) | Not tested |
DMSO, dimethyl sulfoxide.
Cells and virus.
Crandall Reese feline kidney (CRFK) cells were obtained from the European Collection of Cell Cultures (ECACC). The cells were grown in Glasgow's minimal essential medium (MEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The F9 strain of feline calicivirus was kindly provided by Intervet, United Kingdom.
Optimization of drug concentrations to inhibit endocytosis.
The drugs used are listed in Table 1. CRFK cells (105) were seeded onto 13-mm-diameter glass coverslips in a 24-well plate. The following day, cells were pretreated with drugs (at various concentrations) for 30 min. Drug-treated cells were then incubated with 10 μg/ml Alexa Fluor 594-conjugated transferrin or 5 μg/ml Alexa Fluor 488 cholera toxin subunit B for 30 min on ice. Cells were washed twice with phosphate-buffered saline (PBS) and incubated for 30 min at 37°C. Cells were washed twice with PBS containing 0.2% newborn calf serum (PBS-NCS) and then fixed with PBS containing 4% formaldehyde (PBS-F) prior to examination on a Leica SP confocal microscope using TCS NT software. Laser and microscope settings were according to the manufacturer's instructions.
Optimization of drug concentrations to inhibit endosome acidification.
CRFK cells were seeded onto 13-mm-diameter glass coverslips in a 24-well plate. The following day, cells were pretreated with drugs (at various concentrations) for 30 min. Drug-treated cells were then incubated with 2 μM acridine orange for 1 min. Cells were washed three times with PBS, mounted onto glass slides using Citifluor (Citifluor Ltd.), and viewed immediately on a Leica SP confocal microscope.
Drug treatments to block entry.
CRFK cells were seeded onto 13-mm-diameter glass coverslips in a 24-well plate. The following day, cells were pretreated with drugs (at various concentrations as shown in Table 1) for 30 min and then infected with the F9 strain of FCV at a multiplicity of infection (MOI) of 10 in medium containing the appropriate concentration of each drug for a further 60 min. Supernatant containing virus was removed and the cells were washed three times with PBS. Fresh medium containing the drugs was added and the cells were incubated for a further 6 h. Cells were washed twice with PBS-NCS and then fixed with PBS-F prior to immunofluorescence staining.
Purification of F9 RNA and transfection into drug-treated cells.
Virus was purified from CRFK cells infected with F9 using the method described by Zhou et al. (63). Briefly, virus was precipitated using 0.2 M NaCl and 10% polyethylene glycol 3350. Pelleted virus was resuspended in 200 mM boric acid, pH 7.4, containing 0.5 M NaCl. Virions were isolated by isopycnic CsCl gradient centrifugation (1.31 g/ml) for 20 h at 270,000 × g in a Beckman SW40Ti rotor. Fractions containing virus were subjected to further ultracentrifugation to concentrate the samples and remove the CsCl.
RNA was isolated using the method adapted from that described by Burroughs and Brown (9). F9 virus in PBS was extracted three times with phenol and then ethanol precipitated overnight at −20°C. The pellet was washed with 100% ethanol to remove residual traces of phenol.
The purified RNA was used to transfect drug-treated CRFK cells (as described in the method above) using jetPEI (Autogen Bioclear) following the manufacturer's protocol. Briefly, 1 μg RNA was diluted with 0.15 M NaCl, and 2 μl jetPEI was diluted in 0.15 M NaCl. Each tube was vortexed briefly, and they were then mixed together. The RNA-jetPEI transfection mix was incubated at room temperature for 15 to 30 min before adding it to cells. Cells were incubated at 37°C and assessed for infection by immunofluorescence after 16 h.
Immunofluorescence.
Fixed cells were permeabilized by the addition of 0.2% Triton X-100, and the cells were incubated for 5 min at room temperature. Cells were then washed twice with PBS-NCS. Anti-FCV antibodies were added at the required concentration of 1/500 and incubated at room temperature for 30 min. Cells were then washed twice with PBS-NCS, the secondary antibody (diluted to 1/1,000) and DAPI (4′,6′-diamidino-2-phenylindole) were added, and the mixture was incubated for a further 30 min. Samples were then washed three times with PBS-NCS and coverslips were removed and mounted onto glass slides using ProLong Gold antifade mountant (Molecular Probes). Samples were examined using a Leica SP confocal microscope and TCS NT software. Laser and microscope settings were according to the manufacturer's instructions.
Virus binding assay.
CRFK cells were seeded into 24-well plates and allowed to grow to become confluent. Before use, the cells were pretreated with drugs for 30 min at 37°C. The plates were then washed twice in serum-free RPMI 1640 medium. Purified [35S]methionine-labeled virus (generated using the method described by Zhou et al. [63]) was added (30,000 cpm) in 100 μl medium containing the appropriate drug. The plates were incubated at 4°C for 45 min. Cells were washed three times with serum-free RPMI medium and lysed with 100 μl 3 M NaOH. Scintillation counting was used to assess virus binding.
Transfection of CRFK cells by plasmids expressing wild-type and mutant rab5 and eps15 followed by infection with F9 virus.
CRFK cells (105) were seeded into a 24-well plate containing 13-mm-diameter coverslips and grown overnight. Cells were transfected with 0.4 μg of either control plasmids (wild-type rab5 or D3D2 deletion of eps15) or plasmids expressing mutant rab5 (S34N [46]) and eps15 (EHΔ95-295 and ΔIII [5, 6]) using Fugene (Roche) transfection reagent. After transfection, cells were incubated at 37°C for 18 h to allow expression of the green fluorescent protein (GFP)-labeled wild-type and mutant proteins. The transfected cells were then infected with F9 virus at an MOI of 10 and incubated at 37°C for 30 min. Supernatant containing virus was then removed, and the cells were washed twice with PBS. The infected cells were then incubated at 37°C for 6 h to allow infection to proceed. Cells were then washed twice with PBS-NCS and fixed with 4% formaldehyde in PBS, ready for immunofluorescent staining.
Cell permeabilization assay.
Confluent monolayers of CRFK cells in 96-well tissue culture plates were infected with FCV at an MOI ranging from 0.1 to 10 in the absence or presence of 100 μg/ml α-sarcin. After cells had been incubated at 37°C for 60 min the virus inoculum and toxin were removed, and fresh serum-free MEM was added for an additional period of 30 min. After this, the medium was replaced with methionine-cysteine-free MEM supplemented with 25 μCi/ml of the [35S]Met-Cys (Promix, Amersham) and the cells were incubated for 1 h at 37°C. After the labeling period, the cells were washed with PBS, treated with 5% trichloroacetic acid for 5 min at room temperature, and washed three times with ethanol. The cell monolayer was allowed to dry before the addition of 50 μl of 0.1% sodium dodecyl sulfate in 0.1 N NaOH. Total radioactivity in the sample was determined by liquid scintillation counting by solubilizing the sample in OptiPhase “HiSafe” 2 (PerkinElmer).
Effect of inhibitors of endosome acidification on FCV-induced permeabilization.
Confluent monolayers of CRFK cells in 96-well tissue culture plates were pretreated with chloroquine (100 μM) or bafilomycin A1 (200 nM) for 30 min. Cells were then infected with 10 PFU of FCV per cell in the presence of 100 μg/ml α-sarcin. At 60 min postinfection at 37°C, the virus and the toxin were removed, and fresh serum-free MEM was added for an additional 30 min. After this, the medium was replaced with methionine-cysteine-free MEM supplemented with 25 μCi/ml of the [35S]Met-Cys (Promix, Amersham) and the cells were incubated for 1 h at 37°C. The cells were washed with PBS, treated with 5% trichloroacetic acid for 5 min at room temperature, and washed three times with ethanol. The cell monolayer was allowed to dry before the addition of 50 μl of 0.1% sodium dodecyl sulfate in 0.1 N NaOH. Total radioactivity in the sample was determined by liquid scintillation counting by solubilizing the sample in OptiPhase “HiSafe” 2 (PerkinElmer).
RESULTS
Optimization of drugs to block endocytosis.
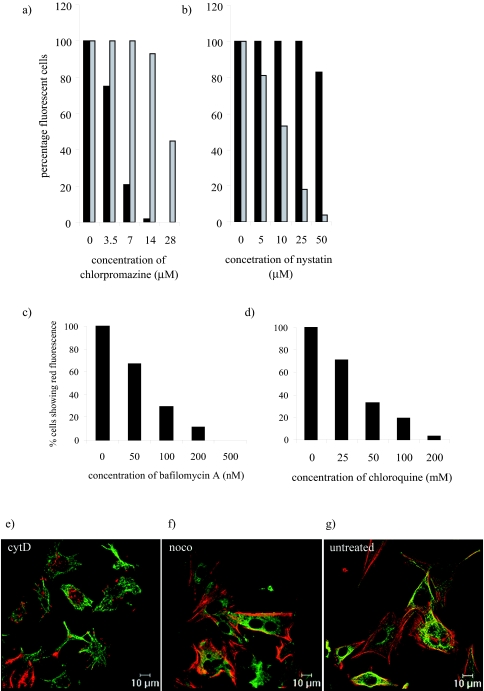
In order to check the efficacy of the various drugs in CRFK cells we carried out dose-response assays using transferrin to examine clathrin-mediated endocytosis, cholera toxin to examine lipid raft/caveola endocytosis, acridine orange to examine endosome acidification, fluorescent phalloidin to examine microfilaments, and a monoclonal antibody to alpha tubulin to examine microtubules. The results shown in Fig. 1a demonstrate that chlorpromazine inhibited the uptake of transferrin by CRFK cells in a dose-dependent manner, confirming the ability of this drug to inhibit clathrin-mediated endocytosis. Maximal inhibition occurred with concentrations of chlorpromazine between 14 and 28 μM. Concentrations of 14 μM and below had no effect on cholera toxin uptake; however, we did observe a 50% inhibition of cholera toxin uptake at 28 μM, suggesting some loss of specificity in the drug at this concentration. At this concentration of chlorpromazine we also observed toxic effects in the CRFK cells; DAPI-stained nuclei showed signs of apoptosis (not shown).
FIG. 1.
Optimization of drug concentrations to inhibit endocytosis. CRFK cells were treated with drugs at various concentrations: (a) chlorpromazine, (b) nystatin, (c) bafilomycin A1, (d) chloroquine, (e) cytochalasin D (cytD), (f) nocodazole (noco), or (g) untreated. (a and b) Cells were then incubated on ice for 30 min with transferrin (black bars) or cholera toxin (grey bars) in the presence or absence of the various drugs. Samples were then transferred to 37°C for a further 30 min. The cells were fixed and examined by fluorescent microscopy. (c and d) Cells were incubated with 2 μM acridine orange for 1 min before washing three times with PBS. Samples were mounted using Citifluor and examined immediately by fluorescent microscopy. (e, f, and g) Cells were fixed and examined by fluorescent microscopy. This experiment was repeated twice and this figure represents one experiment.
Figure 1b shows a similar assay on cells treated with nystatin. Here, we observed an inhibition of cholera toxin uptake but not transferrin, demonstrating the ability of nystatin to inhibit lipid raft/caveola-mediated endocytosis. Maximal inhibition was observed at 50 μM; however, this concentration also partially inhibited transferrin uptake. It has been published previously that excessive cholesterol depletion can also inhibit clathrin-mediated endocytosis (52). Subsequent experiments were carried out with 25 μM nystatin to avoid potential side effects.
Acridine orange is a weak base and is trapped and concentrated in acidic compartments such as endosomes and lysosomes. Incubation of cells with acridine orange results in the emission of green fluorescence where acridine orange is at low concentrations and red fluorescence at higher concentrations in the acidic endosomes. Figures 1c and d show the results of experiments to study the ability of bafilomycin A and chloroquine to inhibit endosome acidification by the lack of red fluorescence in treated cells. Bafilomycin A (Fig. 1c) and chloroquine (Fig. 1d) both inhibited the number of cells displaying red fluorescence in a dose-dependent manner, demonstrating their ability to inhibit endosome acidification. Both drugs showed some degree of toxicity at the highest concentration used (from the appearance of irregular/apoptotic DAPI-stained nuclei). The concentrations used in subsequent experiments were 200 nM bafilomycin A and 200 μM chloroquine. This experiment was also carried out with ammonium chloride and resulted in a similar dose-dependent decrease in red fluorescence in the presence of increasing ammonium chloride (data not shown).
The effects of cytochalasin D and nocodazole were monitored by immunofluorescence of microfilaments with fluorescent phalloidin and those of microtubules with an antibody to alpha tubulin. The effects of the optimal concentrations, 4 μM cytochalasin D and 20 μM nocodazole, are shown in Fig. 1e and f, respectively, and can be compared to results for untreated cells in Fig. 1g. Cytochalasin D treatment resulted in the loss in filamentous actin as detected by Alexa Fluor 594 phalloidin, while microtubules were still visible by indirect immunofluorescence with anti-alpha tubulin primary antibody and Alexa Fluor 488 anti-rat secondary antibody. Nocodazole treatment caused a loss of microtubules, but filamentous actin could still be detected. The concentrations used for the other drugs (amiloride, brefeldin A, and PP2) were based on previously published data (20, 36, 48).
Drug treatment of CRFK cells demonstrates that FCV infects cells via clathrin-coated pits and requires endosome acidification.
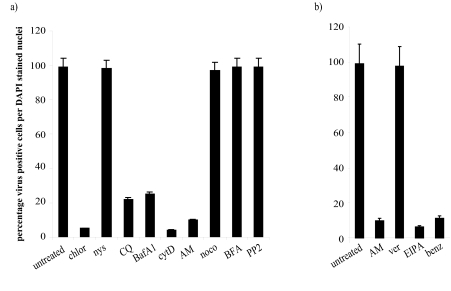
Previous studies of FCV infection suggested that the virus required acidification in endosomes (31). We have examined the infection route of FCV in more detail using a number of drugs known to inhibit various endocytosis routes (shown in Table 1). CRFK cells were pretreated with each of the drugs and then infected with the F9 strain of FCV at an MOI of 10 in the presence of each drug for the duration of the assay (6 h). Infection was examined by immunofluorescence of the viral capsid protein. The effects of the drugs were assessed by counting the percentages of virus-positive cells per DAPI-stained nucleus, and the results are shown in Fig. 2a.
FIG. 2.
(a) Effect of endocytosis inhibitors on FCV infection. CRFK cells were treated with either chlorpromazine (chlor), nystatin (nys), chloroquine (CQ), bafilomycin A1 (BafA1), cytochalasin D (cytD), amiloride (AM), nocodazole (noco), brefeldin A (BFA), or PP2 at the concentrations shown in Table 1. They were then infected with FCV at an MOI of 10 in the presence or absence of the various drugs. At 5 h postinfection (p.i.), the cells were fixed and viral capsid antigen was detected by immunofluorescence. The cells were scored as virus-positive cells per DAPI-stained nucleus. This experiment was repeated four times and this figure represents one experiment. (b) Effect of ion channel blockers on FCV infection. CRFK cells were treated with amiloride (AM), verapamil (ver), EIPA, or benzamil (benz) at the concentrations shown in Table 1. They were then infected with FCV at an MOI of 10 in the presence or absence of the various drugs. At 5 h p.i., the cells were fixed and viral capsid antigen was detected by immunofluorescence. The cells were scored as virus-positive cells per DAPI-stained nucleus. This experiment was repeated three times and this figure represents one experiment.
It can be seen that pretreatment of CRFK cells with chlorpromazine, which blocks endocytosis via clathrin-coated pits, inhibited FCV infection, reducing the proportion of virus-positive cells from 99% in untreated cells to 5% in chlorpromazine-treated cells. FCV infection is also inhibited by ammonium chloride (data not shown), chloroquine, and bafilomycin A1, all of which block endosome acidification. The proportion of virus-positive cells was reduced from 99% to 22% (chloroquine) and 25% (bafilomycin A). We also examined the role of actin microfilaments in FCV entry and demonstrated that disruption of microfilaments with cytochalasin D and jasplakinolide (data not shown) inhibited FCV infection, reducing the proportion of positive cells from 99% to 4% (cytochalasin D) and 5% (jasplakinolide). A potential role for macropinocytosis in FCV infection was examined using amiloride. Pretreatment of cells with amiloride inhibited FCV infection, reducing it from 99% to 10%.
Pretreatment of cells with nystatin, which blocks lipid raft/caveola endocytosis, PP2, which blocks Src family tyrosine kinases, brefeldin A, which inhibits vesicle transport in the secretory pathway, or nocodazole, which disrupts microtubules, has no effect on the infection by FCV.
Inhibition of FCV infection by other ion channel inhibitors.
Having observed the effect of amiloride, we then examined the effect of other ion channel inhibitors: 5-(N-ethyl-N-isopropyl)amiloride (EIPA) and benzamil, which also block Na+/H+ ion channels, and verapamil, which blocks Ca+ channels. We demonstrated (shown in Fig. 2b) that all the inhibitors of Na+/H+ ion channels could inhibit FCV infection: EIPA reduced infection from 99% to 6% and benzamil reduced infection from 99% to 11%. Verapamil pretreatment had no effect on FCV infection.
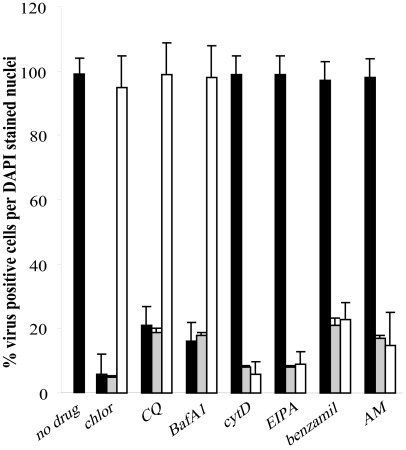
Effect of time of drug addition suggests a role in either entry or replication.
The initial experiments were carried out with drugs present throughout infection. In order to examine when the various drugs might be affecting FCV infection, we infected cells under three different conditions: (i) we infected drug-treated cells, and the drug remained present for an hour postinfection before washing off; (ii) we infected drug-treated cells, and the drug remained present for the duration of the assay; and (iii) we infected untreated cells, and the drug was added 1 hour postinfection. We used the drugs that had shown activity in the previous assay: chlorpromazine, chloroquine, bafilomycin A, cytochalasin D, amiloride, EIPA, and benzamil.
From the results shown in Fig. 3 we can see that chlorpromazine, chloroquine, and bafilomycin all act at an early stage in infection and are effective only when used prior to infection, i.e., they are acting during an entry step. However, cytochalasin D and the Na+/H+ channel inhibitors all appear to be acting at a later time during infection, as can be seen in Fig. 3: no inhibition is observed when the drug is present for the first hour, but when it is added postinfection, a level of inhibition is observed similar to that when the drug is present for the duration of the assay. This suggests that the drugs may be influencing a stage in replication such as RNA or protein production. Due to the nature of the assay we can exclude a role in assembly, as the capsid antibody will detect capsid monomers as well as mature virions.
FIG. 3.
Stage of FCV infection affected by the various drugs. CRFK cells were incubated with drugs during entry only (filled bars), after entry (open bars), or throughout infection (gray bars). At 5 h p.i., the cells were fixed and viral capsid antigen was detected by immunofluorescence. The cells were scored as virus-positive cells per DAPI-stained nucleus. This experiment was repeated four times and this figure represents one experiment. chlor, chlorpromazine; CQ, chloroquine; BafA1, bafilomycin A1; cytD, cytochalasin D; AM, amiloride.
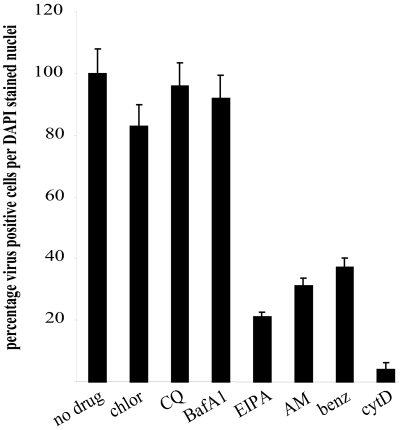
Effect of drugs on transfected RNA.
In order to clarify the effects of the drugs seen in the previous assay, we purified virus RNA from FCV virions for use in transfection of drug-treated cells. This assay examined any role of the drugs in replication alone, as the RNA is delivered artificially in order to circumvent the entry process. We examined the drugs that had shown an effect on FCV infection from the previous assays. Drug-treated cells were transfected with 1 μg FCV RNA and incubated for 16 h. We examined infection by immunofluorescence of the FCV capsid antigen, and the results from a representative assay are shown in Fig. 4. From these results we observed that pretreatment of cells with chlorpromazine, chloroquine, and bafilomycin A1 resulted in no change compared to untreated controls, indicating that these drugs act at a stage prior to virus uncoating and replication. Pretreatment of cells with cytochalasin D and amiloride and its derivatives, however, did result in a decrease in the detected infectivity from transfected RNA. Cytochalasin D treatment reduced the observed infectivity from 100% in the control samples to 5%; amiloride, benzamil, and EIPA reduced infectivity to 33%, 29%, and 21%, respectively.
FIG. 4.
Effect of inhibitors on replication from transfected FCV RNA. CRFK cells were pretreated with various inhibitors at the concentrations shown in Table 1. They were then transfected with 1 μg purified FCV RNA. Cells were fixed after 16 h and stained for virus capsid antigen by immunofluorescence. Results are presented as the percentage of infected cells compared to untreated controls (which are scored at 100%). This experiment was repeated twice and this figure represents one experiment. chlor, chlorpromazine; CQ, chloroquine; BafA1, bafilomycin A1; AM, amiloride; benz, benzamil; cytD, cytochalasin D.
FCV binding is unaffected by drugs which inhibit clathrin-mediated endocytosis.
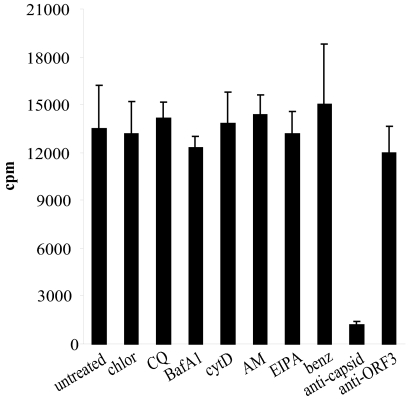
Additional studies were undertaken to discriminate between drug effects on virus binding and entry. A potential effect of the drugs could be to alter virus binding by downregulation of receptor and accessory proteins (a known action of chlorpromazine is to downregulate receptor recycling [61]). We examined the drugs that had shown an effect on FCV infection from the previous assays. Drug-treated cells were incubated with highly purified [35S]methionine-cysteine-labeled FCV; the results from a representative assay are shown in Fig. 5. Pretreatment of cells with chlorpromazine, chloroquine, bafilomycin A, amiloride, EIPA, benzamil, or cytochalasin D had no effect on the ability of FCV to bind to CRFK cells. When virus was preincubated with a polyclonal anticapsid antibody, however, binding was inhibited 10-fold; a control polyclonal antibody to FCV ORF3 had no effect on virus binding.
FIG. 5.
Effect of inhibitors on FCV binding. CRFK cells were pretreated with the various inhibitors at the concentrations shown in Table 1. Cells were then incubated with [35S]methionine-cysteine-labeled purified FCV (30,000 cpm) on ice for 45 min. Samples were washed with serum-free medium and then harvested by lysing the cells with 3 M NaOH. Virus binding was assessed by scintillation counting. This experiment was repeated twice and this figure represents one experiment. chlor, chlorpromazine; CQ, chloroquine; BafA1, bafilomycin A1; AM, amiloride; benz, benzamil; cytD, cytochalasin D.
Dominant-negative mutants of rab5 and eps15 demonstrate that clathrin-mediated endocytosis is required for FCV entry.
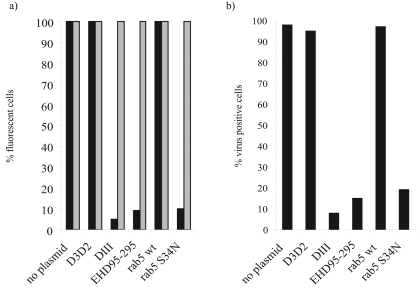
The use of drugs to examine viral endocytosis routes has been well documented; however, there does remain the possibility of side effects from the use of drugs, and we therefore wished to confirm the role of clathrin-mediated endocytosis using a more precisely targeted inhibitor. We obtained wild-type and dominant-negative mutants of eps15 (from Alexandre Benmerah) and rab5 (from Stephen Ferguson). The wild-type proteins of both are important in clathrin-mediated endocytosis. To check their efficacy in feline cells, plasmids expressing the dominant-negative mutants and controls were transfected into CRFK cells that were then subsequently incubated with fluorescent transferrin or cholera toxin. Figure 6a shows that transfection of CRFK cells with no plasmid or the control plasmids (D3D2 and rab5 wild type) had no effect on the uptake of transferrin. However, transfection with ΔIII, EHΔ95-295, or rab5 S34N inhibited transferrin uptake by 95, 91, or 90%, respectively, demonstrating that these dominant-negative mutants function in the feline cells. None of the wild-type or mutant proteins had any effect on cholera toxin B uptake.
FIG. 6.
Dominant-negative mutants inhibiting clathrin-mediated endocytosis block FCV infection. CRFK cells were transfected with either wild-type rab5 or a dominant-negative mutant of rab5 (S34N) or control eps15 (D3D2) or one of two dominant-negative mutants of eps15 (DIII or EHD95-295) and incubated for 16 h. Cells were then either (a) incubated with 10 μg/ml Alexa Fluor 594 transferrin (black bars) or 5 μg/ml Alexa Fluor 488 cholera toxin B (gray bars) for 30 min on ice followed by 30 min at 37°C or (b) infected with FCV at an MOI of 10 and incubated for a further 5 h. They were then fixed and infection was assessed by immunofluorescence staining of virus capsid antigen. All plasmids express the proteins as GFP fusion proteins. Results are shown as the percentages of virus-positive cells per GFP-positive cell. This experiment was repeated four times, and this figure represents one experiment.
The results from subsequent experiments involving FCV infection of transfected cells are shown in Fig. 6b. Transfection of the control plasmids D3D2 (for eps15) and the rab5 wild type had no effect of the number of infected cells. However, when mutants of eps15 (ΔIII and EHΔ95-295) were transfected, a reduction in the proportion of virus-positive cells was observed: 95% infected cells to 8% and 15%, respectively. A similar result was observed when the rab5 dominant-negative mutant, S34N, was transfected, with a reduction from 97% to 19% infected cells.
FCV entry permeabilizes CRFK cells and allows coentry of the translation inhibitor α-sarcin.
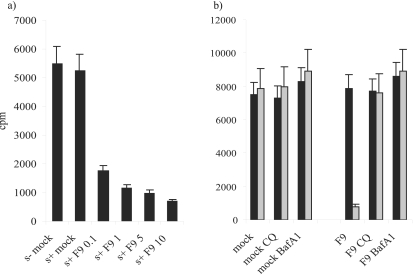
Viruses such as poliovirus and rotavirus have been shown to permeabilize cells to toxins such as α-sarcin and hygromycin B. We examined FCV infection in the presence of α-sarcin to see if this phenomenon occurred. The results shown in Fig. 7a demonstrate that α-sarcin is unable to enter mock-infected CRFK cells. However, when increasing amounts of FCV were added, the levels of host cell translation were reduced compared to uninfected cells and untreated cells, indicating that the toxin was able to gain entry during FCV infection. Effects could be seen even when 0.1 PFU/cell FCV was added. [35S]methionine incorporation was reduced by 67%. The maximum effect was observed at 10 PFU/cell, when [35S]methionine incorporation was reduced by 87%. No further reduction is achieved by adding more virus, as infecting with 200 PFU/cell does not inhibit [35S]methionine incorporation to any greater extent (data not shown).
FIG. 7.
FCV infection permeabilizes cells to toxins. (a) CRFK cells were infected with FCV at MOIs ranging from 0.1 to 10 in the absence (s−) or presence (s+) of α-sarcin at various concentrations. After 60 min virus and toxin were removed and the cells were incubated with methionine-cysteine-free MEM. This medium was removed after 30 min and replaced with methionine-cysteine-free MEM supplemented with [35S]methionine-cysteine (Promix). Cells were incubated for a further 60 min before washing and harvesting. Total radioactivity in the samples was determined by scintillation counting. This experiment was repeated twice, and this figure represents one experiment. (b) Endosome acidification inhibitors block FCV-induced permeabilization of cells to α-sarcin. CRFK cells were treated with bafilomycin A1 or chloroquine and then either infected with FCV at an MOI of 10 or mock infected in the absence (black bars) or presence (gray bars) of 100 μg/ml α-sarcin. Samples were processed as in the legends to the previous figures. CQ, chloroquine; BafA1, bafilomycin A1.
Drugs that block endosome acidification inhibit membrane permeabilization induced by FCV uncoating.
Cells preincubated with the inhibitors were incubated with 10 PFU/cell FCV and 100 μg/ml α-sarcin. The results are shown in Fig. 7b. Neither chloroquine nor bafilomycin A permeabilized the cells to α-sarcin, as seen by the fact that there was no decrease in host cell translation. FCV incubated with cells in the presence of sarcin but in the absence of bafilomycin A or chloroquine reduced incorporation by 90%, as seen previously. When cells were pretreated with either bafilomycin A or chloroquine before infection, they incorporated levels of [35S]methionine similar to those of the controls, demonstrating that acidification was required during virus-induced intoxication.
DISCUSSION
Early events during virus infection involve the interaction of virions with cell surface molecules, their subsequent internalization, uncoating of the virus genome, and penetration of the cytoplasm. A number of internalization and trafficking routes have been demonstrated for different viruses. Our present study has involved examining the entry route taken by feline calicivirus to infect cells.
We examined the entry route of FCV initially using drugs that have been shown to inhibit various entry routes. These drugs (shown in Table 1) inhibited clathrin-mediated endocytosis (chlorpromazine), lipid raft/caveola-mediated endocytosis (nystatin), macropinocytosis (amiloride), and endosome acidification (chloroquine and bafilomycin A1), or disrupted the cytoskeleton in order to inhibit trafficking routes (cytochalasin D and nocodazole). The drugs were first tested at a range of concentrations to identify the optimum dose to use to inhibit uptake of fluorescent transferrin, cholera toxin, and acridine orange (markers for clathrin-mediated endocytosis, caveolae, and endosome acidification, respectively) and also to look for any possible additional effects at higher doses. We discovered that the optimum concentrations in our system were as follows: nystatin at 25 μM, chlorpromazine at 14 μM, chloroquine at 100 μM, bafilomycin A1 at 200 nM, amiloride at 200 μM, nocodazole at 20 μM, and cytochalasin D at 4 μM. Previous studies on picornavirus replication have shown that the optimal concentration for brefeldin A to inhibit replication was 18 μM. We also observed that at higher concentrations, nonspecific effects of the drugs could be observed. Doses of nystatin at 50 μM and above interfere with other endocytosis pathways, as inhibition of transferrin entry could be observed. It has been reported previously that excess depletion of cholesterol from the membrane leads to inhibition of clathrin-coated pit endocytosis as well as uptake through lipid rafts/caveolae (45, 52). At higher doses of chlorpromazine, bafilomycin A, chloroquine, and cytochalasin D, an increase in cell death could be detected by DAPI staining, demonstrating that the reduction in the number of infected cells observed was due to decreased cell viability and not the inhibition of specific entry pathways.
We have demonstrated that FCV infection was inhibited by chlorpromazine, chloroquine, and bafilomycin A1. These data showed that FCV entered cells via clathrin-mediated endocytosis and required endosome acidification. A previous study by Kreutz and Seal (31) had also observed the inhibition of FCV infection by chloroquine. No effect was seen with cells treated with nystatin, nocodazole, or brefeldin A, demonstrating that caveolae/lipid rafts, trafficking along microtubules, and the requirement for Arf1 in generating vesicles for viral RNA replication (4) are not involved in FCV infection. Cytochalasin D and amiloride and its derivatives did inhibit FCV infection and will be discussed in more detail below.
Further experiments to identify the time of action of the drugs included the addition of the drugs at times prior to infection and during entry only, during replication only, or throughout the entire infection. These data suggested that chlorpromazine, chloroquine, and bafilomycin A were acting at early times only, as no inhibition could be seen when the drugs were added 1 hour postinfection. These data were confirmed when purified viral RNA was transfected into drug-treated cells and showed no difference in the levels of virus replication compared to untreated controls. Further controls were carried out to study the effect of the drug treatments on virus binding to the cells. This experiment showed that chlorpromazine, chloroquine, and bafilomycin A do not affect FCV binding to cells. These data confirmed that chlorpromazine, chloroquine, and bafilomycin A were acting at an early stage during entry, postbinding but prior to virus RNA release.
In order to examine more precisely the role of clathrin-mediated endocytosis in FCV entry, we obtained GFP fusion proteins of dominant-negative mutants of rab5 (from Steven Ferguson, Ontario, Canada) and eps15 (from Alexandre Benmerah, Paris, France), both of which are known to inhibit clathrin-mediated endocytosis. Experiments were carried out to check the efficacy of these mutants in feline cells by monitoring the uptake of fluorescent transferrin and cholera toxin B. We demonstrated that dominant-negative mutants of eps15 and rab5 inhibited the uptake of fluorescent transferrin but not cholera toxin B. Transfection of the dominant-negative mutants into cells markedly decreased the ability of FCV to infect the transfected population, thus confirming the importance of clathrin-mediated endocytosis in FCV infection. Ideally, we would have checked the effect of the dominant-negative mutants on binding and virus replication after virus RNA transfection. However, in order to do this we would have had to generate stable cell lines, as the transfection efficiency in our experiments was around 50% and any results would have reflected this mixed population of mutant-expressing and untransfected cells.
We studied the ability of FCV to permeabilize cells to toxins during entry. This phenomenon has been observed for a number of enveloped and nonenveloped viruses including Semliki Forest virus, reovirus, rotavirus, canine parvovirus, hepatitis C virus, Sindbis virus, and poliovirus (19, 32, 34, 35, 40, 41, 62). This permeabilization is thought to coincide with either fusion of the virus envelope with cell membranes in enveloped viruses or the entry of capsids and/or virus RNA into the cytosol in nonenveloped viruses. We studied the ability of FCV (with increasing MOIs) to permeabilize cells using α-sarcin. We demonstrated that even at 0.1 PFU/cell FCV was able to permeabilize cells and allow the uptake of α-sarcin. This resulted in an inhibition of 67% in the incorporation of [35S]methionine-cysteine. This is greater than would be expected, as only 10% of the cells should be infected (based on PFU count). It is possible that the effect observed is due to the action of noninfectious particles that would not generate a plaque but could still undergo entry and permeabilize the cells, allowing intoxication. Maximal intoxication, with around 90% inhibition of [35S]methionine-cysteine incorporation, was seen at 10 PFU/cell as would be expected, as statistically every cell should be infected. No further decrease was seen when a count of 200 PFU/cell was used (data not shown). Drugs such as chloroquine or bafilomycin A1 that raise the endosomal pH could inhibit this permeabilization. These data suggested that FCV undergoes structural changes in the low-pH environment of the endosome to allow the release of its RNA (or capsid) into the cytoplasm. We are currently examining the mechanism of RNA release to see whether FCV creates a pore, like picornaviruses, to release its RNA or massively disrupts the endosome, like adenovirus, to release the contents (43).
The experiments to examine the time of action of the drugs showed that cytochalasin D and amiloride and its derivatives acted at later times during infection and inhibited virus replication. This was confirmed by transfection of FCV RNA, where drug-treated cells showed a marked reduction in virus replication. We have used this assay previously to examine the effect of a similar set of drugs on echovirus 11 entry (51). In that experiment cytochalasin D (amiloride was not used in the study) had no effect on the abilities of two human cell lines (HT29 and RD cells) to replicate EV11 from transfected RNA. This suggests that the effect we have observed here, where cytochalasin D is able to inhibit FCV replication from transfected RNA, is unlikely to be due simply to any toxic effects of the drug. Both cytochalasin D and amiloride and its derivatives had no effect on FCV binding to cells. These data suggested that these drugs were acting later during infection on a stage during virus replication, either during virus RNA replication or translation or capsid assembly and release.
A possible explanation for the inhibition resulting from cytochalasin D and amiloride is an effect on macropinocytosis, which both of these drugs are known to affect. Meier et al. (37) described a role for macropinocytosis in adenovirus infection. However, in this system macropinocytosis was involved in the release of adenovirus from endosomes. This is unlikely to be the case with FCV, as cytochalasin D and amiloride and its derivatives are involved at a step after the RNA has been released from endosomes.
Previous studies have suggested that amiloride and its derivatives could inhibit the replication of picornaviruses due to their effects on intracellular pH. Human rhinovirus type 14 was shown to be inhibited by EIPA in tracheal epithelial cells; the mechanism of action put forward in that study was a block in endosome acidification induced by EIPA and the subsequent failure to release HRV RNA from endosomes (53). Our data do not, however, support this mechanism for FCV, as virus replication from transfected RNA was still susceptible to the effects of the drugs. Gazina et al. (20) studied the effect of EIPA on rhinovirus type 2 replication and suggested that it inhibited virus production and release with a more pronounced effect on virus release. Our data are in agreement with an effect on virus production; however, our assay did not examine virus release. Another study by Holsey et al. (26) demonstrated that poliovirus infection results in an elevation in cytoplasmic pH which was necessary for efficient virus replication. This rise in intracellular pH could be blocked by EIPA and led to a decrease in poliovirus replication.
An alternative possibility is that the effect of EIPA may be due to interactions with viral proteins. Ewart et al. (18) and Premkumar et al. (44) have demonstrated that derivatives of amiloride could block the ion channels formed by human immunodeficiency virus (HIV) Vpu and hepatitis C virus (HCV) p7, respectively. These derivatives were also shown to inhibit HIV replication (17). It was suggested by Gazina et al. (20) that the picornaviral transmembrane protein 2B could be the target for EIPA. This protein has some structural similarity to Vpu and p7 and is important for viral RNA replication. It may also play a role in virus release (1, 2, 16, 20, 28, 55, 56, 57, 58). It is not known if FCV encodes an ion channel similar to 2B, but it does encode a number of polypeptides with sequence motifs characteristic of picornaviral proteins, such as the helicase, proteinase, and polymerase (38).
This is the first report of cytochalasin D inhibiting the replication of a nonenveloped RNA virus. There are examples of the importance of an intact actin network in the transcription/replication of human parainfluenza virus type 3, canine distemper virus, respiratory syncytial virus, and Newcastle disease virus (8, 12, 13, 14, 23, 24, 29, 30). Early ultrastructural studies of FCV infection noted the formation of filaments in infected cells and the association of virions with these filaments (33). During infection we have observed, using immunofluorescence, that while cortical actin remained intact and some fibers could also be observed in the cytoplasm, the majority of stress fibers disappeared (data not shown). Further experiments are ongoing to investigate the effects of both cytochalasin D and amiloride and its derivatives in FCV replication.
Acknowledgments
We thank Alexandre Benmerah for eps15 plasmids and Stephen Ferguson for rab5 plasmids.
This work was supported by the BBSRC and the Wellcome Trust.
REFERENCES
- 1.Aldabe, R., A. Barco, and L. Carrasco. 1996. Membrane permeabilization by poliovirus proteins 2B and 2BC. J. Biol. Chem. 271:23134-23137. [DOI] [PubMed] [Google Scholar]
- 2.Aldabe, R., A. Irurzun, and L. Carrasco. 1997. Poliovirus protein 2BC increases cytosolic free calcium concentrations. J. Virol. 71:6214-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belov, G. A., M. H. Fogg, and E. Ehrenfeld. 2005. Poliovirus proteins induce membrane association of GTPase ADP-ribosylation factor. J. Virol. 79:7207-7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 6.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berryman, S., S. Clark, P. Monaghan, and T. Jackson. 2005. Early events in integrin αvβ6-mediated cell entry of foot-and-mouth disease virus. J. Virol. 79:8519-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke, E., L. Dupuy, C. Wall, and S. Barik. 1998. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology 252:137-148. [DOI] [PubMed] [Google Scholar]
- 9.Burroughs, J. N., and F. Brown. 1978. Presence of a covalently linked protein on calicivirus RNA. J. Gen. Virol. 41:443-446. [DOI] [PubMed] [Google Scholar]
- 10.Carter, M. J., I. D. Milton, P. C. Turner, J. Meanger, M. Bennett, and R. M. Gaskell. 1992. Identification and sequence determination of the capsid protein gene of feline calicivirus. Arch. Virol. 122:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung, S. K., J. Y. Kim, I. B. Kim, S. I. Park, K. H. Paek, and J. H. Nam. 2005. Internalization and trafficking mechanisms of coxsackievirus B3 in HeLa cells. Virology 333:31-40. [DOI] [PubMed] [Google Scholar]
- 12.De, B. P., A. Lesoon, and A. K. Banerjee. 1991. Human parainfluenza virus type 3 transcription in vitro: role of cellular actin in mRNA synthesis. J. Virol. 65:3268-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De, B. P., A. L. Burdsall, and A. K. Banerjee. 1993. Role of cellular actin in human parainfluenza virus type 3 genome transcription. J. Biol. Chem. 268:5703-5710. [PubMed] [Google Scholar]
- 14.De, B. P., and A. K. Banerjee. 1999. Involvement of actin microfilaments in the transcription/replication of human parainfluenza virus type 3: possible role of actin in other viruses. Microsc. Res. Tech. 47:114-123. [DOI] [PubMed] [Google Scholar]
- 15.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doedens, J. R., and K. Kirkegaard. 1995. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 14:894-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewart, G. D., N. Nasr, H. Naif, G. B. Cox, A. L. Cunningham, and P. W. Gage. 2004. Potential new anti-human immunodeficiency virus type 1 compounds depress virus replication in cultured human macrophages. Antimicrob. Agents Chemother. 48:2325-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewart, G. D., K. Mills, G. B. Cox, and P. W. Gage. 2002. Amiloride derivatives block ion channel activity and enhancement of virus-like particle budding caused by HIV-1 protein Vpu. Eur. Biophys. J. 31:26-35. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Puentes, C. 1983. Permeability to alpha sarcin in virus-infected cells. Mol. Cell. Biochem. 50:185-191. [DOI] [PubMed] [Google Scholar]
- 20.Gazina, E. V., D. N. Harrison, M. Jefferies, H. Tan, D. Williams, D. A. Anderson, and S. Petrou. 2005. Ion transport blockers inhibit human rhinovirus 2 release. Antivir. Res. 67:98-106. [DOI] [PubMed] [Google Scholar]
- 21.González, M. E., B. Alarcón, and L. Carrasco. 1987. Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob. Agents Chemother. 31:1388-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunert, H. P., K. U. Wolf, K. D. Langner, D. Sawitzky, K. O. Habermehl, and H. Zeichhardt. 1997. Internalization of human rhinovirus 14 into HeLa and ICAM-1-transfected BHK cells. Med. Microbiol. Immunol. 186:1-9. [DOI] [PubMed] [Google Scholar]
- 23.Gupta, S., B. P. De, J. A. Drazba, and A. K. Banerjee. 1998. Involvement of actin microfilaments in the replication of human parainfluenza virus type 3. J. Virol. 72:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaguchi, M., K. Nishikawa, T. Toyoda, T. Yoshida, T. Hanaichi, and Y. Nagai. 1985. Transcriptive complex of Newcastle disease virus. II. Structural and functional assembly associated with the cytoskeletal framework. Virology 147:295-308. [DOI] [PubMed] [Google Scholar]
- 25.Herbert, T. P., I. Brierley, and T. D. Brown. 1997. Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J. Gen. Virol. 78:1033-1040. [DOI] [PubMed] [Google Scholar]
- 26.Holsey, C., E. J. Cragoe, Jr., and C. N. Nair. 1990. Evidence for poliovirus-induced cytoplasmic alkalinization in HeLa cells. J. Cell. Physiol. 142:586-591. [DOI] [PubMed] [Google Scholar]
- 27.Joki-Korpela, P., V. Marjomäki, C. Krogerus, J. Heino, and T. Hyypiä. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, K. L., and P. Sarnow. 1991. Three poliovirus 2B mutants exhibit noncomplementable defects in viral RNA amplification and display dosage-dependent dominance over wild-type poliovirus. J. Virol. 65:4341-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallewaard, N. L., A. L. Bowen, and J. E. Crowe, Jr. 2005. Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 331:73-81. [DOI] [PubMed] [Google Scholar]
- 30.Katayama, H., M. Hori, K. Sato, M. Kajita, H. Ozaki, H. Karaki, K. Ohashi, and C. Kai. 2004. Role of actin microfilaments in canine distemper virus replication in vero cells. J. Vet. Med. Sci. 66:409-415. [DOI] [PubMed] [Google Scholar]
- 31.Kreutz, L. C., and B. S. Seal. 1995. The pathway of feline calicivirus entry. Virus Res. 35:63-70. [DOI] [PubMed] [Google Scholar]
- 32.Liprandi, F., Z. Moros, M. Gerder, J. E. Ludert, F. H. Pujol, M. C. Ruiz, F. Michelangeli, A. Charpilienne, and J. Cohen. 1997. Productive penetration of rotavirus in cultured cells induces coentry of the translation inhibitor alpha-sarcin. Virology 237:430-438. [DOI] [PubMed] [Google Scholar]
- 33.Love, D. N., and M. Sabine. 1975. Electron microscopic observation of feline kidney cells infected with a feline calicivirus. Arch. Virol. 48:213-228. [DOI] [PubMed] [Google Scholar]
- 34.Madan, V., M. A. Sanz, and L. Carrasco. 2005. Requirement of the vesicular system for membrane permeabilization by Sindbis virus. Virology 332:307-315. [DOI] [PubMed] [Google Scholar]
- 35.Martinez, C. G., R. Guinea, J. Benavente, and L. Carrasco. 1996. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J. Virol. 70:576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maynell, L. A., K. Kirkegaard, and M. W. Klymkowsky. 1992. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 66:1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neill, J. D. 1990. Nucleotide sequence of a region of the feline calicivirus genome which encodes picornavirus-like RNA-dependent RNA polymerase, cysteine protease and 2C polypeptides. Nucleic Acids Res. 17:145-160. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell, V., M. LaRocco, H. Duque, and B. Baxt. 2005. Analysis of foot-and-mouth disease virus internalization events in cultured cells. J. Virol. 79:8506-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otero, M. J., and L. Carrasco. 1987. Proteins are cointernalized with virion particles during early infection. Virology 160:75-80. [DOI] [PubMed] [Google Scholar]
- 41.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 43.Prchla, E., C. Plank, E. Wagner, D. Blaas, and R. Fuchs. 1995. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J. Cell Biol. 131:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Premkumar, A., L. Wilson, G. D. Ewart, and P. W. Gage. 2004. Cation-selective ion channels formed by p7 of hepatitis C virus are blocked by hexamethylene amiloride. FEBS Lett. 557:99-103. [DOI] [PubMed] [Google Scholar]
- 45.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seachrist, J. L., P. H. Anborgh, and S. S. Ferguson. 2000. β2-Adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by Rab GTPases. J. Biol. Chem. 275:27221-27228. [DOI] [PubMed] [Google Scholar]
- 47.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 48.Sorkina, T., F. Huang, L. Beguinot, and A. Sorkin. 2002. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalisation of epidermal growth factor receptor. J. Biol. Chem. 277:27433-27441. [DOI] [PubMed] [Google Scholar]
- 49.Sosnovtsev, S. A., S. V. Sosnovtsev, and K. Y. Green. 1999. Mapping of the feline calicivirus proteinase responsible for autocatalytic processing of the nonstructural polyprotein and identification of a stable proteinase-polymerase precursor protein. J. Virol. 73:6626-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sosnovtsev, S. V., and K. Y. Green. 2000. Identification and genomic mapping of the ORF3 and VPg proteins in feline calicivirus virions. Virology 277:193-203. [DOI] [PubMed] [Google Scholar]
- 51.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, K. Nakayama, M. Yanai, Y. Numazaki, and H. Sasaki. 2001. Bafilomycin A(1) inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1115-L1127. [DOI] [PubMed] [Google Scholar]
- 54.Triantafilou, K., and M. Triantafilou. 2004. Lipid-raft-dependent Coxsackievirus B4 internalization and rapid targeting to the Golgi. Virology 326:6-19. [DOI] [PubMed] [Google Scholar]
- 55.van Kuppeveld, F. J., A. S. de Jong, W. J. Melchers, and P. H. Willems. 2005. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol. 13:41-44. [DOI] [PubMed] [Google Scholar]
- 56.van Kuppeveld, F. J., J. G. Hoenderop, R. L. Smeets, P. H. Willems, H. B. Dijkman, J. M. Galama, and W. J. Melchers. 1997. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 16:3519-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Kuppeveld, F. J., W. J. Melchers, K. Kirkegaard, and J. R. Doedens. 1997. Structure-function analysis of coxsackie B3 virus protein 2B. Virology 227:111-118. [DOI] [PubMed] [Google Scholar]
- 58.van Kuppeveld, F. J. M., J. M. D. Galama, J. Zoll, and W. J. G. Melchers. 1995. Genetic analysis of a hydrophobic domain of coxsackie B3 virus protein 2B: a moderate degree of hydrophobicity is required for a cis-acting function in viral RNA synthesis. J. Virol. 69:7782-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vihinen-Ranta, M., A. Kalela, P. Makinen, L. Kakkola, V. Marjomaki, and M. Vuento. 1998. Intracellular route of canine parvovirus entry. J. Virol. 72:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wunschmann, S., J. D. Medh, D. Klinzmann, W. N. Schmidt, and J. T. Stapleton. 2000. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J. Virol. 74:10055-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou, L., Q. Yu, and M. Luo. 1994. Characterization of two density populations of feline calicivirus particles. Virology 205:530-533. [DOI] [PubMed] [Google Scholar]