Abstract
The infectious cycle of human papillomavirus type 1 (HPV1) is accompanied by abundant expression of the full-length E1^E4 protein (17-kDa) and smaller E4 polypeptides (16-, 11-, and 10-kDa) that arise by sequential loss of N-terminal E1^E4 sequences. HPV1 E4 inhibits G2-to-M transition of the cell cycle. Here, we show that HPV1 E4 proteins mediate inhibition of cell division by more than one mechanism. Cells arrested by coexpression of E1^E4 (E4-17K) and a truncated protein equivalent to the 16-kDa species (E4-16K) contain inactive cyclin B1-cdk1 complexes. Inactivation of cdk1 is through inhibitory Tyr15 phosphorylation, with cells containing elevated levels of Wee1, the kinase responsible for inhibitory cdk1 phosphorylation. Consistent with these findings, overexpression of Wee1 enhanced the extent to which E4-17K/16K-expressing cells arrest in G2, indicating that maintenance of Wee1 activity is necessary for inhibition of cell division induced by coexpression of the two E4 proteins. Moreover, we have determined that depletion of Wee1 by small interfering RNA (siRNA) alleviates the G2 block imposed by E4-17K/16K. In contrast however, maintenance of Wee1 activity is not necessary for G2-to-M inhibition mediated by E4-16K alone, as overexpression or depletion of Wee1 does not influence the G2 arrest function of E4-16K. Cells arrested by E4-16K expression contain low levels of active cyclin B1-cdk1 complexes. We hypothesize that differential expression of HPV1 E4 proteins during the viral life cycle determines the host cell cycle status. Different mechanisms of inhibition of G2-to-M transition reinforce the supposition that distinct E4 functions are important for HPV replication.
Human papillomaviruses (HPVs) are small-DNA viruses that infect cutaneous and mucosal squamous epithelium and produce benign or malignant tumors. The HPV infectious cycle is initiated in keratinocytes of the basal cell layer (13). In these cells the viral genome is maintained at a low copy number by synchronous replication with chromosomal DNA. When the infected cell moves up from the basal layer and enters the terminal differentiation program, the virus switches to vegetative replication and the HPV genome is amplified to very high levels. Further differentiation is accompanied by expression of the capsid proteins and eventual virion formation in the most differentiated cells. The early protein, E1^E4, expressed from a spliced mRNA transcript, is abundant in cells that have switched to productive infection (29, 30, 33). Loss of E1^E4 expression in experimental systems that recapitulate the infectious cycle correlates with reduced viral genome amplification and late gene expression in differentiating keratinocytes (28, 32, 49), indicating that E1^E4 has an important role in the vegetative cycle of HPVs. Knowledge of E1^E4 functions is limited, however, precluding a complete understanding of the role of this viral protein in the HPV life cycle.
The HPV type 1 (HPV1) E1^E4 protein has been shown to mediate reorganization of the promyelocytic leukemia protein from nuclear dot 10 domains to nuclear inclusion bodies (42). Because formation of E4-promyelocytic leukemia protein inclusions occurs in cells that have switched from maintenance of viral genome replication to genome amplification, this E4-mediated process may play an important role in facilitating the infectious cycle in these cells (7, 42, 44). The E1^E4 proteins also associate with the keratin intermediate filament network, but the function of this interaction is not known (4, 9, 37, 40). Reorganization of the keratin cytoskeleton by E1^E4 proteins may compromise cell integrity and facilitate release of the virus from the upper layers of the lesion (9, 41). However, only a limited degree of cytoskeleton collapse has been observed in vivo (37, 45), and perturbation of the keratin networks is not a function of all E1^E4 proteins (40, 43). Cytokeratin association of E1^E4 proteins may act to tether cellular targets of E1^E4 to the networks, and indeed, cyclin B1-cdk1 complexes are sequestered onto the cytokeratin networks by HPV16 E1^E4 (5). In this scenario, HPV16 E1^E4 induces cells to arrest in the G2 phase of the cell cycle, and failure of active cyclin B1-cdk1 to translocate to the nucleus correlates with the G2 arrest function (5, 6).
We have shown that a modified form of HPV1 E4 also induces a G2 cell cycle arrest (19). In HPV1 infections, multiple species of E4 are produced as a result of posttranslational modification (PTM) (3, 10). Proteolysis is an important facet of E4 PTM and involves a sequential cleavage of residues from the N terminus of the 17-kDa E1^E4 polypeptide to produce species of 16, 11, and 10 kDa (39). A progressive accumulation of the modified forms occurs, so that the more processed species predominate in late stages of the infectious cycle. A mutant protein (E4-16K) containing a deletion of amino acids 2 to 15 of E1^E4, equivalent to one of the modified forms of HPV1 E4 found in vivo (16 kDa), is responsible for a G2 arrest function (19). The region missing from the truncated polypeptide includes an LLXLL motif (10LLGLL14), an element that is critical for interaction with cytokeratin networks (39). The truncated E4-16K protein, therefore, does not associate with the keratin cytoskeleton (39). The full-length protein, E1^E4 (E4-17K), can interact with the cytoskeleton but has no effect on the progression of cells from G2 into mitosis (19, 39, 40). Perturbation of the mitotic cell cycle is therefore a specific function of the processed HPV1 E4 protein and is not dependent on an association with the keratin cytoskeleton. Combined expression of full-length E1^E4 and the truncated protein, E4-17/16K, does not attenuate the inhibitory effect of the processed polypeptide on G2-to-M transition. However, aberrant nuclear morphology and cellular genome rereplication, features associated with the G2 arrest function of E4-16K, were not evident (19). Cooperation of the two HPV1 E4 proteins also repressed cellular DNA synthesis, suggesting that HPV1 E4 mediates additional effects on cell proliferation (19). It was predicted that these actions of HPV1 E4 probably act together to alter the physiology of the infected keratinocyte to one that can support efficient amplification of the viral genome.
Here we have investigated the mechanism by which HPV1 E4 inhibits G2-to-M transition of the cell cycle. Our findings indicate that the mechanism underlying the cell cycle block imposed by coexpression of the two E4 proteins (E4-17/16K) differs from that mediated by expression of E4-16K alone. Inactive cdk1 accumulates in cells expressing E4-17/16K, while cells arrested by expression of E4-16K alone contain low levels of active cyclin B1-cdk1 complexes. Inactivation of cdk1 activity in E4-17/16K-expressing cells is by inhibitory Tyr15 phosphorylation, and E4-17/16K-induced cell cycle arrest is dependent on maintenance of Wee1, the kinase responsible for inhibitory cdk1 phosphorylation. Restoration of cdk1 activity in E4-17/16K-expressing cells by okadaic acid implicates serine-threonine phosphatase activity in E4-17/16K function. That HPV1 E4 uses more than one mechanism to inhibit G2-to-M transition of the cell cycle reinforces the supposition that this E4 action is important for productive viral infection.
MATERIALS AND METHODS
Cell culture.
SCC-12F keratinocytes and the cervical tumor cell line HeLa were grown as previously described (19).
Infection with recombinant adenoviruses (rAds).
Construction of rAds that express HPV1 E4 proteins (AdE4-17K and AdE4-16K) or β-galactosidase (Adβ-Gal) have been described previously (42). Asynchronous cells seeded into 6- or 10-cm dishes were infected at a multiplicity of infection of 30, as described previously (19).
Cell synchronization.
Synchronization of HeLa cells with nocodazole was performed essentially as described previously (19). Cell lysates prepared from pelleted cells were used in Western blot analysis or in vitro kinase experiments.
For synchronization of HeLa cells at the G1/S border, cells were treated with 2 mM thymidine for 16 h, after which the medium was removed and the cells washed with phosphate-buffered saline. Cells were incubated with rAds (multiplicity of infection of 30) for 2 h, and the virus solution was removed and replaced with fresh medium. Following incubation (6 h), 2 mM thymidine was then added for a further 16 h. Cells were released from the thymidine-induced block by harvesting with trypsin and reseeding into 10-cm dishes. At various times postrelease, cells were harvested for analysis by flow cytometry or Western blotting.
Flow cytometric analysis.
Flow cytometry and statistical analysis of data were performed as previously described (19).
In vitro kinase assays.
Cells were lysed in 1% NP-40, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, and insoluble material removed by centrifugation. For immunoprecipitation of cyclin-cdk complexes, 10 μg of either cyclin B1 (Santa Cruz Technologies) or cyclin A (Cancer Research UK) was added to 1 mg lysate and samples incubated at 4°C for 2 h. Immune complexes were collected by rotation at 4°C for 90 min with protein-G Sepharose (Cancer Research UK). Beads were washed four times in lysis buffer and finally incubated in 20 μl of 50 mM Tris-HCl, pH 7.4, 50 μM ATP, 10 μCi [γ-32P]ATP (Amersham Biosciences), and 5 μg of histone H1 (Sigma Chemicals) at 37°C for 1 h. Samples were heated at 95°C and separated on a 12% sodium dodecyl sulfate-polyacrylamide gel. The proteins were fixed and visualized by the addition of 100 ml fixation buffer (1% [wt/vol] Brilliant Blue (Sigma Chemicals), 40% [vol/vol] ethanol, 10% [vol/vol] acetic acid), gels dried, and autoradiography performed.
Wee1 knockdown using siRNA.
The following RNA primers were synthesized and purified (Ambion, Huntingdon, United Kingdom) (51): Wee1 sense, 5′-GAGGCUGGAUGGAUGCAUUdTdT-3; Wee1 antisense, 5′-AAUGCAUCCAUCCAGCCUCdTdT-3′; Luciferase sense, 5′-CUUACGCUGAGUACUUCGAdTdT-3′; Luciferase antisense, 5′-UCGAAGUACUCAGCGUAAGdTdT-3′. HeLa cells grown to a cell density of 70 to 80% in 6-cm culture dishes were transfected with Lipofectamine 2000 (Invitrogen Life Technologies). Two picomoles of either Wee1 or Luciferase RNA primers was added to 250 μl of Optimem medium (Gibco BRL). In a separate tube, 2.5 μl of Lipofectamine 2000 was mixed with 250 μl of Optimem medium. Samples were left at room temperature for 15 min, after which the Lipofectamine 2000 mixture was added to the RNA primer mix and incubated for a further 15 min at room temperature. The small interfering RNA (siRNA) primers/Lipofectamine cocktail was added to the cells. After 6 h, siRNA primers/Lipofectamine solution was removed and cells infected with rAds, and cells were harvested 48 h postinfection.
Wee1 overexpression.
HeLa cells were transfected with 5 μg of CMVWee1 (a kind gift of Nobu Watanabe, Discovery Research Institute, Wako, Japan) using Lipofectamine 2000, as described previously (19). After 6 h, cells were infected with rAds and harvested 48 h postinfection.
Inhibition of PP2A activity.
HeLa cells were infected with the different rAds. After 24 h, 10 μM of okadaic acid dissolved in dimethyl sulfoxide (DMSO) (Calbiochem, Merck Biosciences, Nottingham, United Kingdom) was added to the culture medium and cells incubated for a further 48 h prior to harvest. For controls, cells were treated with DMSO alone.
HA immunoprecipitation and Western blotting.
For analysis of E4 complexes, HeLa cells were transfected, as previously described (19), with pcDNA3.0-based (Invitrogen Life Technologies) plasmids which express the full-length HPV1 E1^E4 protein with a hemagglutinin (HA) epitope tag at the C terminus (pcDNA1E4-17KHA) and/or the truncated HPV1 E4 cDNA Δ2-15 that codes for the E4-16K protein (pcDNA1E4-Δ2-15) (42). Cells were lysed in 50 mM HEPES, pH 7.0, 250 mM NaCl, 0.1% NP-40, supplemented with complete EDTA-free protease inhibitor cocktail (Roche); cell lysates were incubated with HA-tag-directed antibody (Covance, Princeton, NJ, US). Immune complexes, isolated as described above, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane prior to Western blotting.
Western blotting was carried out as previously described (19). A monoclonal antibody (MAb) to cyclin B1 was purchased from Cancer Research UK and used at a dilution of 1/1,000. A rabbit polyclonal antibody against cdk1 (Upstate Biotechnology) was used at a 1/1,000 dilution, and a rabbit polyclonal antibody that recognizes cdk1-P tyrosine15 was obtained from Cell Signaling Technology (1/500 dilution). Wee1 was detected with a MAb purchased from Santa Cruz and used at a dilution of 1/1,000. HPV1 E4 proteins were detected using MAb 4.37 (10) at 1/250. Immune complexes were detected using appropriate secondary horseradish peroxidase-conjugated antibodies (Sigma Chemicals) and developed using enhanced chemiluminescence (ECL; Amersham Biosciences).
RESULTS
E4-16K and E4-17/16K mediate G2 arrest by different pathways.
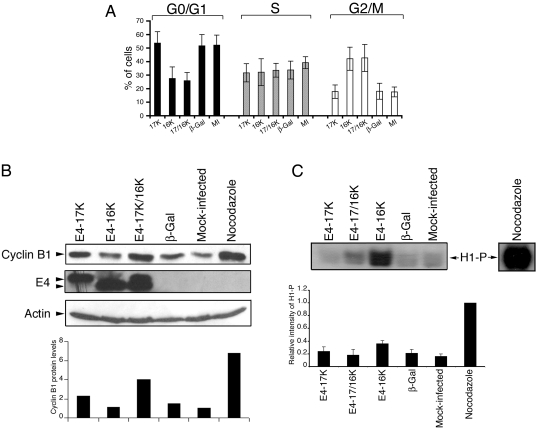
Because activation of the cyclin B1-cdk1 complex is requisite for mitotic progression, we reasoned that failure of cells expressing the truncated protein E4-16K (equivalent to the 16-kDa species expressed in HPV1 warts) or coexpressing E4-16K and the full-length E4-17K protein (E4-17/16K) to progress into mitosis might be due to an aberration in the pathways that regulate cyclin B1-cdk1 activity (19). A rise in cyclin B1 protein levels during G2 is followed by a rapid translocation of the cyclin into the nucleus to stimulate entry into mitosis (35). Therefore, to assess whether the block in G2-to-M transition might be a result of interference in the normal expression of cyclin B1 (19), we examined cyclin B1 protein levels in HeLa cervical tumor cells following infection with rAds that express the different HPV1 E4 polypeptides (AdE4-17K and AdE4-16K) or a control virus expressing β-galactosidase (Adβ-Gal) (19). By 72 h postinfection, analysis by flow cytometry revealed that the cell cycle profiles of cells expressing E4-17K (full-length E1^E4) or β-galactosidase were similar to those of mock-infected cells, with no significant change in G2+M:G1 ratio (Fig. 1A). In contrast, cells infected with AdE4-16K or coinfected with AdE4-17K and AdE4-16K accumulated in G2, with an increase in G2+M:G1 ratio to 1.53 and 1.65, respectively, compared to 0.33 for cells expressing E4-17K alone (Fig. 1A). Western blot analysis of cyclin B1 showed that in E4-16K-expressing cells, the level of cyclin B1 was significantly lower than that in cells that had arrested in G2 following coexpression of E4-17K and E4-16K (Fig. 1B). Indeed, the level of cyclin B1 in E4-17/16K cells approached that of cells arrested in mitosis by the mitotic spindle inhibitor nocodazole, whereas the cyclin B1 levels in E4-16K-expressing cells were similar to those in mock-infected cells or cells expressing the control protein β-galactosidase (Fig. 1B). Cyclin B1 levels in cells expressing the full-length E1^E4 protein (E4-17K) were slightly elevated compared to those in control cells but did not reach the levels attained in cells expressing both E4 proteins.
FIG. 1.
Differences in the level of cyclin B1 protein and cdk1 activity between HeLa cells arrested in G2 by E4-16K expression or combined expression of E4-17K and E4-16K. (A) Cell cycle analysis of HeLa cells either mock infected (MI) or infected with rAds expressing HPV1 E4 proteins (17K, 16K, or both [17/16K]) or the control protein β-galactosidase (β-Gal). Data from seven independent experiments are shown as the means ± standard deviations. The proportion of cells in the G0/G1, S, and G2/M phases were deconvoluted from the frequency histogram by using Multicycle dedicated cell cycle analysis software. The mean G2+M:G1 ratios are 0.33 (E4-17K), 1.53 (E4-16K), 1.65 (E4-17/16K), 0.35 (β-Gal), and 0.34 (MI). (B) Western blot analysis of cyclin B1 and E4 expression in HeLa cell lysates. HeLa cells treated with nocodazole were used as a control population of cells arrested in mitosis. β-Actin represents a protein loading control. The histogram shows the relative levels of cyclin B1, following normalization to actin levels. (C) Level of histone H1 phosphorylation (H1-P) following in vitro phosphorylation ([γ-32P]ATP) using cyclin B1-cdk1 complexes immunoprecipitated from HeLa cell lysates using a cyclin B1 antibody. The histogram shows the relative intensities of H1-P from four independent experiments. Equivalent levels of cyclin B1 and cdk1 were immunoprecipitated for all samples (data not shown).
A difference in cyclin B1 levels between cells expressing either both E4 proteins (E4-17/16K) or the truncated protein (E4-16K) alone was unexpected, since cells accumulated in G2 in both instances. We therefore proceeded to examine cdk1 activity in cells expressing the different HPV1 E4 proteins. Cyclin B1-cdk1 complexes were isolated from asynchronous HeLa cells 72 h postinfection by immunoprecipitation with a cyclin B1 antibody, and cdk1 activity was determined in vitro using exogenous histone H1 as a substrate in kinase assays. Although the extent of G2 arrest in E4-expressing cells (E4-16K and E4-17/16K) and in cells treated with nocodazole was similar (data not shown), cdk1 activity in E4-17/16K-expressing cells was significantly lower than in nocodazole-arrested cells (Fig. 1C). In contrast, cdk1 activity was higher in cells expressing E4-16K alone than in cells expressing the two proteins but surprisingly did not approach the level of activity observed in nocodazole-arrested cells (Fig. 1C). This finding suggested that E4-16K-expressing cells contain a low level of active cyclin B1-cdk1 complexes, a conclusion consistent with our immunofluorescence studies of E4-16K-expressing cells; only a minority of these cells expressed detectable cyclin B1, but it was predominantly nuclear (19). An assessment of cdk activity associated with cyclin A, which also rises throughout the G2 phase of the cell cycle, revealed a high level of histone H1 phosphorylation in both AdE4-16K-infected cells and AdE4-17K/-16K-infected cells, in comparison to that in AdE4-17K-infected and control cells (data not shown). Thus, our data suggest that when expressed together, the E4-17/16K proteins target cyclin B1-cdk1 activity specifically.
G2 arrest mediated by coexpression of E4-17K and E4-16K correlates with inhibitory phosphorylation of cdk1.
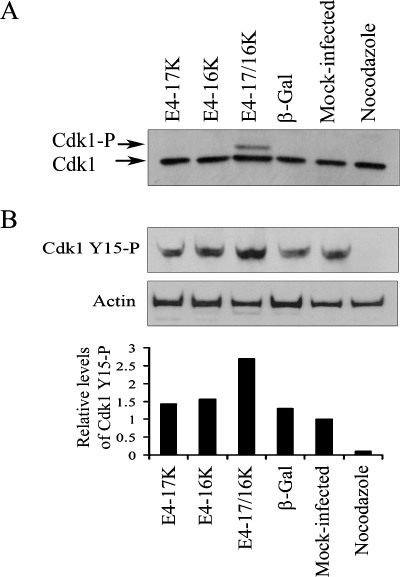
Our data indicate that E4-17/16K-expressing cells contain significant levels of cyclin B1 but low cdk1 activity, suggesting that arrest of the G2-to-M transition might be induced by specific inactivation of cdk1. In interphase, inactivation of cdk1 is mediated by phosphorylation of threonine 14 (Thr14) and tyrosine 15 (Tyr15) by the Wee family of kinases (26, 31, 34). In late G2 through to metaphase, cdk1 is activated by phosphorylation of threonine161 by a cdk-activating kinase, CAK (21), and removal of the phosphate groups from Thr14 and Tyr15 by the phosphatase cdc25c (8). Interestingly, examination of cdk1 protein expression in HeLa cells arrested in G2 following coexpression of E4-17K and E4-16K revealed the presence of an additional slower-migrating form of cdk1 that was not detected in mock-infected cells or cells expressing E4-17K or E4-16K alone or β-galactosidase (Fig. 2A). These data suggest that cdk1 might be preferentially phosphorylated in E4-17/16K-expressing cells. To investigate this possibility, we performed a Western blot analysis using an antibody that recognizes the phosphorylated form (Tyr15) of cdk1 (Fig. 2B). The analysis revealed that the level of cdk1-Tyr15 phosphorylation in E4-17/16K-expressing cells was increased compared to that in cells expressing the individual E4 proteins, β-galactosidase or mock infected, by up to 2.7-fold (Fig. 2B).
FIG. 2.
Inhibition of G2-to-M transition following coexpression of E4-17/16K proteins correlates with increased levels of phosphorylated cdk1. (A) cdk1 protein expression profiles in HeLa cells either mock infected or infected with rAds for 72 h. HeLa cells treated with nocodazole were used as a control population of cells arrested in mitosis. (B) Western blot analysis of cell lysates with an antibody that detects inhibitory phosphorylation of cdk1 on Tyr15 (Cdk1 Y15-P). As a protein loading control, the membrane was probed with an antibody against β-actin. The histogram shows cdk1 Y15-P levels relative to total cdk1 protein.
Since the studies described above were performed with HeLa cells, an HPV18-containing cervical tumor cell line, we wanted to confirm that the action of E4 on cdk1 activity is independent of that of other HPV proteins. Indeed, we have previously shown that E4-17/16K cell cycle arrest function is active in non-HPV-containing cell lines, such as SCC-12F keratinocytes (19). Analysis of cyclin B1 levels and cdk1 activity in SCC-12F cells infected with the different rAds (Fig. 3A) revealed a scenario similar to that for HeLa cells, with E4-17/16K-expressing cells containing increased cyclin B1 protein levels, yet low cdk1 activity, relative to E4-16K-expressing cells or control cells (Fig. 3B and C). Significantly, the marked reduction in cdk1 activity correlated with increased inhibitory cdk1-Tyr15 phosphorylation (Fig. 3C).
FIG. 3.
Activity of cdk1 in SCC-12F keratinocytes arrested by HPV1 E4 proteins. (A) Cell cycle profiles of SCC-12F cells either mock infected or infected with rAds for 72 h. (B) Level of histone H1 phosphorylation (H1-P) following in vitro phosphorylation ([γ-32P]ATP) using cyclin B1-cdk1 complexes immunoprecipitated from SCC-12F lysates with a cyclin B1 antibody. Autoradiograph shown is representative of two experiments. Equivalent levels of cyclin B1 and cdk1 were immunoprecipitated for all samples (data not shown). (C) Western blot analysis of expression of cyclin B1 and cdk1 phosphorylated on Tyr15 (Cdk1 Y15-P) in SCC-12F lysates. As a protein loading control, the membrane was probed with an antibody against β-actin. The histogram shows cdk1 Y15-P levels relative to total cdk1 protein.
Because inhibitory phosphates are removed from cdk1 by cdc25c, we examined the possibility that cdc25c was inactive in E4-17K/16K cells by immunoblotting the HeLa cell lysates with an antibody that recognizes the inactive form of the phosphatase (rabbit antibody that recognizes phosphorylation of Ser216 on cdc25c (no. 9528; Cell Signaling Inc.). We found, however, that there was no significant difference in the level of phosphorylation of Ser216 on cdc25c between cells infected with the different rAds (data not shown), suggesting that the accumulation of inactivated cdk1 in E4-17/16K cells is not due to the inactivation of cdc25c.
Wee1 protein levels are maintained in G2-arrested cells coexpressing the E4-17/16K proteins.
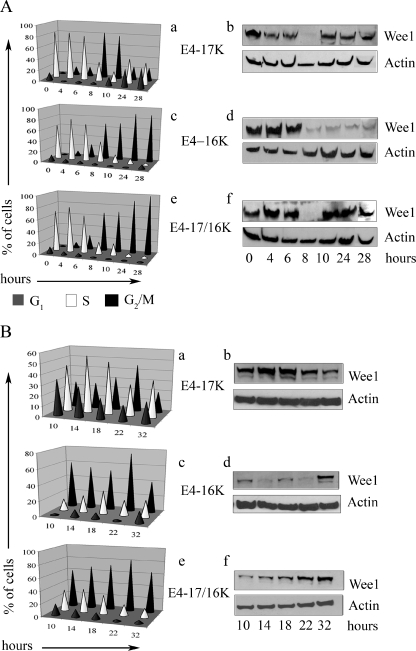
The kinase Wee1, which mediates Tyr15 phosphorylation of cdk1, is cell cycle regulated. In interphase, Wee1 levels are high and the kinase is in an activated state in order to prevent cdk1 from being prematurely activated. As cells enter late G2, Wee1 becomes phosphorylated and targeted for degradation through the proteasome, allowing cdk1 activation (47). We reasoned that if induction of cell cycle arrest in E4-17/16K cells is dependent on inhibitory phosphorylation of cdk1, then normal regulation of Wee1 might be disrupted in these cells. To investigate this possibility, we used HeLa cells induced into synchrony at the G1/S border by thymidine. In order to establish a level of E4 expression in synchronous cells that stimulates cell cycle arrest, HeLa cells were infected with rAds after the first 16-h incubation with 2 mM thymidine. A second thymidine incubation (16 h) followed, and cells subsequently released from the block. Flow cytometry was used to monitor traverse of the cell cycle after release. Progression of cells through the cell cycle was similar for mock-infected cells (data not shown), cells infected with the control virus (Adβ-Gal) (data not shown), or cells infected with the virus expressing the full-length E1^E4 protein (E4-17K) (Fig. 4A, a). Upon release from the cell cycle block (0 h), the cells progressed normally through the cell cycle, and by 8 h after release a majority of cells were in G2/M. By 10 h, cells had begun to leave G2 and reenter G1. At 24 and 28 h postrelease, cells were no longer synchronous (Fig. 4A, a). Western blot analysis of Wee1 levels revealed a cell cycle-dependent fluctuation (Fig. 4A, b). At early times postrelease (0 to 6 h), Wee1 levels were high due to the majority of cells being in S phase. As cells moved into G2 and M at 8 h, Wee1 levels were markedly reduced, reflecting degradation of the Wee1 protein during M phase. By 10 h after release, Wee1 levels increased as cells reentered G1 and S, and by 24 and 28 h, Wee1 levels reflected the asynchronous distribution of cells.
FIG. 4.
Level of Wee1 kinase is stabilized in HeLa cells arrested in G2 following coexpression of the E4-17K and E4-16K proteins. Wee1 protein expression in synchronous HeLa cells infected with rAds expressing E4-17K or E4-16K or coinfected with both viruses 0 to 28 h (A) or 10 to 32 h (B) after release from a double thymidine block. Graphical representation of cell cycle progression is shown in a, c, and e. Western blot analysis of Wee1 levels, with β-actin acting as a protein loading control, is shown in b, d, and f.
Cell cycle progression of cells infected with Ad1E4-16K and that of cells coinfected with Ad1E4-17K and Ad1E4-16K were similar to one another (Fig. 4A, c and e). By 8 h after release, cells had accumulated in G2 and M, and this population steadily increased, so that by 28 h, the majority of cells were in G2/M. Regulation of Wee1 levels, however, was very different between the two infections. In E4-16K-expressing cells, Wee1 levels decreased at 8 h and remained low at 10, 24, and 28 h, reflecting accumulation of cells in G2/M (Fig. 4A, d). In contrast, in E4-17/16K-expressing cells, Wee1 levels decreased at 8 h but increased at 10 h and remained high at 24 and 28 h, even though at these time points a majority of cells were in G2/M (Fig. 4A, f). These data indicate that high Wee1 levels accompany the G2/M arrest induced by coexpression of the two E4 proteins. However, the decrease in Wee1 at 8 h was unexpected (Fig. 4A, f). Perhaps it is an indication that there had been little or no perturbation of the cell cycle by E4-17/16K at this time point, and the loss and reexpression of Wee1 at 8 and 10 h are a reflection of cells moving normally through the cycle. To investigate this possibility, the progression of synchronized cells and Wee1 levels was monitored every 4 h, between 10 and 22 h after release, with a final time point at 32 h (Fig. 4B). In mock-infected cells and cells infected by Adβ-Gal or AdE4-17K, complete synchrony of the cells is no longer evident and Wee1 levels reflect this (Fig. 4B, a and b; also data not shown). The majority of cells infected by Ad1-E416K were arrested in G2/M between 10 and 22 h after release, but cell cycle arrest was not maintained by 32 h (Fig. 4B, c). Consistent with the previous experiment, E4-16K-induced G2/M arrest was accompanied by low Wee1 levels, with only a very small rise at 10 and 18 h, most likely reflecting cell cycle progression of a small population of uninfected cells (Fig. 4B, d). Wee1 rose to a more normal level at 32 h, when the cells were no longer maintained in a G2/M arrest. In cells coinfected with AdE4-17K and AdE4-16K, a population of cells moved from G1 and S into G2/M between 10 and 18 h postrelease, with a majority of cells accumulating in G2/M between 18 and 22 h, and cells remained in cell cycle arrest at 32 h (Fig. 4B, e). Wee1 levels rose as cells accumulated in G2/M (10 to 18 h) and remained high at 22 and 32 h, even though the majority of cells are in G2/M between 18 and 32 h (Fig. 4B, f).
Thus, our analysis of the induction of cell cycle arrest of synchronous HeLa cells by the HPV1 E4 proteins indicates that although expression of the E4-16K or E4-17/16K protein induced a G2 arrest, the nature of the cell cycle arrest is different between the two sets of proteins. So, while the accumulation of E4-16K-expressing cells in G2 occurs between 10 to 14 h postrelease and is accompanied by loss of Wee1 expression, induction of G2 arrest by E4-17/16K occurs at a later time (≥18 h) with the arrested cells containing elevated levels of Wee1. Taken together, these data suggest that the maintenance of high levels of Wee1 may be directly responsible for the failure of E4-17/16K-expressing cells to progress through mitosis.
Wee1 is essential for E4-17/16K-induced inhibition of G2-to-M transition.
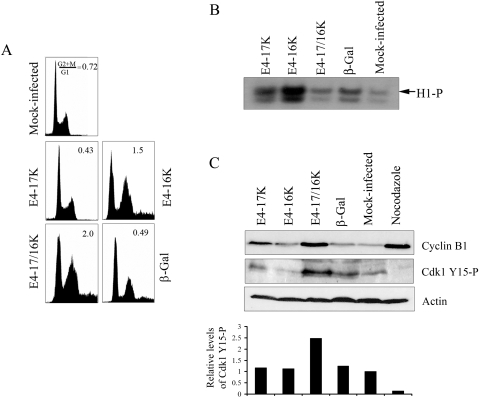
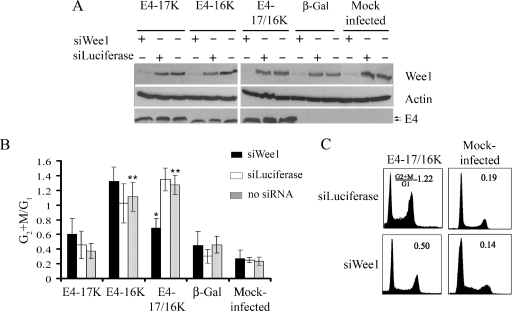
To test whether Wee1 contributes to E4 function, Wee1 protein levels were specifically reduced by using siRNA directed to wee1 mRNA (siWee1) (51). Asynchronous HeLa cells were transfected with siWee1 or an siRNA directed against the enzyme luciferase (siLuciferase). At 6 h posttransfection, cells were then infected with the different rAds and harvested 48 h later for Western blot and cell cycle analysis (Fig. 5A and B). Wee1 protein levels were significantly reduced in cells that had been transfected with siWee1 but not in siLuciferase transfectants (Fig. 5A). Flow cytometry profiles of cells infected by Ad1E4-17K were similar to those of mock- and Adβ-Gal-infected cells, and neither Wee1 nor luciferase siRNAs significantly altered G2+M:G1 ratios (Fig. 5B). Depletion of Wee1 did not influence the G2 arrest function of E4-16K, since the G2+M:G1 ratio for cells transfected with siWee1 (1.32 ± 0.19) was not significantly different from that for siLuciferase (1.02 ± 0.26) or mock transfectants (1.11 ± 0.19) (Fig. 5B). In contrast, a reduction of Wee1 in cells coexpressing both E4 proteins induced a significant (99.9%) decrease in the G2/M cell population (Fig. 5B). G2 arrest was maintained for E4-17/16K cells transfected with siLuciferase (G2+M:G1 ratio of 1.34 ± 0.15) but this was not the case in cells transfected with siWee1, which exhibit a loss of cell cycle arrest (G2+M:G1 ratio of 0.68 ± 0.13, equivalent to the ratio of 0.60 ± 0.21 for E4-17K-expressing cells transfected with Wee1) (Fig. 5B and C). The levels of E4 expression between transfectants were comparable, indicating that inhibition of G2 arrest in siWee1 transfections is not due to a decrease in stability of the E4 proteins (Fig. 5A).
FIG. 5.
Depletion of Wee1 by siRNA inhibits G2 arrest induced by E4-17/16K protein expression. (A) Western blot analysis of the Wee1 protein in HeLa cells either mock transfected or transfected with siRNAs specific for Wee1 (siWee1) or Luciferase (siLuciferase), followed by mock infection or infection with rAds expressing HPV1 E4 proteins or β-galactosidase. Expression of E4 was confirmed in the appropriate infections, and β-actin acted as a protein loading control. (B) The histogram shows G2+M:G1 ratios of data from four independent experiments, shown as means ± standard deviations. The single asterisk indicates a significant (99.9%) decrease in the G2+M:G1 ratio of siWee1-transfected E4-17/16K cells compared to that of mock-transfected (no siRNA) or siLuciferase-transfected E4-17/16K cells. The double asterisk indicates a significant (99.9%) G2 arrest in cells expressing the E4-16K or E4-17/16K protein compared to cells expressing β-galactosidase (no siRNA) or mock-infected cells (no siRNA). (C) Cell cycle profiles of E4-17/16K-expressing cells and control cells treated with siLuciferase or siWee1.
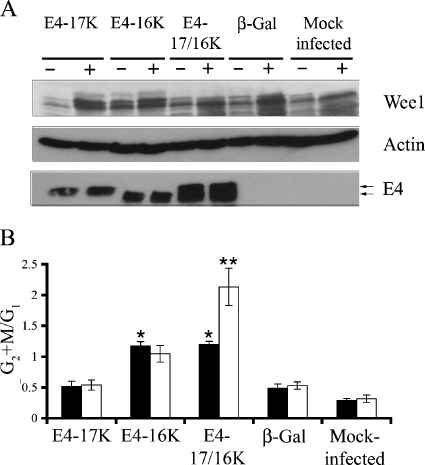
Since maintenance of the Wee1 protein seems necessary for G2 arrest induced by the combined expression of the E4-17/16K proteins, we investigated whether overexpression of Wee1 would enhance the mitotic cell cycle block. A Wee1 expression plasmid was transfected into asynchronous HeLa cells, and after 6 h, cells were infected with rAds (Fig. 6A). Flow cytometry revealed that overexpression of Wee1 in cells dually infected with AdE4-17K and AdE4-16K induced a significant (99.99%) increase in cells accumulating in G2/M, with the G2+M:G1 ratios increasing from 1.20 ± 0.47 (mock-transfected E4-17/16K cells) to 2.13 ± 0.30 (Wee1-transfected E4-17/16K cells) (Fig. 6B). A similar enhancement in a G2 cell cycle block was not apparent following Wee1 overexpression in cells expressing E4-16K alone (G2+M:G1 ratio, 1.04 ± 0.14 in Wee1-transfected cells, compared to 1.17 ± 0.13 in mock transfectants) (Fig. 6B). Wee1 overexpression had no significant effect on cell cycle progression of mock-infected cells or cells infected by Adβ-Gal or AdE4-17K (Fig. 6B).
FIG. 6.
Extent of G2 arrest is enhanced in E4-17/16K-expressing cells by overexpression of Wee1. (A) Western blot analysis of Wee1 protein levels in HeLa cells either mock transfected (−) or transfected (+) with Wee1 expression plasmid. Fluorescence microscopy of cells cotransfected with a green fluorescent protein expression plasmid indicated efficient transfection of 66.7% ± 13.1% (of GFP-positive cells). Expression of E4 was confirmed in the appropriate infections, and β-actin acted as a protein loading control. (B) Histogram of G2+M:G1 ratios in mock (closed bars) and Wee1 (open bars) transfectants. Data from four independent experiments are shown as means ± standard deviations. The single asterisk indicates a significant (99.9%) G2 arrest of cells expressing E4-16K or E4-17/16K compared to cells expressing β-galactosidase. The double asterisk indicates a significant (99.99%) increase in the G2+M:G1 ratio of Wee1-transfected E4-17/16K cells compared to that of mock-transfected E4-17/16K cells.
Taken together, our data (Fig. 4, 5 and 6) indicate that high levels of Wee1 are necessary for induction of G2 arrest by E4-17/16K proteins but are not required for E4-16K-mediated G2 arrest.
A possible role for PP2A in E4-17/16K-induced mitotic cell cycle arrest.
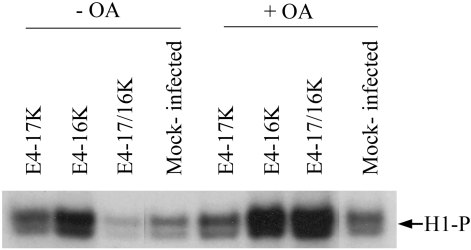
The regulation of Wee1 activity is complex and is subject to multiple levels of regulation, including proteolysis and reversible phosphorylation (24, 46, 48). We have no conclusive evidence that either the full-length or the truncated HPV1 E4 polypeptide interacts with Wee1, and so it is unlikely that E4 affects Wee1 activity by a direct interaction with the kinase. The protein phosphatase 2A (PP2A) negatively regulates progression through mitosis by direct and indirect modes of inactivation of cdk1, including the removal of inhibitory phosphates from Wee1 (20, 25). It is therefore possible that E4-17/16K proteins might affect Wee1 activity by up-regulating PP2A activity. To determine whether inactivation of PP2A might influence the G2 arrest function of HPV1 E4 proteins, asynchronous rAd-infected HeLa cells were treated with okadaic acid, an inhibitor of PP2A. Because removal of PP2A activity by okadaic acid accelerates progression into mitosis (14), there was an increase in the population of cells in G2/M in all infections and of mock-infected cells (data not shown), and therefore, it was not possible to identify cell cycle changes between treated and untreated cells by flow cytometry. We therefore examined cdk1 activity by in vitro histone H1 phosphorylation (Fig. 7). In comparison to untreated samples, incubation with okadaic acid induces a small increase in cdk1 activity in mock- and AdE4-17K-infected cells, as well as in cells arrested by the E4-16K-expressing virus (Fig. 7). However, in cells arrested in G2 by coinfection with both E4 viruses and which contain very low levels of cdk activity, there was a marked increase in the level of histone H1 phosphorylation and this exceeded the level of activity observed in okadaic acid-treated E4-16K cells (Fig. 7). These data suggest that regulation of PP2A activity might have a role in E4-17/16K-mediated inactivation of cdk1 activity.
FIG. 7.
Inhibition of PP2A abrogates inactivation of cdk1 activity in cells coexpressing E4-17/16K proteins. HeLa cells either mock infected or infected with rAds expressing the different E4 proteins were treated with 10 μM okadaic acid (+OA) or DMSO control (−OA). The level of cdk1 activity was determined by histone H1 phosphorylation (H1-P) using cyclin B1-cdk1 complexes immunoprecipitated by a cyclin B1 antibody. Equivalent levels of cyclin B1 and cdk1 were immunoprecipitated for all samples (data not shown). The autoradiograph shown is representative of three experiments.
Formation of a complex containing E4-17K and E4-16K polypeptides in E4-17/16K-expressing cells.
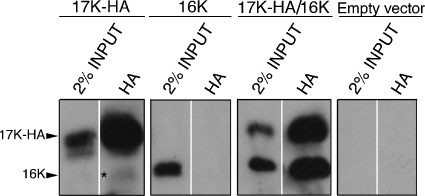
Our studies indicate that the full-length E4-17K polypeptide expressed together with E4-16K interferes with the normal G2-to-M transition of cells, but the mechanism of inhibition is different from that of E4-16K when expressed alone. To begin to understand how cell growth arrest is mediated by E4-17/16K, it became important to determine whether the two E4 proteins associate with one another. Using an anti-E4 MAb, 1D11, that recognizes a leucine-rich motif found at the N terminus of the full-length E1^E4 polypeptide but which is absent in the E4-16K polypeptide (39, 42), E4-17K-containing complexes were immunoprecipitated from HeLa cells infected with the different rAds. The E4 content of 1D11 immune complexes was then analyzed by Western blotting using MAb 4.37, an anti-E4 antibody that detects both E4-17K and E4-16K. Surprisingly, 1D11 immunoprecipitated E4-17K from HeLa cells infected with AdE4-17K alone but was unable to isolate E4-17K from dually infected (AdE4-17K and -16K) cells (data not shown), an observation that suggests the 1D11 epitope is in some way masked when the full-length protein is coexpressed with E4-16K. Therefore, to overcome this problem, E4-17K with a HA tag at its C terminus (17K-HA) was transiently expressed in HeLa cells with untagged E4-16K polypeptide and immunoprecipitated with HA-tag-directed antibodies, and complexes were analyzed by Western blotting with MAb 4.37. The specificity of the HA tag antibody is confirmed by immunoprecipitation of 17K-HA, but not E4-16K, from cells transfected with each of the different plasmids alone (Fig. 8). However, in cells cotransfected with both expression plasmids, the HA tag antibody immunoprecipitates a 17K-HA complex that contains equivalent amounts of E4-16K (Fig. 8), indicating that E4-17K and E4-16K polypeptides associate with one another to form a heteromeric complex. Interestingly, HA complexes isolated from 17K-HA-transfected cells do contain a very low level of a truncated 16-kDa polypeptide, indicating that there is some processing of the full-length E1^E4 protein, consistent with our previous findings (19, 40).
FIG. 8.
E4-17K and E4-16K form a heteromeric complex. Western blot analysis (MAb 4.37) of HA tag immune complexes isolated from HeLa cells transfected with a 17K-HA or 16K expression plasmid or cells cotransfected with both plasmids (17K-HA plus 16K). Control cells were transfected with empty vector (pcDNA3.0). Note that the MAb 4.37 detects both the 17K and 16K forms of E4. The asterisk in the HA immunoprecipitation from 17K-HA-transfected cells indicates the presence of a low level of processed 16-kDa E4 polypeptide.
DISCUSSION
We have shown that cells arrested in G2 following the combined expression of the full-length E1^E4 (E4-17K) protein and an in-vivo-like truncated protein, E4-16K, contain a significant level of inactive cyclin B1-cdk1 complexes, and inactivation appears to be through inhibitory Tyr15 phosphorylation of cdk1. These cells contain an elevated level of Wee1, the kinase responsible for inhibitory cdk1 phosphorylation. Since further overexpression of Wee1 enhanced the extent of G2 arrest we suspect that maintenance of Wee1 activity is necessary for E4-17/16K-mediated inhibition of cell division. Indeed, depletion of Wee1 by siRNA technology alleviated the E4-17/16K block. Taken together, our data indicate a requirement for increased levels of the Wee1 protein and Wee1 kinase activity for E4-17/16K induction of cell cycle arrest. Inhibition of PP2A, a phosphatase that has been implicated in keeping Wee1 active (20, 25), by the microbial toxin okadaic acid relieves the inhibition of cdk1 activity in E4-17/16K cells. This observation suggests that E4 might regulate Wee1 through PP2A. Interestingly, the G2 arrest function of the human immunodeficiency virus type 1 (HIV-1) Vpr protein is dependent on increased Wee1 activity, and a role for PP2A in the mechanism of G2 arrest has been reported, whereby Vpr up-regulates PP2A activity (12, 38, 51, 52). Likewise, the adenovirus E4orf4 and human T-cell leukemia virus Tax proteins also induce G2 arrest, and both influence PP2A activity (52). The possibility that HPV1 E4 proteins might also inhibit cell division through regulation of PP2A activity warrants further investigation; it is important to note, however, that okadaic acid inhibits, in addition to PP2a, other serine-threonine phosphatases, and therefore, it is possible that these may contribute to the E4 effect on cell cycle (2). Cells also fail to progress through G2 phase and into mitosis when the truncated E4-16K protein is expressed alone (19). There is, however, no requirement for an increase in Wee1 levels for inhibition of cell division, as overexpression or depletion of Wee1 does not influence the E4-16K-mediated G2 arrest function. In fact, in E4-16K-expressing cells, although the level of cyclin B1-cdk1 complexes is reduced compared to that of cells expressing both proteins, the complexes are active. Thus, at this stage of our investigations we do not fully understand how E4-16K inhibits the progression of cells from G2 phase into mitosis. The low level of cyclin B1 in these cells, together with our previous finding that exogenous cyclin B1 reversed E4-16K-induced G2 arrest (19), may indicate that E4-16K can somehow affect or delay the accumulation of the mitotic cyclin. So, although E4-16K or E4-17/16K expression both prevent G2-to-M transition of the cell cycle, the cell cycle blocks are not identical (19), and furthermore, they appear to be induced through different mechanisms.
HPV1 E4 polypeptides have been shown to multimerize, both in tissue culture-grown cells and in vivo (1, 11). Therefore, the E4-17K and E4-16K polypeptides, when expressed independently of each other, most likely form homomeric (trimers/tetramers and hexamers/octamers) complexes. Our data indicate that when the two proteins are expressed together, an abundant heteromeric 17/16K complex is formed. Therefore, a plausible hypothesis for the different actions of E4-16K and E4-17/16K on cell division relates to the formation of different E4 multimers. Thus, the E4-17/16K complex may interact with a different set of cellular targets than E4-16K multimers, perhaps by revealing and/or masking specific binding and/or PTM sites within the complex. Because we find that the leucine motif, known to be necessary for the association between E1^E4 and the keratin cytoskeleton (39), is accessible to an antibody that recognizes this motif in E4-17K complexes but not in E4-17/16K complexes (G. L. Knight and S. Roberts, unpublished data), we would argue that this is indeed possible.
The G2 arrest function is conserved between E4 proteins of unrelated HPV types (cutaneous viruses HPV1 and -2 and anogenital viruses HPV11, -16, and -18) (6, 19, 27). The arrest functions of HPV1 E4-16K and HPV16 E1^E4 share a degree of similarity. They are both dependent on a threonine residue contained within proline-rich domains of the E4 protein, and cells expressing these proteins exhibit an aberrant nuclear morphology and chromosomal rereplication (6, 19). Also, like HPV16 E1^E4 (5), HPV1 E4-16K does not mediate G2 arrest by inhibition of cdk1 activity. HPV16 E1^E4 has been shown to bind to cyclin B1 and sequester active cyclin B1-cdk1 complexes in the cytoplasm, thereby preventing their nuclear accumulation and activation of mitosis (5). However, the G2 arrest function of HPV1 E4-16K does not appear to be linked to cytoplasmic compartmentalization of cyclin B1-cdk1 complexes (19). Also, HPV1 E4-16K does not form a robust association with cyclin B1, although a glutathione S-transferase fusion protein of E4-16K is a substrate for cdk1 in vitro (G. Knight, unpublished data). Elucidation of the G2 arrest mechanism of HPV1 E4-16K will be necessary to understand fully the reason for the apparent difference in the mechanism of action. Perhaps the heterogeneity between the life cycles of type 1 and 16 viruses may have necessitated that the viruses evolve nonidentical mechanisms to alter the physiology of the host cell to support virus replication. HPV1, a hugely efficient virus, begins productive replication in the parabasal cells, while for the less-efficient HPV16, this phase is delayed until cells reach the upper spinous layers (33). Also, a far greater proportion of HPV1-infected E4-positive cells remain competent for genome replication as they migrate upwards, which is not the case in HPV16 infections.
Other human viruses (e.g., HIV-1, human T-cell leukemia virus, Epstein-Barr virus, adenovirus, and reovirus) encode proteins that induce G2 arrest of the cell cycle (17, 18, 22, 23, 36, 38). The functional role of interference of cell division in the life cycle of these viruses is as yet unclear, but that so many different viruses do so suggests that it is probably advantageous to successful replication. Indeed, analysis of experimental HIV-1 replication systems has shown that establishment of G2 arrest facilitates efficient virus production (15, 16). Vpr, the HIV-1 protein responsible for inducing cell cycle arrest, instructs two mechanisms to inhibit G2-to-M transition (50). Our finding that HPV1 E4 also mediates inhibition of cell division by more than one mechanism reinforces the supposition that this function is important for the virus. In HPV infections, amplification of viral DNA in nondividing keratinocytes is dependent on activation of S-phase gene activity in these cells, while the later events of capsid expression and virion assembly require cellular differentiation and take place in superficial cells that are not competent to support HPV genome replication (33). Abundant E4 expression occurs at the onset of viral genome amplification, and the protein persists throughout the vegetative phase of the life cycle. Perturbation of pathways that control G2-to-M transition may act to alter the host cell milieu to one that can stimulate maintenance of the replication capacity of suprabasal keratinocytes or, alternatively, to signal a switch to activate late events of the productive cycle. Studies of cell culture systems of keratinocytes containing papillomavirus genomes have identified an important role for E4 in HPV replication (28, 32, 49). Manipulation of these systems to abrogate the G2 arrest function of E4 is imperative for understanding the physiological relevance of this activity. Unfortunately, the availability of such systems does not extend to the benign type 1 virus.
The different HPV1 E4 polypeptides accumulate in a progressive manner as the infected keratinocyte migrates upwards in the wart (3). So, while the full-length E1^E4 polypeptide exists by itself in the deepest wart layers, both the 17- and 16-kDa forms are present in the intermediate layers. Superficial layers no longer contain the full-length E1^E4 protein but retain the 16-kDa polypeptide, accompanied with increasing amounts of the 11/10-kDa proteins. The transition of the full-length 17-kDa HPV1 E1^E4 protein to the truncated forms of 16, 11, and 10 kDa gradually removes N-terminal sequences that have been shown to be critical in distinct biological actions of E4, such as keratin association (17-kDa → 16-kDa) (39) and G2 arrest (16-kDa → 11/10-kDa) (19, 39). This scenario, taken together with the pattern of E4 expression in wart tissue, suggests that E4 functions mediated by N-terminal sequences are lost as the productive cycle proceeds. For example, the association with the keratin intermediate filaments would be active only in cells containing the 17-kDa species and as such may be required only for a limited period of the infectious cycle. Mediation of inhibition of G2-to-M transition by different E4 species suggests that this function might be required for a greater part of the HPV1 life cycle, as it would be active in cells containing both 17-kDa and 16-kDa proteins, and also later in the infectious cycle, in cells that still express the 16-kDa species but in which the level of the 17-kDa species either has fallen below a critical threshold level or is absent altogether.
The implication of PTM for E4 function is only beginning to be evaluated, and this and our previous studies (19, 39, 42) have shown that PTM of the HPV1 E4 protein has considerable impact on its function. Examination of expression of E4 proteins of anogenital HPV types 16 (41), 18 (A. Pugh, G. L. Knight, and S. Roberts, unpublished data), and 31b (37) suggests that these viruses might also produce cleaved forms of the E1^E4 polypeptide, and it will be interesting to investigate the actions of these processed forms, both in isolation and combined with the full-length species. It seems likely that E4 proteins have a pleiotropic role in the infectious cycle (28) and PTM is a way of obtaining functional diversity by generating different E4 species which, either in isolation or by cooperation with one another, exhibit distinct biological actions.
Acknowledgments
We thank N. Watanabe for his kind gift of the Wee1 expression vector. Special thanks go to Ian Bell for constructing the HA-tagged HPV 1 E1^E4 expression plasmid. We are grateful to Phillip H. Gallimore for his encouragement during the early phase of this work.
A Cancer Research UK Programme grant supported this study (C427/A3919 to S.R.).
REFERENCES
- 1.Ashmole, I., P. H. Gallimore, and S. Roberts. 1998. Identification of conserved hydrophobic C-terminal residues of the human papillomavirus type 1 E1E4 protein necessary for E4 oligomerisation in vivo. Virology 240:221-231. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau, R. T., and D. W. Hoskin. 2005. The use of okadaic acid to elucidate the intracellular role(s) of protein phosphatase 2A: lessons from the mast cell model system. Int. Immunopharmacol. 5:1507-1518. [DOI] [PubMed] [Google Scholar]
- 3.Breitburd, F., O. Croissant, and G. Orth. 1987. Expression of human papillomavirus type-1 E4 gene products in warts, p. 115-122. In B. M. Steinberg, J. Brandsma, and L. B. Taichman (ed.), Papillomaviruses: cancer cells, vol. 5. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 4.Brown, D. R., C. R. Brown, and E. E. Lehr. 2004. Intracellular expression patterns of the human papillomavirus type 59 E1/E4 protein in COS cells, keratinocytes, and genital epithelium. Intervirology 47:321-327. [DOI] [PubMed] [Google Scholar]
- 5.Davy, C. E., D. J. Jackson, K. Raj, W. L. Peh, S. A. Southern, P. Das, R. Sorathia, P. Laskey, K. Middleton, T. Nakahara, Q. Wang, P. J. Masterson, P. F. Lambert, S. Cuthill, J. B. Millar, and J. Doorbar. 2005. Human papillomavirus type 16 E1^E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes. J. Virol. 79:3998-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davy, C. E., D. J. Jackson, Q. Wang, K. Raj, P. J. Masterson, N. F. Fenner, S. Southern, S. Cuthill, J. B. Millar, and J. Doorbar. 2002. Identification of a G(2) arrest domain in the E1Ê4 protein of human papillomavirus type 16. J. Virol. 76:9806-9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, P. M., R. B. Roden, D. R. Lowy, and J. T. Schiller. 1998. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. J. Virol. 72:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donzelli, M., and G. F. Draetta. 2003. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 4:671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorbar, J., S. Ely, J. Sterling, C. McLean, and L. Crawford. 1991. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature 352:824-827. [DOI] [PubMed] [Google Scholar]
- 10.Doorbar, J., H. S. Evans, I. Coneron, L. V. Crawford, and P. H. Gallimore. 1988. Analysis of HPV-1 E4 gene expression using epitope-defined antibodies. EMBO J. 7:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doorbar, J., E. Medcalf, and S. Napthine. 1996. Analysis of HPV1 E4 complexes and their association with keratins in vivo. Virology 218:114-126. [DOI] [PubMed] [Google Scholar]
- 12.Elder, R. T., M. Yu, M. Chen, X. Zhu, M. Yanagida, and Y. Zhao. 2001. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology 287:359-370. [DOI] [PubMed] [Google Scholar]
- 13.Fehrmann, F., and L. A. Laimins. 2003. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene 22:5201-5207. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh, S., D. Schroeter, and N. Paweletz. 1996. Okadaic acid overrides the S-phase check point and accelerates progression of G2-phase to induce premature mitosis in HeLa cells. Exp. Cell Res. 227:165-169. [DOI] [PubMed] [Google Scholar]
- 15.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 16.Gozlan, J., J. L. Lathey, and S. A. Spector. 1998. Human immunodeficiency virus type 1 induction mediated by genistein is linked to cell cycle arrest in G2. J. Virol. 72:8174-8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haoudi, A., R. C. Daniels, E. Wong, G. Kupfer, and O. J. Semmes. 2003. Human T-cell leukemia virus-I tax oncoprotein functionally targets a subnuclear complex involved in cellular DNA damage-response. J. Biol. Chem. 278:37736-37744. [DOI] [PubMed] [Google Scholar]
- 18.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight, G. L., J. R. Grainger, P. H. Gallimore, and S. Roberts. 2004. Cooperation between different forms of the human papillomavirus type 1 E4 protein to block cell cycle progression and cellular DNA synthesis. J. Virol. 78:13920-13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechward, K., O. S. Awotunde, W. Swiatek, and G. Muszynska. 2001. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim. Pol. 48:921-933. [PubMed] [Google Scholar]
- 21.Lew, D. J., and S. Kornbluth. 1996. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8:795-804. [DOI] [PubMed] [Google Scholar]
- 22.Liang, M. H., T. Geisbert, Y. Yao, S. H. Hinrichs, and C. Z. Giam. 2002. Human T-lymphotropic virus type 1 oncoprotein tax promotes S-phase entry but blocks mitosis. J. Virol. 76:4022-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauser, A., E. Holley-Guthrie, D. Simpson, W. Kaufmann, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 induces both a G(2) and a mitotic block. J. Virol. 76:10030-10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan, C. H., and P. Russell. 1995. Cell cycle regulation of human WEE1. EMBO J. 14:2166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 26.Mueller, P. R., T. R. Coleman, A. Kumagai, and W. G. Dunphy. 1995. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270:86-90. [DOI] [PubMed] [Google Scholar]
- 27.Nakahara, T., A. Nishimura, M. Tanaka, T. Ueno, A. Ishimoto, and H. Sakai. 2002. Modulation of the cell division cycle by human papillomavirus type 18 E4. J. Virol. 76:10914-10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahara, T., W. L. Peh, J. Doorbar, D. Lee, and P. F. Lambert. 2005. Human papillomavirus type 16 E1^E4 contributes to multiple facets of the papillomavirus life cycle. J. Virol. 79:13150-13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasseri, M., R. Hirochika, T. R. Broker, and L. T. Chow. 1987. A human papilloma virus type 11 transcript encoding an E1-E4 protein. Virology 159:433-439. [DOI] [PubMed] [Google Scholar]
- 30.Palermo-Dilts, D. A., T. R. Broker, and L. T. Chow. 1990. Human papillomavirus type 1 produces redundant as well as polycistronic mRNAs in plantar warts. J. Virol. 64:3144-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker, L. L., S. Atherton-Fessler, and H. Piwnica-Worms. 1992. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. USA 89:2917-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peh, W. L., J. L. Brandsma, N. D. Christensen, N. M. Cladel, X. Wu, and J. Doorbar. 2004. The viral E4 protein is required for the completion of the cottontail rabbit papillomavirus productive cycle in vivo. J. Virol. 78:2142-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peh, W. L., K. Middleton, N. Christensen, P. Nicholls, K. Egawa, K. Sotlar, J. Brandsma, A. Percival, J. Lewis, W. J. Liu, and J. Doorbar. 2002. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 76:10401-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pines, J. 1995. Cell cycle. Confirmational change. Nature 376:294-295. [DOI] [PubMed] [Google Scholar]
- 35.Pines, J., and C. L. Rieder. 2001. Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 3:E3-E6. [DOI] [PubMed] [Google Scholar]
- 36.Poggioli, G. J., T. S. Dermody, and K. L. Tyler. 2001. Reovirus-induced σ1s-dependent G2/M phase cell cycle arrest is associated with inhibition of p34cdc2. J. Virol. 75:7429-7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pray, T. R., and L. A. Laimins. 1995. Differentiation-dependent expression of E1-E4 proteins in cell lines maintaining episomes of human papillomavirus type 31b. Virology 206:679-685. [DOI] [PubMed] [Google Scholar]
- 38.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts, S., I. Ashmole, L. J. Gibson, S. M. Rookes, G. J. Barton, and P. H. Gallimore. 1994. Mutational analysis of human papillomavirus E4 proteins: identification of structural features important in the formation of cytoplasmic E4/cytokeratin networks in epithelial cells. J. Virol. 68:6432-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts, S., I. Ashmole, G. D. Johnson, J. W. Kreider, and P. H. Gallimore. 1993. Cutaneous and mucosal human papillomavirus E4 proteins form intermediate filament-like structures in epithelial cells. Virology 197:176-187. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, S., I. Ashmole, S. M. Rookes, and P. H. Gallimore. 1997. Mutational analysis of the human papillomavirus type 16 E1^E4 protein shows that the C terminus is dispensable for keratin cytoskeleton association but is involved in inducing disruption of the keratin filaments. J. Virol. 71:3554-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts, S., M. L. Hillman, G. L. Knight, and P. H. Gallimore. 2003. The ND10 component promyelocytic leukemia protein relocates to human papillomavirus type 1 E4 intranuclear inclusion bodies in cultured keratinocytes and in warts. J. Virol. 77:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogel-Gaillard, C., G. Pehau-Arnaudet, F. Breitburd, and G. Orth. 1993. Cytopathic effect in human papillomavirus type 1-induced inclusion warts: in vitro analysis of the contribution of two forms of the viral E4 protein. J. Investig. Dermatol. 101:843-851. [DOI] [PubMed] [Google Scholar]
- 44.Swindle, C. S., N. Zou, B. A. Van Tine, G. M. Shaw, J. A. Engler, and L. T. Chow. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Q., H. Griffin, S. Southern, D. Jackson, A. Martin, P. McIntosh, C. Davy, P. J. Masterson, P. A. Walker, P. Laskey, M. B. Omary, and J. Doorbar. 2004. Functional analysis of the human papillomavirus type 16 E1^E4 protein provides a mechanism for in vivo and in vitro keratin filament reorganization. J. Virol. 78:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe, N., H. Arai, J. Iwasaki, M. Shiina, K. Ogata, T. Hunter, and H. Osada. 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA 102:11663-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe, N., H. Arai, Y. Nishihara, M. Taniguchi, N. Watanabe, T. Hunter, and H. Osada. 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA 101:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14:1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson, R., F. Fehrmann, and L. A. Laimins. 2005. Role of the E1^E4 protein in the differentiation-dependent life cycle of human papillomavirus type 31. J. Virol. 79:6732-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshizuka, N., Y. Yoshizuka-Chadani, V. Krishnan, and S. L. Zeichner. 2005. Human immunodeficiency virus type 1 Vpr-dependent cell cycle arrest through a mitogen-activated protein kinase signal transduction pathway. J. Virol. 79:11366-11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan, H., M. Kamata, Y. M. Xie, and I. S. Chen. 2004. Increased levels of Wee-1 kinase in G2 are necessary for Vpr- and gamma irradiation-induced G2 arrest. J. Virol. 78:8183-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao, R. Y., and R. T. Elder. 2005. Viral infections and cell cycle G2/M regulation. Cell Res. 15:143-149. [DOI] [PubMed] [Google Scholar]