Abstract
Murine cytomegalovirus encodes three regulators of antigen presentation to antiviral CD8 T cells. According to current paradigms, all three regulators are committed to the inhibition of the presentation of antigenic peptides. Whereas m152/gp40 catalyzes the retention of peptide-loaded major histocompatibility complex (MHC) class I molecules in a cis-Golgi compartment, m06/gp48 binds stably to class I molecules and directs them into the cellular cargo-sorting pathway of lysosomal degradation. Regulator m04/gp34 also binds stably to class I molecules, but unlike m152 and m06, it does not downmodulate MHC class I cell surface expression. It has entered the literature as a direct inhibitor of T-cell recognition of the MHC-peptide complex at the cell surface. In this work, we have studied the presentation of antigenic viral peptides in cells infected with a comprehensive set of mutant viruses expressing the three regulators separately as well as in all possible combinations. The results redefine m04 as a positive regulator dedicated to the facilitation of antigen presentation. When expressed alone, it did not inhibit T-cell recognition, and when expressed in the presence of m152, it restored antigen presentation by antagonizing the inhibitory function of m152. Its intrinsic positive function, however, was antagonized and even slightly overcompensated for by the negative regulator m06. In an adoptive cell transfer model, the opposing forces of the three regulators were found to govern immune surveillance in the infected host. While negative regulators, also known as immunoevasins, are common, the existence of a positive regulator is without precedent and indicates an intriguing genetic potential of this virus to influence antigen presentation.
Cytomegaloviruses (CMVs) encode proteins that according to current paradigms are specifically committed to the inhibition of major histocompatibility complex (MHC) class I-restricted presentation of antigenic peptides to CD8 T cells (1, 6, 18, 27, 28, 31, 33, 39, 43). These highly specialized viral proteins are currently referred to as immunoevasins (18, 31) or as VIPRs (viral proteins interfering with antigen presentation, also read as viral genes that inhibit antigen presentation to CD8+ T cells) (27, 43). In murine cytomegalovirus (mCMV) infection, three VIPRs, all being type I transmembrane glycoproteins, are expressed in the early phase of viral gene expression and operate at different steps in the MHC class I presentation pathway (6, 27, 31, 33). Normally, MHC class I molecules reach the cell surface via the constitutive secretory pathway. Through transient interaction with class I molecules, m152/gp40, a member of the m145 gene family and the first CMV immunoevasin to be described (2, 17, 38, 44, 45), induces the retention of peptide-loaded MHC class I complexes in the endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC)/cis-Golgi network (2, 44, 45). Interestingly, it also simultaneously downregulates the RAE-1 family ligands of the activating receptor NKG2D expressed by NK cells as well as by activated T cells (16, 19). m04/gp34 and m06/gp48, closely related molecules of the m02 gene family, stably bind to MHC class I molecules and mediate their sorting into vesicular transportation pathways, albeit with diametrically opposed destinations. The m06 glycoprotein routes MHC class I molecules to the late endosome-lysosome pathway for degradation (34, 35). By contrast, the molecular complexes between m04 glycoproteins and MHC class I molecules, which are formed in the ER, migrate via the Golgi apparatus to the cell surface (14, 15).
In a recent review article on viral modulation of antigen presentation, Lilley and Ploegh have summarized the current opinion and state of knowledge (18). Glycoprotein m04 of mCMV is listed there as a prototype of a viral protein that directly interferes at the cell surface with recognition of MHC class I-presented peptides by the T-cell receptor (TCR) of CD8 T cells. It is thought that m04, through binding to MHC class I, either induces a conformational alteration of the class I molecule or inhibits MHC-peptide-TCR complex formation sterically. Both mechanisms discussed imply that m04 can operate as an immunoevasin/VIPR, even though it does not downmodulate MHC class I cell surface expression. In accordance with this current model of m04 function, evidence to suggest cooperation between m04 and m152, as well as between all three immunoevasins, in the inhibition of antigen presentation has been reported (13, 20).
Here we have used the complete set of mCMV immune evasion gene deletion mutants (41) for a comprehensive and systematic functional study of antigen presentation under the influence of the three immunoevasins expressed in the context of infection both individually and in all possible combinations. Surprisingly, our data show that m04 expressed in the absence of the other two immunoevasins does not inhibit antigen presentation and consequent CD8 T-cell recognition. Even more challenging for current opinion, m04 clearly prevented m152-mediated inhibition of antigen presentation in cells infected with deletion mutant mCMV-Δm06. This antagonistic effect of m04 was consistently observed for the complete set of MHC class I molecules of the haplotypes H-2d and H-2b (Kd, Kb, Dd, Db, and Ld) and for a total of 10 different epitopes derived from nine different viral proteins. The list of epitopes also included particular Kb-restricted epitopes for which the literature had predicted the direct opposite (13). Furthermore, the recognition patterns observed with the set of virus mutants applied to fibroblasts, as an example of a stromal cell type, as well as to bone marrow-derived dendritic cells (BMDCs), as an example of a professional antigen-presenting cell type. Most relevantly, in adoptive cell transfer models, the positive and negative effects on antigen presentation applied also to CD8 T-cell-mediated control of mCMV in the infected host.
Our data imply that m04 does not inhibit TCR recognition of peptide-loaded MHC complexes at the cell surface and that it can function as a positive regulator of antigen presentation. To better comply with negative as well as positive functions, we propose to use the generic term vRAP (viral regulator of antigen presentation) as an unbiased new acronym for viral proteins that modulate antigen presentation in either direction.
MATERIALS AND METHODS
Viruses and infection of cells.
Bacterial artificial chromosome (BAC)-derived mCMV MW97.01 (22, 42) has previously been shown to be biologically equivalent to mCMV Smith strain ATCC VR-194 (recently reaccessioned as VR-1399) and is here referred to as mCMV-WT. vRAP gene deletion mutants were constructed previously by BAC mutagenesis (41). Particular care was taken to remove residual BAC vector sequences from the reconstituted viruses by propagation in cell culture. This is essential for in vivo studies, as BAC vector sequences are known to attenuate mCMV with respect to in vivo growth (42). PCR-verified BAC-sequence-free viruses were grown in murine embryonal fibroblasts (MEFs) and were purified by sucrose density gradient ultracentrifugation as described in detail previously (29). Virus stocks were comparable in virus titer (2 × 108 to 6 × 108 PFU/ml), and all viruses showed comparable genome-to-infectivity ratios of ∼500 genomes/PFU.
Second-passage MEFs were prepared as described previously (29). Mouse immature BMDCs were generated from bone marrow of BALB/cJ and C57BL/6N mice according to an established protocol (7) involving selection with 200 U/ml of granulocyte-monocyte colony-stimulating factor. BMDCs were CD14−, and >80% expressed CD11c as determined by cytofluorometric analysis. The CD11c+ BMDCs were further phenotyped as CD40low, CD80low, and CD86low. MEFs and BMDCs were centrifugally infected with 0.2 and 0.4 PFU per cell, respectively. For enzyme-linked immunospot (ELISPOT) assays, cells were infected and incubated for 90 min until they were used as stimulator cells in the 18-h assay. For the cytolysis (51Cr release) assay, cells were infected and incubated for 12 h until they were labeled for 75 min and used as target cells in the 4-h assay.
Antigenic peptides and epitope-specific CTL lines.
The list of antigenic peptides of mCMV for haplotypes H-2d and H-2b has been published in recent review articles (8, 31). For key peptides used in this study, amino acid sequences are specified in the text. Custom peptide synthesis to a purity of >75% was performed by Jerini Peptide Technologies (Berlin, Germany). Epitope-specific polyclonal cytotoxic T-lymphocyte (CTL) lines with a still-broad TCR Vβ usage were generated from memory spleen cells of infected BALB/cJ and C57BL/6N mice (>3 months after infection with mCMV-WT) by repeated stimulation with an optimized molar concentration of the respective synthetic peptides (11, 26). CTL lines were used for the assays after three to five rounds of peptide stimulation. This selection period is required to reach epitope monospecificity of the CTL lines but is still short enough that expression of coreceptor CD8 is not lost (26).
Assays of CD8 T-cell effector function.
Gamma interferon (IFN-γ) ELISPOT assay was used to detect sensitization of CD8 T cells by epitopes presented either after exogenous loading of stimulator cells (MEFs or BMDCs) with synthetic peptide for sensitivity control or after infection of stimulator cells with the indicated viruses. The assay was performed as described previously (11, 26) with 105 stimulator cells per assay culture and with graded numbers of effector cells (300, 200, 100, and 50) seeded in triplicate. After 18 h of cocultivation, plates were developed and spots were counted. Frequencies of IFN-γ-secreting, spot-forming cells were calculated by intercept-free linear regression analysis (effector cell numbers on the abscissa [x] and triplicate spot numbers on the ordinate [y]) using the software Mathematica V4.2.1 Statistics “LinearRegression” (Wolfram Research Inc., Champaign, IL). The calculation gives the slope a of the regression line (y = ax) and its 95% confidence interval (95% CI) as well as the P value for the null hypothesis of random distribution, which has to be <0.01 for a linear function to be accepted. The most probable number (MPN) of IFN-γ-secreting effector cells per 100 effector cells is then the ordinate coordinate y(MPN) = ax, with x = 100. The 95% CI of the MPN is calculated accordingly with the upper and lower limit values of the slope a.
Cytolytic activity was measured in a standard 4-h 51Cr release assay with graded numbers of effector cells seeded in triplicate and 1,000 51Cr-labeled target cells (MEFs) exogenously loaded with synthetic peptide for sensitivity control or infected with the indicated viruses. For the calculation of lytic activity, including all data from the linear portions of the dose-response curves, fractional 51Cr release data f, considered to represent the fraction of lysed target cells, were log transformed into Nαt values according to the formula Nαt = −ln(1 − f). Unlike f, Nαt is proportional to the number (N) of effector cells seeded (23). Linear regression analysis (effector cell numbers on the abscissa and triplicate Nαt data on the ordinate) was then performed as described above. Lytic activity and its 95% CI are expressed as 100 × Nαt.
Cytofluorometric analyses.
Two-color analysis of MHC class I Ld expression on infected MEFs was performed with phycoerythrin-conjugated mouse monoclonal antibody anti-mouse H-2Ld (clone 30-5-7S, catalog no. CL9011PE; Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) and rabbit antibodies specific for the cytoplasmic early-phase glycoprotein m164 (gp38/50) of mCMV. This reagent was generated as follows. Peptide CGVVSRNHQPWRAATNASSRVGRSS, corresponding to the C-terminal end of the m164 protein, was conjugated through the terminal cysteine-to-maleimide-activated keyhole limpet hemocyanin (Pierce Biotechnology, Rockford, Illinois), and the resulting conjugate was used to immunize rabbits. Antipeptide antibodies were purified according to the method of Harlow and Lane (5) by protein A affinity chromatography, followed by affinity chromatography on columns with the immunizing peptide coupled to Sulfo-link coupling gel (Pierce). Specificity was controlled by immunofluorescence as well as by immunoblotting of protein extracts of MEFs infected with mCMV-WT as a positive control and with deletion mutant mCMV-Δm164 as a negative control. In addition, the specificity of the immunoblot was verified by blocking with an excess of peptide (100 μg/ml).
For the blocking of Fcγ III/II receptors, MEFs were first incubated in blocking solution containing purified anti-mouse CD16/CD32 (1 μg/106 cells in 100 μl; BD Biosciences Pharmingen). Specific cell surface staining was performed with the phycoerythrin-conjugated anti-Ld antibody (2 μg/106 cells in 100 μl) and was followed by treatment with BD Cytofix/Cytoperm Plus (BD Biosciences Pharmingen) to fix and permeabilize the cells. Intracellular staining was then performed with the m164-specific antibodies (0.2 μg/106 cells in 100 μl) (see above), followed by Alexa Fluor 488-conjugated second antibody goat anti-rabbit immunoglobulin G (heavy plus light chains) (Molecular Probes, Eugene, Oregon). The measurements were made with a FACSort instrument using CellQuest Pro software for data processing (Becton Dickinson). All viable cells were included in the analyses with no further gating. Fluorescence intensities are displayed as contour plots. Contour lines were defined by scale (log density, 35%), smoothing (setting 1), and threshold (0.5%).
Adoptive cell transfer and quantitation of infection in host tissues.
Eight-week-old female C57BL/6N and BALB/cJ mice were immunocompromised by hematoablative γ-irradiation with single doses of 7.5 Gy (24 h prior to cell transfer and infection) and 6.5 Gy (on the day of cell transfer and infection), respectively. M45-Db and M45-Dd epitope-specific CTLs, respectively, were transferred by intravenous infusion in 0.5 ml of physiological saline. Subcutaneous, intraplantar infection was performed ∼2 h later with 105 PFU of mCMV-WT or of vRAP gene deletion mutant mCMV-Δm04+06 or mCMV-Δm06. Mice were bred and housed under specified-pathogen-free conditions at the Central Laboratory Animal Facility of the Johannes Gutenberg-University. Animal experiments were approved by the Ethics Commission, permission no. 177-07/021-28, in accordance with German federal law.
Organ infection was monitored on day 12 after cell transfer. Infectious virus present in the lungs was quantitated for organ homogenates by a virus plaque assay on MEFs (29). The number of infected cells in liver tissue sections was determined by vRAP gene-specific DNA-DNA in situ hybridization (ISH). ISH probes m04-P, m06-P, and m152-P were generated by PCR and tagged during the PCR by incorporation of digoxigenin-11-dUTP essentially as described previously (41). vRAP gene-specific PCR primers were as follows: for m04-P, m04-for (TGTTGGTGACGGTTGTACTG) and m04-rev (AAGCGGTTTGAAGTTCGAGC), yielding a 767-bp amplificate corresponding to positions 3292 through 4058 of the mCMV Smith strain genome according to Rawlinson et al. (30) (EMBL-ID MCU68299); for m06-P, m06-for (AGCCTCGATGACTTTCCAGATG) and m06-rev (CCATCTCCGTCCGCATTCTCTG), yielding a 731-bp amplificate corresponding to positions 5,395 through 6,125; and for m152-P, m152-for (AGTTGATGTAGACCAGGCGATAC) and m152-rev (GCTATCACCTACTTGCTCCTCTCG), yielding a 1,114-bp amplificate corresponding to positions 210255 through 211368. Viral DNA accumulated within intranuclear inclusion bodies of infected cells was visualized by ISH essentially as described in previous reports (29, 41), except that alkaline phosphatase-conjugated antidigoxigenin antibody (Fab fragments, catalog no. 1093274; Roche) was used with fuchsin as the chromogenic substrate to yield a bright red color.
Significance analysis.
The statistical significance of differences in virus titers was calculated by using distribution-free Wilcoxon-Mann-Whitney (rank sum) statistics. A calculator is provided on the Web site http://www.socr.ucla.edu/Applets.dir/WilcoxonRankSumTable.html (Ivo Dinov, Statistics Online Computational Resources, UCLA Statistics, Los Angeles, California). Samples are considered significantly different if the P value is <0.05 (two-tailed test).
RESULTS
vRAP-modulated pattern of MHC class I cell surface expression in primary fibroblasts.
In previous work by Wagner and colleagues (41), a panel of vRAP gene deletion mutants of mCMV expressing the three vRAPs in all possible combinations (Table 1) was used to determine the impact of these regulatory molecules on MHC class I cell surface expression by infecting cells of the simian virus 40-transformed MEF-derived clonal cell lines B12 (BALB/c derived; H-2d) and C57SV (C57BL/6 derived; H-2b). Due to the transformed state, uninfected B12 and C57SV cells constitutively expressed high levels of MHC class I molecules at the cell surface. A key feature of that system was that uninfected cells and cells infected with the triple vRAP gene deletion mutant mCMV-Δm04+06+152 showed essentially the same class I expression levels, a finding supporting the conclusion that there exist no further vRAPs downmodulating cell surface class I.
TABLE 1.
vRAPs expressed by vRAP gene deletion mutants of mCMV
| Virus | Expression of the following vRAPb:
|
||
|---|---|---|---|
| m04/gp34 | m06/gp48 | m152/gp40 | |
| Δm04+06+152 | − | − | − |
| Δm04+152 | − | + | − |
| Δm06+152 | + | − | − |
| Δm04+06 | − | − | + |
| Δm152 | + | + | − |
| Δm06 | + | − | + |
| Δm04 | − | + | + |
| WTa | + | + | + |
WT, wild type.
+, present; −, absent.
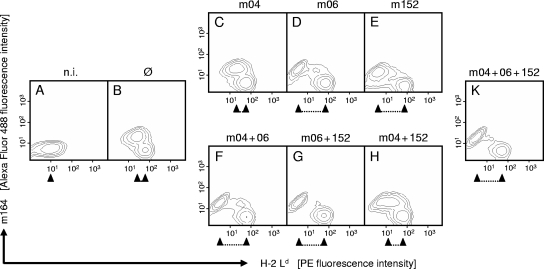
As our study was aimed at investigating the role of vRAPs in the antiviral control in the infected host, a cell culture model that is closer to the in vivo situation was deliberately chosen. Primary cultures of MEFs were used to revisit the impact of vRAPs on MHC class I cell surface expression in untransformed cells. The data are documented for the MHC class I molecule Ld (Fig. 1), with essentially the same results being obtained for Kd and Dd (data not shown). In primary cultures of MEFs, not all cells are in a state permissive for infection. This results in two distinct populations, namely, infected cells and uninfected cells, visible in a two-color cytofluorometric analysis of the expression of class I and the viral cytoplasmic early-phase glycoprotein m164 (gp38/50), used here as a marker for infection. In contrast to the situation for transformed cells (41), class I expression was found to be low in fibroblasts of uninfected cultures (Fig. 1A) and highly upregulated in fibroblasts of cultures infected with mCMV-Δm04+06+152 (Fig. 1B). Notably, there was a particularly strong upregulation of surface class I in uninfected cells present within the infected cultures, whereas the expression was diminished, either less upregulated or actively downmodulated, in cells infected with the triple vRAP mutant. Upregulated class I expression is the elevated baseline for vRAPs to operate.
FIG. 1.
Regulation of MHC class I cell surface expression by vRAPs. Primary BALB/c-derived MEFs were either left uninfected (n.i., no infection) (A) or were infected with mutant mCMV-Δm04+06+152 lacking vRAPs (Ø) (B), with mCMV-WT expressing all three vRAPs (K), or with vRAP gene deletion mutants expressing individual vRAPs and combinations of vRAPs as indicated (C to H). Contamination of MEFs with CD11b+ cells (e.g., macrophages) was below the detection limit of cytofluorometric analysis. Two-color cytofluorometric analysis was performed after 16 h in the late early phase of viral gene expression. Contour plots represent fluorescence intensity levels for ∼35,000 live cells analyzed with no further gating. Ordinate fluorescence data represent expression of the viral cytoplasmic early-phase protein m164 (gp38/50), and abscissa fluorescence data represent cell surface expression of the MHC class I molecule Ld. Upregulated Ld expression in uninfected cells present within the infected cultures served as an internal standard. The regulating effect of vRAPs is highlighted by a caliper rule symbol. PE, phycoerythrin.
With the set of double vRAP gene deletion mutants, the effects of selective expression of single vRAPs m04, m06, and m152 were studied (Fig. 1C to E). Upregulation of MHC class I in uninfected cells present within the infected cultures was identical for all three virus mutants, whereas the vRAPs specifically and differentially regulated class I expression in the m164 antigen-positive, infected cell population. m04 had no detectable impact on cell surface expression of Ld compared with the triple vRAP mutant (Fig. 1, compare panels C and B). In contrast, both m06 (Fig. 1D) and m152 (Fig. 1E) strongly downmodulated class I, with m06 being somewhat more potent. It is worth noting that the downmodulation led to an expression level below that found for fibroblasts of uninfected cultures (Fig. 1, compare panels D and E with panel A).
With the set of single vRAP gene deletion mutants, combinations of two vRAPs were studied (Fig. 1F to H). Again, upregulation of class I in uninfected cells present within the infected cultures was not influenced by the mutations. Coexpression of m04 did not significantly ameliorate the expression of class I as downmodulated by m06 (Fig. 1, compare panels F and D). Coexpression of m152 and m06 led to the strong class I downmodulation predicted by the known synergistic functions of these two vRAPs, namely, class I retention and class I degradation, respectively (Fig. 1G). In contrast, m04 coexpressed with m152 largely, albeit not completely, rescued class I surface expression (Fig. 1H), thus antagonizing the retention function of m152.
To complete the panel, coexpression of all three vRAPs in mCMV-WT led to a strong class I downmodulation (Fig. 1K), indicating that m06 in turn efficiently antagonizes the positive effect of m04.
vRAP-modulated pattern of epitope presentation recognized by CD8 T cells.
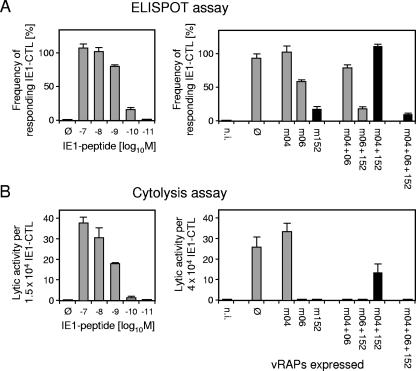
The key question was whether recognition of primary fibroblasts infected with the panel of vRAP gene deletion mutants by CD8 T cells would mirror the pattern observed for MHC class I cell surface expression or would confirm an interference of m04 with TCR recognition at the cell surface, as proposed by the literature (18). In a first approach, we used a CTL line specific for the well-studied, immunodominant IE1 peptide 168-YPHFMPTNL-176 of mCMV (31, 32) that is presented by Ld, so that the functional data (Fig. 2) can be directly related to the Ld cell surface expression data shown above (Fig. 1). Note that the IE1 CTL line used was epitope specific but polyclonal and had broad TCR Vβ usage comprising a wide range of TCR affinities (26). Thus, the frequency of CTLs that recognize infected cells reflects the level of epitope presentation: a high level of presentation stimulates all cells of the CTL line, whereas a low level of presentation stimulates only cells with a high-affinity TCRα/β. The following two effector functions of IE1 CTLs were compared for testing the panel of vRAP gene deletion mutants: IFN-γ secretion in an 18-h ELISPOT assay (Fig. 2A) and cytolytic activity in a 4-h 51Cr release assay (Fig. 2B). Both assays showed comparable sensitivities for the synthetic IE1 peptide exogenously loaded on MEFs, with the detection limits being 10−10 M.
FIG. 2.
vRAP-modulated pattern of IE1 epitope presentation detected with an IE1-specific CTL line. (A) Recognition of infected BALB/c MEFs as stimulator cells in an 18-h IFN-γ-based ELISPOT assay. (B) Recognition of infected and 51Cr-labeled BALB/c MEFs as target cells in a 4-h cytolysis assay. Effector cells were cells of a CTL line specific for the mCMV IE1 peptide 168-YPHFMPTNL-176 presented by Ld. Epitope recognition sensitivities of the IE1 CTLs in the assays were compared by exogenous loading of MEFs with synthetic IE1 peptide. Ø, no peptide added or no vRAP expressed. The vRAPs expressed in cells infected with the respective mutants (see Table 1) are indicated (n.i., no infection). Bars represent MPNs from linear regression analysis of dose-response curves, and error bars represent the 95% upper confidence limits. In the ELISPOT assays, dose-response curves were established with 300, 200, 100, and 50 CTLs throughout. In the cytolysis assays, log2-graded numbers of CTLs were seeded, starting with 40,000 cells in the case of peptide titration and with 60,000 cells in the test of the recognition of infected MEFs. Results for key viruses (see the text) are highlighted in black.
The recognition pattern observed in the ELISPOT assay (Fig. 2A) essentially mirrored the cell surface expression of Ld (recall Fig. 1). Specifically, fibroblasts infected with the triple vRAP gene deletion mutant stimulated all cells of the IE1 CTL line, whereas the phenomenon of immune evasion was seen after infection with mCMV-WT for the majority of CTLs comprising the polyclonal IE1 CTL line. However, this result also shows that the three vRAPs in concert failed to completely prevent presentation of the IE1 peptide, as ∼10% of the cells of this particular CTL line carried TCRs of an affinity sufficient to detect presented IE1 peptide. Single expression of m04 did not reduce epitope presentation and CD8 T-cell stimulation, indicating that this vRAP is not an immunoevasin in its own right. Single expressions of m06 and m152 each reduced epitope presentation and stimulation; however, m152 was significantly more effective in this regard. This is the most notable difference from the inhibitory effect on class I cell surface expression, where m06 was always somewhat stronger than m152 (Fig. 1) (41). Thus, among the three vRAPs expressed individually, m06 is the principal negative regulator of MHC class I cell surface expression, whereas m152 is the principal negative regulator of antigen presentation.
In the set of vRAP gene deletion mutants coexpressing combinations of two of the three vRAPs, m04 had a minor but statistically significant antagonizing effect on the function of m06, and m06 did not noticeably add to the inhibitory effect of m152 (confirmed in an independent second experiment with a different IE1 CTL line [see Fig. 3A]). The most striking result was found for the mutant coexpressing m04 and m152. Here, m04 completely reversed the immunoevasive effect of m152. This result identified m04 as a positively regulating vRAP or “anti-immunoevasin.” As with class I cell surface expression, m06, if coexpressed with m152 and m04 in mCMV-WT, acted as a negatively regulating vRAP, overruling the positive function of m04.
FIG. 3.
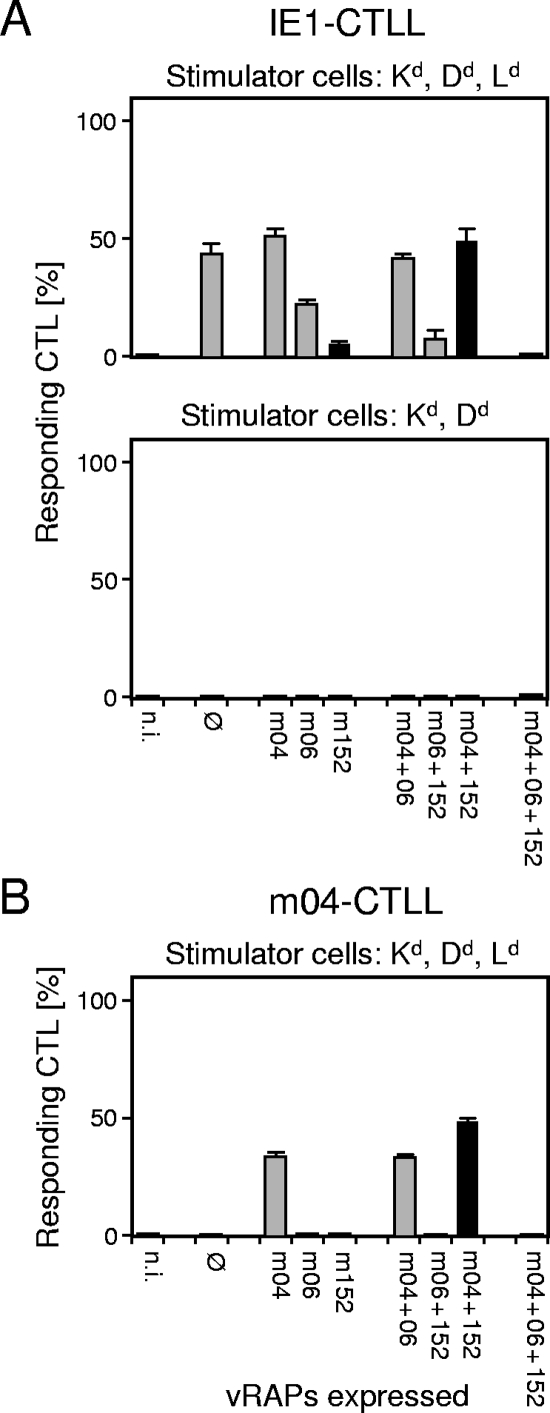
vRAP-modulated recognition patterns reflect epitope presentation. (A) vRAP-modulated recognition patterns of IE1 epitope-specific, Ld-restricted CTLs in ELISPOT assays performed with BALB/c-derived MEFs as stimulator cells expressing all three H-2d haplotype MHC class I molecules Kd, Dd, and Ld (top panel) or with BALB/c-H-2dm2-derived MEFs as stimulator cells expressing only Kd and Dd (bottom panel). (B) vRAP-modulated recognition pattern of m04 epitope-specific, Dd-restricted CTLs in an ELISPOT assay performed with BALB/c-derived MEFs as stimulator cells. For ELISPOT assay details, see the legend to Fig. 2. Ø, no vRAP expressed after infection with mCMV-Δm04+06+152; n.i., no infection.
The pattern for the CTL activity of the very same IE1 CTL line (Fig. 2B) showed an inferior resolution, which may relate to the shorter contact time between CTLs and infected target cells in the assay and the more limited period of viral gene expression scanned by the CTLs. Nonetheless, the essential message of the ELISPOT pattern is confirmed by the CTL activities against cells infected with the “key viruses” indicated in the figure: immunoevasion of cells expressing immunoevasin m152, recognition of cells coexpressing immunoevasin m152 and anti-immunoevasin m04, and immunoevasion of cells expressing all three vRAPs.
The vRAP-modulated CD8 T-cell recognition pattern depends on epitope presentation.
The negative vRAP and principal immunoevasin m152 does not exclusively inhibit the cell surface transport of MHC class I molecules but also downmodulates cell surface expression of RAE-1 family members, ligands of the activating NK cell receptor NKG2D (16, 19). Although activated CD8 T cells also express NKG2D, binding to RAE-1 ligands expressed on infected cells, RAE-1α, -β, and -γ in the specific case of BALB/c, cannot account for the observed ELISPOT reactivity pattern, because it is known that RAE-1/NKG2D interaction, in contrast to what is seen for NK cells, fails to signal in T cells (40). Speculatively, since mCMV infection causes global changes in the cellular transcriptome (37), one could argue that mCMV-infected fibroblasts might express as-yet-unidentified vRAP-regulatable virus-encoded or cellular virus-induced ligands of receptors expressed by activated T cells and capable of signaling for IFN-γ secretion. We chose two complementary genetic approaches to confirm that the observed patterns indeed reflect the presentation of antigenic peptides.
In the first approach (Fig. 3A), the presenting MHC class I molecule was absent from the infected stimulator cells. Specifically, we used MEFs of mutant mouse strain BALB/c-H-2dm2, which cannot present Ld-restricted peptides because of a genetic deletion in the MHC D region encompassing the Ld gene and genes D2d-D4d but are otherwise congenic with BALB/c cells (36). When an Ld-restricted IE1 CTL line was tested in an ELISPOT assay with infected BALB/c-derived MEFs expressing Kd, Dd, and the epitope-presenting Ld molecule, the recognition pattern of the panel of vRAP mutants known from the experiment shown in Fig. 2 was essentially reproduced. In contrast, there was no reactivity at all throughout the panel of viruses when the stimulator cells were BALB/c-H-2dm2-derived MEFs lacking the epitope-presenting Ld molecule.
In the second approach, the presenting class I molecule was expressed but the antigenic peptide to be presented was missing. CTLs specific for the Dd-restricted antigenic peptide 243-YGPSLYRRF-251 derived from protein m04, which is a vRAP and an antigen simultaneously (10), can of course recognize their cognate epitope only on cells infected with viruses in which the m04 gene is not deleted. This necessarily leads to gaps in the pattern, provided that the stimulation to IFN-γ secretion depends on epitope-specific recognition by the T cells. As shown in Fig. 3B, the decisive stimulator cells, namely, MEFs infected with the triple vRAP gene deletion mutant, did not stimulate the m04-specific CTLs.
Combined, these two approaches give reasonable evidence to conclude that the observed reactivity patterns reflect presentation of antigenic peptides by the infected cells.
All known MHC class I H-2d-restricted epitopes of mCMV essentially fit the pattern.
We have so far used presentation of the immunodominant IE1 peptide as a paradigm to show the influence of vRAPs on antigen presentation. It was of course of interest to learn if the negative and positive functions of vRAPs depend qualitatively on the MHC allele and/or on the presented antigenic peptide. vRAPs interact with MHC class I molecules, transiently in the case of m152 and stably in the cases of m04 and m06. Therefore, differences in interaction affinities may certainly account for differences in the relative contributions of vRAPs to antigen presentation, favoring or disfavoring antigen presentation depending on whether positive or negative vRAPs prevail in the competition for class I molecules. The testing of CTL lines specific for all currently known MHC class I H-2d-restricted immunodominant as well as subdominant epitopes of mCMV (8, 31) indeed revealed distinct differences (Fig. 4). To comment just on the more obvious examples, presentation of the Dd-restricted m18 peptide was poorly affected by negative vRAPs m06 and m152, and the effect of m06 was consistently weak for all three tested Dd-restricted epitopes. However, we are hesitant in interpreting these data as MHC allele-specific differences in the intrinsic efficacies of vRAPs. Work in progress (R. Holtappels, unpublished data) indicates that the amount of processed peptide is a major parameter as well. It is clear that negative vRAPs have more difficulty in completely inhibiting the transport of high numbers compared to low numbers of peptide-loaded MHC complexes. In addition, the TCRα/β affinity distributions are identical neither between CTL lines specific for different epitopes nor even between independently generated CTL lines specific for the same epitope. Again, it is clear that high-affinity TCRs can detect small amounts of MHC-peptide complexes at the cell surface, which may lead to the wrong impression of a low vRAP efficacy in preventing antigen presentation. Altogether, one must be cautious with the interpretation of minor differences in selected data.
FIG. 4.
vRAP-modulated recognition patterns of infected BALB/c MEFs for all currently known MHC class I-restricted mCMV epitopes in the H-2d haplotype, except for IE1-Ld and m04-Dd, which are shown in Fig. 3. For definitions and ELISPOT assay details, see the legend to Fig. 2.
Thinking in more-general principles, the relevant key information is that the patterns, without a single exception noted, are consistent with regard to the principal roles of vRAPs, namely, m152 modulating antigen presentation negatively, m04 counteracting the MHC class I retention function of m152, and m06 overruling m04 (Fig. 4).
vRAPs can operate in like manner in different cell types.
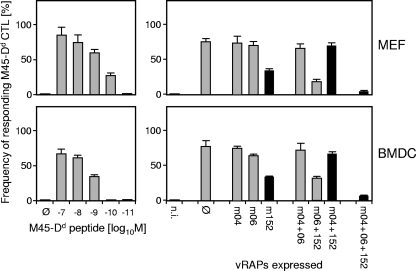
Cell-type-specific differences could possibly determine differences in the relative contributions of vRAPs (for a discussion, see references 20 and 31). We therefore compared the recognition patterns for the panel of vRAP gene deletion mutants for MEFs as an example of a stromal cell type and for BMDCs as an example of a professional antigen-presenting cell type of hematopoietic origin. As a paradigm, the analysis was performed with CTLs specific for the Dd-restricted M45-derived peptide 507-VGPALGRGL-515 (Fig. 5). Although detection of exogenously loaded M45 peptide was a bit more sensitive with MEFs, the recognition patterns were almost congruous.
FIG. 5.
vRAPs operate in like manner in different cell types. In ELISPOT assays, infected BALB/c MEFs were used as an example of a stromal cell type (top panel) and infected BALB/c BMDCs as an example of a professional antigen-presenting cell type of hematopoietic lineage (bottom panel). Effectors were cells of a CTL line specific for the M45-derived peptide 507-VGPALGRGL-515 presented by Dd. The sensitivities of epitope detection by M45-Dd-specific CTLs for the two cell types are compared by exogenous loading with synthetic peptide. Ø, no peptide added or no vRAP expressed; n.i., no infection.
vRAP contributions to the regulation of epitope presentation in cells of the H-2b haplotype.
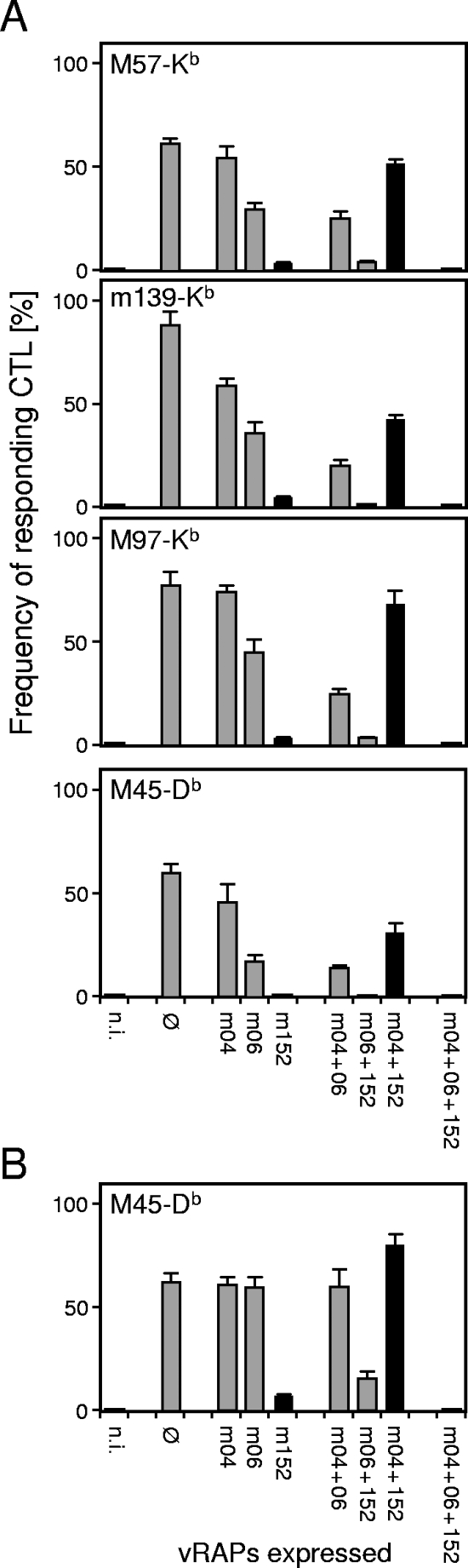
The data for the H-2d haplotype have consistently shown that m04 can act as a positive regulator antagonizing the inhibitory function of m152. In contrast, for the H-2b haplotype, previous work by Kavanagh and colleagues has led to the conclusion that m04 and m152 inhibit peptide presentation in a complementary and cooperative fashion, with the contribution of m04 being required to completely inhibit Kb- but not Db-restricted CTLs (13). To test if there indeed exists a qualitative difference in vRAP functions between the two haplotypes, and in particular for the Kb allele, we have monitored the recognition patterns with the complete set of vRAP gene deletion mutants for a panel of H-2b-restricted antigenic peptides recently identified by Munks and colleagues (24). The list of tested peptides includes Kb-presented peptides derived from proteins M57 and M97 recognized by the CTL clones 5 and 96, respectively (24), two of the CTL clones used in the previous study by Kavanagh and colleagues (13). As shown in Fig. 6A for epitope-specific, polyclonal CD8 T cells comprising a range of TCR affinities, the recognition patterns correspond to the patterns documented above for BALB/c mice in the more basic features. Most notably, even though m04 appears to take a modest negative role when expressed alone (in the case of m139-Kb) and when coexpressed with m06 (in the cases of m139-Kb and M97-Kb), it was clearly found to exert its positive vRAP function when coexpressed with m152, and this was true also for the three Kb-restricted epitopes tested. Thus, there apparently exists no fundamental difference between the two H-2 haplotypes with regard to the negative and positive functions of the three vRAPs.
FIG. 6.
vRAP-modulated recognition patterns for epitopes presented by MHC class I molecules of the haplotype H-2b. (A) Results of ELISPOT assays performed with C57BL/6-derived MEFs as stimulator cells. Epitope specificities of the CTLs and the corresponding presenting class I alleles are indicated. (B) Results of an ELISPOT assay performed with M45-Db epitope-specific CTLs as effector cells and with C57BL/6-derived BMDCs as stimulator cells. For definitions, see the legend to Fig. 2.
In the case of the Db-restricted epitope M45, 985-HGIRNASFI-993 (3), the two negatively modulating vRAPs m152 and m06 were found to be very effective in fibroblasts, with m152 alone being sufficient to completely prevent antigen presentation (Fig. 6A, bottom panel). The presentation of this epitope was found to be less strictly regulated in BMDCs (Fig. 6B). Specifically, vRAP m06 failed completely when expressed alone or in combination with m04 but efficiently counteracted the positive vRAP m04 in BMDCs infected with mCMV-WT. Notably, the anti-immunoevasin function of m04, when coexpressed with immunoevasin m152, was very pronounced in MEFs as well as in BMDCs.
Relevance of vRAPs in regulating in vivo antigen presentation in the infected host.
A priori, neither MEFs nor BMDCs can be regarded as being representative of all of the various cell types infected during CMV disease in host organs. The list of such cell types includes hepatocytes, endothelial cells, pneumocytes and various other types of epithelial cells, myocytes, adipocytes, connective tissue fibrocytes, macrophages, and dendritic cells. Thus, the effect on virus replication in infected organs is the most meaningful assessment criterion for the relevance of vRAPs in the CD8 T-cell-mediated control of CMV disease (for a review, see reference 8).
By using an adoptive cell transfer approach, previous work by our group (9) has shown that CD8 T cells specific for the Db-restricted M45 epitope controlled mCMV replication in spleen, lungs, and liver of C57BL/6 mice infected with vRAP gene deletion mutant mCMV-Δm152 but completely failed in controlling the corresponding revertant virus mCMV-Δm152-rev or mCMV-WT. As revealed by the recognition patterns shown here (Fig. 6), this in vivo finding is in perfect accordance with the dominant inhibitory role of vRAP m152 on M45-Db epitope presentation in MEFs as well as in BMDCs and also with the presentation of the epitope after deletion of the m152 gene. We therefore used this well-established model for testing the in vivo control of the three key viruses, namely, mCMV-Δm04+06 selectively expressing immunoevasin m152, mCMV-Δm06 coexpressing m152 and the anti-immunoevasin m04, and mCMV-WT expressing the m04 antagonizer m06 in addition to m152 and m04. Of particular interest was the question of whether reversal of m152-inhibited epitope presentation by the positive vRAP m04 occurs under in vivo infection conditions also.
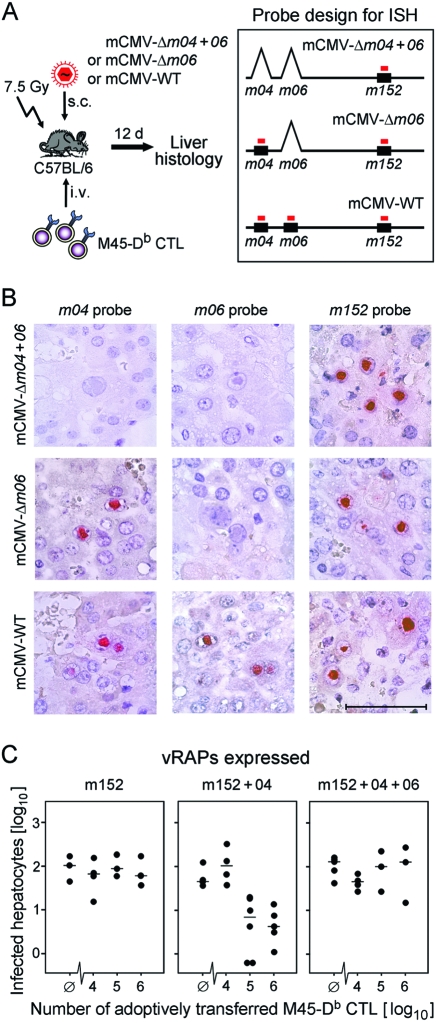
We have chosen the liver, since it represents an organ site of a relevant manifestation of CMV disease, namely, CMV hepatitis. The experimental protocol for adoptive cell transfer and the molecular histology approach for quantitating infection in the liver are sketched for explanation (Fig. 7A). Graded numbers of CTLs specific for the M45-Db epitope were transferred into immunocompromised C57BL/6N recipients infected with the key viruses, and infected cells in the liver were visualized by DNA-DNA ISH using DNA probes specific for the three vRAP genes. As a rigorous quality control, the combinatorial ISH image (Fig. 7B) shows the staining patterns for the groups of recipients that were left without CD8 T cells. The results definitely verified the identity of the three key viruses used for infection. The common molecular denominator of the three viruses, gene m152, was then used for quantitating infected hepatocytes in liver tissue sections on day 12 after the CTL transfer (Fig. 7C). In accordance with the in vitro epitope presentation data for infected MEFs and BMDCs, selective expression of the negatively regulating vRAP m152 prevented the control of liver infection. Coexpression of the positively regulating vRAP m04 restored the control of liver infection, as indicated by a CTL dose-dependent reduction in the number of infected hepatocytes. Additional coexpression of the negatively regulating vRAP m06 again prevented the control of liver infection.
FIG. 7.
Adoptive cell transfer approach for testing vRAP function in vivo. (A) Experimental protocol and probe design for ISH. C57BL/6 transfer recipients were immunocompromised by 7.5-Gy γ-irradiation and were infected subcutaneously (s.c.) with the key viruses indicated. CTLs specific for the M45-derived peptide 985-HGIRNASFI-993 presented by Db were administered in graded numbers intravenously (i.v.). On day (d) 12 after transfer and infection, liver tissue sections were analyzed by ISH for the presence of infected cells, which are primarily hepatocytes. Maps (not drawn to scale) illustrate the positions of vRAP genes (black boxes) and DNA probes (red bars) in the mCMV genome. (B) Combinatorial ISH images of liver tissue sections for the three key viruses and the respective vRAP gene probes verifying the molecular identities of the viruses in the experimental groups with no adoptive transfer (positive control groups; see Ø columns in panel C). Red staining localizes viral DNA accumulated in intranuclear inclusion bodies of infected hepatocytes. Bar marker, 50 μm. (C) Control of virus replication in the liver. The expressed vRAPs are indicated, corresponding (from left to right) to infection with the key viruses mCMV-Δm04+06, mCMV-Δm06, and mCMV-WT, respectively. For quantitation of infected hepatocytes, ISH was performed with the m152 gene-specific probe. The ordinate values represent the numbers of infected hepatocytes present in 50-mm2 areas of liver tissue sections. Black dots represent data for individual adoptive transfer recipients, with the median values marked. Ø, no cell transfer.
Among all epitopes tested so far, M45-Db proved to be an exception in that immunotherapy targeted to it completely failed to control mCMV-WT (9), a finding reproduced here. This particularity of the model was useful to get protection-on and protection-off data. Work in progress is aimed at identifying the molecular reason for the special position of the M45-Db epitope, and there exists evidence for a very small amount of peptide generated in infected cells, so that minimal vRAP function is required to block the transport of the few MHC-peptide complexes to the cell surface (Holtappels et al., unpublished data). The rule defined by our own group's previous work, however, is that transferred CTLs protect immunocompromised recipients in a dose-dependent manner despite the presence of all three vRAPs and regardless of whether the corresponding epitope is classified as dominant or subdominant for CD8 T-cell priming (reviewed in reference 8). That the protein source of the antigenic peptide is not the decisive parameter is intriguingly revealed by the protective M45-Dd epitope.
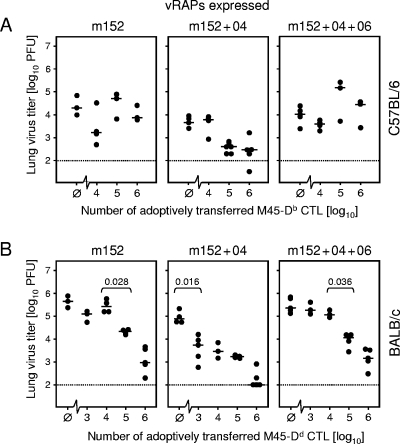
We have therefore used the M45-Db and M45-Dd pair of T-cell specificities to compare the roles of vRAPs in adoptive transfer models, which are refractory and susceptible to the control of mCMV-WT, respectively. For this experiment we have chosen the lungs as another organ site of a relevant manifestation of CMV disease, namely, interstitial CMV pneumonia. Virus replication in the lungs was quantitated by the detection of infectious virus (Fig. 8). The data obtained with C57BL/6 recipients and CTLs specific for the M45-Db epitope (Fig. 8A) fully confirmed the in vivo role of vRAPs as seen before for the liver. Although CTLs specific for the M45-Dd epitope protected all infected BALB/c recipients at high cell numbers, the dose-response curves reflecting antiviral efficacy clearly revealed the function of the vRAPs. Specifically, if m152 was selectively expressed in the infected lungs, significant control of virus replication required at least 105 CTLs. If m04 was coexpressed, significant protection was achieved with as few as 103 CTLs. Finally, additional coexpression of m06 reduced the efficacy of antiviral control again to where at least 105 CTLs were required for significant protection (Fig. 8B).
FIG. 8.
vRAPs regulate the CD8 T-cell-mediated control of virus replication in the lungs. The principle of the in vivo assay of vRAP function is indicated in Fig. 7. The expressed vRAPs are indicated, corresponding (from left to right) to infection with the key viruses mCMV-Δm04+06, mCMV-Δm06, and mCMV-WT, respectively. (A) Adoptive transfer of M45-Db (985-HGIRNASFI-993)-specific CTLs into 7.5-Gy-irradiated and infected C57BL/6N recipients. (B) Adoptive transfer of M45-Dd (507-VGPALGRGL-515)-specific CTLs into 6.5-Gy-irradiated BALB/cJ recipients. Throughout, virus replication in the lungs was assessed on day 12 after infection and transfer by a virus plaque assay of organ homogenates. The ordinate values represent the amounts of infectious virus per lung, expressed as PFU. The dotted line indicates the detection limit of the virus plaque assay. Throughout, black dots represent data for individual adoptive transfer recipients, with the median values marked. Ø, no cell transfer. In panel B, P values for significance (Wilcoxon-Mann-Whitney test; two-tailed; P < 0.05) are indicated for the minimal effector cell doses required for protection.
In conclusion, the regulation of epitope presentation by vRAPs in fibroblasts and BMDCs proved to be a reliable predictor for the regulation of antiviral control in vivo. Most importantly, the data have also revealed a positive vRAP function of m04 in different tissues of the infected host.
DISCUSSION
Employing a comprehensive set of vRAP gene deletion mutants, this functional analysis of vRAP-modulated antigen presentation gives a fascinating view of viral manipulation of host immune surveillance. The important new insight given by our data is that mCMV can regulate antigen presentation not only negatively, which was known before, but also positively. Glycoprotein m04/gp34 is here redefined as a regulator that has the intrinsic propensity to support antigen presentation by transporting peptide-loaded MHC class I complexes to the cell surface for recognition by CD8 T cells. Its capacity to antagonize the MHC class I retention function of vRAP m152/gp40 was consistently observed for all five MHC class I molecules of the haplotypes H-2d and H-2b as well as for 10 different epitopes derived from nine different proteins. It was derived in cell culture for fibroblasts and BMDCs, and in vivo in different tissues of the infected host. Thus, the current opinion that m04 is an immunoevasin/VIPR that operates by blocking the interaction between TCRα/β and the MHC-peptide complexes at the cell surface (18) needs to be revised. By encoding two negative vRAPs as well as one positive vRAP, mCMV has acquired the genetic potential to “decide” by differential gene expression whether the outward appearance of an infected host cell to antiviral CD8 T lymphocytes is “self” or “altered self.”
Our studies have now identified m152 as the principal negatively regulating vRAP for mCMV antigen presentation in the MHC class I pathway. Its deletion restores antigen presentation despite the presence of m04 and m06. This functional consequence of m152 action is molecularly well explained by its capacity to interfere with the default pathway of MHC class I trafficking through catalyzing the retention of peptide-loaded MHC class I molecules in an ERGIC/cis-Golgi compartment (44, 45). vRAP m06 has been shown to bind stably to MHC class I molecules and to route them into the late endosome-lysosome pathway of trans-Golgi network cargo sorting for degradation. This function in cargo sorting has been shown to involve the heterotetrameric cargo-sorting adaptor proteins AP-1A and AP-3A (34, 35). If expressed alone, its impact is greatest on cell surface expression of MHC class I molecules (Fig. 1) (41), but, as shown here, it is only second rank and of variable efficacy in the negative regulation of epitope presentation. Combined with vRAP m152, its function might be described as that of a “trash collector” working hand in hand with the somewhat earlier expressed m152 by transporting the retained complexes to the lysosomes for disposal. Its main role in antigen presentation, however, appears to be to overrule the positive regulatory effect of m04 in mCMV-WT.
The role of vRAP m04 has long remained enigmatic, and some aspects still present us with riddles. Since its discovery by Kleijnen and colleagues (15), it has been clear that vRAP m04 biochemically antagonizes the MHC class I retention function of m152. It was found to bind stably to MHC class I molecules within the ER, with the complex then being transported via the Golgi apparatus to the cell surface (14, 15). As shown by Wagner and colleagues (41), m04 acts as a positive regulator with regard to MHC class I cell surface expression; it was found to reverse the inhibitory effect of m152. Nevertheless, it has entered the literature as a negative vRAP with the idea that its binding to MHC class I molecules would physically interfere with recognition by the TCR at the cell surface (reviewed in reference 18). The current opinion that vRAP m04 is a negative regulator of antigen presentation rests largely on previous work by Kavanagh and colleagues (13). By testing the cytolytic activities of CTL clones in order to compare the recognition levels of IFN-γ-pretreated cells infected with mCMV-WT or mCMV-Δm04 and arrested in the E phase of viral gene expression by phosphonoacetic acid, those authors have shown that immunoevasion is relieved by deletion of the m04 gene in the case of Kb-restricted CTL clones but not in that of Db-restricted CTL clones. The finding that selective deletion of gene m04 showed an antigen presentation phenotype exclusively in cells that were pretreated with IFN-γ (13) raises the question of whether the function of m04 and of vRAPs in general is altered by cytokines. In this context it is important to note that the cells in our assays were deliberately not pretreated with IFN-γ or metabolic inhibitors to avoid any undefined cytokine- or drug-mediated modulation of vRAP interplay.
Nevertheless, our data are not in conflict with previously published data (13); what differs is the interpretation. In fact, an inhibitory contribution made by m04 after infection with mCMV-WT is also revealed by our more comprehensive analysis, which included the epitopes M57-Kb and M97-Kb (Fig. 6A) studied by Kavanagh and colleagues (13). We observed this phenomenon also for M45-Db in BMDCs (Fig. 6B) and in particular for all tested Dd-restricted epitopes in fibroblasts (Fig. 4). However, in all these examples the function of m04 in mCMV-WT was superimposed by the function of m06 and, in our opinion, the data reflect an interplay between m04 and m06 rather than a cooperation between m04 and m152, as was proposed previously (13). For conclusions regarding an interplay between m04 and m152, it is more straightforward to compare virus mutants mCMV-Δm04+06 and mCMV-Δm06, which express m152 alone and in combination with m04, respectively. This comparison has undoubtedly revealed the propensity of m04 to support antigen presentation.
The m152 protein, a member of the m145 gene family, is probably the most ancient of the three vRAPs. This view is supported by the facts that several family members modulate innate immunity to mCMV (for an overview, see reference 12) and that this role is still conserved in m152, enabling it to downmodulate NK cell receptor ligands of the RAE-1 group (19). On the other hand, m04 and m06, closely related members of the m02 gene family, appear to be involved in the disposal of MHC class I complexes retained in the ERGIC as a result of preceding m152 function. In their cytoplasmic tails, m04 and m06 carry sequence motifs, i.e., a YXXΦ motif and a dileucine motif, respectively, which link the m04 class I and m06 class I complexes to cellular adaptor proteins of cargo-sorting pathways (25, 34). This in turn can lead to MHC class I cell surface display mediated by m04 or to lysosomal degradation mediated by m06. It remains an open question why m04, in the presence of m06, can also contribute to negative regulation. One idea is that m04 enhances the efficacy of m06-mediated lysosomal degradation by binding MHC class I molecules in the ER and directing them to the trans-Golgi network for cargo sorting. As both m04 and m06 stably bind to class I molecules, thus competing for the same cargo, the functional data predict that m06 is more efficient in this competition and “steals” the cargo from m04 in the trans-Golgi network for degradation. In this view, the negative vRAP m06 exploits the positive vRAP m04 to have its assistance in the negative function. Experiments to test this hypothesis are in progress.
The evolutionary philosophy of mCMV for employing three proteins belonging to two discrete gene families for modulating antigen presentation is a compelling question. Previously, the question had been why three proteins are required for achieving the same goal. Now, a quite different picture of a sophisticated hierarchic regulation, in which the negative regulator m152 is balanced by the positive regulator m04 that in turn is balanced by the negative regulator m06, is emerging. Under the conditions of productive infection studied so far, the net effect of this hierarchic regulation has been the inhibition of antigen presentation. So, one may ask whether the positive regulator m04 is of any relevance at all. We are convinced that mCMV has not acquired and maintained this gene function for luxury. Future investigations will have to address the possibility of a differential transcriptional and/or posttranscriptional regulation of vRAP gene expression, for instance through signaling by cytokines and ligands of Toll-like receptors or as a result of cell-type- and cell-differentiation-dependent regulation. We propose that m06, through its capacity to overrule m04, is the master regulator of mCMV antigen presentation, and we predict that regulation of m06 gene expression is the molecular switch. Obviously, selective repression of m06 gene expression would enable m04 to exert its positive effect on antigen presentation.
According to current paradigms, vRAPs have little effect on the priming of the immune response and the maintenance of immunological memory in immunocompetent adult mice (3, 4), supposedly because their function is bypassed by antigen cross-presentation through uninfected dendritic cells and alternative mechanisms of immune control (for a discussion, see reference 8). However, vRAPs undoubtedly interfere with clearance of productive infection in stromal and parenchymal tissue cells in the immunocompromised host under conditions of CMV disease when protective CD8 T cells become limiting in number (Fig. 8) (9, 17). Furthermore, recent work by Lu and colleagues suggests that vRAPs may facilitate horizontal virus transmission by inhibiting CD8 T-cell control of virus replication in the salivary glands (21). That evolution of vRAPs preventing antigen presentation is in the interest of the virus appears to be plausible; however, the question of why mCMV expresses a vRAP that supports antigen presentation remains.
CMVs are mostly acquired perinatally and establish persistent infection in the immunologically immature host at a time when immunological self-tolerance is induced in the developing immune system. One intriguing idea with great potential for future investigation is that a shift in the balance between the three vRAPs to the favor of m04 and to the disfavor of m06 in infected tolerogenic cells might help the virus to survive in the long term by presenting antigens for tolerance induction. Thus, both prevention of antigen presentation by negative vRAPs in productively infected tissue cells and support of antigen presentation by the positive vRAP in infected tolerogenic cells can be to the benefit of the virus.
Acknowledgments
We are indebted to Michael W. Munks and Ann B. Hill for information on antigenic peptides prior to publication and to Ulrich H. Koszinowski for the permission to work with the vRAP gene deletion mutants. We thank Kurt Reifenberg and the staff of the CLAF of the Johannes Gutenberg-University for expert animal care.
This work was supported by the Deutsche Forschungsgemeinschaft, SFB 490, individual projects E2 (M.J.R.), E3 (R.H., D.G.-M., D.T., and P.D.), E4 (S.A.O.-K., D.S., and M.J.R.), and E6 (S.H.) as well as by SFB 432, individual project A10 (J.P. and M.J.R.). M.W. was supported by the Bayerische Forschungsstiftung, Forschungsverbund “Neue Strategien der Immuntherapie,” individual project I5.
REFERENCES
- 1.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 9:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Val, M., H. Hengel, H. Haecker, U. Hartlaub, T. Ruppert, P. Lucin, and U. H. Koszinowski. 1992. Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial-Golgi compartment. J. Exp. Med. 176:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold, M. C., M. W. Munks, M. Wagner, U. H. Koszinowski, A. B. Hill, and S. P. Fling. 2002. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL, but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J. Immunol. 169:359-365. [DOI] [PubMed] [Google Scholar]
- 4.Gold, M. C., M. W. Munks, M. Wagner, C. W. McMahon, A. Kelly, D. G. Kavanagh, M. K. Slifka, U. H. Koszinowski, D. H. Raulet, and A. B. Hill. 2004. Murine cytomegalovirus interference with antigen presentation has little effect on the size or the effector memory phenotype of the CD8 T cell response. J. Immunol. 172:6944-6953. [DOI] [PubMed] [Google Scholar]
- 5.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190-197. [DOI] [PubMed] [Google Scholar]
- 7.Herter, S., P. Osterloh, N. Hilf, G. Rechtsteiner, J. Hohfeld, H. G. Rammensee, and H. Schild. 2005. Dendritic cell aggresome-like-induced structure formation and delayed antigen presentation coincide in influenza virus-infected cells. J. Immunol. 175:891-898. [DOI] [PubMed] [Google Scholar]
- 8.Holtappels, R., M. W. Munks, J. Podlech, and M. J. Reddehase. 2006. CD8 T-cell-based immunotherapy of cytomegalovirus disease in the mouse model of the immunocompromised bone marrow transplantation recipient, p. 383-418. In M. J. Reddehase (ed.), Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Wymondham, Norfolk, United Kingdom.
- 9.Holtappels, R., J. Podlech, M.-F. Pahl-Seibert, M. Juelch, D. Thomas, C. O. Simon, M. Wagner, and M. J. Reddehase. 2004. Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J. Exp. Med. 199:131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtappels, R., D. Thomas, J. Podlech, G. Geginat, H. P. Steffens, and M. J. Reddehase. 2000. The putative natural killer decoy early gene m04 (gp34) of murine cytomegalovirus encodes an antigenic peptide recognized by protective antiviral CD8 T cells. J. Virol. 74:1871-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtappels, R., D. Thomas, J. Podlech, and M. J. Reddehase. 2002. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 76:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonjic, S., I. Bubic, and A. Krmpotic. 2006. Innate immunity to cytomegaloviruses, p. 285-319. In M. J. Reddehase (ed.), Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Wymondham, Norfolk, United Kingdom.
- 13.Kavanagh, D. G., M. C. Gold, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2001. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J. Exp. Med. 194:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavanagh, D. G., U. H. Koszinowski, and A. B. Hill. 2001. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J. Immunol. 167:3894-3902. [DOI] [PubMed] [Google Scholar]
- 15.Kleijnen, M. F., J. B. Huppa, P. Lucin, S. Mukherjee, H. Farrell, A. E. Campbell, U. H. Koszinowski, A. B. Hill, and H. Ploegh. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 16:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krmpotic, A., D. H. Busch, I. Bubic, F. Gebhardt, H. Hengel, M. Hasan, A. A. Scalzo, U. H. Koszinowski, and S. Jonjic. 2002. MCMV glycoprotein gp40 confers virus resistance to CD8+ T lymphocytes and NK cells in vivo. Nat. Immunol. 3:529-535. [DOI] [PubMed] [Google Scholar]
- 17.Krmpotic, A., M. Messerle, I. Crnkovic-Mertens, B. Polic, S. Jonjic, and U. H. Koszinowski. 1999. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med. 190:1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilley, B. N., and H. L. Ploegh. 2005. Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol. Rev. 207:126-144. [DOI] [PubMed] [Google Scholar]
- 19.Lodoen, M., K. Ogasawara, J. A. Hamerman, H. Arase, J. P. Houchins, E. S. Mocarski, and L. L. Lanier. 2003. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J. Exp. Med. 197:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LoPiccolo, D. M., M. C. Gold, D. G. Kavanagh, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2003. Effective inhibition of Kb- and Db-restricted antigen presentation in primary macrophages by murine cytomegalovirus. J. Virol. 77:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, X., A. K. Pinto, A. M. Kelly, K. S. Cho, and A. B. Hill. 2006. Murine cytomegalovirus interference with antigen presentation contributes to the inability of CD8 T cells to control virus in the salivary gland. J. Virol. 80:4200-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 94:14759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, R. G., and M. Dunkley. 1974. Quantitative analysis of the 51Cr release cytotoxicity assay for cytotoxic lymphocytes. Cell. Immunol. 14:284-302. [DOI] [PubMed] [Google Scholar]
- 24.Munks, M. W., M. C. Gold, A. L. Zajac, C. M. Doom, C. S. Morello, D. H. Spector, and A. B. Hill. 2006. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 176:3760-3766. [DOI] [PubMed] [Google Scholar]
- 25.Nakatsu, F., and H. Ohno. 2003. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct. Funct. 28:419-429. [DOI] [PubMed] [Google Scholar]
- 26.Pahl-Seibert, M.-F., M. Juelch, J. Podlech, D. Thomas, P. Deegen, M. J. Reddehase, and R. Holtappels. 2005. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J. Virol. 79:5400-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto, A. K., and A. B. Hill. 2005. Viral interference with antigen presentation to CD8 T cells: lessons from cytomegalovirus. Viral Immunol. 18:434-444. [DOI] [PubMed] [Google Scholar]
- 28.Ploegh, H. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 29.Podlech, J., R. Holtappels, N. K. A. Grzimek, and M. J. Reddehase. 2002. Animal models: murine cytomegalovirus. Methods Microbiol. 32:493-525. [Google Scholar]
- 30.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddehase, M. J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831-844. [DOI] [PubMed] [Google Scholar]
- 32.Reddehase, M. J., J. B. Rothbard, and U. H. Koszinowski. 1989. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature 337:651-653. [DOI] [PubMed] [Google Scholar]
- 33.Reddehase, M. J., C. O. Simon, J. Podlech, and R. Holtappels. 2004. Stalemating a clever opportunist: lessons from murine cytomegalovirus. Hum. Immunol. 65:446-455. [DOI] [PubMed] [Google Scholar]
- 34.Reusch, U., O. Bernhard, U. H. Koszinowski, and P. Schu. 2002. AP-1A and AP-3A lysosomal sorting functions. Traffic 3:752-761. [DOI] [PubMed] [Google Scholar]
- 35.Reusch, U., W. Muranyi, P. Lucin, H. G. Burgert, H. Hengel, and U. H. Koszinowski. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubocki, R. J., T. H. Hansen, and D. R. Lee. 1986. Molecular studies of murine mutant BALB/c-H-2dm2 define a deletion of several class I genes including the entire H-2Ld gene. Proc. Natl. Acad. Sci. USA 83:9606-9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shenk, T. 2006. Human cytomegalovirus genomics, p. 49-61. In M. J. Reddehase (ed.), Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Wymondham, Norfolk, United Kingdom.
- 38.Thäle, R., U. Szepan, H. Hengel, G. Geginat, P. Lucin, and U. H. Koszinowski. 1995. Identification of the mouse cytomegalovirus genomic region affecting major histocompatibility complex class I molecule transport. J. Virol. 69:6098-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 40.Vivier, E., E. Tomasallo, and P. Paul. 2002. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr. Opin. Immunol. 14:306-311. [DOI] [PubMed] [Google Scholar]
- 41.Wagner, M., A. Gutermann, J. Podlech, M. J. Reddehase, and U. H. Koszinowski. 2002. MHC class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner, M., S. Jonjic, U. H. Koszinowski, and M. Messerle. 1999. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J. Virol. 73:7056-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler, H., W. Muranyi, H. G. Burgert, E. Kremmer, and U. H. Koszinowski. 2000. The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules. EMBO J. 19:870-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziegler, H., R. Thäle, P. Lucin, W. Muranyi, T. Flohr, H. Hengel, H. Farrell, W. Rawlinson, and U. H. Koszinowski. 1997. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6:57-66. [DOI] [PubMed] [Google Scholar]