Abstract
We describe the characterization of influenza A virus infection of an established in vitro model of human pseudostratified mucociliary airway epithelium (HAE). Sialic acid receptors for both human and avian viruses, α-2,6- and α-2,3-linked sialic acids, respectively, were detected on the HAE cell surface, and their distribution accurately reflected that in human tracheobronchial tissue. Nonciliated cells present a higher proportion of α-2,6-linked sialic acid, while ciliated cells possess both sialic acid linkages. Although we found that human influenza viruses infected both ciliated and nonciliated cell types in the first round of infection, recent human H3N2 viruses infected a higher proportion of nonciliated cells in HAE than a 1968 pandemic-era human virus, which infected proportionally more ciliated cells. In contrast, avian influenza viruses exclusively infected ciliated cells. Although a broad-range neuraminidase abolished infection of HAE by human parainfluenza virus type 3, this treatment did not significantly affect infection by influenza viruses. All human viruses replicated efficiently in HAE, leading to accumulation of nascent virus released from the apical surface between 6 and 24 h postinfection with a low multiplicity of infection. Avian influenza A viruses also infected HAE, but spread was limited compared to that of human viruses. The nonciliated cell tropism of recent human H3N2 viruses reflects a preference for the sialic acid linkages displayed on these cell types and suggests a drift in the receptor binding phenotype of the H3 hemagglutinin protein as it evolves in humans away from its avian virus precursor.
Influenza A viruses cause illness and raised mortality rates in susceptible populations every winter and significantly increase disease burden during pandemic periods, which have occurred at irregular and unpredictable intervals during the last century. The most recent pandemic in 1968 was due to the introduction of a new influenza subtype (H3N2) into the human population. The H3N2 pandemic virus was produced by a reassortment event between the H2N2 influenza subtype that had previously been circulating in the human population and an avian influenza virus. Six gene segments from the H2N2 human strain, including the N2 neuraminidase (NA) gene, combined with the H3 hemagglutinin (HA) and PB1 genes acquired from an avian virus source. The resulting virus with its novel avian HA protein caused widespread infection in the immunologically naive human population (14, 34).
The first stage in influenza virus entry to a host cell is recognition of terminal sialic acid on glycosylated cell surface molecules by the viral HA protein. HA receptor specificity is host species dependent such that human influenza viruses bind via HA to receptor molecules bearing α-2,6-linked sialic acid while avian strains preferentially bind to α-2,3-linked sialic acid (31). A single amino acid change in the HA1 subunit from glutamine to leucine at position 226 has been shown to switch the preference from α-2,3- to α-2,6-linked sialic acid (32), and this mutation is critical in the early adaptation of avian virus HA to humans (4, 20, 25).
In humans, influenza A viruses are primarily respiratory pathogens targeting cells in the respiratory epithelium of the human airway. Early work to identify the cell type targeted by human influenza viruses showed virus binding to the ciliated respiratory epithelium, which displayed the appropriate α-2,6-linked sialic acid receptors in preference to mucin-producing goblet cells containing α-2,3-linked sialic acid (3, 6). More recently, Matrosovich and coworkers demonstrated that the initial cell target of human influenza viruses in a cell culture model of the human airway epithelium is the nonciliated cell type while, in contrast, influenza viruses from avian species infect ciliated cells, and this reflects the distinct expression of sialic acid linkages on nonciliated (α-2,6) and ciliated (α-2,3) cells, respectively (21).
Respiratory viruses such as influenza virus must surmount obstacles in the human airway to reach the cells of the ciliated respiratory epithelium, where infection and replication occur. The mucociliary epithelium presents a physical barrier in terms of active cilial function and the presence of a complex array of glycocalyx components such as tethered and soluble mucins displaying sialyloligosaccharides, which can function as false receptors impeding virus delivery to the surface of the epithelium. In addition, the availability of appropriate receptor molecules on the apical cell surface also drives evolution of the virus-receptor interaction and adaptation to the human host. The importance of the array of sialyloligosaccharides presented on the human airway epithelium in the selection of HA receptor specificity is suggested by the shift in receptor specificity in human viruses compared to that in their avian progenitors (20, 31). The abundance of α-2,6-linked sialic acid on the ciliated epithelium provides a selective pressure for viruses infecting humans to evolve a receptor preference for this sialic acid linkage (9).
Since the 1968 pandemic, the H3N2 subtype has become established in the human population, evolving in the new host subject to immune selective pressure. The addition of glycosylation to HA has been described as a mechanism for antigenic drift and consequent virus escape from antibody binding by shielding antigenic sites on the globular head of the molecule (1). The additional glycosylation can also decrease receptor binding activity, presumably due to occlusion of the receptor binding site pocket (1, 27). Evolution of influenza A viruses in humans has brought about changes in receptor binding specificity and affinity; specifically, it has been observed that recent H1N1 and H3N2 clinical isolates no longer agglutinate chicken red blood cells (2, 22, 24, 26, 37). The fine receptor binding specificity of human influenza viruses is beginning to be understood, and indeed, a new preference of modern H3N2 isolates for binding Neu5Acα2,6Galβ1-4GlcNAc (6′SLN) over Neu5Acα2,6Galβ1-4Glc (6′SL) was recently described (23). Therefore, although a major change in receptor binding specificity occurs early in the adaptation of influenza virus to humans, the interaction of HA with its sialic acid receptor is constantly evolving.
In this study, we describe the cell tropism of recent H3N2 influenza viruses compared to a human H3N2 virus closely related to the 1968 pandemic strain and avian influenza viruses. Using a well-characterized in vitro model of the human airway epithelium (43, 44), we found that recent H3N2 viruses infect a higher proportion of nonciliated cells than an early H3N2 influenza virus, which, like avian influenza strains, preferentially infected ciliated cells. The evolving preference from ciliated cells toward the nonciliated cell type reflects the major distribution of α-2,6-linked sialic acid, the receptor for human viruses, on this cell type.
MATERIALS AND METHODS
Influenza A viruses.
Influenza A H3N2 human viruses were selected from a larger panel of clinical isolates that has previously been characterized and that represents the evolutionary span of the virus in humans from 1968 to the present day (37). Viruses were obtained from archives at the Health Protection Agency (HPA) Centre for Infections, Colindale, London, United Kingdom. Archived samples that had been passaged in cell culture from the original respiratory sample before storage at −80°C were selected and amplified by two to three passages in MDCK cells. Infectious supernatants were stored at −80°C and thawed immediately before use. Avian influenza viruses A/Duck/England/62 (H4N6), A/Duck/Ukraine/1/63 (H3N8), and A/Duck/Singapore/5/97 (H5N3) were originally obtained from the HPA collection held at Colindale and have been extensively passaged in MDCK cells and in eggs. The construction of PIV3-GFP has been described previously (43).
Cell lines for virus titration.
MDCK cells were routinely passaged in Dulbecco's modified Eagle's medium (DMEM; Gibco, New York) with l-glutamine, sodium pyruvate, and pyridoxine hydrochloride supplemented with 10% fetal bovine serum (Atlanta Biologicals, Georgia) and penicillin-streptomycin (Gibco, New York).
HAE cell cultures.
Human airway tracheobronchial epithelial cells were obtained from airway specimens resected at lung transplantation under UNC Institutional Review Board-approved protocols by the UNC Cystic Fibrosis Center Tissue Culture Core. Briefly, primary cells derived from single patient sources were expanded on plastic to generate passage 1 cells and plated at a density of 250,000 cells per well on permeable Transwell-Col (12-mm diameter) supports. Human airway epithelium (HAE) cultures were generated by provision of an air-liquid interface for 4 to 6 weeks to form well-differentiated, polarized cultures that resemble in vivo pseudostratified mucociliary epithelium as previously described (30). Cultures were derived from patients without underlying lung disease.
Viral inoculation of HAE.
Human and avian influenza A viruses were diluted with phosphate-buffered saline (PBS) to equal titers, as determined by a 50% tissue culture infective dose (TCID50) assay of MDCK cells. HAE cells were washed extensively with PBS to remove mucus secretions on the apical surface prior to infection with a low multiplicity of infection (MOI) (∼0.1 to 0.01) of influenza virus in a 100-μl inoculum. Virus was removed from the apical surface following a 1-h incubation at 37°C, and the cells were then incubated at 37°C for a further 6 to 48 h as required. Viruses released into the apical compartment of HAE were harvested by the apical addition and collection of 300 μl of medium allowed to equilibrate at 37°C for 30 min. Samples were stored at −80°C. Viral titer was determined by a hemagglutination assay and a TCID50 assay. Cultures were washed with PBS and fixed with 4% paraformaldehyde before histological sections of the airway epithelium were prepared by the UNC Cystic Fibrosis Center Histology Core using standard techniques.
Hemagglutination assay.
Hemagglutination assays were carried out in V-bottomed microtiter plates using 50 μl of 0.5% suspensions of turkey red blood cells in PBS (Valley Biomedical, Winchester, VA) added to 50 μl virus serially diluted in PBS. Assays were read following a 1 h-incubation on ice.
Viral titration by TCID50 assay.
MDCK cells were seeded on 96-well plates and infected the following day for 1 h at 37°C with log or half-log dilutions of virus in serum-free media. Viral inoculum was removed and replaced with serum-free DMEM plus 1 μg/ml TPCK l-(tosylamido-2-phenyl) ethyl chloromethyl ketone)-treated trypsin (Worthington Biochemical Corporation, New Jersey) and incubated at 37°C for 3 days. Cell monolayers were fixed and stained with crystal violet-methanol, and TCID50/0.1-ml values were calculated using the Spearman-Karber formula.
Immunolocalization of epithelial cell carbohydrate components and viral glycoproteins.
Standard protocols were used for lectin- and antibody-based localization of target antigens and terminal sialic acids on HAE and paraformaldehyde-fixed, paraffin-embedded histological cross sections of HAE and excised lung tissue. Lectin staining was performed with Sambucus nigra agglutinin (SNA; Vector Laboratories) for sialic acid α-2,6 linked to galactose and Maackia amurensis agglutinin (MAA; EY Laboratories) for sialic acid α-2,3 linked to galactose. Lectins were conjugated to biotin linkers, and a streptavidin conjugate of Alexa Fluor 488 (Molecular Probes) was used to detect lectin binding.
Histological cross sections were blocked with 3% bovine serum albumin/PBS and probed with anti-β-tubulin IV mouse monoclonal antibody (Sigma-Aldrich), followed by goat anti-mouse immunoglobulin G (IgG) conjugated to Alexa Fluor 594 (Molecular Probes) to identify ciliated cell types of HAE.
For localization of influenza A proteins in HAE, en face staining with mouse anti-influenza A nucleoprotein (Serotec) and goat anti-mouse IgG conjugated to Alexa Fluor 488 (Molecular Probes) was performed.
For localization of influenza A proteins in histological cross sections of HAE, sections were blocked with 3% bovine serum albumin/PBS, stained with rabbit polyclonal anti-influenza A H3N2 serum (R372 raised against A/England/24/94 at HPA, Colindale) or rabbit polyclonal antibody to avian influenza nucleoprotein (Imgenex), and costained with anti-β-tubulin IV mouse monoclonal antibody (Sigma-Aldrich) to identify infected ciliated cells. Goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC; Jackson ImmunoResearch Labs) or anti-rabbit FITC (Oxford Biotechnology, United Kingdom) and goat anti-mouse conjugated to Alexa Fluor 594 or anti-mouse Texas Red (Calbiochem) were used for detection. Slides were mounted in VectorShield (Vector Labs) mounting medium for fluorescence with DAPI (4′,6′-diamidino-2-phenylindole). Photomicrographs were acquired using a Leica Leitz DMIRB inverted fluorescence microscope equipped with a cooled-color charge-coupled device digital camera (MicroPublisher; Q-Imaging). Infected cells were quantified using the Image Processing Tool Kit plug-ins for Photoshop (ISBN no. 1-928808-00-X; John C. Russ).
Neuraminidase treatment of HAE.
The apical surfaces of HAE cultures were washed extensively (10 times) with PBS prior to treatment with neuraminidase (160 mU/ml) from Vibrio cholerae (Sigma) for 3 h at 37°C. Control cultures (without NA) were incubated with serum-free DMEM only. The neuraminidase was removed, and the cultures were washed three times with serum-free DMEM. Cultures were infected via the apical surface with human H3N2 influenza virus A/England/26/99 or PIV3-GFP (106 PFU) for 1 h at 37°C, the virus was removed, and the surface was washed. Cultures were fixed at 24 h postinfection with ice-cold 50:50 methanol/acetone and washed with PBS. Influenza virus-infected cultures were stained en face with mouse anti-influenza A nucleoprotein antibody (Serotec), followed by goat anti-mouse IgG conjugated to Alexa Fluor 488 (Molecular Probes).
Microscopy.
Visualization of virus with transmission scanning electron microscopy was performed using standard techniques. HAE cultures infected with avian (A/Duck/Singapore/5/97) and human (A/England/26/99) influenza A viruses at an MOI of 0.1 were fixed at 12 h postinfection with perfluorocarbon-osmium tetroxide. Sections were processed for electron microscopy and analyzed.
RESULTS
Distribution of influenza virus sialic acid receptors in HAE correlates with that in human airway tissue.
We examined the distribution of the human influenza virus receptor, α-2,6-linked sialic acid, in cultures of human airway tracheobronchial epithelial cells. Using the lectin SNA and costaining with anti-β-tubulin IV, we determined the distribution of α-2,6-linked sialic acid in relation to ciliated cells. Staining for SNA was abundant on the apical surface of nonciliated cells and particularly concentrated at a level corresponding to the microvilli (Fig. 1A). In addition, a lower level of SNA staining was also observed on the apical surface of some ciliated cells, suggesting that α-2,6-linked sialic acid was distributed across both cell types in HAE (Fig. 1B). To determine whether the localization of α-2,6-linked sialic acid on HAE was representative of the distribution in ex vivo tissue samples from humans, paraffin-embedded histological cross sections of excised human airway tissue were stained. As for HAE, SNA staining was observed predominantly on nonciliated cells although ciliated cells were also stained (Fig. 1C).
FIG. 1.
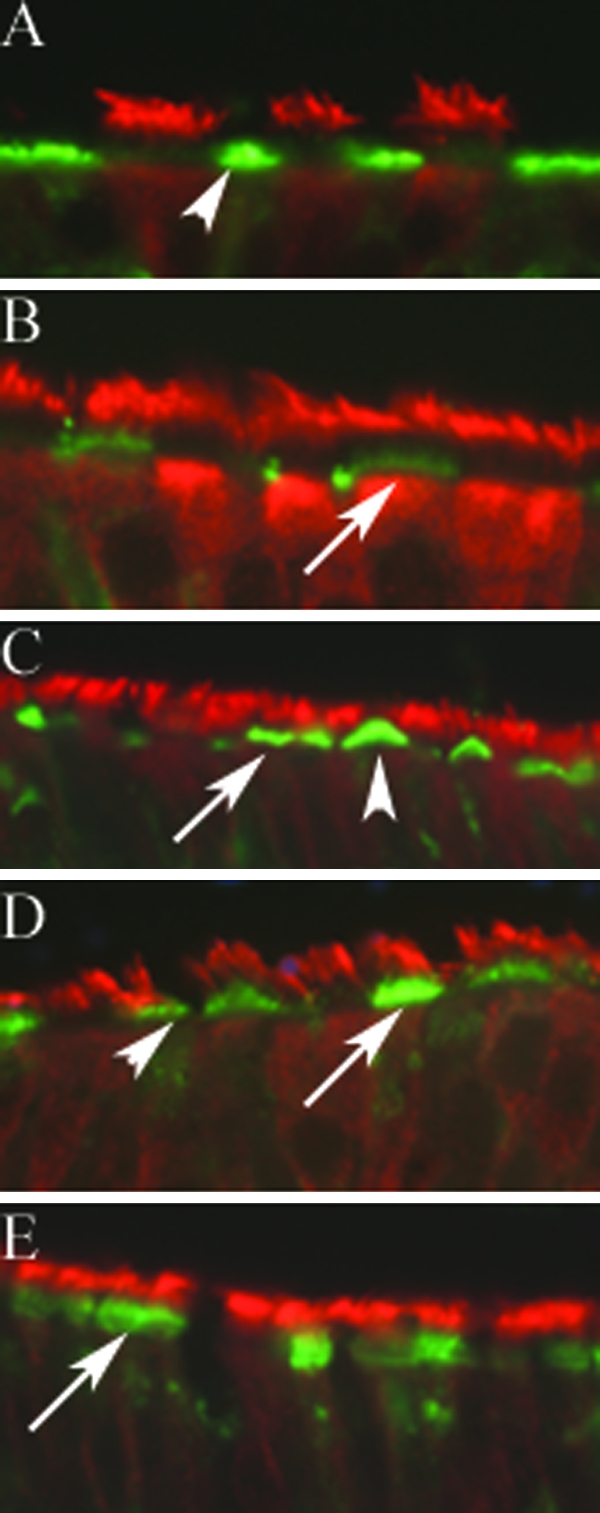
Histological cross sections of HAE and excised human airway tissue samples were probed with anti-β-tubulin IV (red) and the lectin (green) SNA for the human influenza virus receptor (α-2,6-linked sialic acid) or MAA for the avian influenza virus receptor (α-2,3-linked sialic acid). SNA was visible on the apical surface of nonciliated (A) and ciliated cells (B). SNA on ciliated and nonciliated cells was also observed in excised human airway tissue samples (C). MAA was visible on the apical surface of ciliated cells and, to a lesser degree, on nonciliated cells (D). MAA was observed at the apical surface of ciliated cells in paraffin-embedded, excised human airway tissue (E). Arrows, ciliated cells; arrowheads, nonciliated cells.
We also investigated the distribution of the avian influenza virus receptor, α-2,3-linked sialic acid, on HAE and human airway tissue sections by using the α-2,3 linkage-specific lectin MAA. Staining with MAA indicated that α-2,3-linked sialic acid was predominantly on the apical surface of ciliated cells at the base of the cilial shaft in the region of the microvilli (Fig. 1D). Importantly, only a small proportion of ciliated cells in HAE were stained, indicating that not every ciliated cell displays α-2,3-linked sialic acid. In addition, MAA staining was clearly found to a lesser degree on some nonciliated cells (Fig. 1D). Examination of paraffin-embedded histological cross sections of excised human airway tissue also clearly showed staining of some but not all ciliated cells with MAA (Fig. 1E). Again, staining was observed at the immediate apical surface of the cells below the level of the cilia and had a “brush border” appearance, suggesting that the lectin could be localized to the tips of the microvilli. Relative intensity of staining achieved with the lectins was not used as a direct quantitative measure of sialic acid distribution, as the relative affinities of the lectins, the degrees of biotinylation, or the intensities of fluorescent conjugates may differ.
Human influenza A viruses infect both ciliated and nonciliated cells in HAE.
HAE cultures were infected with human strains of H3N2 influenza A virus, for example A/England/26/99, at a low multiplicity of infection and examined for the localization of viral proteins at 6 h postinfection during a single cycle of viral replication. Using β-tubulin IV, a marker for ciliated cells, in conjunction with antibody against viral proteins, virus was detected in both ciliated and nonciliated cell types (Fig. 2). Intense staining of viral proteins localized in the cell nucleus, and spreading through the cytoplasm toward the apical cell surface was observed as early as 6 h postinfection. The polyclonal antisera used detect predominantly nucleoprotein (NP) and HA viral antigens.
FIG. 2.
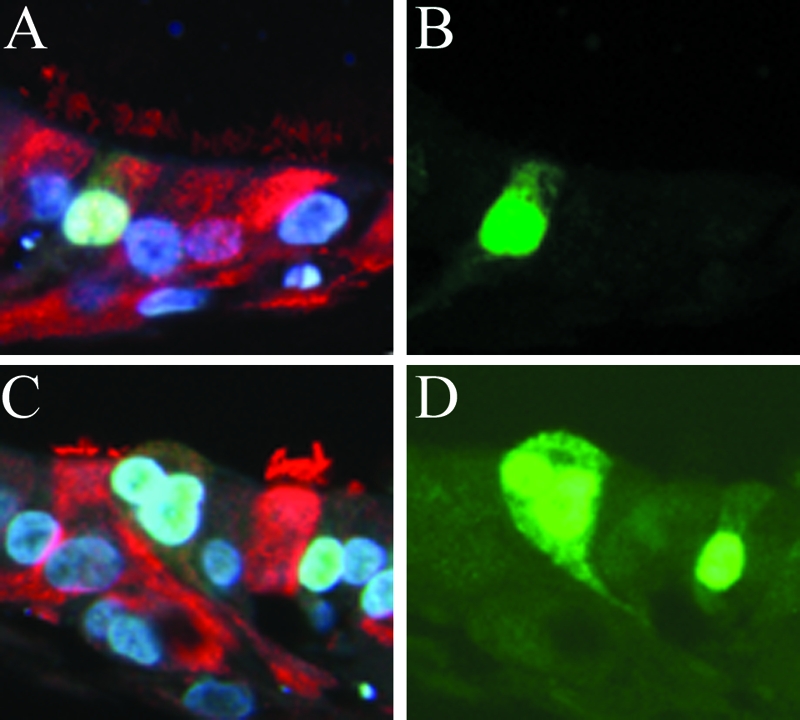
Viral proteins were detected in ciliated (A and B) and nonciliated (C and D) cells in histological cross sections of HAE infected with recent influenza A H3N2 virus strain A/England/26/99 and fixed at 6 h postinfection with 4% paraformaldehyde. Sections were costained with anti-influenza A H3N2 rabbit polyclonal antibody (green) and anti-β-tubulin IV (red) to identify ciliated cells. Cell nuclei were visualized with DAPI staining in VectorShield mounting medium. Representative fluorescent photomicrographs of viral proteins localized in the cell nuclei and cytoplasm are shown costained with β-tubulin IV and DAPI (A and C), and the same images are shown with FITC staining only (B and D).
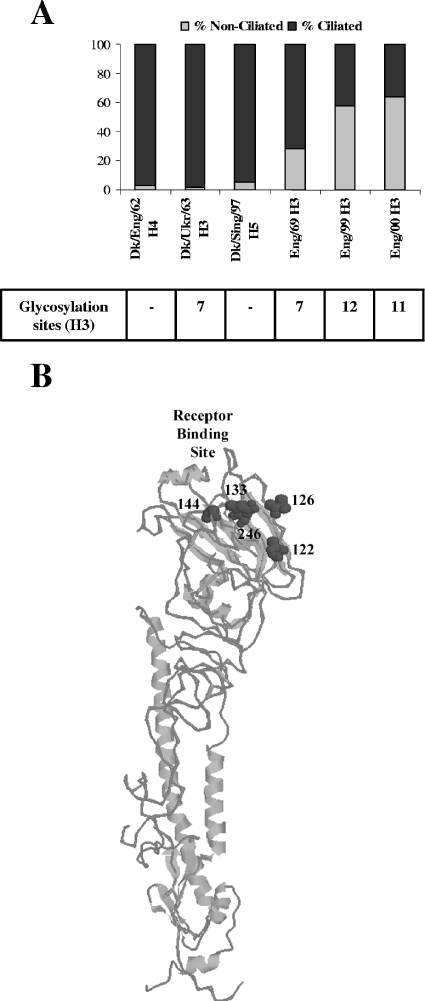
In previous work, we characterized a panel of human H3N2 influenza A viruses isolated between 1969 and 2000 and showed a changing pattern of receptor binding represented by reduced agglutination of red blood cells by recent isolates (37). To determine whether this marked change in receptor binding affected virus cell tropism in HAE, we determined the proportion of ciliated and nonciliated cells infected by an older virus (1969) and by more-recent (1999 to 2000) virus strains (Fig. 3). Interestingly, we observed a trend in cell type tropism in which the older H3N2 strain infected proportionally more ciliated cells than nonciliated cells, whereas recent viruses showed an increasing specificity for nonciliated cells.
FIG. 3.
(A) HAE cultures were infected with human influenza A viruses isolated in England from 1969 to 2000 (A/England/878/69, A/ England/26/99, and A/England/24/00 [H3N2]) and with avian influenza A viruses (A/Duck/England/62 [H4N6], A/Duck/Ukraine/63 [H3N8], and A/Duck/Singapore/5/97 [H5N3]) (MOI, 0.1). Histological cross sections of HAE were immunostained with anti-influenza A H3N2 rabbit polyclonal antibody or anti-avian influenza nucleoprotein rabbit polyclonal antibody and counterstained for β-tubulin IV to identify ciliated cells. Viral proteins were localized at 6 h (human viruses) postinfection in both ciliated and nonciliated cell types, and the proportion of infected ciliated and nonciliated cells for the older virus (1969) and more-recent (1999 to 2000) virus strains was determined. Viral protein from avian strains was found only at 24 h postinfection almost exclusively in ciliated cells. Two independent experiments were performed. The proportion of ciliated and nonciliated cell types with respect to the total number of infected cells in three separate cross sections (10 to 100 infected cells/section) was determined. The number of infected cell types as a proportion of the total number of infected cells from a representative experiment is displayed. The lower table indicates the total number of potential glycosylation site motifs on HA for each virus of the H3 subtype. (B) Three-dimensional structure model of H3 HA (Protein Data Bank identification number 1HGF) (17, 33) produced using Protein Explorer (18), indicating the position of five new potential glycosylation sites (dark gray spacefill) on the globular head of the HA molecule in the vicinity of the receptor binding site. Numbers indicate Asn residues in the first position of the glycosylation site motif. Potential glycosylation site motifs at positions 126 and 246 were found in A/Victoria/3/75-like and A/Mississippi/1/85-like viruses, respectively. Motifs at positions 122 and 133 were found in viruses isolated from 1999 onward; the motif at position 144 was sometimes found in viruses isolated in 1999 and was absent from subsequent strains.
Entry of human influenza viruses into HAE cells is not affected by depletion of cell surface sialic acid.
Both parainfluenza virus 3 (PIV3) and influenza virus utilize cell surface sialic acid as receptors for entry into human airway cells. The entry of PIV3 is exquisitely sensitive to removal of cell surface sialic acid by treatment of HAE with broad-spectrum neuraminidases (43). We investigated the efficiency of entry of human influenza virus A/England/26/99 following pretreatment of HAE with a broad-spectrum neuraminidase from Vibrio cholerae (NA III), which cleaves sialic acid residues with α-2,3, α-2,6, and α-2,8 linkages, and surprisingly found little effect on influenza virus entry although PIV3 infection under identical conditions was abolished (Fig. 4). Although lectin staining revealed that sialic removal is not complete following NA treatment (data not shown), this result demonstrates that unlike PIV3, influenza virus can enter cells which are relatively depleted in cell surface sialic acid.
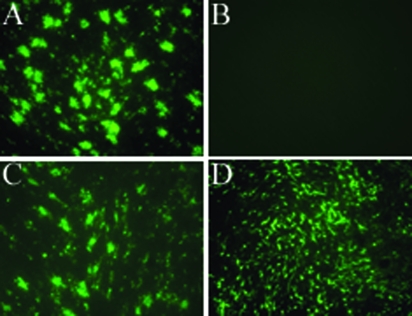
FIG. 4.
HAE cultures were treated with neuraminidase from Vibrio cholerae for 3 h at 37°C (A and B). Control cultures (C and D) were incubated with serum-free DMEM only. Cells were infected via the apical surface with human H3N2 influenza virus A/England/26/99 or PIV3-GFP and fixed at 24 h postinfection. Influenza virus-infected cultures were stained with mouse anti-influenza A nucleoprotein antibody, followed by goat anti-mouse IgG conjugated to Alexa Fluor 488. Representative photomicrographs of neuraminidase-treated HAE cultures infected with influenza virus (A) and PIV3-GFP (B) and control cultures infected with influenza virus (C) and PIV3-GFP (D) are shown.
Avian influenza viruses infect HAE cells.
To determine whether avian strains of influenza A virus could infect human airway cells, as might happen at the start of a pandemic, the apical surfaces of HAE cultures were exposed to equal titers (as determined by a TCID50 assay of MDCK cells) of two different subtypes of avian influenza A virus, A/Duck/Singapore/5/97 (H5N3) and A/Duck/England/62 (H4N6), or the human strain A/England/26/99 (H3N2) for comparison. Following infection and incubation, the cultures were fixed and stained en face with anti-influenza A NP antibody, which recognizes nucleoprotein from both the avian and human viruses. At 6 h postinfection, little or no viral antigen was detected in the avian virus-infected cells. However, after 23 h of incubation, we found clear evidence of avian virus infection of HAE (Fig. 5A and B), although NP staining of avian virus-infected cultures was less widespread than that of human virus-infected cultures (Fig. 5C). Only 5% of the HAE cells were infected by A/Duck/Singapore/5/97 virus and 9% were infected by A/Duck/England/62 virus compared to 12.5% of the cells infected by the human virus under these conditions. The avian virus-infected cultures showed a more punctuate cultures pattern of staining in comparison to the diffuse staining of the human virus-infected cultures, suggesting that the avian virus infection was limited to a focal point of initial infection (Fig. 5A to C).
FIG. 5.
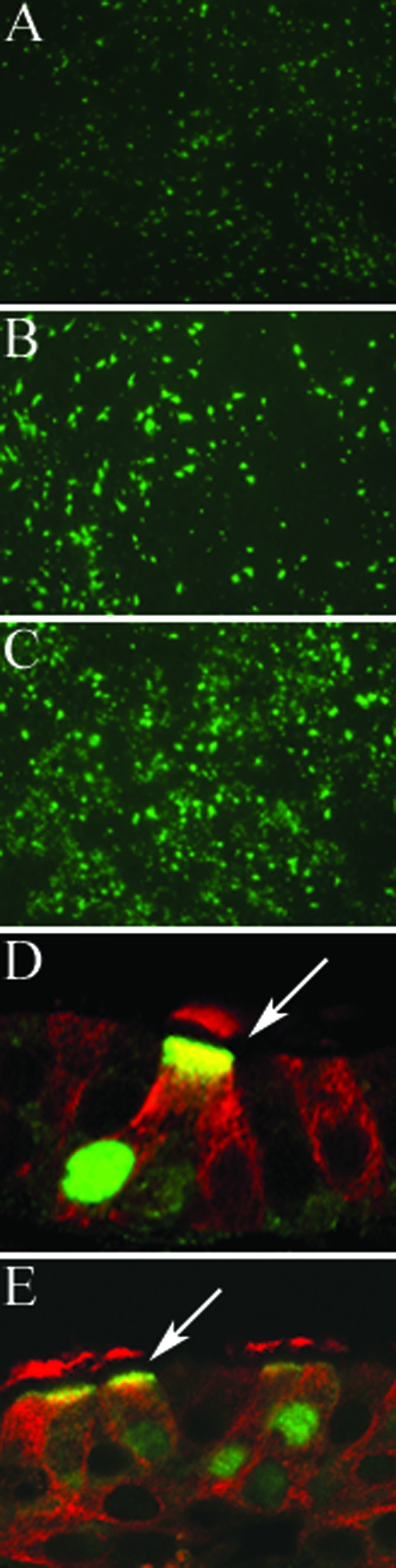
HAE cultures were infected apically with equal titers of two different subtypes of avian influenza virus A/Duck/Singapore/5/97 (H5N3) (A) and A/Duck/England/62 (H4N6) (B) and a human influenza virus A/England/26/99 (H3N2) (C) for 1 h at 37°C. Virus was removed, and after a further 23-h incubation, the cultures were fixed with methanol/acetone (50:50) and stained en face with anti-influenza A nucleoprotein antibody and goat anti-mouse conjugated to Alexa Fluor 488. Representative fluorescent photomicrographs of the stained cultures are shown. Avian influenza viruses A/Duck/Singapore/5/97 (D) and A/Duck/England/62 (E) were found to be localized exclusively in ciliated cells by costaining with rabbit anti-avian influenza A nucleoprotein (green) and mouse monoclonal β-tubulin IV antibody (red) to identify ciliated cells and visualization with anti-rabbit FITC and anti-mouse Texas Red. Arrows, avian virus-infected ciliated cells.
Cell tropism of avian influenza viruses in HAE.
Histological cross sections of HAE fixed at 6 h and 24 h postinfection with avian influenza viruses A/Duck/Singapore/5/97 (H5N3), A/Duck/England/62 (H4N6), and A/Duck/Ukraine/1/63 (H3N8) were examined for the presence of viral nucleoprotein. Viral nucleoprotein was clearly observed in the cell nucleus and distributed through the cytoplasm to the apical cell surface in cultures fixed at 24 h postinfection only (Fig. 5D and E). No avian virus antigen was detected at 6 h postinfection, in contrast to human virus-infected cells which were clearly detected at early times postinfection (Fig. 2), suggesting that human viruses replicated at higher levels than avian viruses in this culture model. Using β-tubulin IV counterstaining, we observed that avian influenza A viruses almost exclusively infected the ciliated cell types (Fig. 3 and 5D and E).
Influenza A viruses replicate productively in HAE.
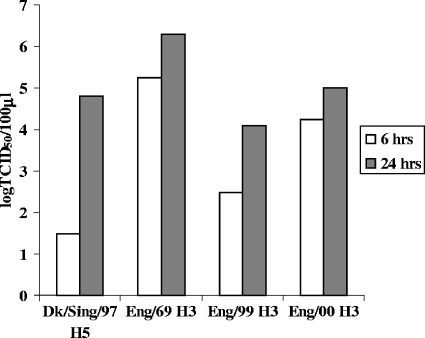
HAE cultures were infected apically with equal titers (MOI, ∼0.1) of old (1969) and modern (1999 to 2000) human H3N2 viruses or with the avian influenza A virus A/Duck/Singapore/5/97, and supernatants were collected from the apical and basolateral compartments of the infected HAE cultures at 6 and 24 h postinfection to measure nascent virus release. Hemagglutination assays with turkey red blood cells indicated that virus was released from the apical but not the basolateral surface of all of the infected cultures (data not shown). TCID50 assays of MDCK cells were used to calculate virus titers released from the apical surface (Fig. 6). All viruses replicated in HAE, although recently isolated viruses (A/England/26/99 and A/England/24/00) produced titers 2 to 3 logs lower than that of the older H3N2 strain (A/England/878/69). Avian virus replication was slower than replication for the human viruses. Only low virus titers were detected at 6 h postinfection, but by 24 h, avian virus titers had accumulated to a level similar to that of the recent human strains (Fig. 6). Accordingly, it was difficult to find avian influenza virus particles in thin sections fixed at 12 h postinfection and analyzed by electron microscopy. One small patch of budded virus was evident after we scanned many fields (Fig. 7A). In contrast, released human influenza virus particles were readily detected above the surfaces of cells fixed at this time (Fig. 7B). The released virus morphology was predominantly spherical, and virions were found largely at the level of microvillus tips (Fig. 7C).
FIG. 6.
HAE cultures were infected with human H3N2 influenza A viruses isolated in England between 1969 and 2000 (A/England/878/69, A/England/26/99, and A/England/24/00) and with avian H5N3 influenza A strain A/Duck/Singapore/5/97 at an MOI of 0.1. Virus released from the apical surface of infected cultures at 6 and 24 h postinfection was quantified by a TCID50 assay of MDCK cells. Representative results from two independent experiments are shown. Viral titers are expressed as log TCID50/100 μl.
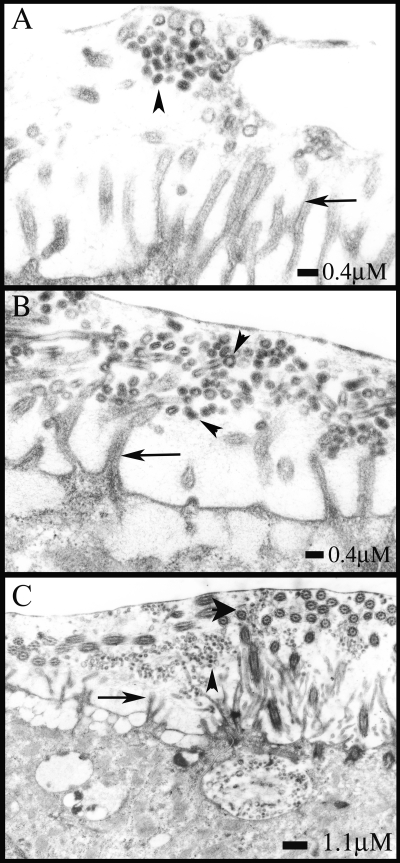
FIG. 7.
HAE cultures were infected with avian (A/Duck/Singapore/5/97) and human (A/England/26/99) influenza A viruses at an MOI of 0.1 and fixed at 12 h postinfection with perfluorocarbon-osmium tetroxide that preserves the air-surface microenvironment of HAE. Sections were processed for electron microscopy and analyzed. For the avian influenza strain, only one small cluster of budded virus particles was visible after many fields were scanned (A). Released human influenza virus particles were readily detected above the surfaces of cells. The released virus morphology was predominantly spherical (B), and virions were found to be accumulated at the level of microvillar tips (C). Scale bars are shown for each panel. Arrows, microvilli; small arrowheads, virus particles; large arrowhead in panel C, cilia.
DISCUSSION
In this study, using well-differentiated human airway tracheobronchial epithelial cells, we have demonstrated that influenza A viruses isolated from human and avian species show a general cell tropism which reflects the predominant distribution of appropriate sialic acid receptors on nonciliated and ciliated cells, respectively. A human influenza A virus isolated during the pandemic of 1968 to 1969 infected a high proportion of ciliated cells, and avian viruses almost exclusively infect this cell type, which displays mainly the preferred avian virus receptor α-2,3-linked sialic acid. Early in the adaptation of the virus to humans, it must acquire the ability to bind α-2,6-linked sialic acid receptors as this is the predominant sialic acid species in the human airway. However, the presence of lesser amounts of α-2,3-linked sialic acid receptors perhaps aids the initial crossover from avian species. Infection of a mixture of cell types by the pandemic-era virus A/England/69 might reflect binding to α-2,3-linked sialic acid on ciliated cells and perhaps also binding to α-2,6-linked sialic acid receptors distributed across both cell types. Recent H3N2 viruses tended to infect nonciliated cells, reflecting the predominance of the preferred human virus receptor α-2,6-linked sialic acid on this cell type. The trend from infection of ciliated cells toward increasing infection of nonciliated cells as the virus evolves in humans might reflect either a decreased affinity for α-2,3-linked sialic acid on ciliated cells or preferential use of α-2,6-linked sialic acid found on nonciliated cell types.
Our results show that the removal of a significant proportion of sialic acid by treatment with a broad-spectrum neuraminidase still allowed influenza infection even when PIV3 infection was completely abrogated. Similarly, using a primary culture of human bronchial epithelial cells, it was recently shown that sialidase treatment reduced infection by human PIV1 but not by influenza virus (15). These data suggest that influenza viruses require only a minimal amount of sialic acid to achieve cellular entry or that influenza virus and PIV3 use sialic acids on different molecules that are differentially cleaved by exogenous neuraminidase. Recent influenza virus A/England/26/99 infects a mixture of cell types in HAE (Fig. 3A), while PIV3 is restricted to ciliated cells (43). The different infection profiles for each virus following neuraminidase treatment may reflect the relative accessibility of ciliated and nonciliated cells for exogenous neuraminidase. If sialic acid is more effectively stripped from ciliated cells than from nonciliated cells, this will abrogate PIV3 infection while influenza virus may be less affected, as it can infect both ciliated and nonciliated cell types.
As the H3N2 subtype has circulated in humans over the last four decades, HA has constantly evolved via antigenic drift as viruses carrying mutations in antigenic sites on the globular head of HA are positively selected. Changes in antigenic sites can also affect the receptor interaction. In particular, the addition of carbohydrate chains to asparagine residues in the vicinity of the receptor binding pocket can obscure sialic acid access and may influence the nature of the sialic acid interaction. Modern H3N2 viruses carry four or five new potential glycosylation sites on the globular head of HA compared to a pandemic-era virus such as A/England/69 (Fig. 3B). Recently isolated viruses with greater numbers of potential glycosylation sites are known to have reduced binding to sialic acid on red blood cells (37) and might therefore have a weaker interaction with sialic acid residues in the airway. HAE cells accurately recapitulate human airway cellular morphology and the highly complex glycocalyx layer; however, the presentation of sialic acid on different cell types and the accessibility of cell surface sialic acid for the viral receptor binding site are poorly understood. In addition, there are secreted soluble sialylated molecules in the airway, including mucins known to carry high levels of α-2,3-linked sialic acid (3), and these may also drive evolution of HA receptor binding by acting as decoy receptors (19).
The heterogeneity of sialic acid expression in the human population is not known, and although we found consistent patterns of sialic acid expression on different HAE cell types, some variation has been reported for other similar models (21). Different culture conditions in otherwise similar models may lead to various degrees of terminal glycosylation on cell surface glycoconjugates. Sialic acid expression may also depend upon age or degree of differentiation of the cell. Further, sialyl transferases have been shown to be upregulated during inflammation (16), and this factor may influence the range of expression in a particular cell model depending upon the source of the primary cells. Levels of expression of a particular sialic acid species may be a factor in virus transmission, host range restriction, and adaptation of influenza virus to infection of humans (11, 41).
The present study agrees with that of Matrosovich et al. (21) in that we clearly show α-2,3-linked sialic acid on the surface of ciliated cells. However, we also found some staining of ciliated cells with the lectin for α-2,6-linked sialic acid, indicating the presence of receptors for both avian and human viruses on ciliated cells. Furthermore, viral antigen was found to be localized in ciliated cells in both avian and human virus infections of HAE, suggesting that viruses from both species are capable of entering this cell type in the human airway epithelium. A complete segregation of cell types initially infected by avian and human viruses has been reported previously (21), and in agreement with this, we also found avian viruses to be almost exclusively in ciliated cells. However, the wider distribution of α-2,6-linked sialic acid expression seen in our model, which was confirmed in histological sections of excised human airway tissue, is reflected in the observed mixed cell tropism of the human viruses. The potential for coinfection of ciliated cells suggested by these data provides a possible mechanism for generation of genetic reassortants of viruses from different origins during infection of humans.
Conflicting reports in the literature show human influenza viruses to be exclusively localized in ciliated (6) or nonciliated (21) cells. The earlier study was made with laboratory variants of pandemic-era H3N2 viruses passaged in the allantoic sac of embryonated chicken eggs, and it has since been shown that this method of cultivation will select receptor binding mutants with avian-like characteristics due to the predominance of α-2,3-linked sialic acid receptors in this egg compartment (10, 12). It is unsurprising, therefore, that these viruses exclusively infected ciliated cells, similar to avian viruses, as shown here and previously (21). The present study, in common with that of Matrosovich and colleagues (21), used human viruses minimally passaged in cell culture to maintain the HA sequence and characteristics of the original clinical isolate (13) and should more accurately report the characteristics of the human viruses studied.
In contrast to other respiratory viruses also studied in this HAE cell model, such as respiratory syncytial virus and PIV3 (43, 44), human influenza virus infection proceeded to complete destruction of the culture, loss of cilial beat, and shedding of the columnar epithelial cells generally within 48 h of infection (44; data not shown). HAE cultures were infectible with avian influenza viruses, even using a low-inoculum titer (MOI, <1); however, avian virus antigen was not detected at early times (6 h) postinfection, in contrast to human virus antigen which was abundant at this time. Further, HAE cultures infected with avian viruses showed considerably less cytopathic effect than human viruses at 48 h postinfection, again suggesting that avian viruses are less able to spread. This perhaps reflects the wider distribution of α-2,6-linked sialic acid, the preferred human virus receptor, and/or the attenuated replication levels of avian viruses in HAE and the ability of the human viruses to infect a mixture of cell types.
Avian influenza virus infection of humans was first described relatively recently during the outbreak of highly pathogenic avian influenza H5N1 virus in 1997 and subsequently in 2004 to 2005 (5, 7, 29, 36, 38). The cellular and tissue tropism of the avian virus in humans is poorly defined, although a recent study of autopsy material from an H5N1-infected patient suggested that despite a generalized clinical manifestation, viral replication may be restricted to the lung and intestine (39). Further, staining for viral antigen was shown exclusively in nuclei of alveolus-lining cells, reported to be type II pneumocytes, while there was no antigen in the columnar epithelium at the time of autopsy (17 days following onset of illness), possibly suggesting that in contrast to infection with human influenza virus, the major cellular site of avian viral replication is the pneumocyte. Two recent studies using human tissue suggest that expression of the avian virus receptor α-2,3-linked sialic acid is abundant in the lower respiratory tract compared to that in the upper respiratory tract. Correspondingly, H5N1 virus was shown to attach to ciliated cells in bronchiolar sections of human lung and to type II pneumocytes in the alveolus but not to the trachea (35, 40). In the current study, ciliated cells derived from bronchial airway epithelium displayed α-2,3-linked sialic acid receptors and were infected by avian viruses. The study of the distribution of sialic acid receptors throughout the human airway epithelia is important in allowing predictive analysis of the threat posed by emerging avian influenza viruses.
PIV3 F glycoprotein has been found to be localized in the membranes of the cilial shafts of HAE cells, suggesting that virus budding and shedding may occur from these structures (43). In the present study of influenza virus-infected HAE cells, viral antigen was not detected in cilial shafts although it was extensively present throughout the cytoplasm and at the apical cell surface. Similarly, observation of infected cultures by electron microscopy at 12 h postinfection showed virus accumulating at the tips of the microvilli below the level of the cilial shafts. HAE cells display sialic acid at the apical cell surface in the region of the microvilli, and lectin staining was not observed on the cilial shaft. This distribution of sialic acid highlights the requirement for a balance between influenza virus hemagglutinin and neuraminidase activities (42). If the virus is released into a region where the viral receptor is present at a high density, then there is an essential requirement for a balanced neuraminidase function to enable virus release and spread (28). The location of budding reflects the difference in necessity for neuraminidase between influenza virus and PIV3. In contrast to influenza virus, PIV3 may rely less on neuraminidase function as it buds from a region in which little sialic acid is displayed.
The morphology of influenza viruses budding from HAE was predominantly spherical, even when viruses with a strongly filamentous phenotype in MDCK cells were observed (8; data not shown). Whether all influenza viruses budding from cells in the human airway naturally show a spherical morphology remains to be determined.
Well-differentiated cultures of the respiratory epithelium, such as the HAE described here, provide an appropriate cell model for the characterization of influenza virus infection of humans and should drive toward a better understanding of virus-receptor interactions, host range restrictions, and transmission of this important pathogen.
Acknowledgments
We thank the directors and teams of the UNC Cystic Fibrosis Center Tissue Culture Core and the Morphology and Morphometry Core for supplying reagents and technical expertise. Human influenza viruses were originally obtained from the Health Protection Agency (HPA), Colindale, London, United Kingdom, and recombinant parainfluenza viruses were obtained from Peter Collins (NIAID).
C.I.T. was funded through an NIH/NHLBI Pulmonary and Critical Care Medicine training grant (HL007106-30).
REFERENCES
- 1.Abe, Y., E. Takashita, K. Sugawara, Y. Matsuzaki, Y. Muraki, and S. Hongo. 2004. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J. Virol. 78:9605-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abed, Y., A.-M. Bourgault, R. J. Fenton, P. J. Morley, D. Gower, I. J. Owens, M. Tisdale, and G. Boivin. 2002. Characterization of two influenza A (H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the hemagglutinin gene. J. Infect. Dis. 186:1074-1080. [DOI] [PubMed] [Google Scholar]
- 3.Baum, L. G., and J. C. Paulson. 1990. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40:35-38. [PubMed] [Google Scholar]
- 4.Bean, W. J., M. Schell, J. Katz, Y. Kawaoka, C. Naeve, O. Gorman, and R. G. Webster. 1992. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J. Virol. 66:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chotpitayasunondh, T., K. Ungchusak, W. Hanshaoworakul, S. Chunsuthiwat, P. Sawanpanyalert, R. Kijphati, S. Lochindarat, P. Srisan, P. Suwan, Y. Osotthanakorn, T. Anantasetagoon, S. Kanjanawasri, S. Tanupattarachai, J. Weerakul, R. Chaiwirattana, M. Maneerattanaporn, R. Poolsavathitikool, K. Chokephaibulkit, A. Apisarnthanarak, and S. F. Dowell. 2005. Human disease from influenza A (H5N1), Thailand, 2004. Emerg. Infect. Dis. 11:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couceiro, J. N. S. S., J. C. Paulson, and L. G. Baum. 1993. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 29:155-165. [DOI] [PubMed] [Google Scholar]
- 7.de Jong, J. C., E. C. J. Class, A. D. M. E. Osterhaus, R. G. Webster, and W. L. Lim. 1997. A pandemic warning? Nature 389:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elleman, C. J., and W. S. Barclay. 2004. The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 321:144-153. [DOI] [PubMed] [Google Scholar]
- 9.Gagneux, P., M. Cheriyan, N. Hurtado-Ziola, E. C. M. Brinkman van der Linden, D. Anderson, H. McClure, A. Varki, and N. M. Varki. 2003. Human-specific regulation of α2-6-linked sialic acids. J. Biol. Chem. 278:48245-48250. [DOI] [PubMed] [Google Scholar]
- 10.Gambaryan, A. S., J. S. Robertson, and M. N. Matrosovich. 1999. Effects of egg-adaptation on the receptor-binding properties of human influenza A and B viruses. Virology 258:232-239. [DOI] [PubMed] [Google Scholar]
- 11.Ito, T. 2000. Interspecies transmission and receptor recognition of influenza A viruses. Microbiol. Immunol. 44:423-430. [DOI] [PubMed] [Google Scholar]
- 12.Ito, T., Y. Suzuki, A. Takada, A. Kawamoto, K. Otsuki, H. Masuda, M. Yamada, T. Suzuki, H. Kida, and Y. Kawaoka. 1997. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J. Virol. 71:3357-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz, J. M., M. Wang, and R. G. Webster. 1990. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J. Virol. 64:1808-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaoka, Y., S. Krauss, and R. G. Webster. 1989. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 63:4603-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogure, T., T. Suzuki, T. Takahashi, D. Miyamoto, K. I. P. J. Hidari, C.-T. Guo, T. Ito, Y. Kawaoka, and Y. Suzuki. 2006. Human trachea primary epithelial cells express both sialyl(α2-3)Gal receptor for human parainfluenza virus type 1 and avian influenza viruses, and sialyl(α2-6)Gal receptor for human influenza viruses. Glycoconj. J. 23:101-106. [DOI] [PubMed] [Google Scholar]
- 16.Lamblin, G., S. Degroote, J.-M. Perini, P. Delmotte, A. Scharfman, M. Davril, J.-M. Lo-Guidice, N. Houdret, V. Dumur, A. Klein, and P. Roussel. 2001. Human airway mucin glycosylation: a combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconj. J. 18:661-684. [DOI] [PubMed] [Google Scholar]
- 17.Macken, C., H. Lu, J. Goodman, and L. Boykin. 2001. The value of a database in surveillance and vaccine selection, p. 103-106. In A. D. M. E. Osterhaus, N. Cox, and A. W. Hampson (ed.), Options for the control of influenza IV. Elsevier Science, Amsterdam, The Netherlands.
- 18.Martz, E. 2002. Protein Explorer: easy yet powerful macromolecular visualization. Trends Biochem. Sci. 27:107-109. [DOI] [PubMed] [Google Scholar]
- 19.Matrosovich, M., and H.-D. Klenk. 2003. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev. Med. Virol. 13:85-97. [DOI] [PubMed] [Google Scholar]
- 20.Matrosovich, M., A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M. R. Castrucci, I. Donatelli, and Y. Kawaoka. 2000. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74:8502-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H.-D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros, R., N. Escriou, N. Naffakh, J.-C. Manuguerra, and S. van der Werf. 2001. Hemagglutinin residues of recent human A (H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology 289:74-85. [DOI] [PubMed] [Google Scholar]
- 23.Mochalova, L., A. Gambaryan, J. Romanova, A. Tuzikov, A. Chinarev, D. Katinger, H. Katinger, A. Egorov, and N. Bovin. 2003. Receptor-binding properties of modern human influenza viruses primarily isolated in Vero and MDCK cells and chicken embryonated eggs. Virology 313:473-480. [DOI] [PubMed] [Google Scholar]
- 24.Morishita, T., S. Kobayashi, T. Miyake, Y. Ishihara, S. Nakajima, and K. Nakajima. 1993. Host-specific hemagglutination of influenza A (H1N1) virus. Microbiol. Immunol. 37:661-665. [DOI] [PubMed] [Google Scholar]
- 25.Naeve, C. W., V. S. Hinshaw, and R. G. Webster. 1984. Mutations in the hemagglutinin receptor-binding site can change the biological properties of an influenza virus. J. Virol. 51:567-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nobusawa, E., H. Ishihara, T. Morishita, K. Sato, and K. Nakajima. 2000. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology 278:587-596. [DOI] [PubMed] [Google Scholar]
- 27.Ohuchi, M., R. Ohuchi, A. Feldmann, and H.-D. Klenk. 1997. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J. Virol. 71:8377-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palese, P., K. Tobita, M. Ueda, and R. W. Compans. 1974. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology 61:397-410. [DOI] [PubMed] [Google Scholar]
- 29.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers, G. N., and J. C. Paulson. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361-373. [DOI] [PubMed] [Google Scholar]
- 32.Rogers, G. N., J. C. Paulson, R. S. Daniels, J. J. Skehel, I. A. Wilson, and D. C. Wiley. 1983. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304:76-78. [DOI] [PubMed] [Google Scholar]
- 33.Sauter, N. K., J. E. Hanson, G. D. Glick, J. H. Brown, R. L. Crowther, S. J. Park, J. J. Skehel, and D. C. Wiley. 1992. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry 31:9609-9621. [DOI] [PubMed] [Google Scholar]
- 34.Scholtissek, C., W. Rohde, V. von Hoyningen, and R. Rott. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87:13-20. [DOI] [PubMed] [Google Scholar]
- 35.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Influenza virus receptors in the human airway. Nature 440:435-436. [DOI] [PubMed] [Google Scholar]
- 36.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, C. I., W. S. Barclay, and M. C. Zambon. 2004. Changes in in vitro susceptibility of influenza A H3N2 viruses to a neuraminidase inhibitor drug during evolution in the human host. J. Antimicrob. Chemother. 53:759-765. [DOI] [PubMed] [Google Scholar]
- 38.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, C. Dolecek, T. T. Tran, M. de Jong, C. Schultsz, P. Cheng, W. Lim, P. Horby, J. Farrar, and the World Health Organization International Avian Influenza Investigation Team. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 39.Uiprasertkul, M., P. Puthavathana, K. Sangsiriwut, P. Pooruk, K. Srisook, M. Peiris, J. M. Nicholls, K. Chokephaibulkit, N. Vanprapar, and P. Auewarakul. 2005. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 11:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. M. Fouchier, A. D. M. E. Osterhaus, and T. Kuiken. 2006. H5N1 virus attachment to lower respiratory tract. Science 312:399. [DOI] [PubMed] [Google Scholar]
- 41.Vines, A., K. Wells, M. Matrosovich, M. R. Castrucci, T. Ito, and Y. Kawaoka. 1998. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J. Virol. 72:7626-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner, R., M. Matrosovich, and H.-D. Klenk. 2002. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 12:159-166. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, L., A. Bukreyev, C. I. Thompson, B. Watson, M. E. Peeples, P. L. Collins, and R. J. Pickles. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 79:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 76:5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]