Abstract
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary adenocarcinoma (OPA). The expression of the JSRV envelope (Env) alone is sufficient to transform a variety of cell lines in vitro and induce lung cancer in immunodeficient mice. In order to determine the role of the JSRV Env in OPA tumorigenesis in sheep, we derived a JSRV replication-defective virus (JS-RD) which expresses env under the control of its own long terminal repeat (LTR). JS-RD was produced by transiently transfecting 293T cells with a two plasmid system, involving (i) a packaging plasmid, with the putative JSRV packaging signal deleted, expressing the structural and enzymatic proteins Gag, Pro, and Pol, and (ii) a plasmid which expresses env in trans for JS-RD particles and provides the genomes necessary to deliver JSRV env upon infection. During the optimization of the JS-RD system we determined that both R-U5 (in the viral 5′ LTR) and the env region are important for JSRV particle production. Two independent experimental transmission studies were carried out with newborn lambs. Four of five lambs inoculated with JS-RD showed OPA lesions in the lungs at various times between 4 and 12 months postinoculation. Abundant expression of JSRV Env was detected in tumor cells of JS-RD-infected animals and PCR assays confirmed the presence of the deleted JS-RD genome. These data strongly suggest that the JSRV Env functions as a dominant oncoprotein in the natural immunocompetent host and that JSRV can induce OPA in the absence of viral spread.
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary adenocarcinoma (OPA) (29). OPA is one of the most common viral diseases of sheep in many regions of the world (38) and is a unique large-animal model for lung carcinogenesis (14, 26).
Among oncogenic retroviruses, JSRV appears to employ unique mechanisms to induce cell transformation. The JSRV envelope glycoprotein (Env) is an oncoprotein (1, 20, 33) which induces transformation of a variety of primary and established cell lines in vitro (1, 9, 18-20, 33, 44) via the activation of the phosphatidylinositol 3-kinase/Akt and MEK/mitogen-activated protein kinase (MAPK) pathways by as-yet-uncharacterized mechanisms (18, 19, 27). In addition, immunodeficient mice inoculated with an adeno-associated virus vector expressing the JSRV Env develop lung adenocarcinoma, indicating that the JSRV Env can also behave as an oncoprotein in vivo, although the same vector is not efficient in inducing tumors in immunocompetent mice (41).
The role of the JSRV Env in OPA tumorigenesis in sheep, the natural host of JSRV infection, is not completely clear. OPA is experimentally reproducible when lambs are inoculated intratracheally with concentrated virus preparations obtained from lung secretions (or lung fluid) of OPA-affected sheep or from supernatant of cells transfected with JSRV infectious molecular clones (12, 29, 35, 37). The incubation period, in this experimental model of OPA, can be as short as a few weeks, suggesting that the viral Env can function as a dominant oncogene in vivo as well as in vitro. However, naturally occurring OPA is characterized by a very long incubation period lasting even a few years (6, 39, 40). Diseases with a long incubation time, in retrovirus-induced tumorigenesis, are often associated with the classical mechanisms of insertional activation. This is the case with, for example, mice, chickens, and cats with leukemias induced by Moloney murine leukemia virus, avian leukosis virus, and feline leukemia virus, respectively (34). However, common integration sites have not consistently been found in OPA (7) and tumors appear to be multiclonal (30).
We have established recently that, under natural conditions, the majority of JSRV-infected animals do not develop OPA lesions during their commercial lifespan (6). In addition, it is relatively difficult to find JSRV in the lungs of infected sheep with no tumor lesions, suggesting that the target cells for JSRV transformation might not necessarily be the reservoirs of virus infection in many cases (6). Thus, many aspects of the pathogenesis of OPA need further clarification.
In this study, we wanted to investigate if expression of the JSRV Env was sufficient to induce lung adenocarcinoma in sheep, the natural host of JSRV infection. To this end, we derived a JSRV replication-defective virus (JS-RD) that upon integration expresses only the viral env under the control of the JSRV long terminal repeat (LTR). JS-RD was able to induce OPA lesions in 4/5 of the inoculated animals, demonstrating that the viral envelope is the primary determinant of oncogenesis in the natural host of JSRV infection.
MATERIALS AND METHODS
Plasmids.
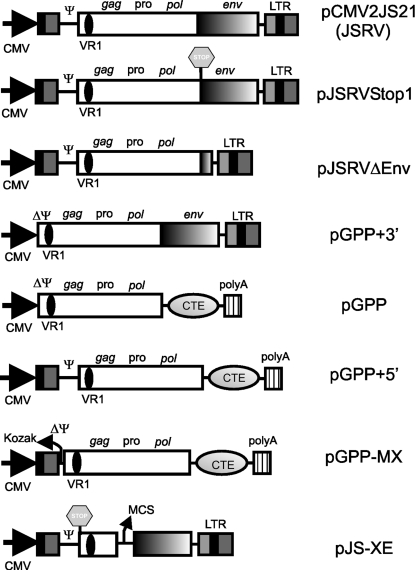
Plasmids employed in this study, unless stated otherwise, were derived by standard molecular biology techniques (2) from the expression plasmid of the JSRV21 infectious molecular clone, pCMV2JS21 (29). pJS-XE is derived from pJS21m (23). pJS-XE has part of gag and the entire pro and pol genes deleted (nucleotides [nt] 1278 to 5269 of JSRV21, GenBank accession number AF105220); in addition, the first ATG (nt 264 to 266) of gag has been replaced with a stop codon (TAG) by site-directed mutagenesis. A multiple cloning site at the end of the truncated gag has also been added in pJS-XE. A schematic diagram of the various plasmids is shown in Fig. 1. In pJSRVstop1, the first ATG and the splicing acceptor site of the JSRV env gene have been disrupted by silent mutagenesis. pJSRVΔenv has most of env deleted (between nt 5479 and nt 7074). pGPP+3′ has the R-U5 region and the untranslated gag region (nt 129 to 263) deleted. pGPP is derived from pGPP+3′, in which the JSRV env and 3′ LTR have been replaced by the constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) and the simian virus 40 polyadenylation signal; in addition, a Kozak-like sequence has been added in front of the gag open reading frame. The MPMV CTE was derived from plasmid pGem7fz(−)MPMV250, a gift from Marie-Louise Hammarskjöld. pGPP+5′ is identical to pGPP, with the exception of the addition in the former of the R-U5 and untranslated gag regions. pGPP-MX is derived from pGPP+5′ by the deletion, from the latter, of the 5′ gag untranslated region (between nt 129 and 263). pCDNA3-HA-Sam68 is an expression plasmid for the RNA binding protein Sam68 (43) and was a gift from David Shalloway.
FIG. 1.
Schematic representation of the plasmids used in this study. JS-RD particles were derived by transfecting 293T cells with pJS-XE and pGPP-MX. VR1, variable region 1; CMV, cytomegalovirus immediate early promoter; Ψ, packaging signal; MCS, multiple cloning site.
Cell culture and virus expression.
293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37°C, with 5% CO2 and 95% humidity. JSRV particles were produced in 293T cells by transfection with the expression plasmid pCMV2JS21 as already described (29). JS-RD particles were produced by transfecting 293T cells with plasmids pGPP-MX, pJS-XE, and pCDNA3-HA-Sam68. Viral particles were collected from supernatants of transfected cells, 24 and 48 h posttransfection and virus was concentrated (200×) by ultracentrifugation as described previously (29). For analysis of intracellular viral proteins, cells were lysed 48 h posttransfection following standard techniques (2). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed on cell lysates (50 μg of protein extract) and supernatants (10 μl of concentrated supernatants) essentially as previously described (29) using ECL Plus (Amersham). Virus expression in cells transfected by various plasmids (or a combination of plasmids) as described in Results was quantified, when necessary, by Western blotting by scanning membranes and measuring chemifluorescence with a Molecular Dynamics Storm 840 imaging system using ImageQuant TL software (Molecular Dynamics). Results were normalized with respect to JSRV p23 obtained by transfecting cells with pCMV2JS21. Experiments (from transfections to Western blotting) were performed independently at least three times and are presented as the mean value for each sample (± standard error); statistical significance was determined by applying the two-sample t test. A P value of <0.05 was considered statistically significant. JSRV was detected by using rabbit polyclonal sera against JSRV p23 (matrix protein) (23) or the surface domain of Env (35).
Animals.
Animal experiments were performed at the Moredun Research Institute (Penicuik, Scotland) and at the Istituto Zooprofilattico Sperimentale dell'Abruzzo e Molise (Teramo, Italy) in accordance with local approved protocols regulating experimental use of animals. Two independent studies were performed using a total of 11 lambs. In study 1, two lambs were inoculated with JS-RD and two lambs with concentrated supernatants derived from 293T transfected with the JSRV packaging plasmid alone. In study 2, three lambs were inoculated with JS-RD, two with JSRV, and two with concentrated supernatants derived from 293T transfected with the JSRV packaging plasmid alone. All lambs were inoculated intratracheally during the first week after birth. Animals of study 1 were kept for 12 months before being euthanized. In study 2, animals were euthanized at 8 months postinoculation with the exception of one of the lambs injected with JSRV and one of those injected with JS-RD, which were killed at 7 weeks and 4.5 months postinoculation, respectively, because they were showing clinical signs of respiratory distress. At the postmortem examination, tissues were collected from seven distinct anatomical regions of the lungs: the cranial part of the left cranial lobe, caudal part of left cranial lobe, left diaphragmatic lobe, right diaphragmatic lobe, right middle lobe, right cranial lobe, and accessory lobe. Tissue samples were excised from the organ and cut into two portions: one was snap-frozen and stored at −80°C, while the other was fixed in formalin for subsequent histopathological and immunohistochemical examination.
Histopathology and immunohistochemistry.
Four- to six-micrometer lung sections were stained with hematoxylin and eosin and examined by light microscopy for tumor lesions. Sections were taken from paraffin blocks of tissues collected from seven anatomically distinct regions, as described above. Sections were examined also by immunohistochemistry for the presence of JSRV Env as previously described, using the EnVision (DAKO) detection system (6, 35). OPA tumor sections were used as positive controls. The activation of MAPK Erk1/2 signaling pathway was detected in tumors induced by JS-RD and JSRV by immunohistochemistry employing rabbit polyclonal anti-phospho-Erk1/2 (Cell Signaling) as described previously (11).
JS-RD- and JSRV-specific PCR assays.
The presence of the JS-RD in the inoculated lambs was assessed by PCR with primers designed to amplify specifically the JS-XE genome or JSRV. Four PCR tests were used: two were JS-XE specific (PCRs no. 1 and 2) and the other two were JSRV specific (PCRs no. 3 and 4). Both the JS-XE- and JSRV-specific PCRs employed the same forward primers designed in variable region 1 of JSRV. Reverse primers for the JS-XE-specific PCRs exploited the presence of a multiple-cloning-site region present upstream of the env gene only in JS-XE. For the JSRV-specific PCRs (PCRs no. 3 and 4), reverse primers were designed for a portion of gag not present in JS-XE. Sequences of the PCR primers employed in this study are available on request. The presence of PCR inhibitors or degraded DNA was ruled out for all samples that were tested by amplifying JSRV-related endogenous retroviruses (enJSRVs) gag (PCR no. 5) as already described (24).
RESULTS
JS-RD design and development.
The main goal of this study was to determine if expression of the JSRV Env in sheep (the natural host of JSRV infection) is sufficient to induce ovine pulmonary adenocarcinoma. Accordingly, we devised a JSRV replication-defective virus that could faithfully mimic the unique tropism and expression properties of JSRV (25) and that delivered a genome with deletions expressing only the viral env. We aimed to produce JS-RD particles by transiently transfecting cells with two plasmids: (i) one expressing the JSRV structural and enzymatic proteins Gag, Pro, and Pol and with the putative packaging signal deleted, and (ii) one functioning both to express Env in trans for the JS-RD particles and to provide the genomes necessary to deliver JSRV env upon infection.
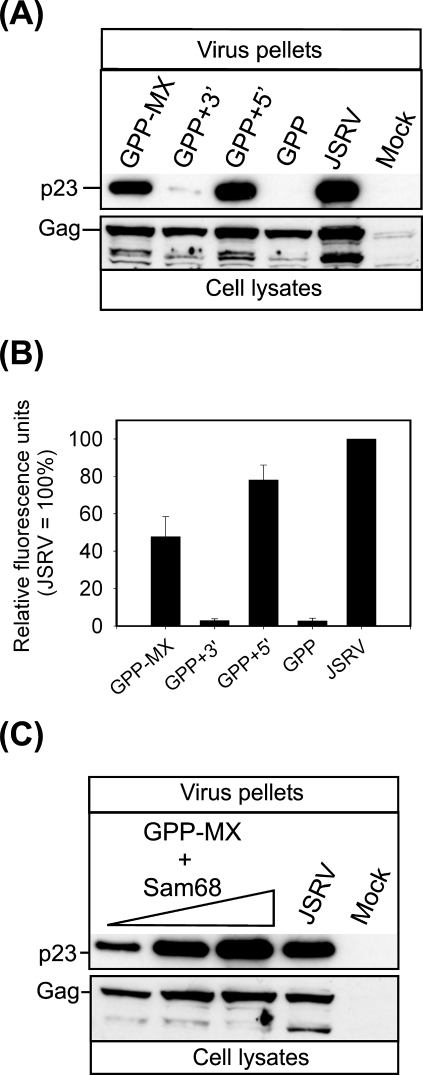
We initially optimized the combination of constructs that could produce the largest amount of JS-RD virions in vitro. A schematic representation of the plasmids used is shown in Fig. 1. Because of the lack of a suitable tissue culture system for the propagation of JSRV, we measured virion production by quantifying virus particles released into the supernatant of transfected cells by Western blotting. The first packaging construct tried was pGPP, which has a cytomegalovirus promoter driving expression of JSRV Gag, Pro, and Pol followed by the MPMV CTE (for optimal RNA export) and a heterologous poly(A) signal (Fig. 1). However, production of viral particles in cells transfected by pGPP was approximately 50 times less abundant than that in cells transfected with the expression plasmid for JSRV (Fig. 2A and B). Restoration of the 3′ end of the JSRV genome in pGPP+3′ did not increase the production of viral particles, suggesting that the lack of env and 3′ LTR in pGPP was not responsible for the defect shown by this construct. Indeed, transfection of 293T cells with pGPP+5′ produced quantities of viral particles comparable to JSRV (78% ± 8.1%) as judged by the content of p23 in the supernatants of transfected cells. We modified pGPP+5′ by deleting the untranslated region upstream of the gag open reading frame in order to use the resulting plasmid (called pGPP-MX) as a packaging construct. pGPP-MX RNA would presumably not be efficiently packaged by JSRV virions, considering the fact that the untranslated gag region is invariably a major part of a retroviral packaging signal (32), although this has not been precisely mapped in JSRV. Cells transfected with pGPP-MX produced approximately half (47.8% ± 10.5%; P = 0.03) the quantity of viral particles produced by JSRV, suggesting that either the untranslated gag region has some beneficial role for JSRV expression or the lack of viral genomic RNA negatively influences virus assembly/exit. The latter seems most likely because Gag expression in the cell lysates is comparable between the various constructs. In order to enhance the production of viral particles by pGPP-MX, we provided the RNA binding factor Sam68 in trans, as it was previously shown that Sam68 increases the cytoplasmic utilization of RNA containing the MPMV CTE (8). Indeed, cells cotransfected with pGPP-MX and pCDNA3-HA-Sam68 produced quantities of viral particles comparable to JSRV (Fig. 2C).
FIG. 2.
Optimization of the JSRV packaging construct. Cell lysates (bottom panel) and virus pellets from supernatants (top panel) of transfected cells were analyzed 48 h posttransfection by SDS-PAGE/Western blotting employing an antiserum against the JSRV p23 (matrix). (A) 293T cells were transfected with the plasmids indicated above each lane. Note that only cells transfected with plasmids containing the R-U5 region of the JSRV-proximal LTR released viral particles into the supernatant. Note that the bands below Gag in the cell lysates are likely the product of partially cleaved Gag (present also in panel C). (B) Quantification of virus release was done by chemifluorescence on Western blots of viral pellets. Shown are the means (± standard errors) obtained in three independent experiments. (C) A positive effect on JSRV particle release was observed in 293T cells cotransfected with pGPP-MX and increasing amounts (from 0.5 to 14 μg) of an expression plasmid for Sam68.
The JSRV env influences viral particle production.
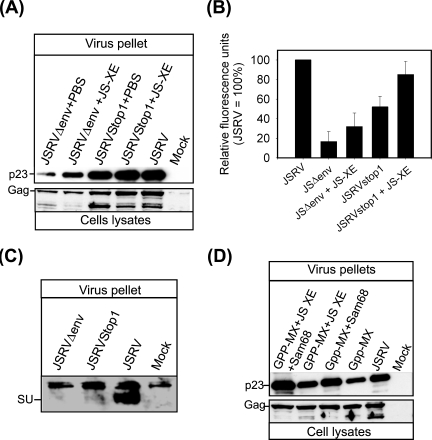
We observed that cotransfecting pJS-XE with pGPP-MX increased viral particle production relative to transfection with pGPP-MX alone. In order to analyze this observation further, we constructed and analyzed pJSRVΔEnv, in which we removed most of the env region of JSRV, and pJSRVstop1, in which we introduced two mutations that altered the splice acceptor and the first ATG of env (Fig. 1). Both these constructs were able to produce viral particles with no detectable envelope (Fig. 3A to C). However, cells transfected with pJSRVΔEnv and pJSRVstop1 produced, respectively, 16% ± 10.4% and 52% ± 10.9% viral particles compared to cells transfected with the expression plasmid for JSRV; in both cases, differences with JSRV were statistically significant (P = 0.001 and 0.01, respectively). Cotransfection of pJS-XE with pJSRVstop1 or pJSRVΔEnv almost doubled the amount of viral particles compared to cells transfected by pJSRVstop1 or pJSRVΔEnv alone, although in the latter case, differences were above significance (P = 0.1). Thus, available data suggest that the JSRV env facilitates viral particle release in cis and likely in trans.
FIG. 3.
The JSRV env region is important for optimal viral particle production. (A) 293T cells were transfected with the plasmids indicated above each lane. Virus pellets from cell supernatants (top panel) and cell lysate (50 μg) were analyzed 48 h posttransfection by SDS-PAGE/Western blotting. Where indicated, Env was supplied in trans by the JS-XE plasmid. Note that the bands below Gag in the cell lysates are likely the product of partially cleaved Gag (present also in panel D). (B) Quantification of virus release was performed by chemifluorescence on Western blots of virus pellets. Shown are the means (± SE) obtained in three independent experiments. (C) 293T cells were transfected with the plasmids indicated. Forty-eight hours posttransfection supernatants were harvested. Virus pellets were analyzed by SDS-PAGE/Western blotting employing an antiserum raised against the JSRV Env. As expected, neither JSRVΔEnv nor JSRVStop1 expresses detectable Env. (D) 293T cells were transfected with the indicated plasmids and p23/Gag was detected as described above. Note that cotransfection of pGPP-MX, pJS-XE, and pCDNA3-HA-Sam68 yields JS-RD virus particles in quantities similar to those of replication-competent JSRV.
Experimental induction of ovine pulmonary adenocarcinoma with JS-RD.
Because of the lack of an in vitro system to efficiently test and titrate JSRV infectivity, we proceeded directly to the experimental inoculation of newborn lambs with JS-RD. Two independent studies were performed, and the results obtained are summarized in Table 1.
TABLE 1.
Summary of the results obtained in the in vivo studiesa
| Lamb no. | Inoculum | OPA lesionsb | IHCc | Result for indicated PCR
|
|||
|---|---|---|---|---|---|---|---|
| JS-XE
|
JSRV
|
||||||
| 1 | 2 | 3 | 4 | ||||
| Study 1 | |||||||
| 77 | JS-RD | + | + | + | + | − | − |
| 74 | JS-RD | + | + | + | + | − | − |
| 71 | GPP-MX | − | − | − | − | − | − |
| 73 | GPP-MX | − | − | − | − | − | − |
| Study 2 | |||||||
| B694 | JS-RD | + | + | + | + | − | − |
| B683 | JS-RD | + | + | + | + | − | − |
| B695 | JS-RD | − | − | + | + | − | − |
| B660 | pGPP-MX | − | − | − | − | − | − |
| B661 | pGPP-MX | − | − | − | − | − | − |
| B685 | JSRV | + | + | − | − | + | + |
| B689 | JSRV | + | + | ND | ND | ND | ND |
+, positive; −, negative; ND, not done.
Presence of histological OPA lesions.
Detection of JSRV Env by immunohistochemistry (IHC).
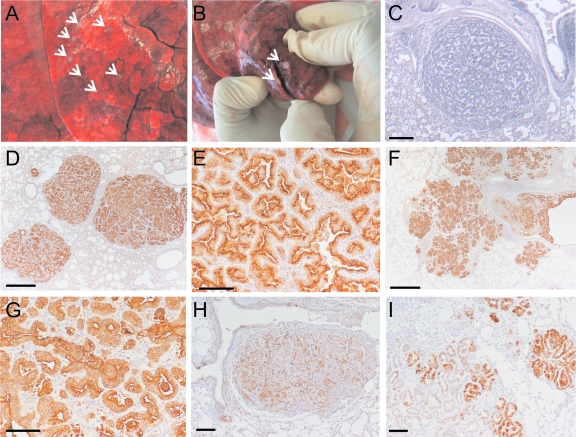
In the first study, two lambs (no. 74 and 77) were inoculated with JS-RD, while two lambs were used as negative controls (no. 71 and 73) and inoculated with the supernatant of cells transfected with the packaging plasmid alone. Lambs were kept for 12 months and were then euthanized without showing signs of clinical distress with the exception of one of the JS-RD-inoculated animals which showed dyspnea (labored respiration). At the postmortem examination, neither negative-control lamb had pulmonary lesions. On the contrary, one of the JS-RD-inoculated animals (no. 77) had numerous small whitish nodules (a few millimeters in diameter) which were well delimitated from the surrounding lungs (Fig. 4A). Few larger nodules (approximately 1 to 2 cm in diameter) were also observed (Fig. 4B). Macroscopic lesions were absent from the second lamb (no. 74) inoculated with JS-RD although the latter had small areas of the lungs with an abnormal color and areas of consolidation (increased density of the pulmonary tissue). Histological examination confirmed the presence of OPA lesions in both the JS-RD-inoculated lambs (see below) (Fig. 4C and D).
FIG. 4.
Macroscopic and microscopic lesions induced by JS-RD. Three of the five JS-RD-inoculated lambs showed numerous isolated lesions creamy in color and of hard consistency (A and B). (C) Histology from a lung section of a JS-RD-inoculated lamb shows the presence of a papillary to acinar expanding nodule that compresses the surrounding normal alveoli. (D and E) Immunohistochemistry in lung sections from a JS-RD-inoculated lamb shows expression of the JSRV Env in tumor cells (characterized by the intracytoplasmic brown color). Micrographs are shown at both low (D) and high (E) magnification. (F and G) Immunohistochemistry of lung sections of JSRV-inoculated lamb no. B689. Note the presence of neoplastic foci of various sizes in close proximity. Micrographs are shown at both low (F) and high (G) magnification. (H and I) Phosphorylated Erk1/2 is detectable both in JSRV- and JS-RD-induced tumors. Detection of phospho-Erk1/2 was achieved by immunohistochemistry both in lung sections from JSRV-inoculated lambs (I) and JS-RD-inoculated lambs (H). A positive reaction is characterized by a cytoplasmic brown color. Bars, 100 μm (panels E, G, H, and I) and 500 μm (panels C, D, and F).
In study 2, three animals were inoculated with JS-RD, two with cell supernatants transfected with the packaging plasmid alone (negative controls), and two with JSRV (positive controls). One animal inoculated with JSRV was euthanized 7 weeks postinoculation when it showed respiratory distress (no. B689). Another animal inoculated with JS-RD (no. B683) had dyspnea 4.5 months postinoculation and was therefore euthanized. All the other animals of study 2, including both negative controls and the remaining positive control, were euthanized approximately 8 months postinoculation. At necropsy, the lungs of one of the animals inoculated with JSRV (no. B685) showed OPA-like lesions, while the other JSRV-inoculated lamb (no. B689) had no gross pathology but had multiple neoplastic foci upon histopathological examination. One of the JS-RD-inoculated animals (no. B694) showed multiple small nodular lesions, as observed with lamb no. 77 in study 1. The other two JS-RD-inoculated lambs (no. B683 and B695) showed some small lesions that looked comparable to OPA lesions. Histopathological examination confirmed the presence of multiple OPA focal lesions in 2/3 JS-RD-inoculated lambs. No neoplastic lesions were observed with lamb B695. In summary, in both studies, OPA was induced in 4/5 JS-RD-inoculated lambs and in 2/2 JSRV-inoculated lambs but not in any of the 4 negative-control animals.
Expression of the JSRV Env in all the experimental animals was assessed by immunohistochemistry (Fig. 4D to G). As expected, abundant expression of JSRV Env was detected in tumor cells of JS-RD- and JSRV-inoculated animals, while no JSRV Env-expressing cells were detected in the negative-control animals (data not shown).
Histopathological appearance of OPA lesions in JSRV- and JS-RD-inoculated sheep.
OPA lesions in JS-RD-infected animals appeared to be mostly formed by isolated tumor nodules composed by cuboidal or columnar proliferating cells growing in an acinar or papilliform pattern and compressing the surrounding normal alveoli (Fig. 4C and D). Larger neoplastic foci were observed occasionally in lamb no. 77. Occasionally, myxomatous tissue (probably of mesodermal origin) was also present around the neoplastic lesions (not shown). The myxomatous component is often seen in naturally occurring OPA, but its origin (neoplastic versus nonneoplastic) is not clear (10).
Lesions in lambs experimentally infected with JSRV were mostly formed by neoplastic foci with characteristics similar to those induced by JS-RD. However, neoplastic foci of various sizes were surrounded by satellite often coalescing nodules (Fig. 4F). The histopathological features and appearance of neoplastic cells were essentially the same in JSRV- and JS-RD-induced tumors, as can be appreciated by the high-magnification micrographs shown in Fig. 4E and G.
Involvement of the H/N-Ras-MEK-MAPK pathway has been shown in JSRV-induced cell transformation in vitro and in vivo (11, 19). In order to test whether virus replication and spread could determine differences in MAPK (Erk1/2) activation, we performed immunohistochemistry in tissues from both JS-RD- and JSRV-inoculated animals. Lung sections were stained with an antibody specific for pErk1/2. Tumor cells in neoplasms induced by both JS-RD and JSRV showed activation of the MAPK pathway (Fig. 4H and I).
JS-XE is detectable in JS-RD-inoculated lambs.
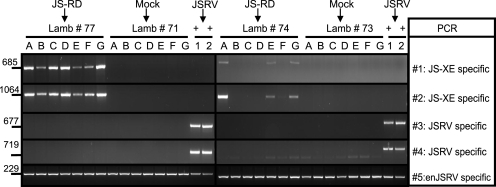
Finally, we investigated whether we could detect JS-XE or JSRV in the lungs of JS-RD-inoculated animals and in the positive- and negative-control animals by developing two PCR assays that specifically amplified the viral genome with deletions (JS-XE) (PCRs no. 1 and 2) and two that detected full-length JSRV (PCRs no. 3 and 4). Given the lack of obvious macroscopic OPA lesions in some of the experimentally infected lambs, we collected tissues from seven distinct anatomical regions in the lungs. Animals were considered positive for JS-RD or JSRV when at least one of seven aliquots was positive with either JS-XE- or JSRV-specific PCR. The results are summarized in Table 1, and representative data are shown in Fig. 5.
FIG. 5.
Detection of JS-RD in experimentally inoculated lambs. Representative examples of PCRs from lambs inoculated with JS-RD, JSRV (positive control), or packaging construct only (mock). DNA was extracted from seven anatomical regions (indicated with letters A to G and corresponding, respectively, to the cranial part of the left cranial lobe, caudal part of left cranial lobe, left diaphragmatic lobe, right diaphragmatic lobe, right middle lobe, right cranial lobe, and accessory lobe) and tested by PCR specific for either JS-XE (no. 1 and 2) or JSRV (no. 3 and 4) as indicated in Materials and Methods. Specific PCR products for JS-XE are found only in JS-RD-infected lambs (no. 77 and 74). JSRV is amplified only by PCRs no. 3 and 4 in DNA extracted from tumor tissue of lamb no. 685 (lanes indicated with +). PCR for enJSRV DNA was used to control for the quality of the DNA preparations.
From all sets of positive and negative controls we obtained the expected results. We detected JSRV in the tumor tissues of the JSRV-inoculated positive-control animals, while we found neither JS-XE nor full-length JSRV in any of the lung tissues collected from the negative controls. In five of five JS-RD-inoculated animals, we detected the viral genome with deletions and did not detect full-length JSRV, confirming that Env expression alone was responsible for the induction of OPA. The integrity of DNA samples was assessed by amplifying enJSRV sequences which are present in approximately 20 copies in the sheep genome (28).
DISCUSSION
In this study, we have shown that expression of the JSRV Env alone is sufficient to induce OPA in sheep. Thus, the JSRV Env is a powerful oncoprotein in vivo, in the natural host of JSRV infection, and viral spread is not necessary for the onset of lung adenocarcinoma.
JS-RD induced OPA in a high percentage of inoculated animals, comparable to experimental JSRV infection (29). The size of the lesions induced by JS-RD was reduced compared to those observed in lambs experimentally infected with JSRV. OPA lesions in JS-RD-inoculated animals appeared usually as well-isolated neoplastic foci compressing the surrounding normal alveoli. In lambs experimentally infected with JSRV (in this and previous studies) (12, 29, 35, 37), neoplastic foci of different sizes were closely together (often coalescing) and lesions gave the impression of being more invasive. We attribute the histopathological differences noted above to the ability of JSRV, upon infection of target cells, to produce new infectious virus that can then infect (and consequently transform) new target cells compromising the respiratory functions more rapidly. On the contrary, JS-RD, being replication defective, can infect and transform only single cells that expand by cell division, and these growing lesions have fewer chances to coalesce with other lesions unless cells in close proximity are initially infected. Alternatively, it is possible that the neoplastic cells induced by replication competent JSRV have a more malignant phenotype than the JS-RD counterpart. We do not favor the latter scenario, since the histopathological features of the neoplastic cells per se are the same in JSRV- and JS-RD-induced tumors. However, we cannot exclude the possibility that in some instances, proto-oncogenes activated by JSRV by insertional mutagenesis participate in the process of transformation initiated by the viral Env (7).
The histopathologies of field cases of OPA differ, with neoplastic lesions appearing sometimes as isolated nodules and sometimes as large neoplastic foci with smaller satellite foci around them; both types of lesions can be observed in the same animal.
We believe that the age of the animal at the time of infection and/or the physiological state of the lungs (e.g., concurrent bacterial or parasitic infection) influences the histopathological pattern shown in naturally occurring OPA. Most retroviruses, with the notable exception of lentiviruses, require actively replicating cells in order to allow the viral preintegration complex to pass through the nuclear membrane (4). Transformed cells in OPA are derived from the differentiated epithelial cells of the distal lungs, type II pneumocytes, and Clara cells, which in general have been believed to give rise to lung adenocarcinoma in sheep and other species, including humans (10, 21, 31). However, in adult animals, type II pneumocytes and Clara cells have a very low replicative index, and consequently, they would not be easily infectible by JSRV. In contrast, type II pneumocytes/Clara cells divide in the very young lamb or in the adult where the epithelium has been injured in order to repair the lesion; in both cases, these dividing cells would be permissive to JSRV infection.
Recently, bronchioalveolar stem cells (BASCs) have been proposed to maintain the bronchiolar Clara cells and pneumocytes and to be the true origin of lung adenocarcinomas (3, 17). If present also in sheep, BASCs might thus be the true target for JSRV replication in the lungs. It is likely that BASCs are most abundant in the young animal or following a lung injury and thus BASC infection by JSRV would fit the model of OPA development as proposed above.
The development of the JS-RD system has allowed us also to further understand the molecular biology of JSRV. The R-U5 region of the viral 5′ LTR is necessary for optimal JSRV expression/particle formation, possibly by interacting with host proteins similarly to the 5′-terminal RNA elements of other retroviruses or by allowing proper Gag assembly (5, 15). Also, our results suggest that for viral particle production, the env region is important both in cis and in trans. Currently, we are trying to determine if a CTE-like structure (13) is present within env of JSRV, as there is in MPMV, and/or whether a Rev-like export protein (and/or a Rev-responsive element) is present within env analogously to mouse mammary tumor virus (16, 22) and HERV-K (42). Additionally, it is likely that the JSRV Env facilitates viral particle exit in the same way as has been shown for MPMV (another betaretrovirus-like JSRV), for which capsid transport is mediated by Env/Gag interactions (36).
Acknowledgments
We thank Os Jarrett, Vincenzo Caporale, and the anonymous reviewers for suggestions that improved the manuscript. We thank Marie-Louise Hammarskjöld and David Shalloway for generously providing reagents and Mark Dagleish for histopathology of the lambs in Study 2. We are grateful to Emanuela Rossi, Patricia Dewar, and clinical staff of the IZS Abruzzi e Molise and MRI for excellent animal care. We thank Matt Golder and colleagues in the Viral Pathogenesis Laboratory for continuous discussions and assistance.
This work was supported by grant CA95706-01 from the National Cancer Institute of the National Institutes of Health, a grant from the Italian Ministry of Health, and grant FF66/01 from the Scottish Executive Environment and Rural Affairs Department. M.P. is a Wolfson-Royal Society research merit awardee.
REFERENCES
- 1.Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. [DOI] [PubMed] [Google Scholar]
- 2.Ausbel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2000. Current protocols in molecular biology. John Wiley and Son, New York, N.Y.
- 3.Berns, A. 2005. Stem cells for lung cancer? Cell 121:811-813. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P. O. 1997. Integration, p. 161-203. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 5.Butsch, M., S. Hull, Y. Wang, T. M. Roberts, and K. Boris-Lawrie. 1999. The 5′ RNA terminus of spleen necrosis virus contains a novel posttranscriptional control element that facilitates human immunodeficiency virus Rev/RRE-independent Gag production. J. Virol. 73:4847-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caporale, M., P. Centorame, A. Giovannini, F. Sacchini, M. Di Ventura, M. de las Heras, and M. Palmarini. 2005. Infection of lung epithelial cells and induction of pulmonary adenocarcinoma is not the most common outcome of naturally occurring JSRV infection during the commercial lifespan of sheep. Virology 338:144-153. [DOI] [PubMed] [Google Scholar]
- 7.Cousens, C., J. V. Bishop, A. W. Philbey, C. A. Gill, M. Palmarini, J. O. Carlson, J. C. DeMartini, and J. M. Sharp. 2004. Analysis of integration sites of jaagsiekte sheep retrovirus in ovine pulmonary adenocarcinoma. J. Virol. 78:8506-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle, J. H., B. W. Guzik, Y. C. Bor, L. Jin, L. Eisner-Smerage, S. J. Taylor, D. Rekosh, and M. L. Hammarskjold. 2003. Sam68 enhances the cytoplasmic utilization of intron-containing RNA and is functionally regulated by the nuclear kinase Sik/BRK. Mol. Cell. Biol. 23:92-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danilkovitch-Miagkova, A., F. M. Duh, I. Kuzmin, D. Angeloni, S. L. Liu, A. D. Miller, and M. I. Lerman. 2003. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus. Proc. Natl. Acad. Sci. USA 100:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de las Heras, M., L. Gonzalez, and J. M. Sharp. 2003. Pathology of ovine pulmonary adenocarcinoma. Curr. Top. Microbiol. Immunol. 275:25-54. [DOI] [PubMed] [Google Scholar]
- 11.de las Heras, M., A. Ortin, A. Benito, C. Summers, and J. M. Sharp. In situ visualization of MAPK Erk 1/2 signalling pathway in contagious respiratory tumours of sheep and goats. J. Comp. Pathol., in press. [DOI] [PubMed]
- 12.DeMartini, J. C., J. V. Bishop, T. E. Allen, F. A. Jassim, J. M. Sharp, M. De las Heras, D. R. Voelker, and J. O. Carlson. 2001. Jaagsiekte sheep retrovirus proviral clone JSRV(JS7), derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J. Virol. 75:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst, R. K., M. Bray, D. Rekosh, and M. L. Hammarskjold. 1997. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol. Cell. Biol. 17:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, H., M. Palmarini, and J. C. DeMartini. 2003. Transformation and oncogenesis by jaagsiekte sheep retrovirus. Curr. Top. Microbiol. Immunol. 275:139-177. [DOI] [PubMed] [Google Scholar]
- 15.Hull, S., and K. Boris-Lawrie. 2002. RU5 of Mason-Pfizer monkey virus 5′ long terminal repeat enhances cytoplasmic expression of human immunodeficiency virus type 1 gag-pol and nonviral reporter RNA. J. Virol. 76:10211-10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indik, S., W. H. Gunzburg, B. Salmons, and F. Rouault. 2005. Mouse mammary tumor virus infects human cells. Cancer Res. 65:6651-6659. [DOI] [PubMed] [Google Scholar]
- 17.Kim, C. F., E. L. Jackson, A. E. Woolfenden, S. Lawrence, I. Babar, S. Vogel, D. Crowley, R. T. Bronson, and T. Jacks. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823-835. [DOI] [PubMed] [Google Scholar]
- 18.Liu, S. L., and A. D. Miller. 2005. Transformation of madin-darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J. Virol. 79:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda, N., W. Fu, A. Ortin, M. de las Heras, and H. Fan. 2005. Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J. Virol. 79:4440-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 98:4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason, R. J., and J. M. Shannon. 1997. Alveolar type II pneumocytes, p. 543-555. In R. G. Crystal, J. B. West, E. R. Weibel, and P. J. Barnes (ed.), The lung: scientific foundations, vol. 1. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 22.Mertz, J. A., M. S. Simper, M. M. Lozano, S. M. Payne, and J. P. Dudley. 2005. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J. Virol. 79:14737-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mura, M., P. Murcia, M. Caporale, T. E. Spencer, K. Nagashima, A. Rein, and M. Palmarini. 2004. Late viral interference induced by transdominant Gag of an endogenous retrovirus. Proc. Natl. Acad. Sci. USA 101:11117-11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmarini, M., C. Cousens, R. G. Dalziel, J. Bai, K. Stedman, J. C. DeMartini, and J. M. Sharp. 1996. The exogenous form of Jaagsiekte retrovirus is specifically associated with a contagious lung cancer of sheep. J. Virol. 70:1618-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmarini, M., and H. Fan. 2003. Molecular biology of jaagsiekte sheep retrovirus. Curr. Top. Microbiol. Immunol. 275:81-115. [DOI] [PubMed] [Google Scholar]
- 26.Palmarini, M., and H. Fan. 2001. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst. 93:1603-1614. [DOI] [PubMed] [Google Scholar]
- 27.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan. 2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002-11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmarini, M., M. Mura, and T. E. Spencer. 2004. Endogenous betaretroviruses of sheep: teaching new lessons in retroviral interference and adaptation. J. Gen. Virol. 85:1-13. [DOI] [PubMed] [Google Scholar]
- 29.Palmarini, M., J. M. Sharp, M. de las Heras, and H. Fan. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philbey, A. W., C. Cousens, J. V. Bishop, C. A. Gill, J. C. Demartini, and J. M. Sharp. 2006. Multiclonal pattern of Jaagsiekte sheep retrovirus integration sites in ovine pulmonary adenocarcinoma. Virus Res. 117:254-263. [DOI] [PubMed] [Google Scholar]
- 31.Platt, J. A., N. Kraipowich, F. Villafane, and J. C. DeMartini. 2002. Alveolar type II cells expressing jaagsiekte sheep retrovirus capsid protein and surfactant proteins are the predominant neoplastic cell type in ovine pulmonary adenocarcinoma. Vet. Pathol. 39:341-352. [DOI] [PubMed] [Google Scholar]
- 32.Rabson, A. B., and B. J. Graves. 1997. Synthesis and processing of viral RNA, p. 205-261. In J. M. Coffin, S. H. Hughes, and H. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 33.Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg, N., and P. Jolicoeur. 1997. Retroviral pathogenesis, p. 475-586. In J. M. Coffin, S. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 35.Salvatori, D., L. Gonzalez, P. Dewar, C. Cousens, M. de las Heras, R. G. Dalziel, and J. M. Sharp. 2004. Successful induction of ovine pulmonary adenocarcinoma in lambs of different ages and detection of viraemia during the preclinical period. J. Gen. Virol. 85:3319-3324. [DOI] [PubMed] [Google Scholar]
- 36.Sfakianos, J. N., and E. Hunter. 2003. M-PMV capsid transport is mediated by Env/Gag interactions at the pericentriolar recycling endosome. Traffic 4:671-680. [DOI] [PubMed] [Google Scholar]
- 37.Sharp, J. M., K. W. Angus, E. W. Gray, and F. M. Scott. 1983. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch. Virol. 78:89-95. [DOI] [PubMed] [Google Scholar]
- 38.Sharp, J. M., and J. C. DeMartini. 2003. Natural history of JSRV in sheep. Curr. Top. Microbiol. Immunol. 275:55-79. [DOI] [PubMed] [Google Scholar]
- 39.Sigurdsson, B. 1958. Adenomatosis of sheep lungs. Experimental transmission. Arch. Gesamte Virusforsch. 8:51-58. (In German.) [DOI] [PubMed] [Google Scholar]
- 40.Sigurdsson, B. 1954. Rida, a chronic encephalitis of sheep: with general remarks on infections which develop slowly and some of their special characteristics. Br. Vet. J. 110:341-354. [Google Scholar]
- 41.Wootton, S. K., C. L. Halbert, and A. D. Miller. 2005. Sheep retrovirus structural protein induces lung tumours. Nature 434:904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, J., H. P. Bogerd, S. Peng, H. Wiegand, R. Truant, and B. R. Cullen. 1999. An ancient family of human endogenous retroviruses encodes a functional homolog of the HIV-1 Rev protein. Proc. Natl. Acad. Sci. USA 96:13404-13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaffran, S., M. Astier, D. Gratecos, and M. Semeriva. 1997. The held out wings (how) Drosophila gene encodes a putative RNA-binding protein involved in the control of muscular and cardiac activity. Development 124:2087-2098. [DOI] [PubMed] [Google Scholar]
- 44.Zavala, G., C. Pretto, Y. H. Chow, L. Jones, A. Alberti, E. Grego, M. de las Heras, and M. Palmarini. 2003. Relevance of Akt phosphorylation in cell transformation induced by Jaagsiekte sheep retrovirus. Virology 312:95-105. [DOI] [PubMed] [Google Scholar]