Abstract
Human immunodeficiency virus type 1 (HIV-1) utilizes Vpu, Env, and Nef to down-modulate its primary CD4 receptor from the cell surface, and this function seems to be critical for the pathogenesis of AIDS. The physiological relevance of CD4 down-modulation, however, is currently not well understood. In the present study, we analyzed the kinetics of CD4 down-modulation and the susceptibility of HIV-1-infected T cells to superinfection using proviral HIV-1 constructs containing individual and combined defects in vpu, env, and nef and expressing red or green fluorescent proteins. T cells infected with HIV-1 mutants containing functional nef genes expressed low surface levels of CD4 from the first moment that viral gene expression became detectable. In comparison, Vpu and Env had only minor to moderate effects on CD4 during later stages of infection. Consistent with these quantitative differences, Nef inhibited superinfection more efficiently than Vpu and Env. Notably, nef alleles from AIDS patients were more effective in preventing superinfection than those derived from a nonprogressor of HIV-1 infection. Our data suggest that protection against X4-tropic HIV-1 superinfection involves both CD4-independent and CD4-dependent mechanisms of HIV-1 Nef. X4 was effectively down-regulated by simian immunodeficiency virus and HIV-2 but not by HIV-1 Nef proteins. Thus, maximal protection seems to involve an as-yet-unknown mechanism that is independent of CD4 or coreceptor down-modulation. Finally, we demonstrate that superinfected primary T cells show enhanced levels of apoptosis. Accordingly, one reason that HIV-1 inhibits CD4 surface expression and superinfection is to prevent premature cell death in order to expand the period of effective virus production.
Human immunodeficiency virus type 1 (HIV-1) utilizes three of its gene products, Vpu, Env, and Nef, to down-modulate its primary CD4 receptor (reviewed in references 21, 37, and 55). The diverse mechanisms underlying the effects of these three viral proteins on CD4 surface expression have been extensively studied. Nef is expressed at high levels early during infection and down-modulates CD4 by enhancing its internalization and directing the receptor to lysosomes for degradation (1, 10, 19, 27, 45, 54). In contrast, Vpu and Env are coordinately expressed from a Rev-dependent single-spliced mRNA late during the viral life cycle and interfere with the transport of newly synthesized CD4 to the cell surface (24, 69, 70). Thus, only Nef acts on CD4 molecules that were already at the cell surface prior to HIV-1 infection, and it plays the most prominent role in CD4 down-modulation (17). The contribution of Vpu and Env has been demonstrated using expression constructs or in the context of nef-defective HIV-1 mutants (reviewed in reference 38).
A number of studies suggest that Nef-mediated CD4 down-modulation plays a critical role in the pathogenesis of AIDS (reviewed in reference 67). nef alleles derived from some long-term nonprogressors of HIV-1 infection are unable to down-modulate CD4 but are fully capable of performing other functions, such as down-regulation of major histocompatibility complex class I molecules (13, 46, 64). Furthermore, point mutations or deletions in Nef disrupting its ability to down-regulate CD4 but not most of its other functions attenuate simian immunodeficiency virus (SIV) replication and delay or prevent disease progression in infected rhesus macaques (9, 33). Moreover, nef alleles derived from AIDS patients and from SIV-infected macaques after the development of immunodeficiency show increased activity in CD4 down-modulation (4, 14, 50). Much less is known about the relevance of Vpu- and Env-dependent CD4 down-modulation for the pathogenesis of AIDS. Unlike nef, which is present in the genomes of all primate lentiviruses, vpu is present only in the HIV-1/SIVcpz group and some closely related SIVs found in Cercopithecus monkeys (reviewed in reference 30). It has been suggested that Vpu contributes to the virulence of HIV-1/SIV chimeras (SHIVs) in infected macaques (31). However, Vpu is multifunctional (30), and it remained elusive whether its effect on the release of viral particles or on CD4 was responsible for this phenotype. Moreover, it has been shown that SHIVs expressing truncated and likely nonfunctional Vpu proteins can cause disease in pigtailed macaques (61).
It is currently unclear which of the multiple consequences of diminished CD4 cell surface expression are critical for lentiviral pathogenesis. For example, CD4 down-modulation may weaken the antiviral immune response, because CD4 interacts with major histocompatibility complex class II on antigen-presenting cells (APCs) and is an important costimulatory factor of T-cell receptor-mediated T-cell activation (68). Furthermore, it has been reported that CD4 down-modulation enhances the release and infectivity of HIV-1 particles (8, 18, 38, 40, 56). These effects may explain why the efficiency of Nef-mediated CD4 down-modulation correlates with its ability to enhance HIV-1 replication in primary T cells and in ex vivo-infected human lymphoid tissues (25, 43). However, the relevance of these activities is somewhat unclear, because Nef also enhances the viral infectivity of HIV-1 particles produced in CD4-negative cells (2, 63), and the effect of Nef on viral particle production was observed with artificially high Nef expression levels (56).
Another possible reason that it may be advantageous for HIV-1 to down-modulate its primary receptor is to avoid superinfection (6, 39, 47). Superinfection immunity may reduce cell killing caused by the accumulation of unintegrated viral DNA (20), enhance the efficiency of viral spread, and reduce the frequency of HIV-1 recombination (37). However, although it is conceivable that CD4 down-modulation may protect HIV-1-infected cells against superinfection, there is surprisingly little experimental evidence to support this hypothesis. Previous studies have used chronically infected cells and cells stably or transiently transfected with constructs expressing Env or Nef (6, 39, 42, 47, 53). To our knowledge, however, it has never been experimentally demonstrated that CD4 down-modulation diminishes superinfection of T cells productively infected with HIV-1.
To further study how HIV-1 modulates CD4 surface expression, we first analyzed the kinetics of receptor down-modulation in T cells and peripheral blood mononuclear cells (PBMC) infected with HIV-1 mutants containing individual and combined defects in Vpu, Env, and Nef. Similar experiments were performed in an elegant early study (17). However, CD4 down-modulation was shown only for a single time point, and Nef was expressed not from its original genomic localization but by an internal ribosome entry site (IRES) element. Thus, the kinetics of CD4 down-modulation and the relative contribution of Vpu, Env, and Nef to this effect during different stages of HIV-1 infection remained elusive. Next, we investigated how effectively Vpu, Env, and a variety of HIV-1, HIV-2, and SIV Nef proteins protect HIV-1-infected cells against superinfection. We found that Nef plays the major role in superinfection immunity and protects cells by both CD4-dependent and CD4-independent mechanisms. In agreement with their higher activity in CD4 down-modulation (4, 14), nef alleles from AIDS patients were more effective than those derived from a long-term nonprogressor of HIV-1 infection (28, 36, 46). Recently, it has been reported that Nef also down-modulates the major HIV-1 coreceptors CCR5 (R5) and CXCR4 (X4), and it has been suggested that R5 down-modulation contributes to the suppression of superinfection (32, 47). Our results suggest, however, that maximal Nef-mediated protection against superinfection by X4-tropic HIV-1 is achieved by an as-yet-unknown mechanism that does not involve CD4 or coreceptor down-modulation.
MATERIALS AND METHODS
Proviral constructs.
The HIV-1 NL4-3 proviral constructs carrying intact nef alleles or a disruptive nef gene followed by an IRES and the enhanced green fluorescent protein (eGFP) gene have been previously described (58, 59). To generate proviral IRES-eGFP vectors containing a defective vpu or env gene, the intergenic region of NL4-3 mutants containing mutations in these genes (57) was cloned into the original proviral reporter constructs using the unique PflmI and StuI restriction sites. The wild-type NL4-3 nef allele was replaced by different HIV-1, HIV-2, and SIV nef alleles using standard PCR mutagenesis and cloning techniques, essentially as described previously (58, 59). To generate reporter viruses expressing a red fluorescent protein, we PCR amplified the DsRed2 gene from pDsRed2-C1 (Clontech) using the 5′ primer pRed2-5 (5′-CACCATGGCCTCCTCCGAGAACGTC-3) and the 3′ primer pRed2-3 (5′-GTCCCGGGTTATCTCGATCCGGTGGATCCTGGGC-3) and replaced the eGFP gene in the proviral constructs by the DsRed2 gene using the unique NcoI and XmaI restriction sites (underlined). All PCR-derived sequences were verified by sequence analysis.
Cells.
Jurkat cells were maintained in RPMI 1640 medium and 293T cells were grown in Dulbecco's modified Eagle medium, both supplemented with 10% fetal calf serum (FCS) and antibiotics. PBMC were isolated using Biocoll separating solution (Biocoll AG), and primary CD4+ T cells were purified using a RosetteSep kit (StemCell Technologies, Inc.) according to the manufacturer's protocol. PBMC and CD4+ T cells were both stimulated for 3 days with 3 μg phytohemagglutinin per ml and cultured in RPMI 1640 medium with 10% FCS, antibiotics, and 10 ng/ml interleukin 2.
Virus stocks.
Virus stocks were generated by transient transfection of 293T cells as described previously (48, 59). To generate pseudotyped viral particles, 293T cells were cotransfected with 5 μg NL4-3 proviral constructs carrying open or defective vpu, env, and nef reading frames followed by an IRES and the eGFP or DsRed2 gene and 1 μg of a plasmid (pHIT-G) expressing the vesicular stomatitis virus G protein (VSV-G) as described previously (59). The p24 antigen concentrations were quantified using an HIV-1 enzyme-linked immunosorbent assay provided by the NIH AIDS Research and Reference Program.
Flow cytometric analysis.
One million Jurkat cells or two million PBMC were transduced with 500 μl VSV-G-pseudotyped HIV-1 IRES-eGFP virus stocks containing 50 ng/ml p24 antigen. At different time points, one quarter of the cells were washed with phosphate-buffered saline (PBS) and stained with anti-CD4-phycoerythrin (clone RPA-T4; Serotec) or anti-CxCR4-phycoerythrin (clone 1265; BD) for 30 min at 4°C. The cells were washed again with PBS, fixed with 2% paraformaldehyde, and analyzed by a fluorescence-activated cell sorter (FACS).
Superinfection.
Jurkat T cells were transduced with VSV-G pseudotyped HIV-1 NL4-3 IRES-DsRed2 or -eGFP particles varying in their vpu, env, and/or nef genes and cultured in RPMI 1640 medium (supplemented with 10% FCS and antibiotics) for 3 days. Thereafter, eGFP-expressing cells were analyzed for CD4 and X4 expression by FACS as described above. Cells transduced with the DsRed2 reporter viruses were challenged with HIV-NL4-3-IRES-eGFP virus stocks containing normalized amount of p24 antigen (300 ng), cultured for another 2 days, washed once with PBS, fixed with 2% paraformaldehyde, and analyzed by flow cytometric analysis. The percentages of superinfected cells were calculated by dividing the number of cells expressing both DsRed2 and eGFP (eGFP+/DsRed2+ cells) by the number of cells expressing both fluorescent proteins plus the number of cells expressing DsRed2 only (eGFP+/DsRed2+ and DsRed2+ cells). To determine the percentages of apoptotic cells in the noninfected, single-infected, and superinfected fractions, Jurkat and primary CD4+ T cells were transduced with nef-defective VSV-G pseudotyped HIV-NL4-3-IRES-DsRed2 particles and challenged with wild-type HIV-NL4-3-IRES-eGFP viruses 3 days later. After another 2 days in culture, the cells were washed once with PBS, stained with Annexin V-APC (Caltag) in Annexin binding buffer for 15 min at room temperature, fixed with Annexin binding buffer containing 2% paraformaldehyde, and analyzed by FACS. Percentages of apoptotic Annexin V+ cells were determined for the DsRed2+, eGFP+, eGFP/DsRed2+, and uninfected cell populations.
Western blot analysis.
293T cells were transfected with 5 μg DNA from the proviral HIV-1 NL4-3 IRES-eGFP vectors as described previously (48, 59). At 40 h after transfection, the viral supernatant was removed and the cells were harvested and lysed with 200 μl lysis buffer (1% Triton X-100; 0.15 M NaCl; 50 mM Tris-HCl, pH 7.4; 5 mM EDTA; 1 mM NaF; 1 mM Na2VO3). Virus stocks containing 150 ng p24 antigen were used for the detection of the viral gp120 envelope glycoprotein in the cell-free culture supernatants. Virions were pelleted in a microcentrifuge and resuspended in 80 μl of lysis buffer. For the detection of Vpu, HeLa cells were used, because a nonspecific band interfered with the Vpu signal for 293T cells. Briefly, HeLa cells were transduced with VSV-G-pseudotyped HIV-1 NL4-3 IRES-eGFP particles, and cellular lysates were generated 3 days later. Viral proteins were separated by 10 to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected by immunoblotting using the following antibodies: human anti-HIV-1 gp120 Ab2G12 and rabbit anti-HIV-1 p24 (provided by the NIH AIDS Research and Reference Reagent Program), mouse anti-HIV-1 Nef (amino acids 151 to 170) (ABI), the polyclonal rabbit anti-HIV-1 Vpu Ab 32-81 (kindly provided by Ulrich Schubert), rabbit anti-GFP, and rabbit anti-β-actin (Abcam). For detection, the appropriate alkaline phosphatase-labeled secondary antibodies were used.
Statistical analysis.
GraphPad Prism version 4.0 statistical software was used for statistical analysis.
RESULTS
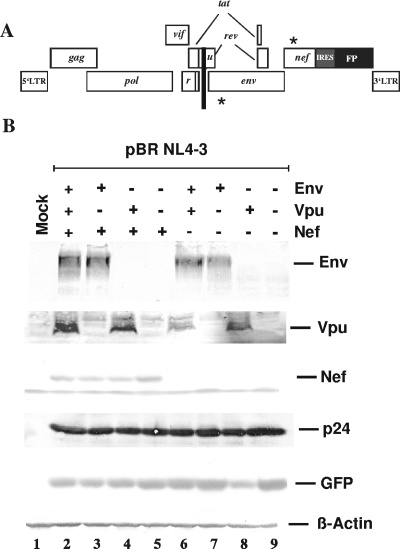
To assess the contribution of Nef, Vpu, and Env to CD4 down-modulation in HIV-1-infected Jurkat T cells, we used HIV-1 NL4-3 IRES-eGFP reporter viruses containing individual and combined defects in these three genes (Fig. 1A). As previously described (58, 59), these proviral HIV-1 constructs coexpress Nef and eGFP from a bicistronic RNA, allowing us to readily distinguish between infected and uninfected cells. Western blot analysis demonstrated that the constructs showed the expected differences in their ability to express Vpu, Env, and Nef (Fig. 1B). In contrast, all proviral HIV-1 constructs expressed similar levels of the p24 core antigen and eGFP (Fig. 1B).
FIG. 1.
Proviral constructs and viral gene expression. (A) Modifications in the HIV-1 NL4-3 genome. The stars indicate the location of the premature stop codons in the env and nef genes and the black bar specifies the deletion in vpu. All proviral constructs express eGFP or DsRed2 via an IRES element (58, 59). (B) Expression of viral proteins. Cells were transfected with the proviral constructs shown schematically in panel A. The gp120 in glycoprotein, the cell-free culture supernatants, and the Nef and p24 core antigens in the cellular extracts of transfected 293T cells were detected by immunoblotting as described in Materials and Methods. eGFP was detected to control for transfection efficiencies and β-actin for the total amount of cellular protein. LTR, long terminal repeat; r, vpr; u, vpu; FP, fluorescent protein; Mock, mock infected.
Next, we measured the kinetics and efficiencies of CD4 down-modulation for Jurkat T cells transduced with the eight different HIV-1 NL4-3 IRES-eGFP constructs. VSV-G-pseudotyped HIV-1 particles were used to allow the analysis of env-defective proviruses and to overcome Nef-dependent differences in viral infectivity (16). The viral doses used for transduction resulted in infection of ≤30% of the Jurkat cells. Thus, the proviral copy numbers in the transduced T cells should be low, as seen with wild-type HIV-1 infection. Notably, all but the env-defective HIV-1 mutants were fully competent for subsequent rounds of replication. Flow cytometric analysis revealed that HIV-1-infected eGFP+ cells first became detectable at 24 h posttransduction (Fig. 2A). All cells infected with HIV-1 mutants containing intact nef genes showed reduced levels of CD4 surface expression once viral gene expression first became noticeable (Fig. 2A, columns 2 and 4 to 6). The expression of the viral Env glycoprotein also resulted in diminished CD4 surface expression in both the presence and the absence of Nef, particularly at high levels of eGFP and (indirectly) viral gene expression (Fig. 2A, columns 3 and 7). In comparison, Vpu had only marginal effects on the surface levels of CD4 expression (Fig. 2A, column 8).
FIG. 2.
Kinetics of CD4 down-modulation in HIV-1-infected Jurkat T cells. (A) Flow cytometric analysis of Jurkat cells transduced with HIV-1 constructs containing individual or combined defects in vpu, env, and nef at 24 and 48 h posttransduction. The numbers give the rMFIs for the indicated ranges of eGFP expression. (B) Quantitative assessment of CD4 down-modulation. The rMFI obtained for Jurkat T cells infected with the HIV-1 mutant containing combined defects in vpu, env, and nef was divided by the corresponding number obtained for cells transduced with the viruses expressing at least one of these three viral proteins. Calculations were performed for cells expressing low (left) or medium (right) levels of eGFP (ranges indicated in panel A) at the indicated time points. E, env; U, vpu; N, nef.
To quantitate the effect of Vpu, Env, and Nef on CD4 expression, we determined the red mean fluorescence intensities (rMFIs) for cells expressing different levels of eGFP (ranges are indicated in Fig. 2A). The rMFIs obtained for Jurkat T cells infected with the HIV-1 mutant containing combined defects in vpu, env, and nef were divided by the corresponding numbers obtained for cells expressing different combinations of these three accessory proteins to calculate CD4 down-modulation (n-fold). The results confirmed that Nef had the strongest effect on CD4 (Fig. 2B). The expression of Env further enhanced CD4 down-modulation at medium levels of eGFP expression (Fig. 2B, right panel). Furthermore, cells infected with HIV-1 mutants containing an intact env gene also showed up to fivefold-diminished CD4 expression levels in the absence of Nef. Unexpectedly, we observed only negligible effects of Vpu on CD4 surface expression by HIV-1-infected Jurkat T cells (Fig. 2B).
The efficiency of CD4 down-modulation and the relative contribution of Vpu, Env, and Nef to this effect may differ between transformed and primary cells. Therefore, we next analyzed the kinetics of CD4 down-modulation in HIV-1-infected human PBMC. HIV-1-infected eGFP+ cells were first observed at 32 h posttransduction (Fig. 3A). Both CD4+ and CD4−/low PBMC were transduced with the VSV-G-pseudotyped HIV-1 constructs. The two cell populations were readily discernible, and no CD4 down-modulation was observed after infection with the Env-, Vpu-, and Nef-defective (E− U− N−) HIV-1 mutant even at high levels of eGFP and, hence, viral gene expression (Fig. 3A, panel 1). In contrast, the CD4+ cell population essentially disappeared in the PBMC cultures transduced with the four proviral HIV-1 constructs containing intact nef genes (Fig. 3A, panels 2 and 4 to 6). Thus, similar to the results obtained using Jurkat T cells (Fig. 2), Nef played the major role in CD4 down-modulation in PBMC. However, the expression of both Vpu and Env also clearly diminished CD4 surface expression at higher expression levels (Fig. 3A, panels 7 and 8). At low levels of viral gene expression, the contribution of Vpu to CD4 down-modulation was evident only at 71 h posttransduction (Fig. 3B, left panel). In contrast, Vpu enhanced the efficiency by which Nef+/Env− HIV-1 constructs down-modulated CD4 about twofold at high expression levels, even at early time points (Fig. 3B, right panels). In the presence of both Env and Nef, the effects of Vpu became most apparent during later time points after transduction (Fig. 3B, right panel). Individual expression of the three viral genes resulted in moderate (Nef) to minor (Vpu, Env) receptor down-modulation. Thus, Vpu contributes more significantly to CD4 down-modulation in primary PBMC than in transformed Jurkat T cells.
FIG. 3.
Kinetics of CD4 down-modulation in HIV-1-infected PBMC. (A) Prestimulated PBMC were transduced with the various HIV-1 NL4-3 IRES-eGFP constructs and analyzed by flow cytometric analysis at the indicated time points. (B) The efficiencies of CD4 down-modulation were calculated essentially as described in the legend to Fig. 2. The mean rMFIs were calculated for all cells expressing the indicated ranges of eGFP. The rMFI of unlabeled control cells was consistently below 5. E, env; U, vpu; N, nef.
It is well established that Nef enhances CD4 internalization and subsequent degradation in lysosomes, whereas Env and Vpu interfere with the transport of newly synthesized CD4 to the cell surface (21, 37, 54). The surface half-life time of CD4 in lymphatic cells has been estimated at about 12 h (45). Thus, even if the transport of newly synthesized CD4 to the cell surface was completely blocked, only a fourfold reduction of CD4 expression would be expected after 24 h of viral gene expression. Therefore, we next analyzed the effects of the three viral proteins under experimental conditions allowing us to detect primarily the effects on the transport of newly synthesized CD4 receptor molecules to the cell surface. We cotransfected 293T cells with the different proviral constructs and a CD4 expression vector. In the absence of Vpu, Env, and Nef, the transfected 293T cells expressed very high levels of CD4 (Fig. 4A, column 1). Cotransfection with the wild-type HIV-1 NL4-3 proviral construct suppressed CD4 surface expression about fivefold (Fig. 4A, column 2). Expression of Nef and Vpu resulted in about two- to threefold diminished levels of surface CD4, whereas Env had no significant effect under these experimental conditions (Fig. 4A, columns 6 to 8; Fig. 4B).
FIG. 4.
Suppression of CD4 expression in transfected 293T cells. (A) 293T cells were cotransfected with the indicated HIV-1 IRES-eGFP proviral constructs and a CD4 expression construct and analyzed by flow cytometric analysis 2 days later. Numbers are the rMFIs at the indicated ranges of eGFP expression. (B) Average suppression of CD4 expression (± SD, n = 3) by expression of the indicated HIV-1 proviral genomes. The rMFI obtained for the vpu-, env-, and nef-defective construct (panel A, lane 1) was divided by the rMFI obtained for the remaining proviral constructs to calculate suppression (n-fold) of CD4 surface expression. The results were confirmed in two independent experiments. (C) Correlation between down-modulation of CD4 in HIV-1-infected Jurkat T cells (measured at 48 h posttransduction at medium levels of eGFP expression) (Fig. 2A) and suppression of CD4 expression by transfected 293T cells. (D) Inhibitory effects of Vpu, Env, and Nef at different levels of CD4 expression. 293T cells were cotransfected with 4 μg of the proviral constructs and 0.1, 0.5, and 1.0 μg of the CD4 expression plasmids. E, env; U, vpu; N, nef; uninf., uninfected; Mock, mock infected; suppr., suppressed; down-mod., down-modulated.
In agreement with the fact that Vpu, Env, and Nef diminish CD4 surface expression by different mechanisms (21, 37, 55), the efficiencies by which the proviral HIV-1 constructs down-modulated CD4 from infected Jurkat T cells and suppressed receptor expression for transfected 293T cells did not correlate significantly with one another (Fig. 4C). The result that Env had no effect in the 293T cell-based assay was unexpected, because it is well established that Env interferes with the transport of CD4 to the cell surface (reviewed in reference 38). However, the levels of CD4 expression in transfected 293T cells are substantially higher than in Jurkat cells or primary T cells (data not shown). Therefore, we examined whether the lack of an Env phenotype was due to CD4 overexpression. Our analysis revealed that intact vpu and nef genes diminished CD4 expression when both 0.1 μg and 0.5 μg of CD4 expression plasmid were cotransfected with the proviral constructs (Fig. 4D). In contrast, Env expression resulted in significantly reduced levels of surface CD4 only when 0.1 μg of the CD4 expression construct was cotransfected. Taken together, these results confirm that the three viral proteins down-modulate CD4 independently (17) and further suggest that the Env-mediated mechanism becomes saturated at low levels of CD4 expression compared to Vpu- and Nef-mediated receptor down-modulation.
One of the reasons that HIV-1 down-modulates CD4 may be to protect cells that are already infected against superinfection. However, experimental evidence to support this hypothesis was derived only from studies using cells transfected with vectors expressing Nef or Env or from chronically infected cells (6, 23, 39, 42, 47, 53). To assess the role of Vpu, Env, and Nef in superinfection resistance of HIV-1-infected T cells, we generated a second set of HIV-1 NL4-3 proviral constructs containing the DsRed2 fluorescent protein under the control of an IRES element and used a FACS-based assay to determine the frequency of superinfected cells (Fig. 5). First, we transduced the Jurkat T cells with VSV-G-pseudotyped HIV-1 NL4-3 constructs containing defects in vpu, env, and/or nef. Pseudotyped HIV-1 particles were used in order to get a comparable number of infected cells for all proviral constructs (i.e., to circumvent the Nef-dependent effects on HIV-1 infectivity) and to analyze env-defective HIV-1 infection. The DsRed2 protein was used for initial HIV-1 infection, because it is active as a tetramer and matures less rapidly than eGFP. Several other red fluorescent proteins, including a monomeric form (12), were also tested, but either their fluorescence was too low or they could not be detected by FACS analysis (data not shown). Thus, despite some limitations, such as relatively low fluorescence intensity and slow maturation kinetics, DsRed2 proved to be most useful for our studies. Notably, the target Jurkat T cells were not overinfected, and most of them would be expected to harbor just a single or a few integrated proviruses. Three days posttransduction, the Jurkat cells harboring the different NL4-3 DsRed2 proviruses were challenged with the eGFP-expressing wild-type X4-tropic HIV-1 NL4-3 reporter virus, and the percentages of superinfected cells were determined by flow cytometric analysis 2 days later (experimental outline given in Fig. 5).
FIG. 5.
Schematic representation of the system to measure HIV-1 superinfection. Jurkat T cells transduced with VSV-G-pseudotyped HIV-1 vpu, env, or nef mutants expressing the DsRed2 reporter gene were challenged with wild-type HIV-1 NL4-3 expressing eGFP. Superinfected T cells were detected by two-color flow cytometric analysis 2 days later.
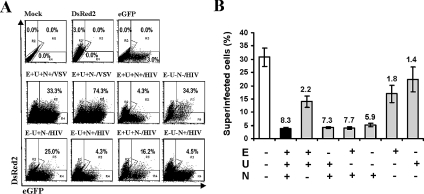
As shown in Fig. 6A (upper row), about 3% of DsRed2+ HIV-1-infected cells were detected at 5 days posttransduction. Inoculation with high doses of the HIV-1 NL4-3 IRES-eGFP reporter virus containing the wild-type X4-tropic gp120 protein also resulted in infection of about 3.0% of the cells. The HIV-1 IRES-DsRed2 construct containing intact vpu, env, and nef genes efficiently protected infected T cells against superinfection by wild-type HIV-1 particles but not against VSV-G pseudotyped virions (Fig. 6A, middle row). Consistent with its critical role in CD4 down-modulation, Nef played the most prominent part in mediating superinfection resistance (Fig. 6A, lower row). Quantitative analysis revealed that an intact nef gene reduced the numbers of double-infected DsRed2+/eGFP+ cells about six- to eightfold even in the absence of intact vpu and env genes (Fig. 6B). In comparison, Vpu and/or Env expression reduced superinfection only about twofold (Fig. 6B). Moreover, the effects of Vpu and Env were evident only with Nef-defective HIV-1 infection.
FIG. 6.
Contribution of Vpu, Env, and Nef to HIV-1 resistance against superinfection. (A) Jurkat T cells were infected with HIV-1 NL4-3 constructs expressing DsRed2 or eGFP via an IRES element from a bicistronic RNA also encoding Nef. Infected cells are characterized by their red or green fluorescence (upper panels, DsRed2 and eGFP). The percentages of superinfected cells indicated in the middle and lower panels were calculated by dividing the number of double-positive cells in gate R3 by the number of cells infected with the VSV-G-pseudotyped HIV-1 IRES-DsRed2 mutants (gate R2) plus those located in gate R3. All Jurkat T cells infected with DsRed2-expressing viruses were challenged with the same dose of HIV-1 NL4-3 IRES-eGFP, either pseudotyped with the VSV G glycoprotein (VSV) or containing the original NL4-3 gp120 Env protein (HIV). The results were confirmed in three to four independent experiments. (B) Percentages of double-positive Jurkat T cells infected with the indicated HIV-1 NL4-3 IRES-DsRed2 Vpu, Env and Nef mutants after challenge with HIV-1 NL4-3 IRES-eGFP. The results are average values (± SD) obtained from triplicate infections. The numbers indicate reductions (n-fold) of the percentages of double-positive cells compared to the control HIV-1 NL4-3 IRES-DsRed2 virus containing combined defects in vpu, env, and nef. Mock, mock infected; E, env; U, vpu; N, nef.
To further assess the role of Nef-mediated CD4 down-modulation in preventing viral superinfection, we analyzed 10 additional HIV-1 nef alleles known to differ in their effects on CD4 expression. This panel comprised mutants of the highly active and well-characterized NA7 Nef (26, 27, 34, 58) containing changes reducing (82A, 105A) or disrupting (174AA) its effects on CD4 surface expression; a primary nef allele (LTNP4-1) from a long-term survivor of HIV-1 infection (LTNP4) that does not efficiently down-modulate CD4 (28, 46) and a mutant nef thereof (LTNP4-8) containing amino acid changes of D56A and K174E, which partly restore its ability to down-modulate CD4 (46); and nef genes obtained from an HIV-1-infected individual with rapidly progressing disease prior to (P2-87) and after (P2-93) development of AIDS (14, 36, 58). Finally, NPex and Pex represent the consensus sequences derived from a large number of HIV-1 subtype B sequences and differed only by changes of T15A, K39R, N51T, H102Y, N157T, S163C, S169N, L170Q and E182M, previously shown to be associated with differential rates of disease progression (14, 36). The NPex and Pex nef alleles were both highly active in CD4 down-modulation (data not shown), most likely because these consensus Nefs contain the “optimal” amino acid at most positions.
In previous studies, these HIV-1 nef alleles were functionally characterized using Nef expression constructs (14, 26, 27, 46, 58). Thus, we first confirmed that they also show differential ability to down-modulate the CD4 receptor in the context of proviral HIV-1 constructs. Flow cytometric analysis showed that the NL4-3, NA7, P2-93, and RPex nef alleles were most effective in down-modulating CD4; the NA7 82A, 105A, P2-87, and NPex nef genes were slightly less active; and the NA7 174AA and LTNP4-1 Nefs were severely impaired for this function (Fig. 7A and data not shown). All these nef alleles protected infected T cells against superinfection, albeit with differential levels of efficiency (Fig. 7B). The NL4-3, NA7, NA7 82A, NA7 105A, P2-87, P2-93, NPex, and RPex Nefs were highly effective and reduced the percentages of superinfected Jurkat T cells about 10-fold (Fig. 7B). Compared to wild-type NA7 Nef, the 82A and 105A Nefs showed slightly diminished protective activity. Similarly, the P2-87 Nef, obtained during the chronic phase of HIV-1 infection, was slightly less effective than the P2-93 Nef obtained after progression to AIDS (Fig. 7B). In comparison, the expression of the NA7 174AA, LTNP4-1, and LTNP4-8 Nefs resulted in only three- to fourfold reduced percentages of superinfected cells. Notably, Jurkat T cells infected with HIV-1 expressing the primary nef allele derived from LTNP4 became superinfected about three times more frequently than those expressing nef genes derived from the progressing individual P2 (Fig. 7B).
FIG. 7.
Effect of HIV-1 nef alleles with differential ability to down-modulate CD4 for superinfection resistance. (A) Down-modulation of CD4 (upper panel) and percentages of superinfected Jurkat cells expressing the indicated HIV-1 nef alleles (lower panel). The numbers in the upper panel are the rMFIs, and those in the lower panel are the percentages of superinfected cells. The controls show uninfected cells (upper panel) and Jurkat T cells (lower panel) that were transduced only with the HIV-1 NL4-3 IRES-DsRed2 construct. (B) Percentages of T cells infected with the indicated HIV-1 nef variants which became superinfected. The numbers are average values (± SD) from three independent experiments. LTNP, long-term nonprogressor; Pr, progressor of HIV-1 infection; Cons., consensus nef alleles. The numbers give the reduction of superinfection (n-fold) compared to the nef-defective control virus. (C) Correlation between CD4 down-modulation and superinfection. The open symbol indicates the percentage of cells infected with nef-defective HIV-1 that became superinfected. The inset shows the correlation between superinfection and CD4 down-modulation for the HIV-1 recombinants containing intact nef genes. CD4 down-modulation was calculated for Jurkat T cells expressing medium levels of eGFP as described previously (14, 48).
The LTNP4-1 and NA7 174AA Nefs were essentially inactive in down-modulating CD4 (Fig. 7A) but still exerted significant protective effects against superinfection (Fig. 7B). Taken together, our data suggest that Nef may reduce the frequency of HIV-1 superinfection about threefold, independently of its effect on CD4 (Fig. 7C). The ability of Nef to inhibit superinfection even further, however, correlated significantly with its ability to down-modulate CD4 (Fig. 7C, inset). It has recently been proposed that Nef may protect cells against superinfection by down-modulating both CD4 and the coreceptor R5 (47). Since we utilized the X4-tropic HIV-1 NL4-3 molecular clone, modulation of R5 should not affect superinfection resistance in our assays. It has been established, however, that Nef also down-modulates X4, although SIV and HIV-2 Nefs are usually more effective than those of HIV-1 (32). Taking advantage of the fact that nef alleles have differential effects on CD4 and X4, we examined whether diminished levels of coreceptor expression may explain the CD4-independent effects on superinfection by X4-tropic HIV-1. As expected from previous studies (26, 27, 32), wild-type NA7 Nef efficiently down-modulated CD4 but had only marginal effects on X4 (Fig. 8A, panel 3). Mutation of 174AA disrupted both of these Nef activities (Fig. 8A, panel 4). The SIVmac239 Nef effectively down-modulated both CD4 and X4 (Fig. 8A and B). In contrast, the highly divergent SIVcol Nef (AF301156) had no significant effect on CD4 surface expression but down-modulated X4 with high efficiency (Fig. 8A and B). Consistent with our previous results (Fig. 7), the NA7 Nef reduced the frequency of superinfected cells about 12-fold and the 174AA mutant Nef about 3-fold (Fig. 8C). The 174AA Nef has only marginal effects on CD4 and is inactive in down-modulating X4 (Fig. 8A, B). Therefore, our data suggest that Nef-mediated protection against HIV-1 superinfection is partly mediated by an as-yet-unknown mechanism that is independent of CD4 and coreceptor down-modulation. However, the expression of the SIVcol Nef effectively protected HIV-1-infected cells against superinfection by X4-tropic HIV-1, although it does not affect CD4 surface expression. Thus, X4 down-modulation by some SIV Nef proteins may efficiently protect infected T cells against infection by X4-tropic lentiviruses. Since several studies have identified dual infections with HIV-1 and HIV-2 (reviewed in references 3, 7, 29, 49, and 60), we also examined whether heterogenous HIV-2 and SIV Nef proteins can protect HIV-1-infected T cells against superinfection. It has been previously shown that the HIV-2 BEN and SIVagm TAN1 Nefs efficiently down-modulate both CD4 and X4 (32, 48; data not shown). In agreement with their ability to down-modulate these receptors, both nef alleles protected HIV-1-infected Jurkat T cells against a second infection (Fig. 8C) suggesting that heterogenous Nef expression would protect T cells infected with HIV-2 or other primate lentiviruses against superinfection by HIV-1.
FIG. 8.
Contribution of X4 down-modulation to lentiviral interference. Flow cytometric analysis (A) and quantitative effect (B) of various nef alleles on CD4 and X4 cell surface expression. Symbols: ▪, NA7; □, 174AA; ⧫, mac239; and ▴, Col Nef. Ranges for green fluorescence on cells defined as expressing no (N), low (L), medium (M), or high (H) levels of eGFP are indicated in panel A. (C) Percentages of Jurkat T cells infected with HIV-1 NL4-3 IRES-DsRed2 constructs expressing the indicated nef alleles and becoming superinfected with HIV-1 NL4-3 IRES-eGFP. Numbers are the reduction of superinfection (n-fold) compared to the nef-defective control virus. Similar results were obtained in an independent experiment.
It has been reported that unintegrated proviral DNA may be perceived by the HIV-1-infected cell as DNA damage and induce apoptosis (20). Based on this observation, it has been speculated that inhibition of superinfection and, hence, the accumulation of unintegrated viral DNA due to the second HIV-1 infection may prevent premature cell death (37), but direct experimental evidence to support this hypothesis is missing. To address this, we examined whether superinfected cells show higher levels of apoptosis than cells infected only once. Jurkat T cells first transduced with VSV-G pseudotyped HIV-1 NL4-3 IRES-DsRed2 were challenged with eGFP reporter virus expressing the wild-type X4-tropic Env protein at 3 days posttransduction and analyzed by flow cytometric analysis 2 days later (Fig. 9A, left). The results demonstrated that Jurkat T cells infected with the DsRed2 reporter virus showed about twofold higher levels of apoptosis than uninfected cells (35.3 ± 0.3% versus 15.9 ± 0.8%, n = 3, P < 0.0001; values give average numbers ± standard deviations [SD]) (Fig. 9A, right). Importantly, superinfected Jurkat T cells showed significantly higher levels of apoptosis (52.0 ± 0.4%; P < 0.0001). These differences were even more pronounced in primary CD4+ T cells (Fig. 9B, left). HIV-1 infection increased the percentages of apoptotic cells about fivefold (4.9 ± 0.9% versus 24.8 ± 4.2% and 29.9 ± 4.2%, respectively, for DsRed2+ and eGFP+) (Fig. 9B, right). Superinfected primary CD4+ T cells showed about threefold-increased levels of programmed death (81.4 ± 3.7%, n = 3, P < 0.0001). These results are direct evidence that inhibition of HIV-1 superinfection protects infected T cells against premature apoptotic death.
FIG. 9.
Superinfected Jurkat T cells show increased levels of apoptosis. (A) Jurkat T cells or (B) primary CD4+ T cells were transduced with nef-defective VSV-G pseudotyped HIV-1 NL4-3 IRES-DsRed2 particles and challenged with wild-type HIV-1 NL4-3 IRES-eGFP viruses 3 days later. Two days postchallenge, the percentages of apoptotic cells in the DsRed2+, eGFP+, and double-positive cells populations were determined by flow cytometric analysis. The right panels give the quantitative analysis of programmed death in single or superinfected Jurkat T cells. Shown are average values (± SD) derived from triplicate (A) infections.
DISCUSSION
Using HIV-1 reporter viruses expressing green or red fluorescent proteins, we demonstrate that CD4 down-modulation protects HIV-1-infected T cells against superinfection and programmed death. Vpu, Env, and Nef contributed to CD4 down-modulation and superinfection immunity, but Nef clearly played the major role. Moreover, we provide evidence suggesting that Nef-mediated superinfection immunity against X4-tropic HIV-1 isolates involves CD4-dependent and CD4-independent mechanisms.
Our result that Nef plays the prominent role in CD4 down-modulation but that Vpu and Env contribute to this effect later during the viral life cycle is in agreement with the data of Chen et al. (17), who used reporter viruses expressing the placental alkaline phosphatase to assess the role of these viral genes in the regulation of CD4. However, some minor discrepancies exist. Unlike the previous study, the present study found that Vpu and Env contribute more significantly to CD4 down-modulation in primary PBMC than in Jurkat T cells. It has also been suggested that CD4 down-modulation is more efficient in transformed Jurkat T cells than in primary human PBMC (17). In comparison, we found that the kinetics of viral gene expression and receptor down-modulation are slightly slower in infected PBMC than in Jurkat T cells. At 2 to 3 days posttransduction, however, the levels of CD4 down-modulation detected in HIV-1-infected PBMC cultures and in Jurkat T cells were comparable (Fig. 2 and 3). In agreement with our data, it has recently been shown that Nef drastically down-modulates CD4 in primary CD4+ T cells (35). Differences in the experimental procedures and/or proviral constructs used may explain the apparent discrepancies, e.g., we expressed Nef from its original genomic location, whereas Chen and coworkers used an IRES element.
It takes about 16 to 24 h after infection until down-modulation of CD4 first becomes noticeable for infected Jurkat T cells (Fig. 2) (37) and even longer for primary human T cells (Fig. 3). The estimated half-life time of HIV-1-infected activated CD4+ T cells, which produce most of the plasma virus in infected individuals, is about 12 to 24 h (15, 51, 52). Because the time period in which CD4 down-modulation confers superinfection resistance before the cells die is very short, whether the effect is physiologically relevant has been questioned (37). However, this temporal regulation may be highly advantageous for the virus. Viral recombination, which requires coinfection of cells with divergent strains, accelerates HIV-1 diversification, thereby helping the virus to escape the host immune response and to become resistant to antiretroviral therapies (reviewed in references 7 and 44). HIV-1 is highly recombinogenic (41), and many examples of recombination between different clades of HIV-1 M, the M and O groups, and different SIV strains have been reported (7, 66). Even the precursor of HIV-1, SIVcpz, most likely resulted from recombination by two SIV strains from smaller monkeys (5). Thus, there are good reasons to believe that co- or superinfections are beneficial for effective viral spread in vivo as long as the divergent HIV-1 strains can complete their entire replication cycle and recombine to generate potentially more fit virus variants. In contrast, superinfection during the later stages of the viral life cycle is presumably highly detrimental for effective viral replication, because it induces premature cell death, thereby further shortening the brief time period of productive viral particle release. The reason HIV-1 utilized three of its gene products to ensure effective down-modulation of CD4 during the late stages of infection is most likely that it serves multiple purposes, such as promoting the effective release of viral particles (8, 56), enhancing the infectivity of progeny virions (4, 18, 38, 40) and, as shown in the present study (Fig. 9), preventing superinfection and hence premature cell death before viral production can be accomplished.
CD4 down-modulation may not have a major impact on the frequency of HIV-1 recombination when the great majority of virally infected cells have a short life span. However, the situation may be different in patients on highly active antiretroviral therapy (HAART), where the virus persists in cell types with longer half-lives, such as macrophages or resting T lymphocytes (62). Nef-mediated CD4 down-modulation is highly effective, and a recent study demonstrated that the HIV-1 Nef protein down-modulates CD4 in resting T lymphocytes (35). Thus, while further studies are clearly required to obtain definitive proof, our current knowledge suggests that CD4 down-modulation may significantly reduce the frequency of superinfection and hence delay the emergence of drug-resistant viruses when HAART shifts the resources of viral replication to long-lived macrophages or T cells.
We and others have previously shown that nef alleles from AIDS patients are frequently particularly active in CD4 down-modulation, whereas those from some HIV-1-infected individuals with nonprogressive infection are inactive in this function (4, 13, 14, 46, 64). Expanding these studies, we now demonstrate that nef alleles from AIDS patients (NA7, P2) are about three- to sevenfold more effective in preventing superinfection than those derived from a LTNP4 (Fig. 7). These differences in Nef function may be advantageous for the virus in a differential host environment. During the late stage of disease, it may be particularly beneficial for the virus to mediate superinfection resistance, because uninfected CD4+ target T cells become limited. In long-term nonprogressors, HIV-1 is usually effectively controlled by the antiviral immune response. Under this scenario, higher frequencies of viral recombination may facilitate the emergence of novel HIV-1 variants and, hence, viral immune evasion.
We demonstrate that even expression of the NA7-174AA or LTNP4-1 nef alleles, which show little if any activity in CD4 down-modulation, reduce superinfection of T cells by X4-tropic HIV-1 about threefold (Fig. 7). It has recently been shown that Nef proteins from diverse groups of primate lentiviruses down-modulate X4 (32), and another study proposed that down-regulation of the R5 coreceptor contributes to protection against R5-tropic HIV-1 superinfection (47). The possibility that some residual effects of the NA7-174AA or LTNP4-1 Nefs on CD4 or X4 surface expression may reduce the susceptibility of HIV-1-infected cells to a secondary infection can certainly not be entirely dismissed. Based on our results, however, we feel that it is more likely that HIV-1 Nef interferes with superinfection by a yet-to-be-identified mechanism that does not involve down-modulation of both X4 and CD4. Several known effects of Nef could potentially affect superinfection resistance, i.e., Nef modifies the composition of the host cell membrane (65, 71), induces changes of the cytoskeleton (11, 22), and alters intracellular trafficking pathways (21, 37).
We have analyzed a large panel of primary HIV-1 nef alleles and found that none of them diminishes surface expression of X4 on both Jurkat T cells and PBMC more than twofold (M. Schindler, F. Kirchhoff, and S. Wildum, unpublished observations). In contrast, some HIV-2 and SIV Nefs down-modulate X4 with high efficiency (32), and the results obtained using the SIVcol Nef, which diminishes X4 but not CD4 surface expression (Fig. 8), clearly show that coreceptor down-modulation can inhibit X4-tropic HIV-1 superinfection. Notably, however, nef alleles that are most effective in X4 down-modulation were derived from primate lentiviruses that do usually not utilize X4 as an entry cofactor (32). Thus, the physiological relevance of X4 down-modulation is obviously not to prevent superinfection but to exert other effects, e.g., inhibition of T-cell migration (32). Following up on the results of a recent report (47), we also attempted to assess the contribution of Nef-mediated R5 down-modulation to HIV-1 superinfection resistance. We confirmed that many nef alleles diminish R5 expression in stably transfected CHO cells overexpressing this receptor. However, in agreement with the findings reported by Michel and coworkers (47), we found that R5 surface expression levels are only marginally reduced in a variety of HIV-1-infected indicator cell lines or in human PBMC (0 to 25%; data not shown). Thus, further studies are required to clarify whether the rather weak effects of HIV-1 Nef on both coreceptors contribute to superinfection resistance of HIV-1-infected primary cells.
Accumulating evidence suggests that coinfections of cells with different HIV-1 strains may be common in vivo and that genetic recombination is a major force driving HIV-1 diversification and escape from HAART or host immunity (7, 44). As long as HIV-1 replicates mainly in short-lived activated T cells, superinfection immunity due to CD4 down-modulation will, presumably, hardly reduce recombination frequency. Our finding that superinfected T cells show approximately two- to threefold increased levels of apoptosis suggests that this function may significantly prolong the time period of virus particle release and, hence, the efficiency of viral replication. Further studies of HIV-1-infected primary T cells and macrophages seem to be required to better understand the physiological role of virus-induced CD4 down-modulation.
Acknowledgments
We thank Thomas Mertens for support, Nicola Bailer and Daniela Krnavek for excellent technical assistance, Ingrid Bennett for critical readings of the manuscript, Ulrich Schubert for Vpu antibody, and Martine Peeters and Valerie Courgnaud for providing the SIVcol nef gene.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) and the Wilhelm-Sander-Stiftung.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., and M. Altfeld. 2003. HIV-1 superinfection. J. Allergy Clin. Immunol. 112:829-835. [DOI] [PubMed] [Google Scholar]
- 4.Argañaraz, E. R., M. Schindler, F. Kirchhoff, and J. Lama. 2003. Enhanced CD4 down-modulation by late-stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 36:33912-33919. [DOI] [PubMed] [Google Scholar]
- 5.Bailes, E., F. Gao, F. Bibollet-Ruche, V. Courgnaud, M. Peeters, P. A. Marx, B. H. Hahn, and P. M. Sharp. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. [DOI] [PubMed] [Google Scholar]
- 6.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 177:1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackard, J. T., D. E. Cohen, and K. H. Mayer. 2002. Human immunodeficiency virus superinfection and recombination: current state of knowledge and potential clinical consequences. Clin. Infect. Dis. 34:1108-1114. [DOI] [PubMed] [Google Scholar]
- 8.Bour, S., C. Perrin, and K. Strebel. 1999. Cell surface CD4 inhibits HIV-1 particle release by interfering with Vpu activity. J. Biol. Chem. 274:33800-33806. [DOI] [PubMed] [Google Scholar]
- 9.Brenner, M., J. Münch, M. Schindler, S. Wildum, N. Stolte, C. Stahl-Hennig, D. Fuchs, K. Mätz-Rensing, M. Franz, J. Heeney, P. Ten Haaft, T. Swigut, K. Hrecka, J. Skowronski, and F. Kirchhoff. 2006. Importance of the N-distal AP-2 binding element in Nef for simian immunodeficiency virus replication and pathogenicity in rhesus macaques. J. Virol. 80:4469-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 78:5745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carl, S., R. Daniels, A. J. Iafrate, P. Easterbrook, T. C. Greenough, J. Skowronski, and F. Kirchhoff. 2000. Partial “repair” of defective NEF genes in a long-term nonprogressor with human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:132-140. [DOI] [PubMed] [Google Scholar]
- 14.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavert, W., D. W. Notermans, K. Staskus, S. W. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, Z. Q. Zhang, R. Mills, H. McDade, J. Goudsmit, S. A. Danner, and A. T. Haase. 1997. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 276:960-964. [DOI] [PubMed] [Google Scholar]
- 16.Chazal, N., G. Singer, C. Aiken, M. L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, B. K., R. T. Gandhi, and D. Baltimore. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J. Virol. 70:6044-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes, M. J., F. Wong-Staal, and J. Lama. 2002. Cell surface CD4 interferes with the infectivity of HIV-1 particles released from T cells. J. Biol. Chem. 277:1770-1779. [DOI] [PubMed] [Google Scholar]
- 19.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel, R., R. A. Katz, and A. M. Skalka. 1999. A role for DNA-PK in retroviral DNA integration. Science 284:644-647. [DOI] [PubMed] [Google Scholar]
- 21.Doms, R. D., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 22.Fackler, O. T., W. Luo, M. Geyer, A. S. Alberts, and P. M. Peterlin. 1999. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3:729-739. [DOI] [PubMed] [Google Scholar]
- 23.Federico, M., F. Nappi, R. Bona, P. D'Aloja, P. Verani, and G. B. Rossi. 1995. Full expression of transfected nonproducer interfering HIV-1 proviral DNA abrogates susceptibility of human He-La CD4+ cells to HIV. Virology 206:76-84. [DOI] [PubMed] [Google Scholar]
- 24.Geleziunas, R., S. Bour, and M. A. Wainberg. 1994. Cell surface down-modulation of CD4 after infection by HIV-1. FASEB J. 8:593-600. [DOI] [PubMed] [Google Scholar]
- 25.Glushakova, S., J. Münch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg, M. E., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8:1239-1242. [DOI] [PubMed] [Google Scholar]
- 28.Greenough, T. C., M. Somasundaran, D. B. Brettler, R. M. Hesselton, A. Alimenti, F. Kirchhoff, D. Panicali, and J. L. Sullivan. 1994. Normal immune function and inability to isolate virus in culture in an individual with long-term human immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 10:395-403. [DOI] [PubMed] [Google Scholar]
- 29.Gross, K. L., T. C. Porco, and R. M. Grant. 2004. HIV-1 superinfection and viral diversity. AIDS 18:1513-1520. [DOI] [PubMed] [Google Scholar]
- 30.Hout, D. R., E. R. Mulcahy, E. Pacyniak, L. M. Gomez, M. L. Gomez, and E. B. Stephens. 2004. Vpu: a multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1. Curr. HIV Res. 2:255-270. [DOI] [PubMed] [Google Scholar]
- 31.Hout, D. R., M. L. Gomez, E. Pacyniak, L. M. Gomez, S. H. Inbody, E. R. Mulcahy, N. Culley, D. M. Pinson, M. F. Powers, S. W. Wong, and E. B. Stephens. 2005. Scrambling of the amino acids within the transmembrane domain of Vpu results in a simian-human immunodeficiency virus (SHIVTM) that is less pathogenic for pig-tailed macaques. Virology 339:56-69. [DOI] [PubMed] [Google Scholar]
- 32.Hrecka, K., T. Swigut, M. Schindler, F. Kirchhoff, and J. Skowronski. 2005. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to SDF-1 chemokine. J. Virol. 79:10650-10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iafrate, A. J., S. Carl, S. Bronson, C. Stahl-Hennig, T. Swigut, J. Skowronski, and F. Kirchhoff. 2000. Disrupting surfaces of Nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol. 74:9836-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iafrate, A. J., S. Bronson, and J. Skowronski. 1997. Separable functions of Nef disrupt two aspects of T-cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 16:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keppler, O. T., N. Tibroni, S. Venzke, S. Rauch, and O. T. Fackler. 2006. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol. 79:616-627. [DOI] [PubMed] [Google Scholar]
- 36.Kirchhoff, F., P. J. Easterbrook, N. Douglas, M. Troop, T. C. Greenough, J. Weber, S. Carl, J. L. Sullivan, and R. S. Daniels. 1999. Sequence variations in human immunodeficiency virus type 1 Nef are associated with different stages of disease. J. Virol. 73:5497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lama, J. 2003. The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr. HIV Res. 1:167-184. [DOI] [PubMed] [Google Scholar]
- 38.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 39.LeGuern, M., and J. Levy. 1992. Human immunodeficiency virus (HIV) type 1 can superinfect HIV-2-infected cells: pseudotype virions produced with expanded cellular host range. Proc. Natl. Acad. Sci. USA 89:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levesque, K., Y. S. Zhao, and E. A. Cohen. 2003. Vpu exerts a positive effect on HIV-1 infectivity by down-modulating CD4 receptor molecules at the surface of HIV-1-producing cells. J. Biol. Chem. 278:28346-28353. [DOI] [PubMed] [Google Scholar]
- 41.Levy, D. N., G. M. Aldrovandi, O. Kutsch, and G. M. Shaw. 2004. Dynamics of HIV-1 recombination in its natural target cells. Proc. Natl. Acad. Sci. USA 101:4204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Little, S. J., N. L. Riggs, M. Y. Chowers, N. J. Fitch, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1994. Cell surface CD4 downregulation and resistance to superinfection induced by a defective provirus of HIV-1. Virology 205:578-582. [DOI] [PubMed] [Google Scholar]
- 43.Lundquist, C. A., M. Tobiume, J. Zhou, D. Unutmaz, and C. Aiken. 2002. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J. Virol. 76:4625-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malim, M. H., and M. Emerman. 2001. HIV-1 sequence variation: drift, shift and attenuation. Cell 104:469-472. [DOI] [PubMed] [Google Scholar]
- 45.Mangasarian, A., M. Foti, C. Aiken, D. Chin, J.-L. Carpentier, and D. Trono. 1997. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity 6:67-77. [DOI] [PubMed] [Google Scholar]
- 46.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michel, N., I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 15:714-723. [DOI] [PubMed] [Google Scholar]
- 48.Munch, J., M. Schindler, S. Wildum, E. Rucker, N. Bailer, V. Knoop, F. J. Novembre, and F. Kirchhoff. 2005. Primary sooty mangabey simian immunodeficiency virus and human immunodeficiency virus type 2 nef alleles modulate cell surface expression of various human receptors and enhance viral infectivity and replication. J. Virol. 79:10547-10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nethe, M., B. Berkhout, and A. C. van der Kuyl. 2005. Retroviral superinfection resistance. Retrovirology 2:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel, P. G., M. T. Yu Kimata, J. E. Biggins, J. M. Wilson, and J. T. Kimata. 2002. Highly pathogenic simian immunodeficiency virus mne variants that emerge during the course of infection evolve enhanced infectivity and the ability to downregulate CD4 but not class I major histocompatibility complex antigens. J. Virol. 76:6425-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 52.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 53.Pham, H. M., E. R. Arganaraz, B. Groschel, D. Trono, and J. Lama. 2004. Lentiviral vectors interfering with virus-induced CD4 down-modulation potently block human immunodeficiency virus type 1 replication in primary lymphocytes. J. Virol. 78:13072-13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piguet, V., Y.-L. Chen, A. Mangasarian, M. Foti, J.-L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the μ chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 56.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 57.Rucker, E., J. Munch, J. C. Grivel, F. Kirchhoff, and L. Margolis. 2004. Vpr and Vpu are important for efficient human immunodeficiency virus type 1 replication and CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 78:12689-12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schindler, M., S. Wuerfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Münch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 77:10548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schindler, M., J. Münch, and F. Kirchhoff. 2005. HIV-1 inhibits DNA damage triggered apoptosis by a Nef-independent mechanism. J. Virol. 79:5489-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith, D. M., D. D. Richman, and S. J. Little. 2005. HIV superinfection. J. Infect. Dis. 192:438-444. [DOI] [PubMed] [Google Scholar]
- 61.Stephens, E. B., S. V. Joag, D. Sheffer, Z. Q. Liu, L. Zhao, S. Mukherjee, L. Foresman, I. Adany, Z. Li, D. Pinson, and O. Narayan. 1996. Initial characterization of viral sequences from a SHIV-inoculated pig-tailed macaque that developed AIDS. J. Med. Primatol. 25:175-185. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson, M. 2003. HIV-1 pathogenesis. Nat. Med. 9:853-860. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz, O., V. Marechal, O. Danos, and J. M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tobiume, M., M. Takahoko, T. Yamada, M. Tatsumi, A. Iwamoto, and M. Matsuda. 2002. Inefficient enhancement of viral infectivity and CD4 downregulation by human immunodeficiency virus type 1 Nef from Japanese long-term nonprogressors. J. Virol. 76:5959-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van't Wout, A. B., J. V. Swain, M. Schindler, U. Rao, M. S. Pathmajeyan, J. I. Mullins, and F. Kirchhoff. 2005. Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells. J. Virol. 79:10053-10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wain-Hobson, S., C. Renoux-Elbe, J. P. Vartanian, and A. Meyerhans. 2003. Network analysis of human and simian immunodeficiency virus sequence sets reveals massive recombination resulting in shorter pathways. J. Gen. Virol. 84:885-895. [DOI] [PubMed] [Google Scholar]
- 67.Wei, B., V. K. Arora, J. L. Foster, D. L. Sodora, and J. V. Garcia. 2003. In vivo analysis of Nef function. Curr. HIV Res. 1:41-50. [DOI] [PubMed] [Google Scholar]
- 68.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76:263-274. [DOI] [PubMed] [Google Scholar]
- 69.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J. Virol. 66:226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng, Y. H., A. Plemenitas, C. J. Fielding, and B. M. Peterlin. 2003. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA 100:8460-8465. [DOI] [PMC free article] [PubMed] [Google Scholar]