Abstract
Entry of herpes simplex virus into cells occurs by fusion and requires four glycoproteins. gD serves as the receptor binding glycoprotein. Of the remaining glycoproteins, gH carries structural and functional elements typical of class 1 fusion glycoproteins, in particular α-helix 1 (α-H1), with properties of a candidate fusion peptide, and two heptad repeats. Here, we characterized α-H2 and compared it to α-H1. α-H2 (amino acids 513 to 531) is of lower hydrophobicity than α-H1. Its deletion or mutation decreased virus infection and cell fusion. Its replacement with heterologous fusion peptides did not rescue infection and cell fusion beyond the levels exhibited by the α-H2-deleted gH. This contrasts with α-H1, which cannot be deleted and can be functionally replaced with heterologous fusion peptides (T. Gianni et al., J. Virol. 79:2931-2940, 2005). Synthetic peptides mimicking α-H1 and α-H2 induced fusion of nude lipid vesicles. Importantly, they increased infection of herpes simplex virus, pseudorabies virus, bovine herpesvirus 1, and vesicular stomatitis virus. The α-H1 mimetic peptide was more effective than the α-H2 peptide. Consistent with the findings that gH carries membrane-interacting segments, a soluble form of gH, but not of gD or gB, partitioned with lipid vesicles. Current findings highlight that α-H2 is an important albeit nonessential region for virus entry and fusion. α-H1 and α-H2 share the ability to target the membrane lipids; they contribute to virus entry and fusion, possibly by destabilizing the membranes. However, α-H2 differs from α-H1 in that it is of lower hydrophobicity and cannot be replaced with heterologous fusion peptides.
Viruses that enter cells by fusion with cell membranes carry fusion glycoproteins with distinctive features (13, 31, 34). A key element is the fusion peptide, a hydrophobic segment located N terminally in cleaved glycoproteins or internally in uncleaved glycoproteins. The sequential steps that lead to fusion execution include extensive refolding of the fusion glycoprotein, the exposure and relocation of the fusion peptide, and its insertion in the target cell membrane; this generates an intermediate simultaneously anchored to the virion envelope and the cell membrane. The insertion of the fusion peptide in cell membranes is thought to facilitate fusion through a destabilizing effect on the lipids. In class 1 fusion glycoproteins, a key step in the refolding process is the formation of a coiled coil between trimers of heptad repeat 1 (HR-1) and HR-2—also called a six-helix bundle, or hairpin. This relocates the fusion peptide and the transmembrane domain to the same end of the coiled coil, such that the viral and the cellular membranes are brought in close juxtaposition and fusion starts.
Recently, it has become evident that the ectodomain of certain class 1 fusion glycoproteins, including human immunodeficiency virus (HIV) gp41 and glycoproteins from some paramyxoviruses, exhibits additional elements that add to their complexities. HIV gp41 carries a membrane-proximal pre-transmembrane domain, whereas the Sendai virus and other paramyxovirus fusion glycoproteins carry a hydrophobic segment downstream of HR-1, named internal fusion peptide, or HR-3 (14, 15, 24, 41, 52). The properties of these elements have been investigated mainly by means of mimetic peptides which, in most cases, induce fusion of model lipid membranes (16, 24, 52).
The molecular mechanism of entry of herpesviruses into the cell differs from that of well-known viruses, in that it requires a multicomponent fusion system. Herpes simplex virus (HSV), the paradigm of viruses encoding a multicomponent fusion machinery, enters target cells by fusion of the envelope with either the plasma membrane or the endocytic vesicle. Four viral glycoproteins—gD, gB, gH, and gL—are absolutely required for virus entry and cell-cell fusion (6, 9, 18, 35, 47, 51, 53). The role of gD is twofold. It serves as the receptor-binding glycoprotein able to interact with two alternative receptors: nectin1 and herpesvirus entry mediator (11, 23, 40). It also serves as the trigger of fusion. The current model envisions that gD action is exerted through conformational changes (10). The unliganded gD adopts a closed conformation where the ectodomain C terminus, which carries the profusion domain, folds back towards the N terminus. At receptor binding, gD adopts an opened conformation, where the profusion domain is displaced from its binding site on the N terminus and the latter becomes part of the receptor binding site (20, 33).
The role of the highly conserved gH, gL, and gB, which altogether are responsible for carrying out fusion in the Herpesviridae family, has remained elusive until recently, when structural and functional elements typical of fusion glycoproteins were identified in gH, in particular an α-helix (α-H1) and two HRs (Fig. 1) (21, 22, 25-27). The essential α-H1 is located between amino acid (aa) residues 377 and 397 and is positionally conserved in all the gH alleles examined, and in HSV-2 gH (25), α-H1 is located in an antigenic loop made of cysteines 2 and 4 (7, 8). It is highly hydrophobic, and when transplanted at the C terminus of a soluble glycoprotein (gD1-260t), it renders the soluble gD membrane bound. Its replacement with heterologous fusion peptides partially rescues infection and fusion of the α-H1-deleted gH. Because of these properties, it has been proposed that α-H1 be considered the candidate fusion peptide. gH HR-1 and HR-2 are located downstream of the candidate fusion peptide and upstream of the transmembrane segment, respectively (Fig. 1) (26, 27). Their properties are as follows. Replacement of 2 aa in HR-1, predicted to abolish coiled coil formation, abolishes infection and cell-cell fusion. Replacement of 3 aa in HR-2, predicted to increase the coiled coil propensity, increases infection and cell-cell fusion. Synthetic peptides mimicking HR-1 and HR-2 inhibit virus infection and form a stable complex which exhibits a higher α-helix content than those of the two single peptides, suggesting that HR-1 and -2 may form a six-helix bundle (22, 27). Functional HRs are present in gH of human cytomegalovirus and Epstein-Barr virus (36, 43).
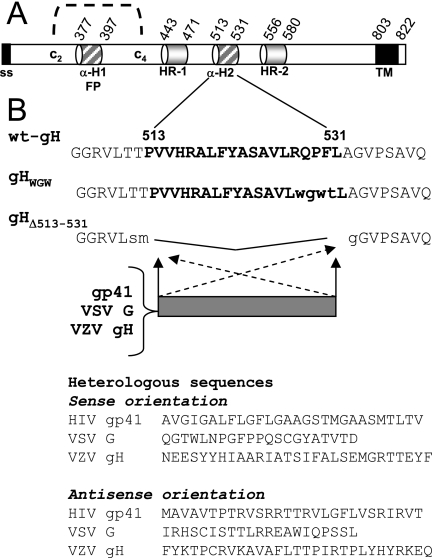
FIG. 1.
(A) Linear map of HSV gH showing the amino acid positions of α-H1 (i.e., candidate fusion peptide; FP), α-H2, HR-1 and HR-2, signal sequence (SS), and the transmembrane (TM) domain. C2 and C4, cysteines 2 and 4. (B) Amino acid sequence of α-H2 and adjacent sequences in wt-gH, in gHWGW, and in α-H2-deleted gH (gHΔ513-531). Heterologous sequences show the amino acid sequences of the fusion peptides of HIV gp41, VSV G, and the putative fusion peptide of VZV gH or the amino acid sequences obtained by cloning the DNA fragments in the antisense orientation. The hererologous sequences were cloned in gHΔ513-531, in the sense or antisense orientation, thus generating the plasmids gH-αH2VSVG, gH-αH2AS-G, gH-αH2gp41, gH-αH2AS-gp41, gH-αH2VZV, and gH-αH2AS-VZV.
In addition to α-H1, gH carries an α-H2 located between aa 513 and 531 (25) (Fig. 1). α-H2 exhibits a weaker predicted interaction with membranes than α-H1, and when transplanted at the C terminus of gD1-260t, it confers to gD1-260t only partial ability to become membrane bound. Synthetic peptides that include a portion of α-H1 or the entire α-H2 interact with lipid membranes, induce their fusion, and also adopt an α-helical conformation in buffers of decreasing polarity (21). Inasmuch as flexibility and ability to adopt different secondary structures are key properties of fusion peptides (34), these findings further support the contention that α-H1 may serve as the fusion peptide and raised the question of the functional significance of α-H2 in virus infection and cell fusion. An additional sequence of interest, located at gH aa 626 to 644, was predicted by its tendency to partition at the membrane interface. A peptide mimicking this sequence induces fusion of nude lipid vesicles with high efficiency and adopts an α-helical conformation in buffers of decreasing polarity (21). This sequence remains to be characterized in the context of gH and with respect to virus infection and fusion.
gL is a soluble glycoprotein that forms a heterodimer with gH (29) and is required in order for gH to adopt its correct conformation and to be transported from endoplasmic reticulum to the plasma membrane. It carries neutralizing epitopes (54). The significance of gB in fusion execution is unclear at the moment. Numerous lines of evidence indicate that gB is absolutely required for HSV to enter cells and for fusion to occur (6, 53). gB plays a role in virus attachment to the cell surface heparan sulfate (28). It also binds cells devoid of heparan sulfate in a saturable manner. A soluble form inhibits infection, suggesting that it may interact with a receptor (4).
The objective of this study was to characterize α-H2 and compare its ability to interact with membranes with that of α-H1. Deletion, mutational analysis, and substitution of α-H2 indicate that this is an important but nonessential domain. Synthetic peptides mimicking α-H1 and α-H2 not only induce fusion of nude lipid vesicles but also enhance virus entry in a species-unspecific manner as well as cell-cell fusion.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney (BHK), COS, Madin-Darby bovine kidney, F6, and J-nectin 1 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% to 10% fetal calf serum. F6 cells are a stably transformed Vero cell line that expresses HSV-1 gH under the control of the HSV-1 gD promoter (18). The J1.1-2 cell line (J cells), a derivative of BHKtk− cells with high resistance to HSV infection, and their derivative line, J-nectin 1, stably expressing human nectin1, were described previously (12). In the gH deletion mutant (ΔgH HSV) SCgHZ, the gH gene was replaced with a lacZ gene. SCgHZ was grown and titrated in the complementing F6 cells (18). R8102, a recombinant HSV-1 strain expressing lacZ under control of the α27 promoter, psuedorabies virus (PrV), and the bovine herpesvirus type 1 (BoHV-1) recombinants carrying LacZ were described previously (3, 19, 38). The vesicular stomatitis virus (VSV), New Jersey serotype, was obtained from the American Type Culture Collection.
Plasmids.
Expression plasmids for HSV-1 gD, gB, gH, and gL were described previously (2). EGFR2Δ (named Erb-2) carries the extracellular domain and transmembrane sequences of rat HER-2/neu (nucleotides 25 to 2096) (GenBank accession no. NM_017003) and has had the tyrosine kinase domain deleted (48). Plasmid pCAGT7 contains the T7 RNA polymerase gene under control of the CAG promoter, and the pT7EMCLuc plasmid expresses the firefly luciferase under the T7 promoter (42, 46). Plasmid pcDNA 3.1(−) Myc-His/Lac vector (Invitrogen, Italy) constitutively expresses β-galactosidase (β-Gal).
Constructs.
gHWGW, carrying the RQPF527-530WGWT substitutions, was derived by site-directed mutagenesis with oligonucleotide 5′-CGCCTCGGCTGTCCTCTGGGGGTGGTACCTGGCTGGCGTCCCCTCGGCG-3′. For the deletion of the sequence from aa 513 to 531, an SphI site was first introduced at aa 512 in pMTS-gH, by means of the oligonucleotide 5′-GGGGGCCGCGTGCTGAGCATGCCGGTACCCCACCGGGCGCTATTTTAC-3′, thus generating gH512-SphI. A second SphI site was introduced at aa 532 by means of the oligonucleotide 5′-GGCTGTCCTCCGGCGGCCGCGCATGCCTGGCGTCCCCTCGGCGG-3′. To generate gHΔ513-531, the SphI fragment was then collapsed. The mutagenesis inserted the following substitutions: TT511-512SM and A532G. The gH-αH2VSVG, gH-αH2AS-G, gH-αH2gp41, gH-αH2AS-gp41, gH-αH2VZV, gH-αH2AS-VZV plasmids (carrying the sequences encoding the fusion peptide of HIV gp41, VSV, or putative fusion peptide of varicella zoster virus [VZV] gH in a sense or antisense [AS] orientation) were generated by ligation of the appropriate sequences in gHΔ513-531, as previously detailed (25).
Fusion assay.
The luciferase-based cell-cell fusion assay was performed in COS cells as detailed previously (39, 46), using the luciferase assay system from Promega (Florence, Italy). The total amount of transfected plasmid DNA was made equal by addition of Erb-2 plasmid DNA. All samples were run three times and in triplicates. Subconfluent cultures of BHK cells, grown on glass coverslips in 24-well plates, were transfected with DNA mixtures that contained the expression plasmids for gD, gH, gL, and gB, plus pcDNA 3.1(−) Myc-His/Lac vector (Invitrogen, Milan, Italy), for constitutive expression of β-Gal (80 ng of each plasmid). After incubation at 37° for 24 h in the presence or absence of the indicated synthetic peptides (pep.gH-αH121, pep.gH-αH219, and pep.gD265-289), cells were fixed, and syncytia were detected by staining with 5-bromo-4chloro-3-indolyl-β-galactopyranoside (X-Gal), as described previously (1).
Infectivity complementation assay.
The complementation assay was performed as detailed in reference 10. Briefly, cells in T25 flasks were transfected with the appropriate gH plasmid. The total amount of plasmid DNA transfected per flask was made equal by addition of Erb-2 plasmid DNA. Four hours later, cells were infected with a gH−/+ stock of SCgHZ (7 PFU/cell). Unpenetrated virions were inactivated by washing two times with phosphate-buffered saline (PBS), followed by a 1-min rinse with 40 mM sodium citrate, 10 mM KCl, 135 mM NaCl, pH 3. The monolayers were rinsed twice with PBS and overlaid with medium containing 1% fetal calf serum. Cells were incubated overnight at 37°C, and the progeny virus was titrated in F6 cells.
CELISA and IFA.
Cell enzyme-linked immusorbent assay (CELISA) was performed as described previously (25, 46). For indirect immunofluorescence assay (IFA), COS cells were grown on glass coverslips and transfected with the indicated plasmids by means of Polyfect (QIAGEN, Florence, Italy). After 24 h, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature, followed, when requested, by 0.1% Triton X-100 in PBS. Samples were incubated with the following monoclonal antibodies (MAbs): 52S and 53S to gH (44, 50) and anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC)-conjugated antibody (Jackson Immunoresearch). Samples were observed with a Zeiss microscope, and micrographs were taken with a Kodak DC290 digital camera.
Synthetic peptides.
Lyophilized peptides pep.gH-αH121, pep.gH-αH219, pep.gH-HR125, pep.gH-HR135, pep.gH-HR225, and pep.gD265-289, synthesized by Primm, San Raffaele Biomedical Science Park, Milan, Italy, and by the Peptide Synthesis Facility, University of Wisconsin Biotechnology Center, were dissolved in DMEM without serum at a concentration of 3 mM. The solutions were adjusted to neutral pH by addition of 1 M Tris HCl, pH 10.
Effect of synthetic peptides on virus infection and cell-cell fusion.
The experiments were performed in 96-well plates with extracellular virions of R8102, PrV LacZ, or BoHV-1, at an input multiplicity of infection of 1 PFU/cell. Cells were incubated with increasing concentrations of the peptides and the viral inoculum for 90 min at 37°C. After removal of the inoculum and rinsing of the cells with DMEM containing 1% fetal calf serum, infected cells were incubated for 16 h. Infectivity was measured as β-Gal activity using o-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate (40). The absorbance at 405 nm was read in a Bio-Rad microplate reader. Serial 10-fold dilutions of VSV were allowed to adsorb to Vero cells in the absence or presence of the indicated amounts of peptides. After removal of the inoculum and rinsing of the cells with DMEM containing 1% fetal calf serum, infected cells were incubated for 16 h in the absence of peptides in medium containing methylcellulose. Plaques were scored 24 h later.
Liposome preparation.
Asolectin (l-α-phosphatidylcholine from soybean) (Sigma-Aldrich, Milan, Italy) was dissolved in 3:1 chloroform-methanol and dried under a nitrogen stream to a thin film. The film was hydrated in 140 mM NaCl-10 mM Tris HCl, pH 8.0, vigorously vortexed for 7 min, and subjected to 19 cycles of extrusion through two 200-nm-pore-size polycarbonate membranes, with a Liposofast syringe-type extruder (Avestin, Ottawa, Canada).
Fluorescence measurements.
Fluorescence measurements were performed at 25°C with a Jasco (FP-777) spectrofluorimeter. An aliquot of liposomes was loaded with 3 μM pyrene (Fluka, Milan, Italy) and mixed with nude lipid vesicles. The emission spectra excited at 340 nm was recorded in the 300- to 550-nm range. The fluorescence intensities of the monomer (IM) (390 nm) and excimer (IE) (470 nm) emission peaks were measured. The extent of fusion was expressed as the excimer/monomer fluorescence intensity (IE/IM) ratio (30). The peptide-induced fusion of lipid vesicles was performed by adding nude liposomes to pyrene-loaded liposomes (1:2 [wt/wt]) in the presence or absence of peptides (30 μM for single peptides or 15 μM each for mixtures of two peptides). To quantify the extent of fusion, a replicate lipid vesicle fusion assay was performed in the presence of 8 × 107 PFU of partially purified extracellular HSV-1(F) virions. Fusion induced by peptides was expressed as percentage relative to the fusion induced by virions. Relative to liposomes solubilized with Triton X-100, fusion induced by virions was in the range of 10 to 30%.
Sedimentation analysis of gHt.gL preincubated with liposomes.
The recombinant soluble forms of gHt.gL (gH792t.gL), gBt (gB730Hist), or gDt (gD285t) (5, 32, 45) (generous gift of G. H. Cohen and R. Eisenberg, University of Pennsylvania) were produced by means of baculoviruses, purified by affinity chromatography (49). The negative control consisted of IgG (Sigma-Aldrich, Milan, Italy). Aliquots of 1.5 μg in 15 μl were mixed with 200 μl liposomes (10 mg/ml) in a ratio of 1 mg of protein to 900 to 1,600 μM lipids. The mixtures were incubated for 5 to 10 min at 37°C and layered onto a discontinuous sucrose gradient containing 1 ml of 50% (wt/vol) sucrose, 1.5 ml of 25% sucrose, 1 ml of 15% sucrose, and 0.5 ml of 5% sucrose. Centrifugation was carried out for 22 h at 22,000 rpm at 4°C in a Beckman SW50.1 centrifuge. Four fractions, corresponding to each sucrose concentration, were collected. The amount of lipids in each fraction was measured by a quantitative determination of phosphorus, as described previously (37). The fractions were concentrated to 50 μl by means of Amicon Y10 filters and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were identified by enhanced chemiluminescence with MAb H12 to gH, MAb VIII-62 to gL, MAb H1817 to gB, MAb H170 to gD, and anti-mouse IgG peroxidase.
RESULTS
Deletion and mutational analyses of α-H2.
Figure 1A shows the position of α-H2 in the linear map of gH and its sequence. In order to investigate the properties of α-H2, the sequence was either mutagenized or deleted in an expression vector that contained gH under the immediate-early cytomegalovirus promoter. The substitutions introduced in α-H2 were aimed at decreasing the propensity to form the helix. The resulting mutant was named gHWGW (Fig. 1B). In order to delete α-H2, two SphI restriction sites were inserted at aa 512 and 532 of the gH coding sequence (Fig. 1B). The SphI fragment was then collapsed, and the resulting plasmid was named gHΔ513-531 (Fig. 1B). The two gH mutants were tested in three functional assays: i.e., (i) proper folding and ability to traffic to the cell surface; (ii) ability to induce cell fusion when cotransfected with plasmids encoding gD, gB, and gL; and (iii) ability to complement the infectivity of a gH deletion HSV mutant (infectivity complementation assay).
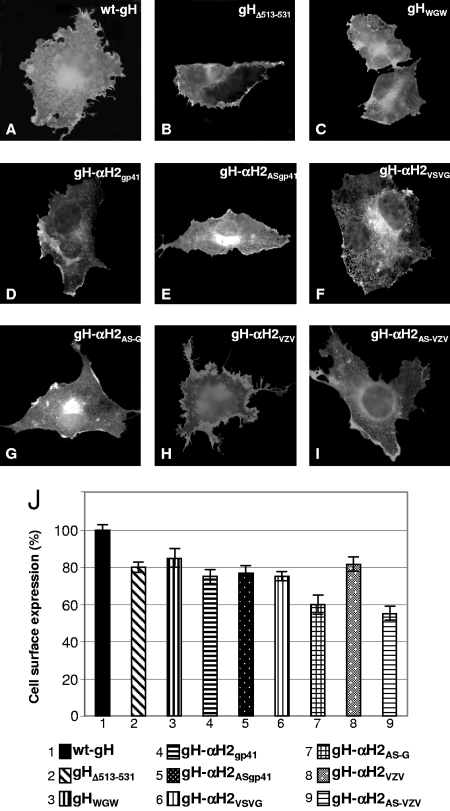
gH dimerizes with gL, in order to achieve proper folding and to be trafficked to the plasma membrane (47). The ability of mutant forms of gH to form a heterodimer with gL and to be trafficked to the plasma membrane was determined by IFA of cells cotransfected with the mutant or wild-type gH (wt-gH) plasmid and a gL expression plasmid. MAb 53S recognizes a discontinuous epitope and strictly requires gL for reactivity. MAb 52S recognizes a gL-independent discontinuous epitope with critical residues at positions 536 to 537 (44, 50). Both gHWGW and gHΔ513-531 maintained the IFA reactivity to MAb 52S, suggesting no major defect in proper folding (data not show). The IFA reactivity of paraformaldehyde-fixed cells to MAb 53S indicates no major defect in trafficking to the plasma membranes and in heterodimer formation with gL (Fig. 2A to C). Cell surface expression, as quantified by CELISA, showed no significant decrease relative to wt-gH (Fig. 2J).
FIG. 2.
Cell surface expression of wt-gH, gHΔ513-531, gHWGW, gH-αH2VSVG, gH-αH2AS-G, gH-αH2gp41, gH-αH2AS-gp41, gH-αH2VZV, and gH-αH2AS-VZV. (A to I) Paraformaldehyde-fixed COS cells transfected with wild-type or mutant gH plasmids and a gL-encoding plasmid and reacted with MAb 53S. The positive reactivity denotes gH-gL heterodimer formation and trafficking to the plasma membrane. (J) CELISA quantification of cell surface expression of gH mutants in COS cells cotransfected with a gL plasmid. Each assay was performed in triplicate. The bars represent the mean percentage relative to wt-gH ± standard deviation.
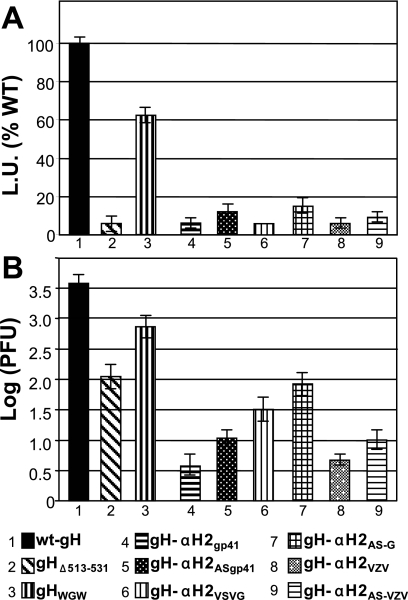
The cell-cell fusion was performed by transfection of effector COS cells with mutant or wt-gH plasmid plus plasmids encoding gD, gB, gL, and the reporter luciferase gene under the T7 promoter. The target cells were transfected with the T7 polymerase. Cells were mixed and allowed to fuse 24 h after transfection. The extent of fusion was quantified by means of the T7 promoter-driven reporter luciferase (46). As shown in Fig. 3 A, gHΔ513-531 exhibited no fusion activity in COS cells; in contrast, gHWGW exhibited 60% activity relative to wt-gH.
FIG. 3.
(A) Cell-cell fusion and (B) infectivity complementation of mutant forms of gH. (A) Cell-cell fusion of COS cells transfected with the gH mutant plasmids plus plasmids encoding gL, gB, and gD. Fusion was quantified by means of a T7 promoter-driven reporter luciferase gene, as luciferase units (L.U.), according to reference 46, and is expressed as a percentage of fusion induced by wt-gH. Each assay was performed in triplicate. The bars represent the mean ± standard deviation. (B) COS cells were transfected with mutant or wt-gH plasmids and superinfected with the gH deletion mutant virus SCgHZ 4 h later. Virus was harvested 24 h after transfection and titrated in F6 cells. The bars represent the mean of triplicates ± standard deviation.
In the infectivity complementation assay, cells were transfected with mutant or wt-gH and superinfected with the gH deletion mutant virus SCgHZ (18). The transgenic gH complements the deletion in the virus, and the complemented virions are or are not infectious, depending on whether gH is or is not functional. The results in Fig. 3B show that virions complemented with gHΔ513-531 exhibited an about 4% infectivity, and virions complemented with gHWGW exhibited a 13% infectivity relative to wt-gH. (Note that the ordinate scales in Fig. 3A and B differ.)
Replacement of α-H2 with heterologous fusion peptides does not rescue virus infection and cell-cell fusion activity relative to α-H2-deleted gH.
Previously, we showed that replacement of α-H1, the candidate fusion peptide with the fusion peptide of HIV gp41 or VSV G partially rescued HSV infectivity and cell-cell fusion (25). The corresponding antisense sequences did not. Here, we asked whether α-H2 could be substituted for with heterologous fusion peptides from HIV gp41, VSV G, or the putative fusion peptide of VZV gH. To this end, the appropriate sequences were cloned into the α-H2-deleted gH (gHΔ513-531) in a sense or antisense direction (Fig. 1B). The resulting constructs were named gH-αH2gp41, gH-αH2AS-gp41, gH-αH2VSVG gH-αH2AS-G, gH-αH2VZV, gH-αH2AS-VZV, respectively. Preliminarily, we ascertained that the chimeric forms of gH were able to form a heterodimer with gL (seen as immunofluorescence reactivity to MAb 53S) and to traffic to the cell surface. The results in Fig. 2D to I indicate that all constructs reacted with MAb 53S and reached the cell surface with only minor decreases (Fig. 2J).
The chimeric gH constructs were assayed for cell-cell fusion activity and for infectivity complementation, as detailed above. The results illustrated in Fig. 3A show that none of the chimeric gH was capable of cell-cell fusion activity. The results of the infectivity complementation assay were essentially similar (Fig. 3B). Surprisingly, the chimeric forms of gH carrying the antisense sequences were somewhat more effective in rescuing infection than the corresponding forms of gH carrying the sense sequences. Altogether, the results of Fig. 3A and B indicate that α-H2 sequence is an important determinant for cell-cell fusion and for HSV infection. Its deletion or replacement of few amino acids drastically reduces both activities; the activity of the α-H2-deleted gH cannot be rescued by heterologous fusion peptides. The properties of α-H2 contrast sharply with those of α-H1, in that deletion or mutation of α-H1 completely abolished infection and fusion (25); the activities were rescued by heterologous fusion peptides.
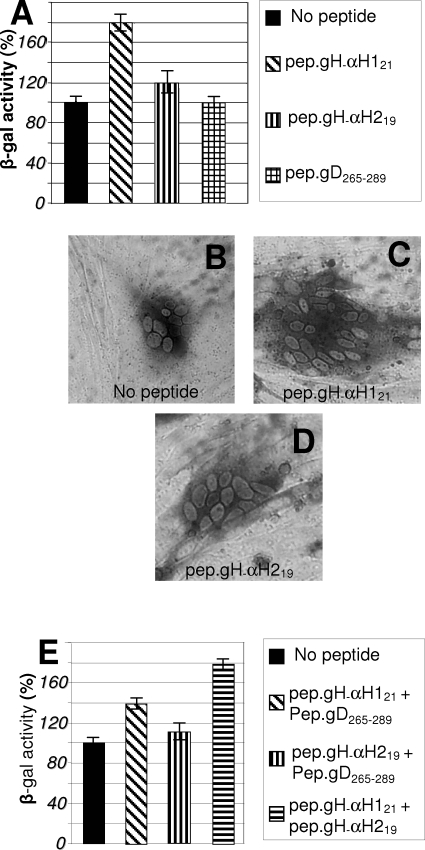
Mimetic peptides with the sequence of α-H2 and α-H1 induce fusion of lipid vesicles.
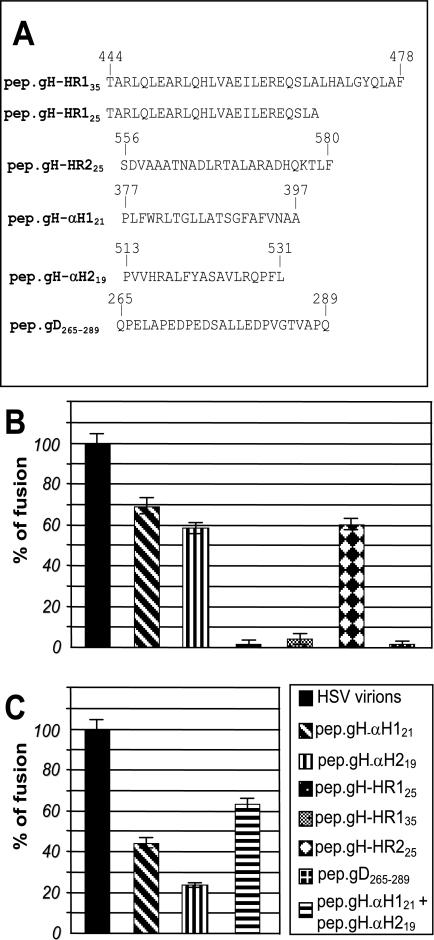
A wealth of studies have made use of synthetic peptides mimicking predicted hydrophobic sequences to characterize their properties: in particular, the ability to interact with lipid vesicles. To investigate further the properties of α-H2 and to compare them to the properties of α-H1, we derived synthetic peptides to the two sequences, named pep.gH-αH219 and pep.gH-αH121 (Fig. 4A), and measured their ability to induce fusion of lipid vesicles. Large unilamellar lipid vesicles were prepared as detailed in Materials and Methods. An aliquot was loaded with pyrene. Lipid vesicles were mixed with pyrene-loaded lipid vesicles in the absence or presence of pep.gH-αH121, pep.gH-αH219, or the unrelated peptide pep.gD265-289 (30 μM each). Lipid mixing was measured as the IE/IM ratio, as detailed in Materials and Methods (Fig. 4B). As a positive control and a quantification of the extent of fusion, we mixed the two populations of lipid vesicles in the presence of partially purified extracellular herpes simplex virions, which caused a readily quantifiable fusion. Fusion induced by the synthetic peptides was expressed as a percentage relative to fusion induced by herpes simplex virions. As can be seen in Fig. 4B, pep.gH-αH121 induced a high extent of fusion; pep.gH-αH219 was somewhat less effective. The effect was specific since the unrelated peptide pep.gD265-289 and synthetic peptides to HR-1 (26) sequences did not exert any fusion activity. The synthetic peptide mimicking gH HR-2 sequence caused fusion of the lipid vesicles, in accordance with previous observation (21). Addition of cholesterol in a 1:1 molar ratio to lipids did not result in increase of the fusion activity of the peptides (data not shown).
FIG. 4.
(A) Sequence and coordinates of synthetic peptides to HSV-1 gH α-H1, α-H2, HR-1, HR-2, and gD. (B and C) Fusion of large unilamellar vesicles induced by mimetic peptides to α-H1 and α-H2, and, as a reference, by herpes simplex virions. Large unilamellar vesicles were mixed with pyrene-loaded vesicles in the presence of herpes simplex virions or the indicated peptides (30 μM) in a 3:1 lipid/peptide ratio. Extent of fusion was measured as decrease of the excimer fluorescence emission (IE) and increase in monomer fluorescence emission (IM) and is expressed as percent IE/IM ratio. Fusion induced by synthetic peptides was expressed as a percentage relative to the fusion induced by herpes simplex virions. (B) Induction of lipid vesicle fusion by single peptides (30 μM). (C) Induction of lipid vesicle fusion by a mixture of peptides mimicking α-H1 and α-H2 (each at 15 μM). All assays were performed in triplicates. The bars represent the mean ± standard deviation.
To measure the effect of the simultaneous presence of pep.gH-αH121 and pep.gH-αH219, the peptides were added to liposomes at lower concentration (15 μM each, rather than 30 μM). As can be seen from Fig. 4C, the mixture of the two peptides exerted a fusion activity somewhat higher than that exerted by the single peptides. No increase was seen when pep.gH-αH121 or pep.gH-αH219 was mixed with unrelated peptides (data not shown). We conclude that peptides mimicking α-H2, α-H1, and HR-2 exhibit an intrinsic ability to interact with lipids and to cause fusion of lipid vesicles.
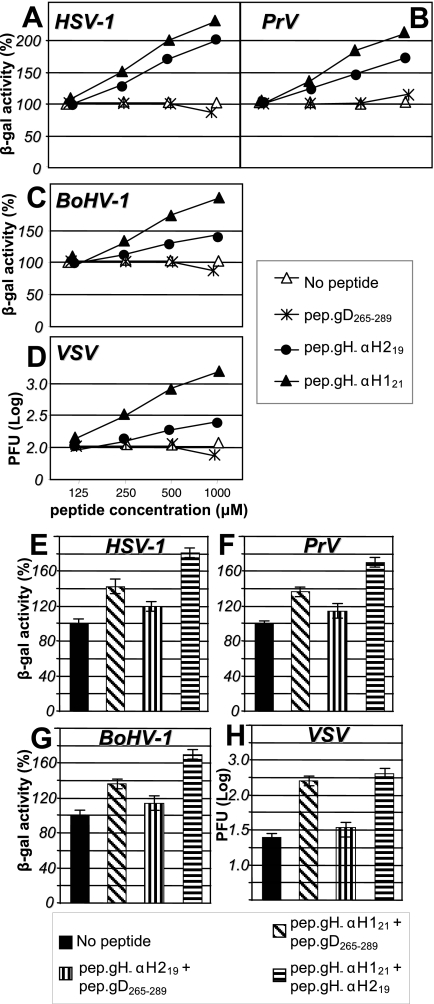
Mimetic peptides with the sequence of α-H2 and α-H1 enhance HSV-1, PrV, BoHV-1, and VSV infection.
The effect of α-H1 and α-H2 mimetic peptides on nude lipid vesicles prompted us to ask whether the fusogenic effect was exerted also in in vivo systems and, consequently, whether virus infection and cell-cell fusion were enhanced. The R8102 recombinant, carrying the Lac-Z reporter gene under the immediate-early α-27 promoter, was used in these experiments. In cells infected with this virus, β-Gal expression is a direct measure of the extent of infection. Surprisingly, we found that both peptides increased infection in a dose-dependent fashion. At a concentration of 1 mM, HSV infection was doubled. pep.gH-αH121 was somewhat more effective than pep.gH-αH219 (Fig. 5A). The effect was specific inasmuch as pep.gD265-289 had no effect on HSV infection.
FIG. 5.
Enhancement of HSV-1, PrV, BoHV-1, and VSV infection by synthetic peptides or a mixture thereof. (A to D) Effect of the peptides on infection with HSV-1(R8102) (A), PrV (B), or BoHV-1 (C) recombinants carrying the LacZ reporter gene and VSV (D). J-nectin1, Madin-Darby bovine kidney, or Vero cells were exposed to the indicated peptides from time zero (start of virus adsorption) until harvest. Infection was quantified as β-Gal activity (R8102, PrV, and BoHV) or as the number of plaques (VSV). The abscissa shows the μM peptide concentration. In panel D, the scale is logarithmic. (E to H) Effect of simultaneous exposure to mixtures of mimetic peptides, each one present at a 200 μM concentration, on HSV-1 (E), PrV (F), BoHV-1 (G), and VSV (H) infection. In panel H, the scale is logarithmic. The bars represent the mean of triplicates ± standard deviation.
The fusion of nude lipid vesicles induced by α-H1 and α-H2 mimetic peptides suggested that the enhancing effect on infection was likely consequent to the effect on lipids. We reasoned that, if this were the case, the enhancing effect should not be restricted to HSV-1, but could be observed also with other viruses: e.g., PrV, BoHV-1, and VSV. For PrV and BoHV-1, recombinant viruses carrying the β-Gal reporter gene were used. For VSV, the effect was measured as an increase in the number of plaques. As shown in Fig. 5B and C, pep.gH-αH121 and pep.gH-αH219 exerted an enhancing effect on PrV and BoHV-1 infection, with somewhat lower increases than those observed with HSV. The increase in the number of VSV plaques was dramatic (15-fold at 1,000 μM) (Fig. 5D). We also asked whether the simultaneous exposure to α-H1 and α-H2 mimetic peptides increased infection more than exposure to single peptides. Suboptimal concentrations (200 μM) of each peptide were used. As shown in Fig. 5E to H, the simultaneous presence of α-H1 and α-H2 mimetic peptides increased infection of R8102, PrV, BoHV-1, and VSV to higher levels than those attained by the single peptides. The simultaneous presence of α-H1 mimetic peptide and pep.gD265-289 did not increase infection.
Next, we examined whether the enhancing effect of the peptides was also detectable in the cell-cell fusion assay. BHK cells, cotransfected with plasmids encoding mutant or wt-gH, gB, gD, or gL, were incubated in the presence of pep.gH-αH219 or pep.gH-αH121, or a mixture of both. As can be seen from Fig. 6A, pep.gH-αH121 almost doubled the fusion induced by the HSV glycoproteins. pep.gH-αH219 exerted only a minor effect (typical examples are shown in Fig. 6C and D). The irrelevant peptide pep.gD265-289 exerted no effect. The mixture of pep.gH-αH219 and pep.gH-αH121 increased fusion to a higher level than the single peptides (Fig. 6E). Thus, the effect of the peptides on fusion is similar to that observed on infections.
FIG. 6.
Enhancement of cell-cell fusion by synthetic peptides or mixtures thereof. Cell-cell fusion in BHK cells transfected with plasmids encoding wt-gH, gL, gB, gD, and LacZ and exposed to the indicated peptides. (A and E) Fusion was quantified by means of β-Gal activity. (A to D) Peptides at 450 μM each. (B to D) Representative examples of polykaryocytes formed in the absence of added peptides (B) or in the presence of the indicated mimetic peptides (C and D). (E) Additive effect on cell-cell fusion of mixtures of mimetic peptides, each one present at a 200 μM concentration. The bars represent the mean of triplicates ± standard deviation.
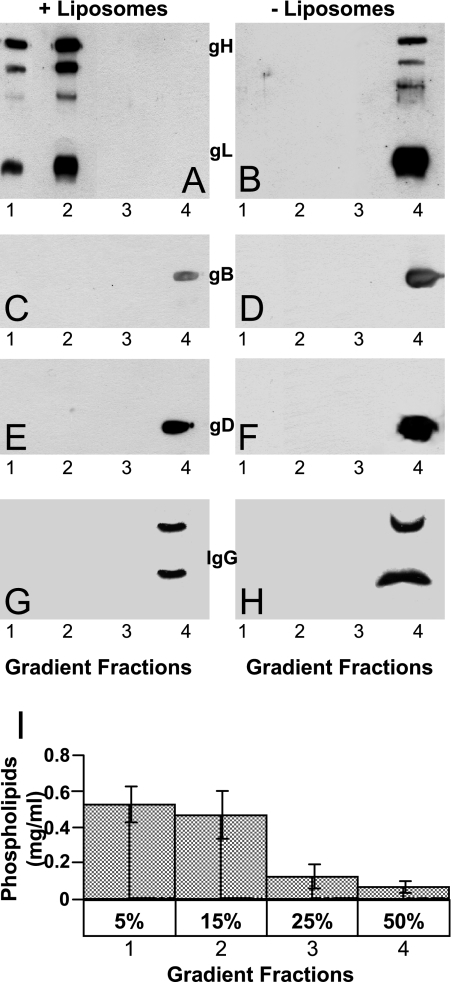
A soluble truncated form of gHt.gL interacts with lipid vesicles.
The finding that peptides with the sequence of gH α-H2, and α-H1 have an intrinsic ability to interact with lipids prompted us to investigate whether a soluble truncated form of the glycoprotein carrying the gH ectodomain (gH792t.gL) associates with lipid vesicles. To control for specificity, the interactions of soluble forms of gD and gB, carrying the ectodomains—gD285t and gB730Hist—or an unrelated glycoprotein, IgG, were analyzed in parallel. Aliquots (1.5 μg) of the soluble glycoproteins were mixed with preformed lipid vesicles at 37°C for 5 to 10 min and then subjected to sedimentation through preformed discontinuous 5 to 15, 15 to 25, and 25 to 50% sucrose gradients. Under the centrifugation conditions, lipid vesicles float in the 5 to 15% sucrose layers, whereas soluble proteins sediment to the bottom of the tube. As can be seen in Fig. 7A, gHt.gL specifically partitioned with the 5 to 15% sucrose gradient fractions, where the lipid vesicles partition. In contrast, gD285t, gB730Hist, and IgG partitioned with the 50% sucrose layer and the pellet. We conclude that the ectodomain of gH792t.gL exhibits a specific ability to interact with lipids, not shared with the ectodomain of the other two HSV fusion glycoproteins.
FIG. 7.
Sedimentation analysis of soluble forms of gH792t.gL (A and B), gB730Hist (C and D), gD285t (E and F), and IgG (G and H), preincubated (A, C, E, and G) or not (B, D, F, and H) with liposomes, through discontinuous preformed sucrose gradients. Gradient fractions 1 to 4 contained 5, 15, 25, and 50% sucrose (wt/vol), respectively. After centrifugation, the fractions were concentrated by Amicon Y10 filters, and the proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and enhanced chemiluminescence. The liposome content in each fraction was determined by means of phosphorous determination. A typical example is represented in panel I. Vertical bars represent standard deviation.
DISCUSSION
We will discuss the key points to emerge from this study in view of the accumulating evidence that gH exhibits structural and functional elements typical of viral fusion glycoproteins. The salient features of our results are as follows.
(i) Deletion of α-H2, its replacement with heterologous fusion peptides in a sense or antisense direction, or the mutation of 4 aa residues decreased infection to less than 10% relative to wt-gH and almost abolished cell-cell fusion. The results show that α-H2 constitutes an important functional domain of HSV gH, albeit a nonessential one.
(ii) α-H1 and α-H2 share important properties, underscored by properties of their mimetic peptides. Thus, synthetic peptides mimicking α-H1 and α-H2 interact with nude lipid vesicles in the absence of adjacent gH sequences and in the absence of membrane proteins in the vesicles (this study and reference 21). They induce fusion of the lipid vesicles, and, more importantly, increase virus infection and cell-cell fusion. Remarkably, the enhancement of infection was not restricted to HSV but was observed also with PrV, BoHV-1, and VSV. To our knowledge, enhancement of infection by mimetic peptides to hydrophobic sequences is novel and has not been described in any system. Taken together, these effects suggest that the interaction of α-H1 and α-H2 with the lipids possibly leads to lipid destabilization in the membranes and thus increases fusion and virus entry. The lipid destabilization may be critical for inducing the curvature of the membrane at the site of fusion and/or for decreasing the energy barrier. Interaction of gH with lipids may be critical also in guiding the glycoprotein refolding.
The synthetic peptides mimicking α-H1 and α-H2 differ from peptides mimicking HR-2 (27). All induce fusion of the lipid vesicles, denoting a certain ability to interact with lipids. However, the first two enhance infection and fusion, whereas the latter inhibits these activities. It is well known that HR-2 mimetic peptides block virus infection by forming a complex with the HR-1 located in the glycoprotein and thus hinder the glycoprotein refolding (17). Therefore, the different behavior of the two groups of mimetic peptides reflects the different molecules they target: i.e., the membrane lipids in the case of α-H1 and α-H2 mimetic peptides and gH itself in the case of the HR-2 mimetic peptide.
(iii) A soluble truncated form of gH, carrying the gH ectodomain, in the form of gHt.gL heterodimer, was capable of associating with lipid vesicles. This property was unique to gHt.gL and was not displayed by the ectodomains of soluble forms of gDt or gBt or by the irrelevant glycoprotein IgG. In the case of gB, which is clearly involved in fusion and virus entry, bioinformatic analyses predicted no candidate fusion peptide or hydrophobic segments and no strong HR sequence, and association with lipids was not expected. Hence, the ability of gHt.gL to interact with lipid membranes correlates with the presence of hydrophobic segments. The results imply that the ability of α-H1, α-H2, and perhaps other gH sequences to interact with lipids is maintained even when the segments are integral parts of gH. Remarkably, when α-H1 or α-H2 sequences were engineered downstream of the truncated soluble gD1-260t, the truncated gD became membrane bound, showing that α-H1 and α-H2 do interact with cell membranes.
(iv) Notwithstanding the above similarities, α-H2 and α-H1 differ in a number of important properties. First, α-H1, but not α-H2, is positionally conserved in all of the gH alleles from different herpesviruses that were examined (25). Second, α-H1, but not α-H2, cannot be deleted without total loss of infection. Third, α-H1 can be functionally replaced with heterologous fusion peptides, whereas α-H2 cannot. Indeed, the highest rescue of the α-H2-deleted gH was observed with constructs carrying the antisense inserts. Fourth, α-H2 is less hydrophobic than the candidate fusion peptide, both by bioinformatic prediction and measured as the ability to render the soluble gD1-260t membrane bound (25). Fifth, the mimetic peptide to α-H1 was more effective than the mimetic peptide to α-H2 in inducing fusion of nude lipid vesicle and in enhancing infection and cell-cell fusion (this work and reference 21). These features differentiate α-H1 from α-H2. They reinforce the view that α-H1, but not α-H2, exhibits properties typical of fusion peptides. Collectively, the results reported here and elsewhere highlight gH as a structurally complex glycoprotein. It carries sequences able to interact with lipids, namely α-H1, with properties of a candidate fusion peptide, in addition to α-H2 and the pretransmembrane sequence with a tendency to partition at the membrane interface. It also carries two functional HRs. These elements are typical of class I fusion glycoproteins.
Acknowledgments
We are grateful to our colleagues for their generosity and continuous supply of reagents. In particular, we thank G. H. Cohen and R. Eisenberg (University of Pennsylvania), T. Minson and H. Browne (Cambridge University), B. Roizman (University of Chicago), P. G. Spear (Northwestern University), and M. Ackerman (University of Zurich) for the gift of recombinant proteins, viruses, and antibodies. We are indebted to Elisabetta Romagnoli for invaluable assistance.
This work was supported by grants from EU (Targetherpes), FIRB autonomous and coordinated projects, Cofin-MIUR 40%, University of Bologna 60%, and Fondo Pallotti.
REFERENCES
- 1.Avitabile, E., G. Lombardi, and G. Campadelli-Fiume. 2003. Herpes simplex virus glycoprotein K, but not its syncytial allele, inhibits cell-cell fusion mediated by the four fusogenic glycoproteins, gD, gB, gH, and gL. J. Virol. 77:6836-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant, and downmodulates their cell surface expression. J. Virol. 78:8015-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic, N., B. G. Klupp, B. Makoschey, A. Karger, A. Flamand, and T. C. Mettenleiter. 1996. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J. Gen. Virol. 77:2277-2285. [DOI] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, W., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. (Erratum, 62:4438.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns, T. M., D. J. Landsburg, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Contribution of cysteine residues to the structure and function of herpes simplex virus gH/gL. Virology 332:550-562. [DOI] [PubMed] [Google Scholar]
- 8.Cairns, T. M., M. S. Shaner, Y. Zuo, M. Ponce-de-Leon, I. Baribaud, R. J. Eisenberg, G. H. Cohen, and J. C. Whitbeck. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J. Virol. 80:2596-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campadelli-Fiume, G. 2000. Virus receptor arrays, CD46 and human herpesvirus 6. Trends Microbiol. 8:436-438. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchi, F., M. Lopez, L. Menotti, M. Aoubala, P. Dubreuil, and G. Campadelli-Fiume. 1998. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc. Natl. Acad. Sci. USA 95:15700-15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin superfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov, A. S., S. S. Rawat, S. Jiang, and R. Blumenthal. 2003. Role of the fusion peptide and membrane-proximal domain in HIV-1 envelope glycoprotein-mediated membrane fusion. Biochemistry 42:14150-14158. [DOI] [PubMed] [Google Scholar]
- 15.Dutch, R. E., G. P. Leser, and R. A. Lamb. 1999. Paramyxovirus fusion protein: characterization of the core trimer, a rod-shaped complex with helices in anti-parallel orientation. Virology 254:147-159. [DOI] [PubMed] [Google Scholar]
- 16.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 18.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraefel, C., M. Ackermann, and M. Schwyzer. 1994. Identification of the bovine herpesvirus 1 circ protein, a myristylated and virion-associated polypeptide which is not essential for virus replication in cell culture. J. Virol. 68:8082-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. USA 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galdiero, S., A. Falanga, M. Vitiello, H. Browne, C. Pedone, and M. Galdiero. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 280:28632-28643. [DOI] [PubMed] [Google Scholar]
- 22.Galdiero, S., M. Vitiello, M. D'Isanto, A. Falanga, C. Collins, K. Raieta, C. Pedone, H. Browne, and M. Galdiero. 2006. Analysis of synthetic peptides from heptad-repeat domains of herpes simplex virus type 1 glycoproteins H and B. J. Gen. Virol. 87:1085-1097. [DOI] [PubMed] [Google Scholar]
- 23.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh, J. K., S. G. Peisajovich, and Y. Shai. 2000. Sendai virus internal fusion peptide: structural and functional characterization and a plausible mode of viral entry inhibition. Biochemistry 39:11581-11592. [DOI] [PubMed] [Google Scholar]
- 25.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simpex virus glycoprotein H contains a membrane α-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianni, T., L. Menotti, and G. Campadelli-Fiume. 2005. A heptad repeat in herpes simplex virus gH, located downstream of the α-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianni, T., A. Piccoli, C. Bertucci, and G. Campadelli-Fiume. 2006. Heptad repeat 2 in herpes simplex virus-1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion. J. Virol. 80:2216-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herold, B. C., R. J. Visalli, N. Susmarski, C. R. Brandt, and P. G. Spear. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 75:1211-1222. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioffe, V., and G. P. Gorbenko. 2005. Lysozyme effect on structural state of model membranes as revealed by pyrene excimerization studies. Biophys. Chem. 114:199-204. [DOI] [PubMed] [Google Scholar]
- 31.Kielian, M., and F. A. Rey. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamb, R. A., R. G. Paterson, and T. S. Jardetzky. 2006. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 344:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopper, M., and T. Compton. 2004. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 78:8333-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinetti, G. V. 1962. Hydrolysis of lecithin with sodium methoxide. Biochemistry 1:350-353. [DOI] [PubMed] [Google Scholar]
- 38.Menotti, L., R. Casadio, C. Bertucci, M. Lopez, and G. Campadelli-Fiume. 2002. Substitution in the murine nectin1 receptor of a single conserved amino acid at a position distal from the herpes simplex virus gD binding site confers high-affinity binding to gD. J. Virol. 76:5463-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milne, R. S. B., S. L. Hanna, A. H. Rux, S. H. Willis, G. H. Cohen, and R. J. Eisenberg. 2003. Function of herpes simplex virus type 1 gD mutants with different receptor-binding affinities in virus entry and fusion. J. Virol. 77:8962-8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 41.Morrison, T. G. 2003. Structure and function of a paramyxovirus fusion protein. Biochim. Biophys. Acta 1614:73-84. [DOI] [PubMed] [Google Scholar]
- 42.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 43.Omerović, J., L. Lev, and R. Longnecker. 2005. The amino terminus of Epstein-Barr virus glycoprotein gH is important for fusion with epithelial and B cells. J. Virol. 79:12408-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 47.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rovero, S., A. Amici, E. D. Carlo, R. Bei, P. Nanni, E. Quaglino, P. Porcedda, K. Boggio, A. Smorlesi, P. L. Lollini, L. Landuzzi, M. P. Colombo, M. Giovarelli, P. Musiani, and G. Forni. 2000. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 165:5133-5142. [DOI] [PubMed] [Google Scholar]
- 49.Rux, A. H., S. H. Willis, A. V. Nicola, W. Hou, C. Peng, H. Lou, G. H. Cohen, and R. J. Eisenberg. 1998. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpesvirus entry mediator. J. Virol. 72:7091-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suarez, T., W. R. Gallaher, A. Agirre, F. M. Gońi, and J. L. Nieva. 2000. Membrane interface-interacting sequences within the ectodomain of the human immunodeficiency virus type 1 envelope glycoprotein: putative role during viral fusion. J. Virol. 74:8038-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitbeck, J. C., Y. Zuo, R. S. B. Milne, G. H. Cohen, and R. J. Eisenberg. 2006. Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM. J. Virol. 80:3773-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]