Abstract
To investigate the early establishment of bovine leukemia virus (BLV) infection, we injected BLV-infected or mock-infected allogeneic cells into the shoulder of sheep in which an efferent lymphatic duct of the draining prescapular lymph node had been cannulated. Rare mononuclear cells acting as centers of BLV infection in culture were present within 4 to 6 days in efferent lymph and within 6 to 10 days in blood. Soon after BLV injection, immunoglobulin M+ (IgM+) and CD8+ cells increased in efferent lymph and oscillated reciprocally in frequency. CD8+ blasts increased on days 4 to 6, when infectious centers increased 100-fold in lymph. On days 6 and 7, both lymph and blood were enriched with CD8+ cells that were labeled late on day 5 with an intravenous pulse of 5-bromo-2′-deoxyuridine (BrdU). Lymph, but not blood, was enriched with BrdU+ B cells on day 7. Capsid-specific antibodies became detectable in efferent lymph on days 6 to 8 and surface glycoprotein-specific antibodies on day 9, preceding their detection in serum by 9 to 14 days. Systemic dissemination of BLV-infected cells was thus accompanied by an increase in proliferating CD8+ cells and the onset of BLV-specific antibodies in lymph. Infectious centers reached maximum frequencies of 0.2% in lymph by days 11 to 13, and then their frequencies increased by 5- to 40-fold in blood cells, suggesting that many infected blood cells do not recirculate back into lymph. Beginning on days 10 to 13, a subpopulation of B cells having high levels of surface IgM increased sharply in peripheral blood. Such cells were not present in lymph. After a day 16 pulse of BrdU, recently proliferated cells that stained intensely for surface IgM appeared in blood within 15 h. Predominantly B lymphocytes contained the viral capsid protein when lymph and blood cells were cultured briefly to allow BLV expression. However, both early in lymph and later in blood, BrdU+ B cells greatly exceeded productively infected cells, indicating that new BLV infections stimulate proliferation of two different populations of B cells.
The early events leading to the establishment of infection by bovine leukemia virus (BLV) and its close relative, human T-cell leukemia virus, are unknown. Allogeneic cells containing the viral genome are the vehicles for entry of these viruses into naïve individuals via blood, semen, or breast milk. The allogeneic cells produce virus that infects cells of the new host. During the first month following experimental injection of BLV into sheep, which are highly susceptible to this virus and form tumors at high frequency, the peripheral blood contains changing levels of cells that express viral genes in culture (24, 33, 46). BLV-infected cells usually become detectable in blood during the second week after introduction of the virus, rapidly reach a peak in number during the third week, and decrease soon thereafter. This pattern suggests that BLV spreads to new host cells in tissues before large numbers of infected cells enter the blood.
The sites where BLV first spreads among cells in a newly infected animal are unknown. Its preferred cellular hosts are B lymphocytes (1, 24, 27, 34, 38, 43, 48), although BLV has been reported to infect cells of other hematopoietic cell lineages in vivo (19, 32, 48, 55). Pioneering studies showed that spleens of yearling calves harbor BLV-infected cells on day 8 after subcutaneous injection of BLV-infected allogeneic cells (51), but further work revealed that splenectomized calves can establish and maintain BLV infection (52). Although the peripheral blood cells used to introduce BLV in these studies were injected into the shoulder, which is drained by the prescapular lymph node, virus-infected cells were not detected in that node until 13 to 14 days after injection, concurrent with their detection in peripheral blood (51, 52). Testing for BLV-infected cells entailed injecting sheep with cell suspensions from the calves, so only limited numbers of samples could be examined. We adopted a different approach to investigate initial BLV infection of lymphoid tissue.
Lymph nodes are important sites for the establishment of infection by the lymphotropic human and simian immunodeficiency viruses and are virus reservoirs during asymptomatic stages of infection (3, 6, 9, 31, 42). Squirrel monkeys injected intravenously with cells producing the oncogenic retrovirus human T-cell leukemia virus type 1 have proviral DNA in lymph nodes, the spleen, and peripheral blood mononuclear cells (PBMCs) beginning on day 12 (26).
Sheep, which are well studied as experimental hosts for BLV, are classical subjects for investigating lymphocyte trafficking and antigen reactivity within lymph nodes (5, 15, 20, 58) because they are large enough to allow collection of lymph by direct cannulation of lymphatic ducts and are amenable to loose restraint. Immune responses to infectious viruses, including maedi-visna virus (2), vaccinia virus (23), and Orf virus (56), and the replication of maedi-visna virus (2) have been investigated using cannulated sheep lymph nodes. To investigate the dissemination of virus-infected cells and the onset of adaptive immunity during de novo BLV infection of sheep, we cannulated an efferent lymphatic duct of a prescapular lymph node and then injected BLV-bearing allogeneic cells into the shoulder drained by that node. We then examined efferent lymph and peripheral blood for 3 to 4 weeks to determine the numbers and types of immune cells, their ability to produce virus in culture, their proliferative capacity at two critical times, and the presence of antibodies specific for virus structural proteins.
MATERIALS AND METHODS
Lymph node cannulation.
Yearling sheep weighing approximately 45 kg were screened for respiratory diseases, parasites, and Q fever antibodies. Sheep were anesthetized with pentothal (20 mg/kg of body weight, intravenous injection) and halothane (1 to 3%, inhaled) for installation of silastic tubing (inner diameter, 0.025 in.; outer diameter, 0.047 in.) into an efferent lymphatic duct of a prescapular lymph node (39, 58). Efferent lymph dripped continuously from the lymphatic cannula. A capped catheter installed in the jugular vein was flushed daily with sterile, injection-quality saline and was left filled with 68 mM sodium citrate, 72 mM sodium chloride, and 10 mg ampicillin per ml. Animals were loosely haltered in individual metabolism cages and were always maintained as pairs to reduce stress; they were fed alfalfa pellets and were provided with mineral salt blocks and unlimited water. All procedures were approved by the University of California, Davis, Animal Use and Care Administrative Advisory Committee.
BLV infection.
Animals were injected with 107 PBMCs from donor sheep 212 (24): uninfected cells (mock infections) were obtained and frozen 2 days before sheep 212 was injected with BLV, whereas infected cells were obtained on day 670 of infection. PBMCs were thawed, washed, and cultured overnight in 4 ml of Iscove's modified Dulbecco medium (IMDM; GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 100 U penicillin per ml, 100 μg streptomycin per ml, and 50 μM 2-mercaptoethanol (IMDM-10FBS). Cultured cells were injected into the neck and shoulder ipsilateral with the cannulated prescapular lymph node. Half the inoculum was injected intradermally in multiple portions of 0.1 to 0.15 ml and the remaining half was injected subcutaneously in portions of 0.5 ml.
BrdU injection.
To label the DNA of cells proliferating in vivo, 5-bromo-2′-deoxyuridine (BrdU; Boehringer Mannheim) was dissolved at 15 mg/ml of injection-quality normal saline. A total of 15 mg BrdU/kg of body weight (36, 37) was slowly injected into the venous catheter over 20 to 25 min; cells were collected 14 to 60 h later as specified for individual experiments.
Sample preparation.
Freely flowing efferent lymph was collected into sterile 15-ml tubes containing 0.5 to 1 ml acid citrate dextrose [75 mM trisodium citrate, 38 mM citric acid, 136 mM d-(+)-glucose] to prevent clotting. Lymph cells were collected by centrifugation at 300 × g for 10 min at room temperature (RT). Pelleted cells were twice resuspended in Hanks buffered saline solution (HBSS) lacking Ca2+ and Mg2+ and centrifuged at 200 × g for 10 min. The reserved lymph supernatant was centrifuged again at 800 × g for 10 min to remove remaining debris and then was frozen.
To prepare PBMCs, peripheral blood was collected from the venous catheter into 20-ml syringes containing 3 ml acid citrate dextrose. After centrifugation of the blood at 1,100 × g for 15 min at RT, buffy coat cells were collected, diluted with HBSS, layered over Histopaque 1077 (Sigma), and centrifuged at 1,100 × g for 30 min at RT. Mononuclear cells removed from the interface were washed twice in HBSS and collected by 10 min of centrifugation, first at 300 × g and then at 200 × g.
PBMCs from sheep 43 that were prepared by this procedure were heavily contaminated with erythrocytes, so the pellet obtained after the first wash of the mononuclear cell layer was treated for 2 min at RT with 2 ml of erythrocyte lysis solution (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA; pH 7.3) (30). The mixture was then diluted with 10 ml of HBSS and centrifuged at 200 × g. Pelleted PBMCs were washed with HBSS and collected by centrifugation at 200 × g.
Blood was collected into 1 mg EDTA-K3 per ml to measure packed cell volume in duplicate 40-μl heparinized capillary tubes. After lysis of erythrocytes, quadruplicate samples of leukocytes and platelets were counted using hemacytometers. At least 800 leukocytes from two stained blood smears were differentially scored under a magnification of ×200. Serum was harvested from whole blood collected into silicon-coated tubes and centrifuged at RT for 15 min at 1,100 × g and was stored at −20°C. No more than 450 ml blood (1% body weight) was drawn from an animal over any 2-week interval.
Cell culture.
Pelleted PBMCs or lymph cells were resuspended in 10 ml of IMDM-10FBS and the density was adjusted to 2 × 106 cells per ml. Culture flasks were seeded at 1 × 106 cells/ml by adding an equal volume of minimal Eagle's medium (MEM) containing 5% FBS. Lipopolysaccharide (LPS) (from Salmonella enterica serovar Minnesota; Sigma) was added at 10 μg/ml as specified for individual experiments. Flasks were maintained at 37°C in a humid atmosphere containing 5% CO2.
Assays of infectious centers.
An assay of infectious centers, in which adherent indicator cells form syncytia when infected with BLV, was modified from a previously described procedure (46). To restrict lateral movement of PBMCs or lymph cells during coculture, the viscosity of the semisolid medium used to suspend test cells was increased by using equal amounts of methylcellulose of 4,000 cP (Serva) and 400 cP (Sigma). Duplicate or quadruplicate samples of test cells were plated onto F81 indicator cells in 1% (wt/vol) methylcellulose in MEM-5FBS supplemented with LPS at 10 μg/ml. Cocultures were incubated at 37°C for 2 days and then were washed one or two times with MEM-5FBS to remove the test cells. The adherent indicator cells were fed with fresh medium; after 2 to 3 days at 37°C, they were washed twice with HBSS, fixed with absolute methanol, and stained with Giemsa. Clusters of syncytia initiated by an infected test cell were counted under a magnification of ×40. Serial twofold dilutions of cells inducing ≤50 clusters per well yielded a linear response in this assay.
Assays for sheep BLV antibodies.
An immunodiffusion assay (Leukassay B; Synbiotics) was used as previously described (45) to test undiluted sera for the presence of precipitating antibodies specific for BLV glycoprotein antigen. Sera from experimental animals were scored as negative, weak positive, or positive. Antibodies specific for the BLV surface (SU) envelope glycoprotein were measured following the manufacturer's instructions for a commercial enzyme-linked immunosorbent assay (ELISA) (Herdcheck bovine leukemia virus antibody test kit: serum/verification; IDEXX). Two hundred-microliter volumes of diluted sera (1:20) or lymph samples (1:5) were tested. Detection of sheep immunoglobulins required the addition of horseradish peroxidase-conjugated donkey anti-sheep immunoglobulin G (IgG) (heavy plus light chains) (The Binding Site) at 1:10,000 to reagent C in the kit. Antibodies specific for the BLV capsid (CA) protein were measured following instructions for the ViraCHEK BLV ELISA kit (a gift from Kathy Bestul, Synbiotics Corporation). Samples (50 μl) of undiluted serum or lymph were added, together with recombinant BLV CA antigen conjugated to horseradish peroxidase, to wells coated with recombinant BLV CA antigen. Previously characterized sheep sera (24) were used as supplemental positive and negative controls.
Immunostaining. (i) Cell surface proteins.
Samples of 106 cells were pelleted for 5 to 6 min at 300 × g and washed in 1 ml staining buffer (phosphate-buffered saline [PBS], pH 7.2 to 7.4, 2.7 mM KCl, 1.5 mM KH2PO4, 140 mM NaCl, 8 mM Na2HPO4 containing 0.5% bovine serum albumin [fraction V; Sigma] and 0.1% sodium azide [Sigma]). Antibodies were added to 100 μl staining buffer and the mixtures were incubated on ice for 30 min in the dark, unless otherwise noted. After each exposure to antibody, cells were washed twice with 1 ml staining buffer and collected by centrifugation for 5 to 6 min at 300 × g. After the final wash, cells were resuspended in staining buffer and stored at 4°C in the dark to await analysis by flow cytometry.
(ii) Double labeling of cell surface and CA proteins.
Cells already exposed to primary antibody specific for a surface antigen (or unstained cells) were washed and incubated for 30 min at RT in 100 μl of fixative (Solution A from Fix & Perm Kit; Caltag) and then were washed twice with staining buffer and incubated for 30 min at RT in 100 μl of permeabilizing buffer (kit Solution B) containing primary CA antibody and a secondary antibody specific for primary surface antibody. To maintain permeabilization, cells were washed twice with staining buffer supplemented with 0.1% saponin (Calbiochem). The secondary antibody specific for the primary CA antibody and the tertiary surface reagent (if required) were added in 100 μl of staining buffer containing 0.3% saponin, and the cell-antibody mixture was incubated for 30 min on ice in the dark. Finally, cells were washed twice in normal staining buffer, resuspended, and stored at 4°C for analysis.
(iii) Simultaneous labeling of cell surface proteins and BrdU.
The DNA of cells that had been labeled for surface antigen was denatured by heating the cells at 37°C for 30 min in 0.5 ml PBS containing 2 mM MgCl2 and 375 units of DNase I (Sigma). Cells were then washed twice with PBS and incubated for 30 min at RT with 100 μl of staining buffer containing 12 μg mouse IgG (Biodesign International) to block remaining reactive sites on antibodies used to detect surface antigens or CA protein. Excess block was removed by one wash with staining buffer. Cells were then incubated with mouse anti-BrdU antibody in staining buffer containing 0.5% Nonidet P-40 detergent (Pierce) (22) for 45 min at RT in the dark. Finally, cells were washed in PBS, resuspended in staining buffer, and stored at 4°C before analysis.
Flow cytometry antibodies and controls.
Primary antibodies to surface proteins were as follows: affinity-purified rabbit-anti-sheep immunoglobulin μ (Kirkegaard & Perry Laboratories) used at 1:200; mouse-anti-bovine CD4 (clone GC1A, IgG2a isotype; cross-reactive with sheep; Veterinary Medical Research and Development, Inc.) used at 1:50; mouse-anti-bovine CD8 (clone CC63, IgG2a isotype; cross-reactive with sheep; Serotec) used at 1:50; mouse-anti-bovine WC1 (clone CC15, IgG2a isotype; cross-reactive with sheep; Serotec) used at 1:50 to detect a major subpopulation of γδ T cells (41); and affinity-purified mouse-anti-sheep CD14 (clone VPM65, IgG1 isotype; Serotec) (16) used at 1:50. For double labeling, mouse-anti-human CD14 (clone TÜK4, IgG2a isotype; cross-reactive with sheep; Serotec) conjugated to phycoerythrin-Cy5 was used at 1:50. Primary mouse monoclonal antibodies specific for BLV CA protein (clones 4′G9 and 5F7, IgG1 isotypes; a gift from Daniel Portetelle, Department of Microbiology, Faculty of Agronomy, Gembloux, Belgium) were used at 1:50. Fluorescein-conjugated monoclonal mouse anti-BrdU (clone B44; Becton Dickinson Immunocytometry Systems [BDIS]) was used at 1:25.
Secondary antibodies (all from Southern Biotechnology Associates, Inc.) were as follows: biotinylated F(ab′)2 goat-anti-rabbit IgG (heavy plus light chains) used at 1:100 to bind the IgM-specific primary antibody; biotinylated F(ab′)2 goat-anti-mouse IgG2a (γ2a chain specific) used at 1:100 to bind the primary antibodies specific for CD4, CD8, and WC1; and phycoerythrin-conjugated F(ab′)2 goat-anti-mouse IgG1 (γ1 chain specific) used to bind the primary antibodies specific for CD14 or BLV CA protein.
The tertiary reagent, streptavidin-conjugated Tricolor (Caltag), was used at 1:100 to 1:200 to bind to biotinylated secondary antibodies.
Controls included with each staining assay included unlabeled cells to set background autofluorescence levels; cells exposed only to secondary and tertiary reagents were used to control for nonspecific binding. Singly stained samples were used to establish proper compensation on the flow cytometer.
Flow cytometric analysis.
Data were collected using a FACScan (BDIS) bench-top flow cytometer and analyzed using Cellquest (BDIS) and FloJo (Tree Star, Inc.) software. Live cells were selected by gating on forward versus side scatter to exclude extremes. For routine samples, 10,000 live-gated events were evaluated; for samples containing rare CA+ or BrdU+ cells, 50,000 or 100,000 events were evaluated.
RESULTS
Cell profiles after cannulation of the prescapular node.
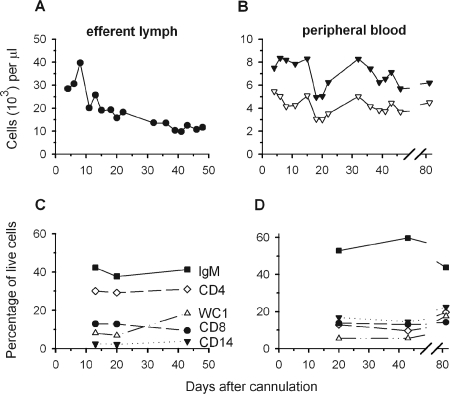
Each of the large, bilateral prescapular nodes of a sheep collects afferent lymphatic vessels that drain an entire forequarter. To ascertain how the loss of cells in efferent lymph affected levels of circulating immune cells, we monitored a cannulated, uninfected sheep for 46 days. Lymph cell counts rose sharply on day 8 after surgery and then slowly decreased (Fig. 1A). Blood leukocyte counts dropped between days 15 and 20 but then rebounded (Fig. 1B). In spite of the continuous loss of lymph cells, blood leukocytes stayed within an average range of 4 × 103 to 12 × 103/μl of peripheral blood (57), and mononuclear cells were consistently ∼70% of peripheral blood leukocytes (Fig. 1B).
FIG. 1.
Leukocytes in efferent lymph and peripheral blood of cannulated, uninfected sheep 455. Cell counts are plotted in thousands per μl as a function of time after cannulation for efferent lymph cells (A) and leukocytes (B) (closed symbols) and mononuclear cells (open symbols) from peripheral blood. Percentages of live-gated, mononuclear cells staining for the surface markers IgM, CD4, CD8, WC1, and CD14 are plotted for efferent lymph (C) and peripheral blood (D).
Efferent lymph cells were almost exclusively mononuclear. Proportions of IgM+, CD4+, CD8+, WC1+ (a marker of γδ T cells), and CD14+ cells were stable during weeks 2 and 3 in the aforementioned animal's lymph (Fig. 1C); by 6 weeks, WC1+ cells increased and CD8+ cells decreased slightly. Relative levels of these cell lineages were stable among PBMCs from weeks 2 through 6 after cannulation (Fig. 1D); 6 weeks after lymph drainage was terminated, the level of IgM+ B cells dropped somewhat. The cell lineage profiles suggested that despite the loss of cells in efferent lymph, the balance of circulating immune cells remained homeostatic for several weeks.
BLV-infected cells appeared first in efferent lymph but reached higher levels in peripheral blood.
Four additional cannulated sheep yielded lymph for up to 27 days (Table 1). The cannula of a fifth sheep dripped erratically and blocked permanently on day 3 after BLV infection; this animal was treated as a sham-cannulated control that had been subjected to surgery and infected in the same way as the cannulated animals.
TABLE 1.
Lymph flow in cannulated sheep
| Sheep no. (characteristic) | Avg lymph flow in ml/h (duration [(days)] |
|---|---|
| Uninfected sheep | |
| 455 (surgical control) | NDa |
| 973 (mock infected) | 35.6 (20) |
| 44 (mock infected) | 25.3 (25) |
| Infected sheep | |
| 454 | 18.0 (23) |
| 972 | 57.9 (27) |
| 43 (sham cannulated) | 1.6 (8)b |
ND, not determined.
Lymphatic cannula blocked irreversibly 8 days after surgery.
After 5 days to resolve immediate inflammatory reactions to the surgery, sheep were injected in the ipsilateral shoulder with allogeneic cells. To differentiate immune responses to nonself cells from those to BLV, two animals were mock infected with 107 PBMCs that had been collected from a donor sheep prior to its injection with BLV. Three sheep received 107 PBMCs obtained from the same sheep after BLV infection; approximately 8 × 104 of those cells contained the viral CA protein after being cultured overnight. The onset of BLV infection was monitored in the new hosts by enumerating infected efferent lymph cells and PBMCs: cell-associated CA protein was revealed by immunostaining (Fig. 2) and the ability of cultured cells to produce BLV was demonstrated in an assay of infectious centers (Fig. 3).
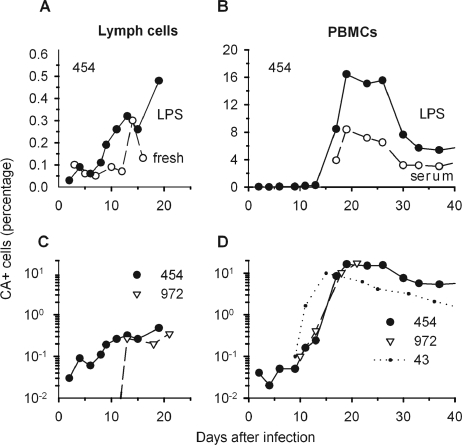
FIG. 2.
CA+ cells present among efferent lymph cells and PBMCs of BLV-infected animals. Cells immunostained for intracellular CA protein were enumerated by flow cytometry and then plotted as percentages of total live-gated cells. (A) Efferent lymph cells from sheep 454 were stained either directly (fresh) after purification or after being cultured overnight in medium supplemented with LPS. (B) PBMCs from sheep 454 were cultured overnight with fetal bovine serum or with added LPS. (C) Lymph cells from sheep 454 and 972 and (D) PBMCs from sheep 454, 972, and 43 (sham cannulated) were cultured overnight with LPS. Note that different scales are used to plot percentages in individual panels.
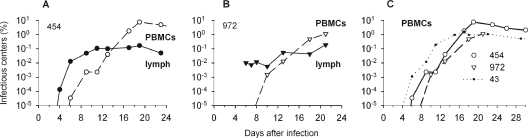
FIG. 3.
Infectious centers among cultured, efferent lymph cells and PBMCs from BLV-infected animals. Cells to be tested were suspended in methylcellulose medium supplemented with LPS. Percentages of cells that produced syncytia among indicator cells are plotted as a function of time after sheep were injected with BLV-infected cells. (A and B) Lymph and PBMCs for sheep 454 (A) and 972 (B). (C) PBMCs from sheep 454, 972, and 43 (sham cannulated).
Although BLV expression cannot be detected in most infected mononuclear cells circulating in the blood of infected sheep or cows (14, 32, 46), cells leaving a newly infected lymph node might actively produce virus. To explore this possibility, we immunostained lymph cells freshly isolated from an infected sheep for BLV CA protein. On day 14, 0.3% of fresh, live-gated efferent lymph cells scored as CA+ (Fig. 2A). In contrast, at other times during the first 2 weeks after BLV injection, only 0.1% or fewer fresh lymph cells appeared to be CA+. This percentage represents very few events in flow cytometric assessments of 10,000 to 50,000 cells, and control samples exposed only to secondary and tertiary reagents could contain similar levels of fluorescing cells. Thus, even during establishment of a new infection, circulating lymph cells rarely contained BLV CA proteins.
Silently infected PBMCs from BLV-infected sheep synthesize CA protein after being cultured for short periods, and LPS stimulation increases the percentage of CA+ cells (12, 28, 32, 44). As expected, LPS augmented the frequency of CA+ cells among cultured PBMCs from a cannulated, infected sheep (Fig. 2B). Among cultured lymph cells stimulated overnight with LPS, CA+ cells were present at levels above background beginning on day 9 (Fig. 2A). Cultured lymph cells from the other infected sheep exhibited only background staining on days 7 and 10 and then included CA+ cells from day 13 onward (Fig. 2C).
Activity as infectious centers is a very sensitive measure of BLV-producing cells because large numbers of cells can be tested (33). Lymph cells first contained infectious centers on day 4 in one cannulated animal (Fig. 3A) and on day 6 in the other (Fig. 3B), when 1.3 × 10−4% and 0.01%, respectively, of 3 × 106 cells transmitted BLV in culture. No infectious centers were obtained on day 2 from the first sheep (Fig. 3A); day 2 and day 4 lymph cells from the other sheep (Fig. 3B) became contaminated with bacteria.
Infectious centers appeared later in peripheral blood than in efferent lymph. Very rare PBMCs (5 × 10−5%) first manifested this activity on day 6 in one cannulated sheep (Fig. 3A), and 0.002% of day 10 PBMCs from the other cannulated animal transmitted virus (Fig. 3B). PBMCs from the sham-cannulated sheep, which was not losing lymph, first included infectious centers (0.001%) on day 6 (Fig. 3C). During days 6 to 10, infectious centers were 5- to 50-fold more abundant among PBMCs from this animal than among those from the two cannulated animals.
PBMCs containing CA protein in short-term cultures appeared with the same timing and frequency for the two cannulated, infected animals: CA+ cells were first detected in cultures of day 10 or 11 cells and increased greatly after day 13 (Fig. 2D). Like infectious centers, the frequency of CA+ PBMCs rose more quickly in the sham-cannulated sheep (Fig. 2D) and they reached a maximum by day 17, slightly earlier than in the cannulated sheep.
Altogether, BLV-positive cells were 5- to 40-fold less abundant among efferent lymph cells than among PBMCs. In the assay of infectious centers, fewer than 0.2% of the lymph cells from the cannulated sheep induced syncytia, whereas 1 to 8% of PBMCs did so (Fig. 3A and B). Lymph cells containing CA protein after short-term culturing never exceeded 0.5%, whereas 10 to 20% of cultured PBMCs were CA+ (Fig. 2C and D).
BLV-specific antibodies were present in lymph beginning on day 6.
Neutralizing and CA-specific antibodies appear in serum within 2 weeks after BLV is injected into sheep, just when virus-bearing cells first become evident in blood (45). As infected cells increase sharply, antibodies specific for the SU glycoprotein become detectable in serum. However, since the prescapular lymph node should contain viral antigens soon after BLV is introduced into nearby tissue, lymph could contain earlier evidence of a humoral immune response. Efferent lymph samples from infected, cannulated animals first contained CA-specific antibodies on days 6 to 8 (Table 2), whereas sera first revealed these antibodies on days 15 to 18. SU-specific antibodies were first detected by ELISA in day 9 lymph but were not evident in serum until days 20 to 23, when precipitating, SU-specific antibodies were also detected by immunodiffusion. CA- and SU-specific serum antibodies appeared on day 11 and day 15, respectively, in the sera of sham-cannulated sheep, sooner than in the sera of the cannulated animals but later than in their efferent lymph samples. Neither lymph samples nor sera from mock-infected sheep generated signals in these assays. Thus, lymph samples from infected animals contained detectable BLV-specific antibodies much earlier than sera did, and in both fluid types, reactivity against CA appeared first.
TABLE 2.
Appearance of anti-CA and anti-SU antibodies in lymph and serum
| Infected sheep no. | Timing (d after infection) of first positive reaction by:
|
||||
|---|---|---|---|---|---|
| CA ELISA
|
SU ELISA
|
BLV IDa for serum | |||
| Lymph | Serum | Lymph | Serum | ||
| 454 | 8 | 18 | 9 | 23 | 23 |
| 972 | 6 | 15 | 9 | 20 | 21 |
| 43b | 11 | 15 | 15 | ||
ID, immunodiffusion assay for precipitating SU antibodies.
Sham-cannulated control.
Numbers of mononuclear cells and blasts following cannulation and BLV infection.
BLV infection stimulated increases in numbers of efferent lymph cells. Initially, these cells decreased slowly in all cannulated animals (Fig. 4A), probably because of continuous cell loss in dripping lymph. In contrast to the continued decline for mock-infected controls, lymph cell counts began to increase 6 days after BLV was injected into cannulated animals, and cell numbers remained higher, on average, for the next 2 weeks.
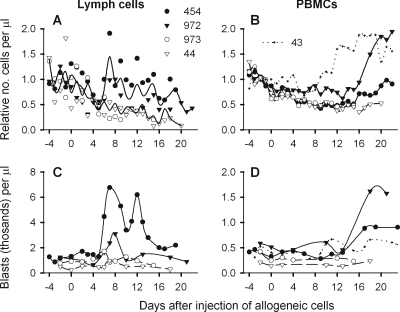
FIG. 4.
Numbers of cells and blasts in efferent lymph and blood. To account for distinctive cell numbers in individual animals, numbers of lymph cells (A) and PBMCs (B) per μl lymph or blood were divided by the average obtained from the same animal during the first 5 days after lymphatic cannulation, before injection of allogeneic cells. The resulting ratios are plotted for BLV-infected sheep 454 and 972 (closed symbols), for mock-infected sheep 973 and 44 (open symbols), and for infected, sham-cannulated sheep 43. In panel A, solid lines depict averages for infected and uninfected animals. (C and D) Blasts were quantified as percentages of live cells by gating on cells with high forward and side scatter. Absolute numbers of blasts per μl are plotted for lymph (C) and blood (D).
BLV infection markedly increased PBMCs as well. In all cannulated sheep, PBMCs declined during the first 5 days after surgery (Fig. 4B), but the decrease slowed in mock-infected controls and cell numbers leveled. Right after BLV injection, PBMCs of the infected, cannulated sheep stabilized or continued to decrease. Then, on days 15 to 16, PBMCs from both infected, cannulated sheep began to increase. In the infected, sham-cannulated sheep, PBMCs began to increase a week earlier, beginning on day 9.
BLV infection increased the numbers of blasts among both lymph cells and PBMCs. When efferent lymph cell output increased on days 6 to 8 in the infected animals (Fig. 4A), many lymph cells displayed high forward and side scatter (Fig. 4C). Blasts increased later, beginning after day 13, among PBMCs from these animals (Fig. 4D). Fewer blasts were present among PBMCs from the sham-cannulated sheep, but more were present than in mock-infected animals, indicating that blast formation was stimulated by BLV infection.
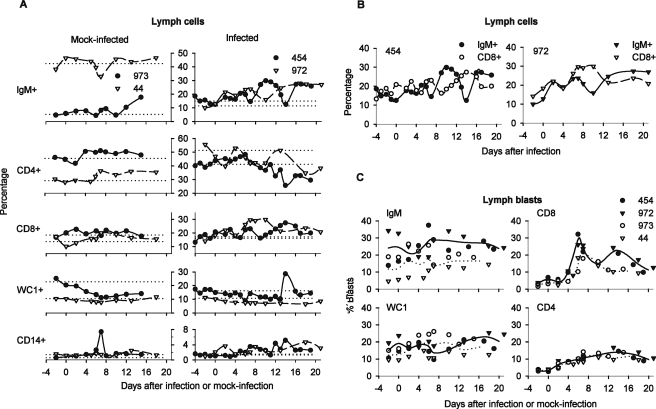
BLV-infected animals displayed early increases in IgM+ and CD8+ lymph cells.
To determine how a new BLV infection affects the balance of immune cells in lymph, we stained cell surface markers on freshly isolated cells. Baseline percentages of cell lineages were established for each animal as the average of measurements made before injection of allogeneic cells. Individuals displayed distinctive baseline profiles, as illustrated by the very different proportions of IgM+ cells, CD4+ cells, and WC1+ cells in lymph samples from the two mock-infected animals (Fig. 5A, left). Levels of most cell subsets were relatively stable in the efferent lymph of mock-infected sheep.
FIG. 5.
Cell lineages and blasts among efferent lymph cells of mock-infected and infected sheep. (A) Percentages of live cell labeling on the surface for IgM, CD4, CD8, WC1, and CD14 are plotted for each animal relative to the day of injection with allogeneic cells. Horizontal reference lines represent the average percentage for a given lineage as measured in each animal before injection of allogeneic cells. (B) Reciprocal oscillation of IgM+ and CD8+ lymph cells in infected, cannulated sheep. (C) Percentages of blasts among lymph cells of different lineages. Blasts were quantified as percentages of lymph cells staining for the indicated surface marker by gating on cells with high forward and side scatter (infected animals, closed symbols; mock-infected animals, open symbols). Averages for infected (solid lines) and mock-infected (dotted lines) animals were calculated using measurements made either on the same day or on adjacent days after injection of allogeneic cells.
In contrast, beginning 2 days after BLV injection, percentages of IgM+ cells rose in efferent lymph of the infected sheep and then oscillated above baseline (Fig. 5A, right). IgM+ cells underwent pronounced decreases in sheep 454 on days 7 and 14. Both times, its lymph cells were fragile: only 63% and 51%, respectively, of immunostained cells were in the live gate, compared its overall average of 85%. IgM+ cells decreased once during days 7 to 10 in sheep 972, but its lymph cells were not especially fragile, retaining close to the overall average of 94% live-gated cells after immunostaining. In both animals, CD8+ cell levels in lymph also rose and oscillated following infection. Notably, CD8+ cell levels were high when IgM+ cell levels were low (Fig. 5B); this pattern was unique to the infected animals. These reciprocal oscillations were the most striking alteration in their lymph cell profiles.
During the interval when infectious centers began to emerge in lymph of the infected sheep, CD8+ blasts increased (Fig. 5C). In mock-infected animals, CD8+ blasts increased to a lesser extent several days later, suggesting a response to the injected allogeneic cells. The earlier rise and higher percentage of CD8+ blasts in infected animals were therefore BLV related. A second increase in CD8+ blasts on day 13 or 14 in infected animals was not paralleled in mock-infected animals, suggesting that it too was BLV related.
Both infected and mock-infected animals had similar proportions of CD4+ blasts among lymph cells, whereas IgM+ and WC1+ blasts varied greatly among individuals. On average, IgM+ blasts accounted for a larger fraction of IgM+ cells in infected animals.
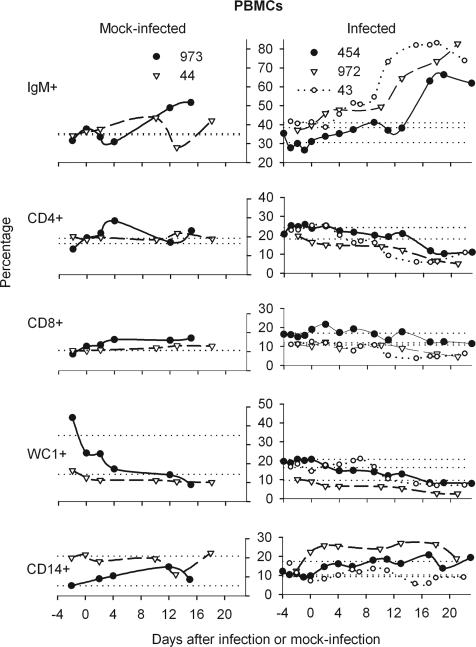
BLV-infected animals displayed late, sharp increases in PBMCs having high levels of surface IgM.
Beginning on day 13, BLV-infected, cannulated animals displayed sharp increases in IgM+ peripheral blood cells (Fig. 6); IgM+ cells increased earlier, after day 9, in the sham-cannulated sheep. Percentages of CD14+ cells were somewhat elevated over baseline in the infected, cannulated animals, but one mock-infected animal also displayed increased CD14+ cells while the infected, sham-cannulated sheep did not, suggesting that cannulation was responsible. Decreased percentages of CD4+ and WC1+ blood cells counterbalanced the large increases in IgM+ cells in infected animals, whereas mock-infected animals had relatively stable percentages of CD4+, CD8+, and WC1+ blood cells. Therefore, the most striking alteration in immune cell balance in the blood of infected animals was the sharp increase in IgM+ cells beginning on days 9 to 13.
FIG. 6.
Cell lineages among PBMCs of mock-infected and infected sheep. Data are presented as described for Fig. 5A with the addition of percentages for sham-cannulated, infected sheep 43.
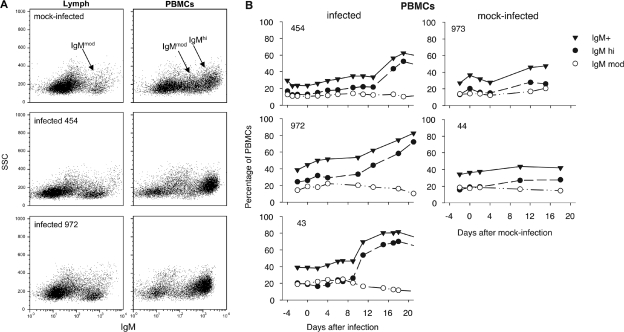
This increase was attributable to a specific subset of B cells. Two populations of IgM+ lymphocytes were present among PBMCs (Fig. 7A): in one population, cells stained intensely for surface IgM (IgMhi) and had slightly more side-scatter than cells in the other population, which stained moderately for surface IgM (IgMmod). BLV infection specifically increased IgMhi B cells in peripheral blood beginning 9 to 13 days after infection (Fig. 7B); mock-infected sheep displayed only mild increases in IgMhi PBMCs. In contrast to blood, lymph did not contain a discrete population of IgMhi cells (Fig. 7A). As is discussed later, the IgMhi peripheral blood cells probably constitute a subpopulation of B cells that does not recirculate through lymph.
FIG. 7.
Accumulation of cells with high levels of surface IgM in blood, but not lymph, of BLV-infected animals. (A) Dot plots of surface-stained, live-gated lymph cells (left) and PBMCs (right) are displayed as side scatter (SSC) versus intensity of IgM staining. IgM+ cell populations from cannulated sheep 973 are from day 15 after mock infection; cells from infected sheep 454 (day 19) and 972 (day 18) are from the days of peak incidence of infectious centers. (B) Percentages of IgM+, IgMhi, and IgMmod cells among live-gated PBMCs from BLV-infected and mock-infected sheep were obtained using FloJo software from additional gates drawn around the populations shown in panel A and are plotted as a function of time after injection of allogeneic cells.
Most CA+ mononuclear cells among cultured lymph and peripheral blood cells were IgM+.
In the peripheral blood of sheep and cattle, IgM+ B cells are the predominant BLV hosts. To determine whether this is also true of efferent lymph cells, we doubly stained cells that had been LPS stimulated in overnight cultures. From lymph samples obtained on the days when animals had maximal levels of BLV-positive cells, ∼0.5% of the cells were CA+ (Table 3) and 64 to 73% of these CA+ cells were IgM+. Only 11 to 12% of the lymph cells were IgM+ at the time of staining, so B cells were enriched five- to sixfold among CA+ cells. Although 3.5 to 14% of CA+ cells in these lymph cell cultures bore a T-cell marker (CD4, CD8, or WC1), the predominant lymph cell containing BLV CA protein was clearly IgM+.
TABLE 3.
Lineage of CA+ cells after overnight culture
| Cell source or type | Sheep no. | Day p.i.a | LPSc | CA+ cells (% of all mononuclear cells) | Distribution of CA+ cells among indicated subsets (%)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| IgMhi | IgMmod | CD4+ | CD8+ | WC1+ | |||||
| Efferent lymph | 454 | 19 | + | 0.47 | 64 | 13 | 13 | 10 | |
| 972 | 21 | + | 0.49 | 73 | 14 | 9.5 | 3.5 | ||
| PBMCs | 454 | 19 | − | 8.36 | 66 | 22 | 5 | 4 | 3 |
| + | 16.44 | 69 | 25 | 2 | 2.5 | 1.5 | |||
| 972 | 21 | + | 17.57 | 81 | 14 | 2 | 2 | 1 | |
| 43b | 15 | + | 9.94 | 83 | 14 | 1.2 | 0.6 | 1.2 | |
Day postinfection (p.i.) when CA+ cells were at maximum frequency.
Sham-cannulated animal.
+, cultured with LPS; −, cultured without LPS.
In cultures of PBMCs obtained on the same days, 88 to 95% of the CA+ cells were IgM+, and most of those were IgMhi. CA+ PBMCs from the sham-cannulated sheep were also mostly IgMhi cells. Supplementation with LPS doubled the number of PBMCs containing CA protein in overnight cultures but did not alter their lineage profile. Cells bearing T-cell markers constituted only minor proportions of CA+ PBMCs, so IgMhi B cells were the predominant CA+ cell in short-term cultures of PBMC prepared when infected cells were most abundant in blood.
Enrichment of recently proliferated CD8+ and IgM+ cells in lymph on day 6 or 7 after BLV infection.
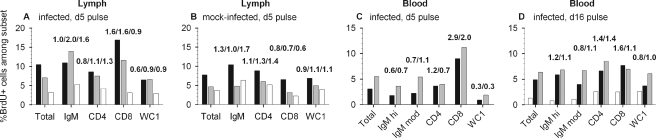
Immune cells responding to BLV antigens should proliferate in the draining lymph node (5). Moreover, as an oncoretrovirus, BLV is thought to require proliferating host cells to establish a stable, productive infection. We first detected infectious centers in lymph on days 4 to 6, and increased numbers of enlarged cells began to emerge on day 6 from the newly infected lymph node. To determine which cells were in S phase of the mitotic cell cycle late on day 5 in vivo, we injected BrdU intravenously. On each of the following three days, we doubly stained efferent lymph cells for lineage markers and for BrdU incorporated into DNA.
Fourteen hours after the pulse, 10.5% of efferent lymph cells from an infected sheep were BrdU+; the percentages decreased progressively in lymph samples collected at 38 and 62 h (Fig. 8A). At 14 h, 16.9% of CD8+ cells were BrdU+, a 1.6-fold enrichment of proliferating cells relative to the proportion present among all lymph cells. At 38 h, CD8+ cells were again enriched 1.6-fold and IgM+ cells were enriched 2.0-fold with BrdU+ cells. In comparison, a mock-infected sheep had 8% BrdU+ cells in an efferent lymph sample collected 14 h after precursor injection (Fig. 8B). CD8+ cells were not enriched with proliferating cells in any sample from this animal. Only mild enrichment with BrdU+ cells was observed for IgM+ cells at 14 h and for CD4+ cells at 38 and 62 h. In the second mock-infected sheep, 7% of lymph cells were BrdU+ when sampled 15 h after precursor injection, and CD4+ and CD8+ cells were only 1.2-fold enriched with BrdU+ cells (data not shown). Thus, CD8+ cells that had recently synthesized DNA were markedly enriched in lymph of a BLV-infected sheep for 2 days following a day 5 pulse of BrdU, and the level of recently proliferated IgM+ cells was increased in lymph collected on the second day. Corresponding increases did not occur in animals injected with uninfected, allogeneic cells.
FIG. 8.
Percentages of BrdU+ cells present among efferent lymph cells and PBMCs after intravenous pulses of BrdU. The percentage of BrdU-stained cells, as determined by flow cytometry, is plotted for the total live-cell population as well as within IgM+, CD4+, CD8+, and WC1+ cell subsets. Numbers above each group of bars indicate enrichment (n-fold) for BrdU+ cells within a subset relative to the frequency of BrdU+ cells within the whole cell population. (A and B) BrdU was injected on day 5 after infection or mock infection, and lymph was collected for staining after 14 h (black bars), 38 h (gray), and 62 h (white). (C) BrdU was injected into sham-cannulated sheep 43 on day 5 after infection and blood was collected 15 h (black bars) and 40 h (gray) later. (D) On day 15, blood was collected from sham-cannulated sheep 43 to evaluate residual BrdU+ cells (white bars): relative enrichments within subsets were 0.6 (IgMhi), 0.8 (IgMmod), 2.0 (CD4+), 1.9 (CD8+), and 2.0 (WC1+). BrdU was then injected on day 16, and blood was collected 15 h (black bars) and 39 h (gray) later.
We assessed blood-borne BrdU+ cells after a day 5 intravenous pulse of the infected, sham-cannulated sheep. At 14 h, 3% of PBMCs were labeled, and 5.5% were positive at 38 h (Fig. 8C). CD8+ PBMCs were markedly enriched (2.9- and 2.0-fold) with BrdU+ cells at these two times. Thus, CD8+ cells that had been in S phase late on day 5 after BLV was introduced into a new host animal were present in both blood and lymph on days 6 and 7. In contrast to results with lymph cells, fewer IgM+ PBMCs were BrdU+ than expected from their proportions in the population, with the exception of IgMmod cells on day 7.
Proliferative cells during the later B-cell increase in peripheral blood.
To determine the contribution of ongoing cell proliferation to the later B-cell increase in peripheral blood, we injected the infected, sham-cannulated sheep with BrdU again on day 16. The previous day, only 1.3% of its PBMCs still contained BrdU that had been introduced during the day 5 pulse (Fig. 8D). Residual BrdU+ cells were notably underrepresented among IgMhi cells, which constituted 66% of the PBMCs at the time. After the second pulse, PBMCs collected on days 17 and 18 included 4.9% and 6.4% BrdU+ cells (Fig. 8D). BrdU+ IgMhi cells appeared rapidly in the blood: on days 17 and 18, 6 to 7% of IgMhi cells were BrdU+. In contrast, the IgMmod subpopulation accumulated BrdU+ cells more slowly, taking until day 18 to reach a level equivalent to that of all PBMCs, collectively. By comparison, in a cannulated, mock-infected sheep that was pulsed with BrdU on day 16, BrdU+ PBMCs did not appear in significant numbers until day 18, and IgMhi cells were not enriched with BrdU+ cells on either day 17 or day 18 (data not shown). In the infected, sham-cannulated sheep (Fig. 8D), CD8+ cells were enriched 1.6-fold with BrdU+ cells on day 17. Therefore, when B cells were increasing rapidly in the peripheral blood of sheep newly infected with BLV, IgMhi cells comprised most of the proliferating cells, and 6 to 7% of IgMhi cells that were present in the circulation on day 17 or 18 had acquired BrdU late on day 16.
DISCUSSION
These results provide unique insights into the dissemination of virus-infected cells and development of the adaptive immune response during a new BLV infection. They also identify possibilities for interplay between virus replication and the immune response. The virological aspects of infection and the humoral immune response to BLV were remarkably similar in the cannulated sheep, whereas individual sheep differed in the timing of alterations in immune cell balances and in cell fragility at key points.
Establishment of infection in cells of the new host.
We did not directly address aspects of infection that preceded the emergence of cells acting as infectious centers from the prescapular lymph node on day 4. The injected allogeneic cells and any virions present in the culture supernatant should have been rapidly transported by dendritic cells to the draining prescapular lymph node. Afferent lymph draining from an injection site contains fluorescent dendritic cells within 20 to 60 min after intradermal injection of fluorescein-labeled protein (18) and within 1 to 4 h after injection of a plasmid encoding green fluorescent protein (54). The events ensuing upon the arrival of the viral genome in the lymph node depend on whether the viral genome was delivered as a DNA provirus in allogeneic cells, as viral RNA in virions, or in some type of viral structure within allogeneic cells. At this juncture, immunoreactivity of graft versus host and host versus graft could play critical roles by stimulating virus production and activating cells to be targets for virus infection. Production and release of virus by the injected allogeneic cells, virus entry into cells of the new host, reverse transcription, proviral integration, and viral gene expression are all required to establish stable infection in the new host. These events could occur in the initially infected lymph node, but host cells harboring newly acquired BLV virions could also emigrate before completing provirus synthesis and integration. If so, such cells are very rare or they harbor very few virions, as we detected fresh efferent lymph cells containing the viral CA protein only once, on day 14.
Dissemination of infected cells and amplification of infection.
The presence of infectious centers in day 4 lymph samples signaled that BLV arrived in distal lymphoid tissue by days 4 to 6. Our measurements of lymph flow indicate that ∼2 × 104 infectious centers emerged through the cannulated lymphatic duct on day 4 and ∼2 × 106 on day 6. Other efferent ducts carried infected cells through lymph to the blood, where factors governing lymphocyte recirculation determined whether the initially very rare BLV-infected cells reentered lymphatic tissue or remained in blood. Only ∼1% of sheep lymphocytes are present in blood at any given time (4). Before infectious centers began to increase rapidly in blood (days 10 to 13), new host cells must have acquired BLV, either as infectious virus produced by cells that had traveled to distal sites via lymph and blood or as proviruses acquired by division of already-infected cells.
Rapid humoral immune response.
Sufficient antigen was present to stimulate the secretion of CA-specific antibodies into lymph as early as day 6 and that of SU antibodies by 1 to 3 days later. The appearance of both antibodies in serum lagged by 1 to 2 weeks. A shorter delay occurred in the sham-cannulated sheep, perhaps because it had not sustained continuous loss of cells and antibodies in dripping lymph. CA-specific antibodies were similarly present as early as day 4 in the lymph of sheep injected with cell-free maedi-visna virus, and SU antibodies followed a week later (2). In each case, virus-infected cells disseminate in lymph along with antiviral antibodies. Moreover, in both lymph and serum, CA-specific antibodies are an earlier signal of infection than the SU antibodies that are conventionally used to define seroconversion for BLV.
Immune cell profiles in lymph.
A cell-mediated response by cytotoxic T lymphocytes should ensue when BLV begins to replicate in cells of the new host. CD8+ peripheral blood cells have been reported to increase just prior to seroconversion after de novo BLV infection of sheep (53), and peptides derived from the BLV surface glycoprotein induce CD8+ cytotoxic T-lymphocyte activity (13, 21, 25). In our BLV-infected sheep, CD8+ cells were activated at times that suggested a virus-induced response. CD8+ blasts increased between days 4 and 8, just when infectious centers began to emerge in efferent lymph, and increased again on day 13 or 14. Fully stimulated lymphoblasts enter S phase; increased BrdU incorporation into CD8+ cells in vivo on day 5 was unique to the BLV-infected animals. Labeled CD8+ cells disseminated into blood as early as day 6.
The episodes in which CD8+ lymph cells increased and IgM+ cells decreased during the first 2 weeks of infection must reflect both direct and indirect effects of BLV infection on B cells. Absolute numbers of CD8+ cells were increased, so it is tempting to speculate that antigen-specific cell killing took place. However, we did not directly test CD8+ cytotoxic activity. CD8+ blasts increased on days 6 to 11 in lymph of sheep injected with maedi-visna virus; however, the cells lacked an activation protein and cells from only one of five animals exhibited direct cytotoxic activity. These deficits potentially contribute to viral evasion of immune defenses (2).
Ruminants have a notable proportion of γδ T cells in their blood and lymph. Blood-derived γδ T cells from cattle that have stable BLV infections, but do not display the lymphoproliferation called persistent lymphocytosis, exhibit non-major histocompatibility complex-restricted cytotoxic activity against the BLV envelope protein in culture (35). In our study, levels of WC1+ cells did not vary markedly during early infection except on day 14 in the lymph of one sheep, when these cells increased strikingly in percentage as well as in absolute numbers. This happened when IgM+ cells were low, raising the possibility that WC1+ γδ T cells may contribute protective responses during acute BLV infection.
BLV host cells.
A measurable fraction of fresh lymph cells from this same infected sheep contained CA protein on day 14, when lymph cells were fragile, large cells were disproportionately reduced, IgM+ cells were reduced, and CD8+ and WC1+ cells were increased. These features may delineate a round of BLV replication and may spread to new cells. The presence of CA protein could indicate recent viral entry into cells; we have found that CA protein, produced by BLV-infected cells in cultures of PBMCs and presumably contained within virions, can spread to new cells within the cultures and be detected by flow cytometry (B. E. Fulton and K. Radke, unpublished data; reference 12).
BLV appears to be able to enter lymph node-derived T cells early after sheep are infected, but B cells are clearly the preferred hosts because the majorities of CA+ lymph cells and PBMCs in cultures prepared at the peak of BLV incidence were IgM+. Only very small percentages of CD4+, CD8+, and γδ cells were CA+ among PBMCs, but CA+ T cells were more prevalent among cultured lymph cells, among which T cells were present in proportions higher than those seen for PBMCs. Lymph-borne T cells could have acquired CA protein as virions during short-term culture, or they could have acquired BLV in the lymph node and then synthesized CA protein after expression of proviral genomes in culture. CD4+ and CD8+ peripheral blood T cells grown from mitogen-stimulated, long-term cultures of bovine PBMCs contain the BLV provirus (49), so these cells are capable of supporting the viral life cycle at least through reverse transcription.
B-cell dynamics following de novo infection.
BLV infection stimulated proliferation on day 5 of IgM+ B cells that were subsequently enriched in a day 7 lymph sample. Proliferating cells are potential hosts for stable retrovirus infections, but at maximum, only 1 in 400 BrdU+ B cells were productively infected on day 7, because only 0.008% of lymph cells were centers of infection when 3.3% of lymph cells were BrdU+ B cells. Additional cells might harbor BLV but not be capable of producing infectious virus in the cultures.
After day 13, infectious centers became more prevalent in blood than in efferent lymph. The pool of lymphocytes recirculating through lymphoid tissue is 10 to 20 times larger than that in blood (47), so either BLV entered a population of nonrecirculating cells or infected cells lost their ability to recirculate. We believe that the IgMhi PBMCs whose increase began on day 9 or 10 in infected animals were nonrecirculating B cells. Sheep have two roughly equal populations of B cells in peripheral blood. One population reenters lymph from blood and is also found in lymph nodes and the follicles of the spleen and Peyer's patches; the cells have low levels of surface IgM and display CD21 and L-selectin, but not CD11b (17, 59). In contrast, nonrecirculating B cells stay in the blood and are also found in the marginal zone of the spleen; these cells have high levels of surface IgM and display CD11b and CD11c, but not L-selectin. The latter cells frequently display CD5, are large, have high proliferative activity, and are similar in these respects to B-1 cells in mice (7). CD11b+, IgMhi sheep B cells exhibit greater forward and side scatter than CD11b− B cells (8). We thus infer that the higher-side-scatter IgMhi B cells accumulating in the blood of our sheep were nonrecirculating.
Both IgMhi and IgMmod B cells can be BLV hosts in sheep: both types of IgM+ cells contained CA protein after PBMCs from our sheep were cultured overnight. Moreover, BLV proviruses are present in populations of both CD11b+ and CD11b− B cells harvested from the blood of sheep at 4 weeks after BLV infection (8). Interestingly, BLV-induced tumors in sheep are CD5-negative B cells (40). It has been speculated that CD5+ B cells are opportunistic hosts for BLV infection because of their increased capacity for self-renewal (10).
We learned that the B cells increased by BLV infection in blood differ from the B cell population in efferent lymph. BLV was probably transmitted from infected lymph cells into IgMhi cells 4 to 13 days after virus was introduced into the skin, but where this happened is unknown. Although IgMhi cells are found in the marginal zone of the spleen, that organ is not required for the establishment of BLV infection in calves or for the maintenance of an infection initiated 10 weeks prior to splenectomy (52).
Blood-borne IgM+ cells, specifically IgM+ CD5+ B cells (10), increase early during BLV infection of sheep and calves (11, 29, 50). The proliferation of IgM+ cells exceeds that for mock-infected controls and contributes to the increasing numbers of blood-borne B cells present at the time of seroconversion. After we exposed cells in vivo to BrdU on day 16, 6% of the IgMhi PBMCs examined 15 h later were labeled, demonstrating that the dividing cells moved rapidly into the circulation. In another study, a sheep injected with BLV provirus was pulsed with BrdU on the day it seroconverted to SU+, and 3 days later, 6% of the IgM+ PBMCs were BrdU+ (10), demonstrating a similar contribution of recently proliferated cells to the circulating B-cell population.
During this transient, early lymphocytosis, BLV-induced proliferation affects more than the number of productively infected cells. In our sheep, only 1 to 8% of PBMCs acted as infectious centers when 60% of PBMCs were IgMhi cells, complementing the finding that proviral load varies markedly among seroconverting sheep exhibiting similar degrees of lymphocytosis (10). Both studies show that the proliferative capacity of sheep B cells is altered in an animal at the time of seroconversion but that not all proliferative cells are productively infected.
In summary, when we infected sheep by intradermal and subcutaneous injection of BLV, virus-infected cells disseminated on days 4 to 6 in lymph emerging from the regional, draining lymph node. Despite the early onset of adaptive immunity, BLV continued to spread to new host cells because increasing numbers of BLV infectious centers appeared in lymph and blood. At a yet to be identified anatomic site, BLV was transmitted into a population of IgMhi B cells that do not recirculate through lymph, and after day 10, IgMhi B cells began to increase markedly in blood. Increased proliferation contributed to the lymphocytosis of IgMhi B cells occurring at this time. Both early in lymph and later in blood, only a minority of proliferating cells were productively infected with BLV. Interesting issues that await investigation include how and where BLV is transmitted among host cells as a new infection amplifies, how BLV evades immune responses during early infection, and how proliferation of uninfected B cells is altered.
Acknowledgments
We thank Rob Gunther, Linda Talken, and Jessica Davis (Experimental Animal Surgery Facility of the UC Davis School of Medicine) for providing invaluable surgical expertise; Debbie Grossman and Eleanor Mangosing for technical assistance; Daniel Portetelle (Department of Microbiology, Faculty of Agronomy, Gembloux, Belgium) for the generous gift of BLV CA antibodies; and Kathy Bestul (Synbiotics Corporation, San Diego, CA) for the gift of reagents to measure antibodies to BLV CA protein.
This research was supported by a grant from the University of California Cancer Research Coordinating Committee and by funds from the University of California Agricultural Experiment Station and the UC Davis Cancer Center. Flow cytometry data were acquired at the UC Davis Optical Biology Shared Resource Facility, supported in part by a Cancer Center support grant from the National Cancer Institute, NIH. This facility was constructed with support from Research Facilities Improvement Program grant C06 RR-12088-01 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Aida, Y., M. Miyasaka, K. Okada, M. Onuma, S. Kogure, M. Suzuki, P. Minoprio, D. Levy, and Y. Ikawa. 1989. Further phenotypic characterization of target cells for bovine leukemia virus experimental infection in sheep. Am. J. Vet. Res. 50:1946-1951. [PubMed] [Google Scholar]
- 2.Bird, P., B. Blacklaws, H. T. Reyburn, D. Allen, J. Hopkins, D. Sargan, and I. McConnell. 1993. Early events in immune evasion by the lentivirus maedi-visna occurring within infected lymphoid tissue. J. Virol. 67:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blacklaws, B. A. 1997. Quantification of the reservoir of HIV-1. Trends Microbiol. 5:215-216. [DOI] [PubMed] [Google Scholar]
- 4.Blunt, M. H. 1975. The blood of sheep: composition and function. Springer-Verlag, Berlin, Germany.
- 5.Cahill, R., J. B. Hay, H. Frost, and Z. Trnka. 1974. Changes in lymphocyte circulation after administration of antigen. Haematologia 8:321-334. [PubMed] [Google Scholar]
- 6.Chakrabarti, L., P. Isola, M. C. Cumont, M. A. Claessens-Maire, M. Hurtrel, L. Montagnier, and B. Hurtrel. 1994. Early stages of simian immunodeficiency virus infection in lymph nodes. Evidence for high viral load and successive populations of target cells. Am. J. Pathol. 144:1226-1237. [PMC free article] [PubMed] [Google Scholar]
- 7.Chevallier, N., M. Berthelemy, V. Laine, D. Le Rhun, F. Femenia, B. Polack, J. Naessens, D. Levy, and I. Schwartz-Cornil. 1998. B-1-like cells exist in sheep. Characterization of their phenotype and behaviour. Immunology 95:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevallier, N., M. Berthelemy, D. Le Rhun, V. Laine, D. Levy, and I. Schwartz-Cornil. 1998. Bovine leukemia virus-induced lymphocytosis and increased cell survival mainly involve the CD11b+ B-lymphocyte subset in sheep. J. Virol. 72:4413-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, O. J., G. Pantaleo, D. J. Schwartzentruber, C. Graziosi, M. Vaccarezza, and A. S. Fauci. 1995. Pathogenic insights from studies of lymphoid tissue from HIV-infected individuals. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 10(Suppl. 1):S6-S14. [PubMed] [Google Scholar]
- 10.Debacq, C., M. T. Sanchez Alcaraz, F. Mortreux, P. Kerkhofs, R. Kettmann, and L. Willems. 2004. Reduced proviral loads during primo-infection of sheep by bovine leukemia virus attenuated mutants. Retrovirology 1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimmock, C. K., W. H. Ward, and K. F. Trueman. 1989. Lymphocyte subpopulations in sheep during the early stage of experimental infection with bovine leukaemia virus. Immunol. Cell Biol. 67:141-145. [DOI] [PubMed] [Google Scholar]
- 12.Fulton, B. E., Jr. 2001. Ph.D. thesis. University of California, Davis, Calif.
- 13.Gatei, M. H., M. F. Good, R. C. Daniel, and M. F. Lavin. 1993. T-cell responses to highly conserved CD4 and CD8 epitopes on the outer membrane protein of bovine leukemia virus: relevance to vaccine development. J. Virol. 67:1796-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaynor, E. M., M. L. Mirsky, and H. A. Lewin. 1996. Use of flow cytometry and RT-PCR for detecting gene expression by single cells. BioTechniques 21:286-291. [DOI] [PubMed] [Google Scholar]
- 15.Glover, D. J., and J. G. Hall. 1976. A method for the collection of lymph from the prescapular lymph node of unanaethetized [sic] sheep. Lab. Anim. 10:403-408. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, V. K., I. McConnell, R. G. Dalziel, and J. Hopkins. 1996. Identification of the sheep homologue of the monocyte cell surface molecule—CD14. Vet. Immunol. Immunopathol. 51:89-99. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, V. K., I. McConnell, R. G. Dalziel, and J. Hopkins. 1998. Two B cell subpopulations have distinct recirculation characteristics. Eur. J. Immunol. 28:1597-1603. [DOI] [PubMed] [Google Scholar]
- 18.Harkiss, G. D., J. Hopkins, and I. McConnell. 1990. Uptake of antigen by afferent lymph dendritic cells mediated by antibody. Eur. J. Immunol. 20:2367-2373. [DOI] [PubMed] [Google Scholar]
- 19.Heeney, J. L., P. J. Valli, R. M. Jacobs, and V. E. Valli. 1992. Evidence for bovine leukemia virus infection of peripheral blood monocytes and limited antigen expression in bovine lymphoid tissue. Lab. Investig. 66:608-617. [PubMed] [Google Scholar]
- 20.Hein, W. R., and P. J. Griebel. 2003. A road less travelled: large animal models in immunological research. Nat. Rev. Immunol. 3:79-84. [DOI] [PubMed] [Google Scholar]
- 21.Hislop, A. D., M. F. Good, L. Mateo, J. Gardner, M. H. Gatei, R. C. Daniel, B. V. Meyers, M. F. Lavin, and A. Suhrbier. 1998. Vaccine-induced cytotoxic T lymphocytes protect against retroviral challenge. Nat. Med. 4:1193-1196. [DOI] [PubMed] [Google Scholar]
- 22.Holm, M., M. Thomsen, M. Hoyer, and P. Hokland. 1998. Optimization of a flow cytometric method for the simultaneous measurement of cell surface antigen, DNA content, and in vitro BrdUrd incorporation into normal and malignant hematopoietic cells. Cytometry 32:28-36. [DOI] [PubMed] [Google Scholar]
- 23.Issekutz, T. B. 1985. Characteristics of lymphoblasts appearing in efferent lymph in response to immunization with vaccinia virus. Immunology 56:23-31. [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston, E. R., M. A. Powers, L. C. Kidd, and K. Radke. 1996. Peripheral blood mononuclear cells from sheep infected with a variant of bovine leukemia virus synthesize envelope glycoproteins but fail to induce syncytia in culture. J. Virol. 70:6296-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabeya, H., K. Ohashi, K. Ohishi, C. Sugimoto, H. Amanuma, and M. Onuma. 1996. An effective peptide vaccine to eliminate bovine leukaemia virus (BLV) infected cells in carrier sheep. Vaccine 14:1118-1122. [DOI] [PubMed] [Google Scholar]
- 26.Kazanji, M., A. Ureta-Vidal, S. Ozden, F. Tangy, B. de Thoisy, L. Fiette, A. Talarmin, A. Gessain, and G. de The. 2000. Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): provirus expression, persistence, and humoral and cellular immune responses. J. Virol. 74:4860-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenyon, S. J., and C. E. Piper. 1977. Cellular basis of persistent lymphocytosis in cattle infected with bovine leukemia virus. Infect. Immun. 16:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidd, L. C., and K. Radke. 1996. Lymphocyte activators elicit bovine leukemia virus expression differently as asymptomatic infection progresses. Virology 217:167-177. [DOI] [PubMed] [Google Scholar]
- 29.Klintevall, K., L. Fuxler, and C. Fossum. 1997. Bovine leukemia virus: early reflections in blood after an experimental infection of calves. Comp. Immunol. Microbiol. Infect. Dis. 20:119-130. [DOI] [PubMed] [Google Scholar]
- 30.Kruisbeck, A. M. 2000. Isolation and fractionation of mononuclear cell populations, p. 3.1.1-3.1.5. In J. E. Coligan, A. M. Kruisbeck, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y.
- 31.Lafeuillade, A., C. Poggi, C. Tamalet, and N. Profizi. 1997. Human immunodeficiency virus type 1 dynamics in different lymphoid tissue compartments. J. Infect. Dis. 176:804-806. [DOI] [PubMed] [Google Scholar]
- 32.Lagarias, D. M., and K. Radke. 1989. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J. Virol. 63:2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagarias, D. M., and K. Radke. 1990. Transient increases of blood mononuclear cells that could express bovine leukemia virus early after experimental infection of sheep. Microb. Pathog. 9:147-158. [DOI] [PubMed] [Google Scholar]
- 34.Levy, D., R. Kettmann, P. Marchand, S. Djilali, and A. L. Parodi. 1987. Selective tropism of bovine leukemia virus (BLV) for surface immunoglobulin-bearing ovine B lymphocytes. Leukemia 1:463-465. [PubMed] [Google Scholar]
- 35.Lundberg, P., and G. A. Splitter. 2000. γδ+ T-lymphocyte cytotoxicity against envelope-expressing target cells is unique to the alymphocytic state of bovine leukemia virus infection in the natural host. J. Virol. 74:8299-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay, C. R., W. L. Marston, and L. Dudler. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171:801-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClure, S. J., and W. R. Hein. 1989. Functional characteristics of 197+ CD4− CD8− sheep T lymphocytes: expansion and differentiation of peripheral T cells. Immunol. Cell Biol. 67:223-231. [DOI] [PubMed] [Google Scholar]
- 38.Mirsky, M. L., C. A. Olmstead, Y. Da, and H. A. Lewin. 1996. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J. Virol. 70:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyasaka, M., and Z. Trnka. 1985. Sheep as an experimental model for immunology: immunological techniques in vitro and in vivo, p. 403-423. In I. Lefkovitz and B. Pernis (ed.), Immunological methods. Academic Press, New York, N.Y.
- 40.Murakami, K., K. Okada, Y. Ikawa, and Y. Aida. 1994. Bovine leukemia virus induces CD5− B cell lymphoma in sheep despite temporarily increasing CD5+ B cells in asymptomatic stage. Virology 202:458-465. [DOI] [PubMed] [Google Scholar]
- 41.Naessens, J., C. J. Howard, and J. Hopkins. 1997. Nomenclature and characterization of leukocyte differentiation antigens in ruminants. Immunol. Today 18:365-368. [DOI] [PubMed] [Google Scholar]
- 42.Pantaleo, G., C. Graziosi, J. F. Demarest, O. J. Cohen, M. Vaccarezza, K. Gantt, C. Muro-Cacho, and A. S. Fauci. 1994. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol. Rev. 140:105-130. [DOI] [PubMed] [Google Scholar]
- 43.Paul, P. S., K. A. Pomeroy, D. W. Johnson, C. C. Muscoplat, B. S. Handwerger, F. F. Soper, and D. K. Sorensen. 1977. Evidence for the replication of bovine leukemia virus in the B lymphocytes. Am. J. Vet. Res. 38:873-876. [PubMed] [Google Scholar]
- 44.Powers, M. A., and K. Radke. 1992. Activation of bovine leukemia virus transcription in lymphocytes from infected sheep: rapid transition through early to late gene expression. J. Virol. 66:4769-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radke, K., D. Grossman, and L. C. Kidd. 1990. Humoral immune response of experimentally infected sheep defines two early periods of bovine leukemia virus replication. Microb. Pathog. 9:159-171. [DOI] [PubMed] [Google Scholar]
- 46.Radke, K., T. J. Sigala, and D. Grossman. 1992. Transcription of bovine leukemia virus in peripheral blood cells obtained during early infection in vivo. Microb. Pathog. 12:319-331. [DOI] [PubMed] [Google Scholar]
- 47.Schnappauf, H., and U. Schnappauf. 1968. Drainage of the thoracic duct and amount of the “easily mobilized” lymphocytes in calves, sheep and dogs. Blut 16:209-220. (In German.) [DOI] [PubMed] [Google Scholar]
- 48.Schwartz, I., A. Bensaid, B. Polack, B. Perrin, M. Berthelemy, and D. Levy. 1994. In vivo leukocyte tropism of bovine leukemia virus in sheep and cattle. J. Virol. 68:4589-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stott, M. L., M. C. Thurmond, S. J. Dunn, B. I. Osburn, and J. L. Stott. 1991. Integrated bovine leukosis proviral DNA in T helper and T cytotoxic/suppressor lymphocytes. J. Gen. Virol. 72:307-315. [DOI] [PubMed] [Google Scholar]
- 50.Ungar-Waron, H., R. Paz, J. Brenner, B. Yakobson, N. Partosh, and Z. Trainin. 1999. Experimental infection of calves with bovine leukemia virus (BLV): an applicable model of a retroviral infection. Vet. Immunol. Immunopathol. 67:195-201. [DOI] [PubMed] [Google Scholar]
- 51.Van Der Maaten, M. J., and J. M. Miller. 1978. Sites of in vivo replication of bovine leukemia virus in experimentally infected cattle. Ann. Rech. Vet. 9:831-835. [PubMed] [Google Scholar]
- 52.Van Der Maaten, M. J., J. M. Miller, and M. J. Schmerr. 1982. Role of the spleen in the pathogenesis of experimentally induced bovine leukemia virus infection. Vet. Microbiol. 7:411-418. [DOI] [PubMed] [Google Scholar]
- 53.Ward, W. H., C. K. Dimmock, and F. W. Eaves. 1992. T lymphocyte responses of sheep to bovine leukaemia virus infection. Immunol. Cell Biol. 70:329-336. [DOI] [PubMed] [Google Scholar]
- 54.Watkins, C., S. Lau, R. Thistlethwaite, J. Hopkins, and G. D. Harkiss. 1999. Analysis of reporter gene expression in ovine dermis and afferent lymph dendritic cells in vitro and in vivo. Vet. Immunol. Immunopathol. 72:125-133. [DOI] [PubMed] [Google Scholar]
- 55.Williams, D. L., O. Barta, and G. F. Amborski. 1988. Molecular studies of T-lymphocytes from cattle infected with bovine leukemia virus. Vet. Immunol. Immunopathol. 19:307-323. [DOI] [PubMed] [Google Scholar]
- 56.Yirrell, D. L., H. W. Reid, M. Norval, G. Entrican, and H. R. Miller. 1991. Response of efferent lymph and popliteal lymph node to epidermal infection of sheep with orf virus. Vet. Immunol. Immunopathol. 28:219-235. [DOI] [PubMed] [Google Scholar]
- 57.Young, A. J. 1998. Sheep immunology and goal peculiarities: leukocyte migration, p. 493-495. In P. G. P. Pastoret, H. Bazin, and A. Govaerts (ed.), Handbook of vertebrate immunology. Academic Press, San Diego, Calif.
- 58.Young, A. J., W. R. Hein, and J. B. Hay. 1997. Cannulation of lymphatic vessels and its use in the study of lymphocyte traffic, p. 2037-2059. In I. Lefkovitz (ed.), Immunology methods manual. Academic Press, San Diego, Calif.
- 59.Young, A. J., W. L. Marston, M. Dessing, L. Dudler, and W. R. Hein. 1997. Distinct recirculating and nonrecirculating B-lymphocyte pools in the peripheral blood are defined by coordinated expression of CD21 and L-selectin. Blood 90:4865-4875. [PubMed] [Google Scholar]