Abstract
Analysis of the interactions of low-risk human papillomavirus type 11 (HPV11) L2 with karyopherin β (Kap β) nuclear import receptors revealed that L2 interacted with Kap β1, Kap β2, and Kap β3 and formed a complex with the Kap α2β1 heterodimer. HPV11 L2 contains two nuclear localization signals (NLSs)—in the N terminus and the C terminus—that could mediate its nuclear import via a classical pathway. Each NLS was functional in vivo, and deletion of both of them abolished L2 nuclear localization. Both NLSs interacted with the viral DNA. Thus, HPV11 L2 can interact with several karyopherins and the viral DNA and may enter the nucleus via multiple pathways.
Human papillomavirus (HPV) virions consist of a single molecule of 8-kb double-stranded circular DNA contained within a spherical capsid composed of 72 L1 capsomers and the L2 minor capsid protein, estimated to have 36 molecules per capsid (10, 15). Although L1 expressed alone in mammalian cells harboring episomal DNA forms virions (18), L2 expression is required for efficient encapsidation of the viral DNA (15, 17). Expression and nuclear import of L2 during the productive stage precede the expression and nuclear translocation of L1 (5). Studies with HPV virions in raft cultures have shown that L2 participates in at least two steps in the production of infectious virus (7). L2 binds to cells (9, 19) and interacts with β-actin and tSNARE syntaxin 18 (1, 20) and also facilitates the escape of the viral genome from the endocytic compartment after viral uncoating (8). Cleavage of L2 at a furin consensus site located in the N terminus was reported to be required for infection (14). Bovine papillomavirus type 1 (BPV1) L2 termini required for infectivity can function as nuclear localization signals (NLSs) mediating nuclear import via a classical pathway, and the C-terminal NLS (cNLS) can also interact with the viral DNA (4). These results, together with the colocalization of the incoming L2 and genome in the nucleus at ND10 (3), suggest that BPV1 L2 may facilitate the nuclear localization of the genome in the initial stages of infection. In a related virus, simian virus 40, nuclear import of simian virus 40 DNA is mediated by the VP3 capsid protein via interaction with the importin heterodimer (12).
Active nuclear import of proteins is mediated by import receptors of the karyopherin β (Kap β)/importin β superfamily that interact with nucleoporins at the nuclear pore complex to transport the proteins into the nucleus. Binding of nuclear RanGTP to the Kap βs causes dissociation of the import complexes, leading to the release of the transported cargoes inside the nucleus (6, 11). In this study we investigated the interactions of the L2 minor capsid protein of low-risk HPV11 with import receptors and viral DNA and mapped its NLSs and DNA binding sites.
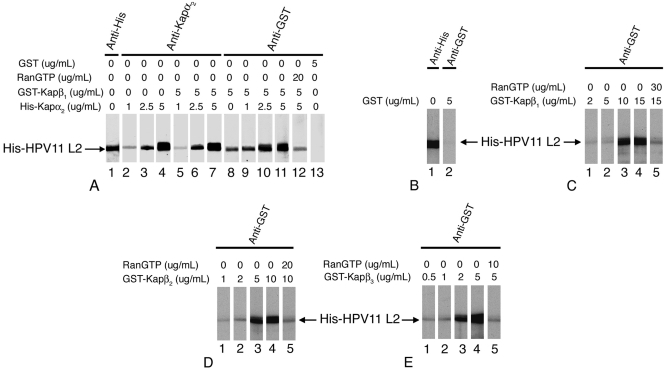
The His-tagged HPV11 L2 contained in the pProEX HTb plasmid vector (16) was expressed in Escherichia coli BL21-CodonPlus and purified as previously described (2). We analyzed the interactions between His-tagged HPV11 L2 and the karyopherins via overlay blotting assays (2). The L2 blots were either detected with an anti-His antibody or incubated with increasing concentrations of different Kaps (Fig. 1). The Kap α2 adapter bound to L2 at concentrations of 2.5 and 5 μg/ml Kap α2 in either the absence or presence of Kap β1 (Fig. 1A, lanes 3, 4, 6, and 7). Glutathione S-transferase (GST)-Kap β1 bound directly to L2, but in the presence of increasing concentrations of Kap α2, the amount of Kap β1 bound increased (Fig. 1A, lanes 8, 10, and 11). Moreover, RanGTP inhibited the formation of the Kap α2β1/L2 complex (Fig. 1A, lane 12), suggesting that the complex can be dissociated in the nucleus by RanGTP. GST did not bind L2 (Fig. 1A, lane 13). These data suggest that HPV11 L2 forms an import complex with Kap α2β1 heterodimers via interaction with the Kap α2adapter.
FIG. 1.
HPV11 L2 forms a complex with the Kap α2β1 heterodimer and interacts with the Kap β1, Kap β2, and Kap β3 import receptors. Purified HPV11 L2 was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. (A) One L2 blot was detected with anti-His antibody (lane 1). Other L2 blots were incubated with increasing concentrations of Kap α2 in the absence (lanes 2 to 4) or in the presence (lanes 5 to 7 and 9 to 11) of 5 μg/ml GST-Kap β1. For lane 12, the L2 blot was incubated with Kap α2 and GST-Kap β1 plus RanGTP. Separate L2 blots were incubated with GST-Kap β1 alone (lane 8) or GST (lane 13). Bound Kap α2 was detected with an anti-Kap α2 antibody (Ab) (lanes 2 to 7), and bound GST-Kap β1 and GST were detected with an anti-GST Ab (lanes 8 to 13). (B) L2 blots were incubated with either anti-His Ab (lane 1) or with 5 μg/ml GST and then anti-GST Ab (lane 2). (C) L2 blots were incubated with increasing concentrations of GST-Kap β1 (lanes 1 to 4) or with GST-Kap β1 plus RanGTP (lane 5), and the bound GST-Kap β1 was detected with an anti-GST Ab. For panels D and E, similar incubations were done with GST-Kap β2 and GST-Kap β3, respectively.
Three import receptors interacted directly with HPV11 L2 but with different affinities: GST-Kap β1 at concentrations of 10 and 15 μg/ml (Fig. 1C), GST-Kap β2 at 5 and 10 μg/ml (Fig. 1D), and GST-Kap β3 at 2 and 5 μg/ml (Fig. 1E). GST did not interact with HPV11 L2 (Fig. 1B). Significantly, RanGTP inhibited the interactions between each Kap β import receptor and L2 (Fig. 1C, D, and E, lanes 5). We have previously shown that high-risk HPV16 L2 interacts with Kap α2β1, Kap β2, and Kap β3 (2). Thus, L2 of low-risk HPV11 has a conserved pattern of interactions with HPV16 L2 but also interacts directly with Kap β1. In contrast, BPV1 L2 interacts with Kap α2β1 heterodimers but not with either Kap β2 or Kap β3 (4).
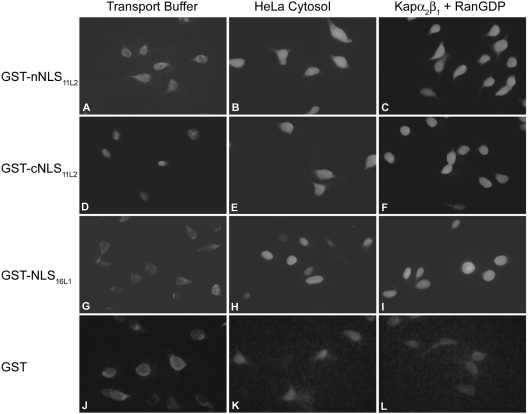
HPV16 L2 contains two NLSs, localized near the N terminus (nNLS) and the C terminus, that can independently mediate nuclear import of L2 (2), and in other HPV L2 proteins, these positively charged sequences are partially conserved. We made GST fusion proteins as previously described (2) with the corresponding N-terminal sequence (nNLS = 1MKPRARRRKRA11) and C-terminal sequence (cNLS = 439ARRRRKRI446) of HPV11 L2 and analyzed them in nuclear import assays in digitonin-permeabilized HeLa cells (2). This analysis revealed that both the nNLS and the cNLS could mediate nuclear import of a GST reporter in the presence of either exogenous cytosol containing the karyopherins or recombinant Kap α2β1 heterodimers plus RanGDP (Fig. 2, panels B, C, E, and F). GST-NLS16L1, used as positive control for the classical pathway (13), was also imported in the presence of either cytosol or Kap α2β1 plus RanGDP (Fig. 2, panels H and I), whereas GST was not (Fig. 2, panels K and L). In agreement with these data, in solution binding assays performed as previously described (2) both the nNLS and cNLS formed complexes with Kap α2β1 heterodimers via interaction with Kap α2, with the nNLS having a higher affinity than the cNLS (data not shown). Assays of L2 or the L2 mutants binding to Kap β2/3 receptors revealed that deletion of the nNLS abolishes the interaction of HPV11 L2 with Kap β2/3 (data not shown), suggesting that the nNLS is required for these interactions, as it is for HPV16 L2 (2).
FIG. 2.
Both the nNLS and the cNLS of HPV11 L2 can mediate nuclear import via a classical pathway. Digitonin-permeabilized HeLa cells were incubated with 0.25 μg of either GST-nNLS11L2 (panels A to C), GST-cNLS11L2 (panels D to F), GST-NLS16L1 (panels G to I), or GST (panels J to L) in the presence of either transport buffer only (panels A, D, G, and J), HeLa cytosol (panels B, E, H, and K), or Kap α2β1 heterodimers (1 μg each) plus RanGDP (3 μg) (panels C, F, I, and L). Note the nuclear import in panels B, C, E, F, H, and I.
To examine the roles of the nNLS and cNLS of HPV11 L2 in vivo, we generated plasmids containing enhanced green fluorescent protein-L2 (EGFP-L2), EGFP-L2ΔN (lacking the nNLS), EGFP-L2ΔC (lacking the cNLS), and EGFP-L2ΔNΔC (lacking both NLSs), using the pEGFP-C1 plasmid (Clontech, Inc.), carried out transfection assays in HeLa cells, and examined the localization of EGFP-L2 fusion proteins via fluorescence microscopy. EGFP-L2 showed a clear nuclear localization, in contrast with the diffuse localization of the EGFP throughout the cell (Fig. 3A, panels A and C). Although the EGFP is small enough to passively diffuse through the nuclear pore complex, EGFP-L2 (as well as L2 itself) is above the limit of passive diffusion and its nuclear localization requires an NLS(s). Both EGFP-L2ΔN and EGFP-L2ΔC had a predominant nuclear localization in HeLa cells (Fig. 3B, panels A and C), suggesting that either NLS can mediate nuclear import of L2 in vivo. In contrast, localization of EGFP-L2ΔNΔC lacking both NLSs was mostly cytoplasmic (Fig. 3B, panel E), suggesting the absence of an additional NLS in HPV11 L2.
FIG. 3.
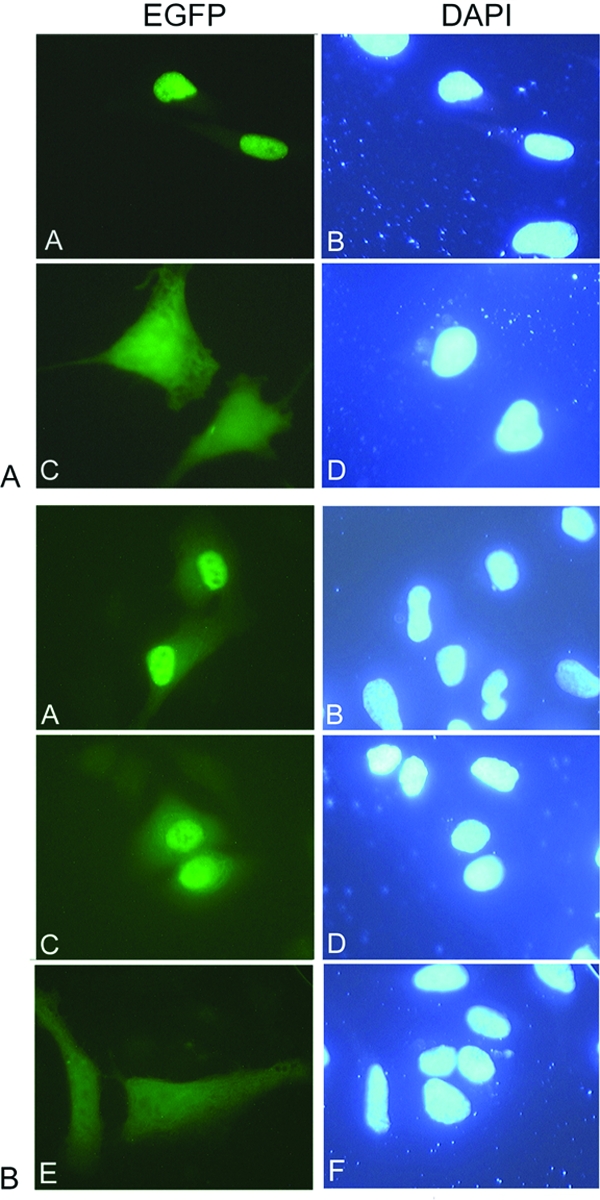
The nNLS and cNLS of HPV11 L2 can independently mediate nuclear localization of L2 in vivo. HeLa cells were transfected with either EGFP-L2 (A, panels A and B), EGFP (A, panels C and D), EGFP-L2ΔN (B, panels A and B), EGFP-L2ΔC (B, panels C and D), or EGFP-L2ΔNΔC (B, panels E and F) plasmids, using FUGENE 6, and were examined by fluorescence microscopy at 24 h posttransfection. The fluorescence of the EGFP is shown in the left column and DAPI (4′,6′-diamidino-2-phenylindole) staining of the nuclei is shown in the right column.
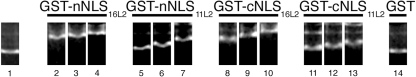
We previously showed that BPV1 L2 interacts via its cNLS with DNA in a DNA sequence-independent manner (4). We analyzed the interactions of the two NLSs of HPV11 L2 and HPV16 L2 with either the HPV16 DNA plasmid or an unrelated DNA using DNA mobility shift assays (4). Each NLS interacted efficiently with the viral DNA and with the unrelated DNA, whereas GST did not (Fig. 4) (data not shown). Deletion of both NLSs in the L2ΔNΔC strongly inhibited the interaction of the L2 proteins with the DNA (data not shown). These data suggest that the NLSs of HPV11 and HPV16 L2 proteins are their DNA binding sites and that this DNA binding occurs without nucleotide sequence specificity.
FIG. 4.
Both the nNLSs and cNLSs of high-risk HPV16 L2 and low-risk HPV11 L2 interact with DNA. An HPV16 DNA plasmid (lane 1) was incubated with increasing amounts (2, 5, or 10 μg of protein) of either GST-nNLS16L2 (lanes 2 to 4), GST-nNLS11L2 (lanes 5 to 7), GST-cNLS16L2 (lanes 8 to 10), or GST-cNLS11L2 (lanes 11 to 13) or 10 μg of GST (lane 14) and analyzed via agarose gel electrophoresis.
Overall, the data show that HPV11 L2 interacts via its two NLSs with several Kaps and the viral DNA and may enter the nucleus via multiple pathways. The high-affinity binding of HPV11 L2 to Kap β2 and Kap β3 suggests that the pathways mediated by these import receptors may be preferentially used by L2 in conditions of competition with host proteins for the classical pathway. Future studies in vivo with HPV L2 proteins and specific mutants will investigate how interference with NLS function affects HPV infection and analyze the potential role of L2 proteins in the nuclear localization of the viral DNA.
Acknowledgments
We thank G. Blobel, Y. M. Chook, S. Adam, G. Dreyfuss, K. Weis, A. Lamond, N. Yaseen, and R. Roden for their generous gifts of expression vectors. We thank Kaitlin Quinn and Anna Leszczynski for technical assistance in preparation of recombinant transport factors and L2 proteins.
This work was supported by a grant from the National Institutes of Health (1-RO1 CA94898-01) to J.M.
REFERENCES
- 1.Bossis, I., R. B. Roden, R. Gambhira, R. Yang, M. Tagaya, P. M. Howley, and P. I. Meneses. 2005. Interaction of tSNARE syntaxin 18 with the papillomavirus minor capsid protein mediates infection. J. Virol. 79:6723-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darshan, M. S., J. Lucchi, E. Harding, and J. Moroianu. 2004. The L2 minor capsid protein of human papillomavirus type 16 interacts with a network of nuclear import receptors. J. Virol. 78:12179-12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day, P. M., C. C. Baker, D. R. Lowy, and J. T. Schiller. 2004. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl. Acad. Sci. USA 101:14252-14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fay, A., W. H. Yutzy IV, R. B. S. Roden, and J. Moroianu. 2004. The positively charged termini of L2 minor capsid protein required for bovine papillomavirus infection function separately in nuclear import and DNA binding. J. Virol. 78:13447-13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florin, L., C. Sapp, R. E. Streeck, and M. Sapp. 2002. Assembly and translocation of papillomavirus capsid proteins. J. Virol. 76:10009-10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried, H., and U. Kutay. 2003. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 60:1659-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren, S. C., N. A. Patterson, M. A. Ozbun, and P. F. Lambert. 2005. The minor capsid protein L2 contributes to two steps in the human papillomavirus type 31 life cycle. J. Virol. 79:3938-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamper, N., P. M. Day, T. Nowak, H. C. Selinka, L. Florin, J. Bolscher, L. Hilbig, J. T. Schiller, and M. Sapp. 2006. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J. Virol. 80:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawana, K., K. Matsumoto, H. Yoshikawa, Y. Taketani, T. Kawana, K. Yoshiike, and T. Kanda. 1998. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology 245:353-359. [DOI] [PubMed] [Google Scholar]
- 10.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moroianu, J. 1999. Nuclear import and export pathways. J. Cell Biochem. Suppl. 32-33:76-83. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi, A., D. Shum, H. Morioka, E. Otsuka, and H. Kasamatsu. 2002. Interaction of the Vp3 nuclear localization signal with the importin α2/ β heterodimer directs nuclear entry of infecting simian virus 40. J. Virol. 76:9368-9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson, L. M., R. C. Rose, and J. Moroianu. 2002. Nuclear import strategies of high risk HPV16 L1 major capsid protein. J. Biol. Chem. 277:23958-23964. [DOI] [PubMed] [Google Scholar]
- 14.Richards, R. M., D. R. Lowy, J. T. Schiller, and P. M. Day. 2006. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 103:1522-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roden, R. B., R. Kirnbauer, A. B. Jenson, D. R. Lowy, and J. T. Schiller. 1994. Interaction of papillomaviruses with the cell surface. J. Virol. 68:7260-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stauffer, Y., K. Raj, K. Masternak, and P. Beard. 1998. Infectious human papillomavirus type 18 pseudovirions. J. Mol. Biol. 283:529-536. [DOI] [PubMed] [Google Scholar]
- 18.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, R., P. M. Day, W. H. Yutzy IV, K.-Y. Lin, C.-F. Hung, and R. B. S. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, R., W. H. Yutzy IV, R. P. Viscidi, and R. B. Roden. 2003. Interaction of L2 with beta-actin directs intracellular transport of papillomavirus and infection. J. Biol. Chem. 278:12546-12553. [DOI] [PubMed] [Google Scholar]