Abstract
Lymphocytes with the regulatory CD4+ CD25+ phenotype frequently suppress memory T-cell responses. Murine herpes simplex virus type 1 (HSV-1) models have shown that CD4+ CD25+ cells can limit immunity-mediated corneal damage but slow viral clearance. We investigated the effect of CD4+ CD25+ cells from healthy HSV-2-infected humans on recall proliferative (CD4) responses to HSV-2. Depletion and reconstitution experiments were consistent with a suppressive effect of autologous blood-derived CD4+ CD25+ cells for whole HSV-2 antigen. Regulatory T cells may modulate human CD4 memory responses to HSV-2 and influence their antiviral and inflammatory functions.
Immune responses to chronic viral infections such as herpes simplex virus type 1 (HSV-1) and HSV-2 are regulated by both host and pathogen feedback mechanisms. Recently, CD4+ T cells expressing the interleukin-2 (IL-2) receptor CD25 in the absence of known recent stimulation have been shown to down-modulate T-cell responses in both antigen-specific and nonspecific fashions and have been labeled T-regulatory (Treg) cells (3). Treg cells may limit pathogen-induced immunopathology and autoimmunity. Modulation of acquired immune responses by CD4+ CD25+ cells has been detected in several human infections (3).
HSV infections cause significant morbidity and mortality (5) and may contribute to human immunodeficiency virus type 1 transmission (6). Improved vaccination and immunotherapy are required, without triggering inappropriate immunity and potentially worsening HSV-induced stromal keratitis (HSK) or herpes-associated erythema multiforme (14). In the HSV system in mice, CD4+ CD25+ cells have contrasting effects on viral clearance and HSK. In vivo depletion of Treg was found to increase acute and memory HSV-specific CD4-T-cell responses in mice (17). This correlated with faster clearance of virus during primary HSV-1 challenge of Treg cell-depleted mice and also with faster clearance of HSV epitope-bearing reporter challenge viruses after previous vaccination with HSV subunit vaccines during Treg cell depletion (18, 20). However, Treg cell depletion was found to increase the systemic and corneal levels of pathogenic Th1-pattern CD4 T cells and worsen clinical scores in murine models of HSK (17).
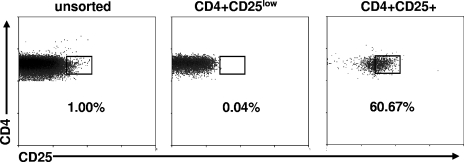
We therefore performed in vitro analyses to investigate whether circulating CD4+ CD25+ cells in HSV-2-infected persons could regulate memory responses to HSV-2 antigen. Subjects with known HSV-2 infection (2) and cytomegalovirus (CMV) infection (12) status who were not symptomatic for HSV at the time of phlebotomy were enrolled according to an institutional review board-approved protocol. Heparin-anticoagulated venous blood was processed (19) for peripheral blood mononuclear cells (PBMC) that were used immediately. Control wells contained either 5 × 104 whole PBMC/well or 5 × 104 γ-irradiated (4,000-rad) PBMC/well and either HSV-2 strain 333 (9) whole antigen (11) (105 PFU prior to UV irradiation) or similarly prepared mock antigen, whole CMV antigen, or phytohemagglutinin (PHA) as described previously (12) in 200 μl T-cell medium (11). PBMC were stained with anti-CD4-tricolor (Caltag, Burlingame, CA), anti-CD25-phycoerythrin, and anti-CD14-fluorescein isothiocyanate (BD Pharmingen, San Jose, CA) for 20 min at 4°C and sorted (MoFlo; DakoCytomation, Fort Collins, CO). Gating excluded nonlymphocytes and CD14+ cells. Total CD4+ cells, CD4+ cells with the brightest 1% CD25 staining, and CD4+ cells with the dimmest 40% CD25 staining were collected separately. Postsorting reanalyses (n = 7) showed that 59.6% ± 10.4% of sorted CD4+ CD25+ cells had CD25 expression in the top 1% region of the unsorted cells, compared to 0.01% ± 0.03% of the purified CD4+ CD25low cells. Representative data are shown in Fig. 1.
FIG. 1.
CD4 and CD25 staining for CD14− lymphocyte-gated, freshly prepared PBMC. Results are from a typical subject. The percentages of cells in the indicated regions are listed. The left panel is before sorting of cells; the middle and right panels are analyses of sorted CD4+ CD25low and CD4+ CD25+ cells, respectively.
The responder cell populations evaluated were whole CD4+ cells (5 × 103 cells/well), CD4+ cells depleted of CD25+ cells (5 × 103 cells/well), or CD25-depleted cells plus CD4+ CD25+ cells (5 × 103 cells each). Responder cells were seeded with 4,000 γ-irradiated PBMC (5 × 104 cells/well) as antigen-presenting cells. Proliferation assays were performed, as described previously (11), in triplicate or quadruplicate with the antigens mentioned above. Supernatants were saved (−80°C), [3H]thymidine was added on day 6, and cells were harvested on day 7. Gamma interferon (IFN-γ) was measured in supernatants as described previously (10). In control experiments, the whole PBMC from each subject responded to HSV-2, PHA, and CMV with a net [3H]thymidine incorporation of >10,000 cpm, with the irradiated PBMC alone having a net response of <1,000 cpm, as expected (data not shown).
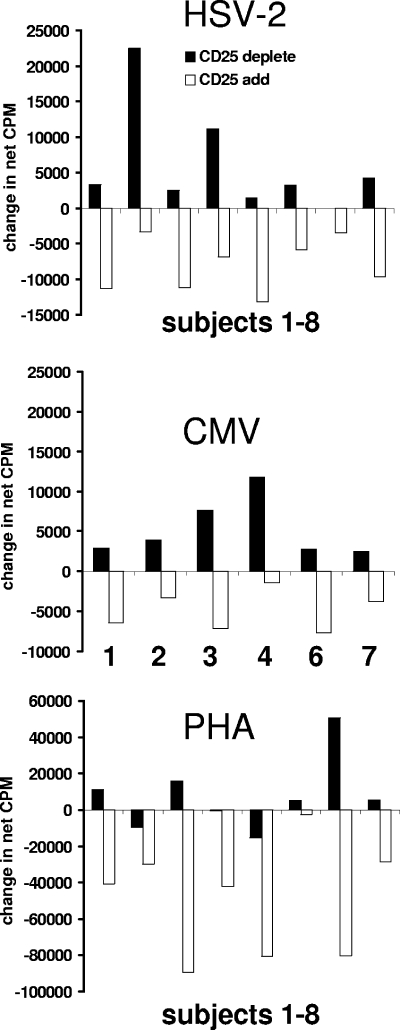
The data available from eight healthy adult subjects were consistent with a modulatory effect of CD4+ CD25+ cells on memory proliferative responses to HSV-2 antigen (Fig. 2). The magnitudes of the changes in proliferation seen after the depletion of CD4+ CD25+ cells, or the readdition of these cells at a 1:1 Treg cell-responder cell ratio, were variable and relatively small. Dose-response titrations of Treg cells would be optimal in follow-up experiments. The Treg cell-responder cell ratio in the add-back experiments, while higher than the in vivo ratio, is typical of this type of in vitro system (21). For seven of eight subjects, there was an increase in proliferation after Treg cell depletion. All subjects showed a decrease after Treg cells were added. We used the two-tailed Wilcoxon matched-pairs signed-rank test (Instat 3.05; GraphPad, San Diego, CA) to compare proliferative responses. For whole CD4 versus CD25-depleted CD4 responder cells, the two-tailed P value was 0.016 for the median difference to be significantly different from zero. By comparing CD25-depleted responders and wells to which Treg cells had been reintroduced, the P value was 0.008. Person-to-person variation was noted in both the increase seen after Treg cell depletion and the decrease seen upon add-back, similar to that noted previously for several human infectious disease models (1, 4) and possibly related to differences in the balance between proliferating antigen-specific cells and different Treg cell subsets between individuals. We feel that the decrease in counts per minute noted after the addition of CD4+ CD25+ cells does not represent simple consumption of medium resources or competition for [3H]thymidine, as the total number of responder cells is very small (5 × 103 to 10 × 103 cells per well).
FIG. 2.
Effect of depletion and addition of CD4+ CD25+ cells on [3H]thymidine incorporation proliferative responses to whole HSV-2 and CMV recall antigens in seropositive donors and to PHA. Data for CD25 depletion (solid bars) are the differences between the mean counts per minute for CD25-depleted CD4 responder cells minus the mean counts per minute for unsplit CD4 responder cells. Data for CD25 add-back experiments (open bars) are the differences between the mean counts per minute for CD4 responder cells with CD4+ CD25+ cells added back minus the mean counts per minute for CD4 responder cells depleted of CD4+ CD25+ cells. The same eight subjects are presented in order for HSV-2 and PHA. For the six CMV-seropositive subjects, the subject numbers corresponding to the HSV-2 and PHA data are shown.
Other laboratories have previously reported that CD4+ CD25+ lymphocytes can regulate memory CD4 responses to CMV, as measured by ex vivo IFN-γ accumulation (1). Recall CMV-specific proliferative responses are also reduced by Treg cells (1, 15). We studied a subset of six subjects who were CMV seropositive. As with HSV-2, the Wilcoxon test indicated significantly increased proliferation after CD4+ CD25+ cells were depleted from whole CD4 cells and decreased responses when Treg cells were added to CD25-depleted responders (P = 0.03 for each comparison). All six CMV-seropositive subjects had an increase in proliferation after the removal of CD4+ CD25+ cells and a decrease upon their addition. A similar effect on CMV-specific proliferation was previously reported for human immunodeficiency virus type 1-infected subjects (22). For PHA, depletion of Treg cells did not increase responses in all subjects, and the statistical test did not reach significance. However, their addition to Treg cell-depleted responders did significantly reduce the observed proliferation (P = 0.008). Only four specimen sets were available for supernatant IFN-γ measurements. For each subject, the addition of Treg cells to CD4+ CD25-depleted responders caused decreased net IFN-γ production by CD25-depleted responders. This reduction of IFN-γ secretion reinforces data from the proliferation assays, reducing the likelihood that decreased [3H]thymidine incorporation was due simply to competition for [3H]thymidine. Additional experiments will be required to define possible differences in the sensitivities of proliferative and IFN-γ responses to regulation by CD4+ CD25+ T cells, as separate memory cell subsets may proliferate and secrete cytokines (7).
The HSV-2-specific responses analyzed in this report are most likely CD4+ memory T-cell responses because we added inactivated, rather than live, HSV-2 as a recall antigen. PBMC that proliferate in response to irradiated HSV-2 and whole CMV antigen are primarily CD4+, although some dilution of CFSE (carboxyfluorescein diacetate succinimidyl ester) indicative of cell division, in CD4-negative cells has been noted (12). Cross-presentation of HSV-2 tegument proteins to memory CD8 T cells has been previously documented (10). We have not yet evaluated the influence of Treg cells on CD8 T cells in humans, although suppressive effects in mice in the settings of primary and memory CD8 responses to infection and immunization were previously noted (18, 20).
The antigenic specificity and mechanisms used by Treg cells in our study are unknown. It is possible that both “natural” regulatory T cells and antigen-specific T cells producing cytokines such as IL-10 could be involved. Previous studies of the Treg cell phenomenon for HSV in mice have implicated IL-10 (17). By using human HSK corneas recovered at transplantation, we recovered HSV-1-specific CD4+ clones with both high and low levels of antigen-specific production of IL-10 in response to HSV antigen (13). These clones were able to proliferate to antigen in vitro. Others have previously reported that antigen-specific Treg cells typically proliferate poorly in culture (3), although there are exceptions (16). While our data showed that CD4+ CD25+ cells can influence memory responses to HSV-2 in vitro, it is possible that other cell types may exercise a regulatory role. Regulatory CD8+ T cells and NKT cells in various systems (8) have been described. The integrated phenomenon documented in this report should be dissectible using further cell fractionation and protein-blocking reagents and may provide insight into HSV-2 pathogenesis if applied to defined cohorts or over time. The disease burdens of recurrent lytic HSV infection and inappropriate corneal inflammation in HSK call for a better understanding of the factors that balance the cellular immune response to HSV.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AI50132 and AI30731.
We thank the research subjects, Lawrence Corey and Anna Wald, the staff of the Virology Research Clinic, Seattle, WA, and Christopher McClurkan and Don Maris for technical assistance.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beissert, S., A. Schwarz, and T. Schwarz. 2006. Regulatory T cells. J. Investig. Dermatol. 126:15-24. [DOI] [PubMed] [Google Scholar]
- 4.Boettler, T., H. C. Spangenberg, C. Neumann-Haefelin, E. Panther, S. Urbani, C. Ferrari, H. E. Blum, F. von Weizsacker, and R. Thimme. 2005. T cells with a CD4+ CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J. Virol. 79:7860-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey, L., and A. Wald. 1999. Genital herpes, p. 285-312. In K. K. Holmes, P. F. Sparling, P. A. Mardh, S. M. Lemon, W. E. Stamm, P. Piot, and J. M. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill, Inc., New York, N.Y.
- 6.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435-445. [DOI] [PubMed] [Google Scholar]
- 7.Divekar, A. A., D. M. Zaiss, F. E. Lee, D. Liu, D. J. Topham, A. J. Sijts, and T. R. Mosmann. 2006. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J. Immunol. 176:1465-1473. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, H., and L. Chess. 2006. Regulation of immune responses by T cells. N. Engl. J. Med. 354:1166-1176. [DOI] [PubMed] [Google Scholar]
- 9.Kit, S., M. Kit, H. Qavi, D. Trkula, and H. Otsuka. 1983. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim. Biophys. Acta 741:158-170. [DOI] [PubMed] [Google Scholar]
- 10.Koelle, D. M., H. Chen, M. A. Gavin, A. Wald, W. W. Kwok, and L. Corey. 2001. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J. Immunol. 166:4049-4058. [DOI] [PubMed] [Google Scholar]
- 11.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificity of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koelle, D. M., J. Huang, M. T. Hensel, and C. L. McClurkan. 2006. Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes. J. Virol. 80:2863-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koelle, D. M., S. N. Reymond, H. Chen, W. W. Kwok, C. McClurkan, T. Gyaltsong, E. W. Petersdorf, W. Rotkis, A. R. Talley, and D. A. Harrison. 2000. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J. Virol. 74:10930-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokuba, H., S. Imafuku, J. W. Burnett, and L. Aurelian. 1999. Longitudinal study of a patient with herpes-simplex-virus-associated erythema multiforme: viral gene expression and T cell repertoire usage. Dermatology 198:233-242. [DOI] [PubMed] [Google Scholar]
- 15.Rushbrook, S. M., S. M. Ward, E. Unitt, S. L. Vowler, M. Lucas, P. Klenerman, and G. J. Alexander. 2005. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J. Virol. 79:7852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suffia, I. J., S. K. Reckling, C. A. Piccirillo, R. S. Goldszmid, and Y. Belkaid. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse. 2004. CD4(+)CD25(+) regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123-4132. [DOI] [PubMed] [Google Scholar]
- 18.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+ CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tigges, M. A., D. M. Koelle, K. Hartog, R. E. Sekulovich, L. Corey, and R. L. Burke. 1992. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J. Virol. 66:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toka, F. N., S. Suvas, and B. T. Rouse. 2004. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J. Virol. 78:13082-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valencia, X., G. Stephens, R. Goldbach-Mansky, M. Wilson, E. M. Shevach, and P. E. Lipsky. 14 March 2006. TNF down-modulates the function of human CD4+CD25hi T regulatory cells. Blood [Online.] doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed]
- 22.Weiss, L., V. Donkova-Petrini, L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249-3256. [DOI] [PubMed] [Google Scholar]