Abstract
Until recently, adenovirus (Ad)-mediated gene therapy was almost exclusively based on human Ad type 5 (Ad5). Preexisting immunity and the limited, coxsackievirus and adenovirus receptor-dependent tropism of Ad5 stimulated attempts to exploit the natural diversity in tropism of the other 50 known human Ad serotypes. Aiming in particular at immunotherapy and vaccination, we have screened representative serotypes from different Ad species for their ability to infect dendritic cells. Ad19a, an Ad from species D, was selected for development as a new vector for vaccination and cancer gene therapy. To clone and manipulate its genome, we have developed a novel methodology, coined “exposon mutagenesis,” that allows the rapid and precise introduction of virtually any genetic alteration (deletions, point mutations, or insertions) into recombinant Ad bacterial artificial chromosomes. The versatility of the system was exemplified by deleting the E3 region of Ad19a, by specifically knocking out expression of a species-specific E3 gene, E3/49K, and by reinserting E3/49K into an E3 null Ad19a mutant. The technology requires only limited sequence information and is applicable to other Ad species. Therefore, it should be extremely valuable for the analysis of gene functions from any Ad species. In addition, a basic, replication-defective E1- and E3-deleted Ad19a vector expressing GFP (Ad19aGFP) was generated. This new vector based on species D Ads exhibits a very promising tropism for lymphoid and muscle cells and shows great potential as an alternative vector for transduction of cell types that are resistant to or only poorly transduced by conventional Ad5-based vectors.
Adenoviruses (Ads) are nonenveloped viruses with a double-stranded DNA genome ranging in size from 29 to 45 kb. All human Ads have the potential to induce acute and persistent infections and are generally associated with mild pathogenesis. However, Ads can also induce severe symptoms or even life-threatening diseases, particularly in immunocompromised patients (29). To date, 51 different human serotypes have been described; these serotypes are classified into the six species A to F (57). Members of species B, C, and E usually cause respiratory infections, those of species A and F are associated with infections of the gastrointestinal tract, and those of species D, harboring the vast majority of serotypes (32), show a tropism for the eye. Three serotypes of this species (Ad8, Ad19a, and Ad37) cause a distinct eye disease called epidemic keratoconjunctivitis (EKC). Interestingly, a prominent subset of species D was isolated from AIDS patients previously (17).
Ads of species C, in particular, the best-characterized serotypes Ad5 and Ad2, have played a crucial role in the discovery and characterization of many important molecular processes, e.g., splicing, replication, and tumor suppression (52, 57). More recently, these viruses have emerged as extremely useful models for the study of the interaction of viruses with the host immune system, unraveling many viral immunomodulatory functions that appear to contribute to immune evasion and persistence (10, 37, 41, 71). Moreover, the species C Ad Ad5 has gained widespread use as a vector for gene therapy and vaccination (16, 30, 32, 52, 63).
In the past few years there has been growing interest in studying Ads from species other than C. The reasons are severalfold. First, the tissue tropism and receptor usage of the various species differ significantly. While species C Ads primarily utilize the coxsackievirus and adenovirus receptor (CAR) as an attachment receptor (4, 51), most species B Ads use CD46 (23, 39, 60) and some possibly use CD80/86 (59; for an up-to-date review, see reference 73). For species D, the identity of the primary attachment receptor remains controversial. Initial evidence suggested that fibers of species D Ads can bind to CAR (51); however, subsequent studies clearly showed that infection by members of this species does not rely on CAR. Some authors have suggested CD46 as primary receptor for the EKC-causing Ad37 (72, 73), whereas others provided strong evidence for α(2-3)-linked sialic acid as a ligand for attachment of EKC-causing Ads (2, 3, 12, 64). Secondly, the composition of genes in the immunomodulatory E3 region differs remarkably between Ad species (10, 11). Thus, it is likely that species-specific E3 genes, together with the differential receptor usages, contribute to the distinct pathogenesis and disease association of the different species (8, 11, 36, 70). Thirdly, it became apparent that Ad5-based gene therapy vectors have significant limitations. Efficient Ad5 transduction requires CAR, and some normal tissues and advanced tumor cells, e.g., hematopoietic cells such as dendritic cells (DCs) (34, 35), exhibit low CAR expression or lack CAR altogether. DCs are key antigen-presenting cells and are exploited for immunotherapy and vaccination (55). Moreover, preexisting immunity, with ∼80% of the human population being seropositive for Ad5, considerably limits the efficacy of Ad5 vector treatments (63, 66). Therefore, exploitation of the natural diversity of Ads may help to overcome some of the obstacles faced in gene therapy and vaccination.
Investigation and exploitation of Ads other than species C was hampered at least in part by the lack of convenient cloning and mutagenesis systems. Traditionally, two approaches have been used to generate recombinant Ads. In both cases, genetic changes are introduced into a subcloned fragment of the Ad genome. Subsequently, this modified fragment is introduced into the Ad genome by in vitro ligation to restriction endonuclease-digested virus DNA or by homologous recombination between the modified fragments and digested viral DNAs in permissive host cells (5). Alternatively, homologous recombination between cotransfected plasmids carrying overlapping and complementary parts of the Ad genome was used to generate recombinant species C Ads (6). These methods are limited by the low efficiency of virus reconstitution and by the need for plaque purification of the recombinant viruses, since wild-type (wt) progenies are frequently generated during virus reconstitution (16, 45).
To overcome the problems associated with homologous recombination in permissive cells and to improve the efficiency of genetic manipulation, full-length genomes of species C Ads have been cloned in Saccharomyces cerevisiae(33) and Escherichia coli (13), allowing mutagenesis under noncomplementing conditions. However, these approaches require subcloning of the modified DNA fragment in special shuttle plasmids, which is labor intensive (13, 15, 26, 33, 42). In addition, most of these mutagenesis procedures are greatly restricted by the limited availability of appropriate restriction sites close to the targeted sequence (13, 33) or by the requirement for a specific host for genetic manipulation (13, 15, 26, 33). A range of methods exists for generating Ad2 and Ad5 vectors containing expression cassettes (see references 1, 16, 45, and 47 and references therein). Genetic manipulation of these vectors is relatively fast and simple but is restricted to a defined, predetermined site. Recombinant Ads based on other human serotypes (e.g., Ad4, Ad7, or Ad35) or animal Ads have been generated by traditional homologous recombination (40) in cells or in E. coli or by classical cloning techniques (21, 22, 31, 48, 62, 66). However, fast, generally applicable, and efficient methods for cloning and precise manipulation of Ad genomes for detailed studies of the various functional activities of different Ad species or for exploration of their potential as vectors are not yet available.
Recently, a novel recombination system, called ET recombination, has been exploited for genetic engineering of recombinant DNA in E. coli (46, 74). ET recombination uses λ phage-derived recombination proteins that mediate effective recombination of linear DNA fragments into the target sequences, requiring only very short (usually 35- to 50-bp) homologous sequences. This allows the introduction of virtually any selectable mutation in a single step. Likewise, large genomic fragments can be cloned by ET recombination into a PCR-derived linear vector carrying short homology arms (75). However, in the latter case, degradation of linear DNA in E. coli must be prevented, and short terminal repeats within the homology arms of the vector may lead to a substantial background due to a high frequency of vector circularization (75). Mutations generated by PCR or even fully synthetic DNA can be introduced by ET recombination, which, unlike other methods based on homologous recombination, does not require special E. coli strains.
Here we demonstrate that ET recombination can be successfully applied to construct recombinant Ads. To ensure correct recombinatorial construction, purified Ad19a DNA was marked prior to ET recombination with an antibiotic resistance gene using a transposon 7 (Tn7)-derived in vitro transposition system (7). Convenient and complete removal of the selection marker makes the resulting recombinant Ad genome-bacterial artificial chromosome (BAC) ready for reverse genetic approaches. In addition, we report on a novel, two-step mutagenesis technique in which the mutation coupled with the Tn7-derived selection marker is first introduced by ET recombination. Subsequently, the operational sequences are completely removed by a simple transposase cleavage-ligase reaction in vitro. The potency of these methodologies is demonstrated by introducing deletions, insertions, and point mutations in a recombinant Ad19a genome. Moreover, a novel Ad19a-derived first-generation gene therapy vector was established that seems to have an interesting tropism for lymphoid cells.
MATERIALS AND METHODS
Cell lines, viruses, and preparation of viral genomic DNA.
The human epithelial lung carcinoma cell line A549 (ATCC CCL-185) and the Ad5-transformed human epithelial kidney cell line 293 (ATCC CRL-1573) were cultured in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine (27). Jurkat E6-1 (ATCC TIB-152), LCL, and T2 (ATCC CRL-1992) cells were maintained in RPMI medium supplemented with 10% FCS and antibiotics. The ME strain of Ad19a (67), a kind gift of G. Wadell (Department of Virology, Umea University, Sweden), was plaque purified and amplified by infecting subconfluent A549 cells at 1 to 3 PFU/cell. We refer to the plaque-purified stock of the ME strain as Ad19aT3 in this report. Virus stocks were prepared using standard protocols (44). The human Ad5-derived E1- and E3-deleted green fluorescent protein (GFP)-expressing vector Ad5GFP was purchased from Qbiogene. Like the Ad19aGFP vector described in this report, Ad5GFP expresses enhanced GFP under the control of the cytomegalovirus (CMV) promoter-enhancer in the deleted E1 region. In addition, both vectors have large deletions within the E3 region; Ad19aGFP lacks 4.5 kb and Ad5GFP lacks 1.88 kb, preventing expression of all E3 genes and of all except E3/12.5K, respectively.
For preparation of genomic Ad19a DNA, A549 cells were infected at a multiplicity of infection of 1 to 3. After the cytopathic effect was complete, the infected cells were washed once in phosphate-buffered saline (PBS), scraped from the plates, and resuspended in PBS. Cells (∼4 × 106 cells/ml) were lysed by adding an equal volume of TST buffer (2% Triton X-100, 400 mM NaCl, 20 mM Tris-HCl, pH 8.0) to the cell suspension followed by incubation on ice for 30 min. Cell debris were removed by centrifugation at 14,000 × g for 10 min at 4°C, and the supernatant was treated with 50 μg/ml proteinase K (Roche) in the presence of 0.5% sodium dodecyl sulfate for 60 min at 56°C. After extraction of the nucleic acids by phenol-chloroform and ethanol precipitation, the extract was treated with RNase A (Sigma). RNA-free viral DNA was again phenol-chloroform extracted and precipitated with ethanol.
Tn labeling of viral DNA and recombinatorial cloning of the wt Ad19a genome.
The BAC entry vector was generated by direct cloning of an assembled PCR product consisting of the two Ad19a inverted terminal repeats (ITRs) linked by a short unique E4 sequence. The left and right Ad19a ITRs were amplified by PCR using oligonucleotides 19aITR-PacI and 19aLrev and 19aRfor, respectively (for primer sequences, see Table S1 in the supplemental material). To amplify the left end of the genome, the forward primer specific to the terminal virus sequence was flanked by a 5′ PacI site (19aITR-PacI) and the reverse primer specific to the 3′ end of the ITR (19aLrev) was tagged with a short sequence homologous to 19aRfor. For amplification of the right Ad19a end, the same terminal primer (19aITR-PacI) and a primer specific to a conserved E4 sequence (19aRfor) were used. The products of the “left” and “right” PCRs were combined and reamplified by use of the terminal primer 19aITR-PacI. The BAC entry vector p19aLR was generated by inserting the assembled PCR products into the PacI site of the pKSO BAC vector (43).
pGPS1.1 (New England Biolabs [NEB]) containing a mini-Tn cassette (Transprimer-1) was used as Tn donor in the Tn-assisted cloning experiments. Purified viral DNA (200 or 300 ng) was labeled with Transprimer-1 in vitro by use of TnsABC* according to the Genome Priming System (GPS) protocol (New England Biolabs). Recombination-proficient electrocompetent E. coli DH10B (Invitrogen) carrying p19aLR and pBADαβγ (74, 75) was prepared as described previously (68) and subsequently transformed with Tn-labeled Ad19a DNA by use of a Bio-Rad GenePulser with the following settings: 2,500 V, 200 Ω, and 25 μF. Upon induction with 0.1% l-arabinose (46, 74), the transformants were incubated for 2 h at 37°C in LB medium and plated onto LB agar plates containing 25 μg/ml chloramphenicol (Cm) and 20 μg/ml kanamycin (Kn). Doubly resistant colonies were isolated, boiled, and screened by PCR using primers specific to the Ad hexon to identify BACs containing Ad19a DNA. To roughly locate the Tn insertion site, recombinant BACs were analyzed by restriction digestion.
ET recombination.
Synthetic oligonucleotide primers used for the generation of the ET recombination fragments were designed as follows. At the 5′ ends all primers carried 40-nucleotide up- or downstream homology arms for ET recombination. These 40 nucleotides were Ad sequences in the vicinity of the location in which the desired mutation should occur. At their 3′ end, all ET primers contained the priming sequences complementary to either the left end (5′-TGT GGG CGG ACA AAA TAG TTG G-3′) or right end (5′-TGT GGG CGG ACA ATA AAG TCT TAA ACT GAA-3′) of the Transprimer-1 cassette of pGPS1.1. Depending on the application, different insertion sequences (e.g., three-nucleotide direct repeats) were included between the homology arms and the Tn priming regions (for the sequences of the primers used in this study, see Table S1 in the supplemental material). The linear recombination fragments were amplified by use of an Expand High Fidelity PCR system (Roche) and 2 ng pGPS1.1 as the template. The PCR and the ET recombination procedure were performed as described previously (68). Briefly, PCR products were purified with a PCR purification kit (QIAGEN). For ET recombination, arabinose-induced electrocompetent E. coli DH10B cells carrying the target BACs and pBAD-αβγ (46) were transformed with 300- to 400-ng purified recombination fragment. After 1.5 h growth in 1 ml LB medium at 37°C the transformants were plated on LB agar plates containing 25 μg/ml Cm and 20 μg/ml Kn.
Transposon excision and exposon mutagenesis.
Purified Tn-containing BACs (140 ng) were treated with 1 μl TnsABC* (NEB) in 1× GPS buffer (250 mM Tris-HCl [pH 8.0], 20 mM dithiothreitol, 20 mM ATP) in the presence of 90 ng of temperature-sensitive plasmid pST76T (50), which serves as a dead-end target. After 10 min incubation at 37°C, a 1/20 vol of 0.3 M MgCl2 was added to initiate Tn end cleavage. Following 60 min incubation at 37°C the reaction was stopped by heat treatment (15 min, 75°C). A total of 400 cohesive end units of T4 ligase were added, and the reaction mixture was incubated overnight at 16°C for recircularization. After heat inactivation of the T4 ligase, the reaction mixture was phenol-chloroform extracted and the BAC DNA was ethanol precipitated. Electrocompetent E. coli DH10B or pUC19RP12-transformed DH10B (49) expressing meganuclease I-SceI was transformed with the purified DNA. The transformants were incubated at 37°C in LB medium and plated on LB agar plates containing 25 μg/ml of chloramphenicol. BACs prepared from isolated colonies according to the standard alkaline lysis procedure were analyzed by restriction digestions.
In the insertion reaction of “exposon mutagenesis,” 200 ng of purified SapI-treated PCR amplified inserts was added to the heat-inactivated TnsABC* reaction prior to T4 ligase treatment. Subsequent treatments were as described above. For construction of the E3-deleted, E3/49K-expressing recombinant virus Ad19aΔE3+49K, the E3/49K open reading frame (ORF), including the 5′ simian virus 40 (SV40)-derived intron sequence of pSG5, was amplified by PCR using Sap49Kfor and Sap49Krev primers and pSG5-E3/49K (70) as the template. The GFP-expressing Ad19a vector was generated in two steps. First, the GFP ORF of pEGFP-N2 (BD Clontech) was cloned into the pBK-CMV expression vector (Stratagene). Subsequently, the GFP transcription unit was amplified with SapGFPfor and SapGFPrev primers (see Table S1 in the supplemental material) and inserted in the E1- and E3-deleted Ad19a genome via ET recombination.
Reconstitution of recombinant viruses.
Recombinant viruses were reconstituted by transfection of approximately 50% confluent 293 cell culture dishes (6 cm) with PacI-linearized Ad19a-BACs by use of a standard calcium phosphate precipitation method (54). Cells were incubated with the transfection mixture overnight and split 48 h posttransfection onto 10 cm dishes. After development of a complete cytopathic effect, the recombinant viruses containing supernatants were further amplified either on A549 cells (E3 mutants) or 293 cells (E1 mutants). Recombinant virus stocks were prepared by standard protocols (44).
Flow cytometry.
Fluorescence-activated cell sorting (FACS) was carried out essentially as described previously (20, 56) except that 3 to 5 × 105 cells/sample were used. Adherent cells (A549) were washed once with PBS and detached with trypsin-EDTA or EDTA alone. DCs were either floating or detached from the plate by vigorous pipetting. Cells were resuspended in Dulbecco's minimal essential medium containing 10% FCS, centrifuged (300 × g, 5 min), and washed in PBS before they were fixed with formaldehyde (CellFIX; BD Biosciences). After quenching with NH4Cl and further washes in PBS, cells were resuspended in ice-cold FACS buffer (FB; PBS, 2.5% FCS, 0.07% sodium azide) or FB supplemented with 0.1% saponin (FB+SAP; Calbiochem). FB+SAP was used for detection of intracellular antigens such as the Ad capsid antigen hexon. Monoclonal antibody (MAb) 2Hx-2 (ATCC HB-8117) against hexon (∼1 μg purified or undiluted hybridoma supernatant supplemented with 0.1% saponin) was added. To detect E3/49K on the cell surface of infected cells, a rat MAb generated by immunization with recombinant Ad19a E3/49K (M. Windheim, E. Kremmer, and H.-G. Burgert, unpublished data) was used in the absence of saponin. Fas expression was monitored with MAb B-G27 (Chemicon) as described previously (27). After incubation for 45 min at 4°C, cells were washed three times with FB or FB+SAP followed by incubation with fluorescein isothiocyanate-labeled goat anti-mouse antibodies (Sigma) or phycoerythrin-coupled goat anti-rat antibodies (Dianova). After 45 min incubation at 4°C in the dark, cells were washed three more times with FB or FB+SAP. Fluorescence profiles were obtained by analyzing 5,000 viable cells by use of a FACSCalibur flow cytometer and CellQuest software (BD Biosciences). Background staining obtained with the secondary antibody alone or an unrelated isotype control for the hexon antibody (34-1-2S directed against the murine MHC Kd molecule) (ATCC HB79) was deducted from the mean value of fluorescence. GFP expression was monitored through its endogenous fluorescence. The percentage of GFP-expressing cells was determined by selecting a region of fluorescence above the background of autofluorescence from uninfected cells.
Culture of human DCs.
DCs were derived from buffy coats (Red Cross blood bank, Munich) by use of standard methods. Briefly, peripheral blood mononuclear cells were isolated by sedimentation by the Ficoll-Hypaque technique and plated in RPMI medium supplemented with 5% human serum and antibiotics. After 1 h adsorption, the floating cells were removed and the adherent cells were incubated for 6 or 7 days with granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sando) (100 IU/ml) and IL-4 (1,000 U/ml). At day 7, most cells were nonadherent, immature DCs (CD14−, CD1+, CD86+). For infection, cells were washed in OptiMEM (Invitrogen) and transferred into OptiMEM. After 1 h, Ad19a or Ad2 (5 to 50 PFU/cell) was added to the cells and incubated for 1 h. Then the medium was removed and replaced by RPMI 1640, 10% heat-inactivated FCS, GM-CSF (Sando) (100 IU/ml or 50 ng/ml), and IL-4 (1,000 U/ml). In parallel, A549 cells or the primary fibroblasts SeBu (20) were infected with the same PFU/cell ratio of virus. Thirty-eight to 44 h later the cells were processed for FACS analysis.
RESULTS
Cloning of the Ad19a genome as BAC.
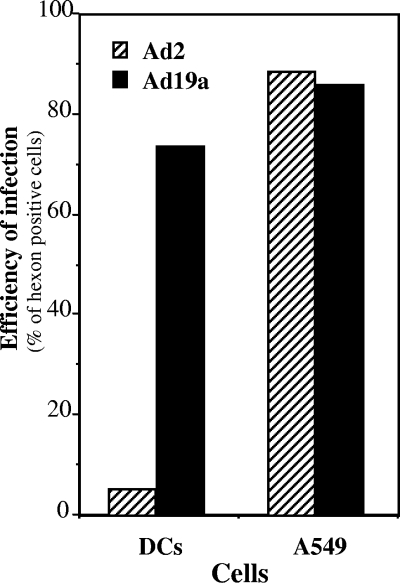
We have screened various serotypes from different Ad species for their infection efficiency of DCs and have identified serotype Ad19a as being particularly efficient for DC infection. Monocyte-derived DCs were infected with Ad19a and, as a control, with the standard species C Ad, Ad2. At 44 h postinfection (pi) the extent of infection was quantitatively assessed by FACS analysis monitoring Ad hexon expression. While >70% of Ad19a-infected cells stained positive for hexon, less than 10% were infected by Ad2. However, the two viruses infect the lung epithelial cell line A549 with similar efficiencies (Fig. 1). This is to be expected, as the viruses were titrated on A549 cells. Thus, the two viruses are similarly effective for infection of lung epithelial cells or primary fibroblasts (data not shown) whereas they differ dramatically in their efficiency of infection of DCs. As the DCs lacked CAR (data not shown), efficient DC infection by Ad19a is CAR independent. This promising feature of Ad19a might thus be exploited to design an alternative vector for efficient transduction of DCs and other CAR-negative cells.
FIG. 1.
Highly efficient infection of dendritic cells by Ad19a compared to Ad2. Immature DCs were generated from peripheral blood monocytes by incubation with GM-CSF and IL-4 for 7 days. DCs were harvested, washed, and infected with 50 PFU/cell of Ad2 or Ad19a. At 44 h later cells were processed for flow cytometry by intracellular staining for Ad hexon by use of MAb 2Hx-2. In parallel, the lung epitheloid cell line A549 was infected for 24 h and subsequently stained for FACS analysis. The bar diagram represents the means of results for hexon-positive cells for Ad19a (black bars) and Ad2 (hatched bars) from two experiments.
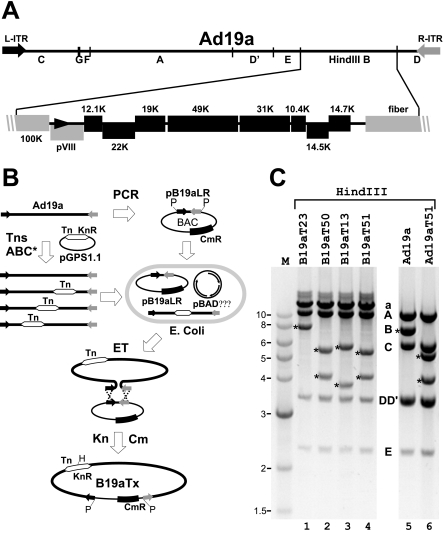
Another aspect that makes Ad19a particular interesting is its specific disease association. Therefore, we decided to clone the Ad19a genome. Our initial attempts to clone subgenomic fragments of Ad19a into conventional cloning vectors revealed that the HindIII B fragment containing the E3 region (Fig. 2A) is unstable in E. coli. However, we were able to maintain this fragment in a BAC vector (data not shown). This prompted us to utilize the BAC vector also for cloning of the full-length Ad19a genome. Rather than using the previously introduced RecABCD recombination system of E. coli (13), we used the ET recombination system (74, 75) for construction of recombinant Ad genomes. The rationale was that ET recombination, in contrast to RecABCD (69), requires only very short homologous sequences (35 to 50 bp). As the Ad19a genome had not been sequenced and even subgenomic fragments were unstable in E. coli, the ET system seemed to be particularly suitable. The entry vector pB19aLR was constructed by inserting the PCR-amplified Ad19a ITRs, separated by a short spacer of viral E4 sequence, into the BAC vector pKSO (43). Two PacI sites were introduced at each vector-ITR boundary, enabling subsequent release of the linear Ad genome for virus reconstitution. E. coli DH10B was cotransformed with the pB19aLR entry vector and the plasmid pBADαβγ encoding the recombination genes redα, redβ, and gam of phage λ (75). Following the procedure for introducing markerless PCR-derived linear DNA fragments by ET recombination (74), we initially used linear Ad19a DNA and a counter-selectable Ad19a ITR-containing entry vector but failed to obtain recombinants containing genomic Ad19a DNA. Even recombination with the more stable Ad2 genome was extremely inefficient (1 recombinant out of 400 clones screened; data not shown). To overcome this problem we established a system that allows the positive selection of recombinant Ad19a genomes. Genomic Ad19a DNA prepared from infected cells was labeled in vitro using Transprimer-1 and the purified gain-of-function mutant transposase complex TnsABC* (NEB). Transprimer-1 is a Tn7-derived mobile DNA element that carries the Kn resistance gene flanked by I-CeuI and I-SceI meganuclease recognition sites and is contained in the Tn donor plasmid pGPS1.1 (NEB). Originally, the Transprimer-1 system was developed for sequencing larger segments of DNA (7), but it can also be successfully utilized to randomly label linear genomic DNA (25). When recombination-competent bacteria carrying the pB19aLR ITR entry plasmid together with pBADαβγ were transformed with the Tn-labeled Ad19a genomes, recombinant BACs could be positively selected by using Cm that selects for the vector and Kn selecting for the labeled Ad genome (Fig. 2B). Using 200 to 300 ng of labeled Ad19a genome we reproducibly obtained 15 to 22 doubly resistant colonies, of which 50% were positive for Ad DNA, as analyzed by Ad hexon-specific PCR (data not shown). Thus, labeling of the linear Ad19a genome with a positive selection marker appeared to be essential for its successful cloning.
FIG. 2.
Tn-assisted cloning of the Ad19a genome. (A) Schematic representation of the Ad19a genome. The linear Ad genome is flanked by 135-bp ITRs (L-ITR, R-ITR; black and gray arrows). The HindIII fragments are marked according to size from A to G, with the HindIII B fragment shown in greater detail. Nonessential E3 ORFs are shown as black boxes and adjacent essential genes (100K, pVIII, fiber) as gray boxes. Numbers above or below the boxes indicate the names of the E3 ORFs based on their calculated molecular weights. (B) Schematic representation of Tn-assisted cloning of the Ad19a genome. The PCR-amplified Ad19a ITRs were cloned into the BAC vector pKSO carrying a chloramphenicol resistance gene (CmR), thereby generating pB19aLR with PacI (P) sites at each ITR-vector border. This entry vector was introduced into E. coli DH10B together with the recombination plasmid pBADαβγ expressing the respective λ genes involved in recombination. Purified Ad19a DNA was labeled in vitro with a Tn (white double arrows) carrying a kanamycin resistance (KnR) gene by use of TnsABC* transposase and the Tn donor plasmid pGPS1.1. Upon transformation with the Tn-labeled Ad19a DNA, ET recombination (ET) with p19aLR was induced and Ad19a-containing recombinant BACs (B19aTx) were selected by Kn and Cm. For simplicity, the HindIII site (H) present in the Tn is only shown in B19aTx. (C) HindIII digests of BAC DNA from selected Tn-positive clones (B19aT23, B19aT50, B19aT13, and B19aT51; lanes 1 to 4) and viral DNA from wt Ad19a and B19aT51-derived reconstituted virus, Ad19aT51 (lanes 5 and 6). The typical Ad19a HindIII fragments are indicated with the letters A to E (lane 5; F and G are not visible). Fragment A, one of the doublet DD′, and fragment E are visualized in all selected BAC clones. Fragment C and the other DD′ fragment are missing due to their linkage to the vector backbone. Together, these form fragment a. Insertion of the Tn in fragment B introduces an additional HindIII site, yielding two new fragments (marked by asterisks). For B19aT23 the second HindIII B-derived fragment is too small to be visible in this gel. Ad19aT51 was reconstituted by transfection of PacI-cleaved B19aT51 BAC DNA into 293 cells. PacI cleavage removes the plasmid vector; hence, viral DNA lacks fragment a and exhibits the normal end fragments (C and one of the DD′ fragments; compare lanes 5 and 6). Please note the presence of the same two extra fragments derived from fragment B (asterisk) in the recombinant Ad19aT51 virus as in the parental B19aT51 BAC (compare lanes 4 and 6).
BACs containing the hexon gene were isolated and digested with HindIII. We focused on clones containing the Tn insertion in the HindIII B fragment (Fig. 2A), because a large part of it represents the E3 region that is dispensable for Ad growth in cell culture. Consequently, clones with Tn insertions in the E3 region should yield infectious virus upon transfection of 293 cells. HindIII digestion of BAC DNA from four independent clones indicated that three of them (B19aT13, B19aT51, and B19aT50) indeed had Tn insertions in the HindIII B fragment, since the typical 7.8-kb HindIII B fragment was missing and instead two smaller fragments were visible (Fig. 2C, lanes 2 to 4). The appearance of these new fragments can be explained by the presence of a known HindIII site approximately in the middle of the introduced Tn. In B19aT23 the Tn-marked fragment migrates slightly slower than the original HindIII B fragment, indicating that the Tn had inserted close to one of the ends of the original fragment. When the four BAC clones were linearized by PacI and transfected into 293 cells, the three clones with two HindIII B-derived fragments (B19aT13, B19aT50, and B19aT51) resulted in viable progeny. Sequencing of the Tn insertion sites of the viable clones revealed that B19aT51 has the Tn insertion at the end of the E3/19K ORF. A comparison of the HindIII restriction patterns of viral DNA derived from reconstituted Ad19aT51 and wt Ad19a showed no difference except that the normal 7.8-kb HindIII B fragment was replaced by two smaller fragments that are identical in size to those seen after digestion of the B19aT51-BAC (Fig. 2C, lanes 4 to 6). This suggests that the Tn-containing fragment is stable and faithfully replicated along with the viral genomic DNA.
Removal of the Tn allows reconstitution of the cloned Ad19a genome.
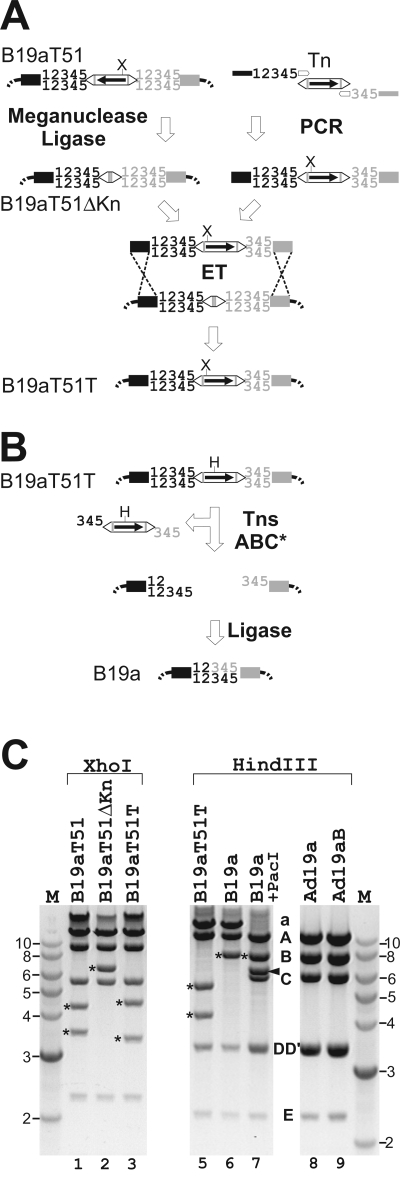
To apply the BAC technology to the analysis of Ad19a biology, the transposon has to be removed and the wt genome has to be reconstituted. One possibility is to replace the Tn-containing DNA fragment by use of the corresponding fragment from wt Ad DNA and traditional cloning techniques. We followed this strategy initially and reconstructed the wt Ad19a genome by partial digestion with AvrII and subsequent insertion of the corresponding Ad19a AvrII fragment (data not shown). However, this method is not generally applicable and requires exact mapping with the restriction enzymes to be used and therefore is laborious. To make the Tn-removal step independent of any further mapping work the nuclease activity of the TnsABC* complex was exploited. Upon insertion of the Tn by use of the Tn7 transposase the inserted Tn was flanked by 5-bp target repeats (12345 in Fig. 3A) 12345 the five nucleotides in the Ad genome directly flanking the Tn insertion site. TnsABC* cleaved one strand of the double-stranded donor DNA exactly at the 3′ ends of the Tn and the other strand 3 bp apart within the target duplications, creating 3-base 5′ overhangs within the target duplications (345 in Fig. 3B) (14). Thus, the wt donor sequence is not restored after transposase excision and simple end joining, since the resulting ends are usually not compatible and the cleavage removes only 3 bp of the 5-bp duplications, leaving a residual 2-bp insertion (14).
FIG. 3.
Generation of the wt Ad19a genome upon Tn removal from B19aT51. (A) Schematic representation of the precise removal of the Tn. The KnR gene (arrow) of the Tn (open double arrows) was removed in vitro by I-SceI/I-CeuI meganuclease double digestion (meganuclease sites are indicated by gray lines) followed by end filling and ligation, generating B19aT51ΔKn. In parallel, a PCR was performed using primers specific to the Tn ends (open arrows) flanked by 40-bp homologies to the target sites in Ad (black and gray boxes). In the forward primer the entire target repeat (12345) was incorporated, whereas only the last 3 bp of the right target repeat were included into the homology region of the reverse primer. Target repeats are indicated on either side of the Tn by black and gray numbers. This Tn-containing PCR fragment was introduced into the B19aT51ΔKn by ET recombination, whereby the orientation of the Tn in the newly generated BAC B19aT51T becomes reversed. (B) Tn removal from B19aT51T. B19aT51T was treated with TnsABC* transposase, which excises the Tn, leaving compatible 3-base-long 5′ overhangs on the BAC ends. Simple ligation reconstitutes the 5-bp wt Ad target sequence, thereby generating a BAC containing the wt Ad19a genome (B19a). (C) Restriction analysis of BAC clones and their derived Ads. The XhoI patterns of B19aT51, B19aT51ΔKn, and B19aT51T are shown in lanes 1 to 3, respectively, with Tn-containing fragments indicated by asterisks. The HindIII pattern of BACs (lanes 5 to 7) and reconstituted viruses (lanes 8 and 9) is also shown. Fragment B-derived bands are indicated by asterisks. A HindIII-PacI double digest of B19a DNA releases the end fragments (C and one of the DD′ fragments) from the vector backbone (black arrowhead), eliminating fragment a (lane 7). BAC-derived Ad19a, Ad19aB; wt Ad19a, Ad19a. M indicates the lane of DNA markers (NEB) with numbers in kilobases.
To overcome this problem we first removed the Kn cassette from the Tn in B19aT51, one of the BACs with the Tn in the E3/19K gene, by double digestion with the meganucleases I-CeuI and I-SceI. Subsequent blunting and religation resulted in an intermediate BAC clone that retained the Tn ends but not the selectable marker (Fig. 3A, B19aT51ΔKn). A new ET recombination fragment was generated by PCR using the Tn donor plasmid as the template and primers specific to the ends of the Tn flanked by homologies to the viral target sequences up- and downstream of the Tn insertion site. The upstream insertion site was left intact, but the first 2 bp of the downstream target duplication were deleted by primer design (Fig. 3A). ET recombination between the redesigned Tn-positive recombination fragment and B19aT51ΔKn as the target should yield BAC B19aT51T, with only 3-bp target repeats at either end of the Tn. Subsequent treatment of B19aT51T with TnsABC* in vitro removes the Tn sequences, creating now-compatible 3-nucleotide 5′ overhangs (345 in Fig. 3B) at both ends of the BAC. These represent the last 3 bp of the original target repeats (Fig. 3B). TnsABC* cleaves the donor site only in the presence of target DNA. Therefore, to provide nonimmune target sites for the excising Tn to transpose into, a plasmid with a temperature-sensitive origin of replication was included in the cleavage reaction. This dead-end plasmid was lost, since it is not replicated at 37°C. After simple ligation, the released BAC ends were joined and the wt sequence was restored (Fig. 3B; B19a). As TnsABC* cleavage and ligation are not 100% efficient, the reaction mixture was transformed into E. coli DH10B carrying an I-SceI expression plasmid (49), thereby selecting against Tn-positive BACs which contain an I-SceI site. Pretesting the resulting colonies for Kn sensitivity identified those that had lost the Tn, since removal of the Tn is accompanied by a loss of the Kn resistance gene. To remove the I-SceI expression plasmid, E. coli DH10B was retransformed with the BAC DNA preparation from the Kn-sensitive colonies. Restoration of the wt sequence can be confirmed by restriction analysis (Fig. 3C) and sequencing.
Restriction digests of BAC intermediates confirmed the success of the individual manipulations. The presence of the Tn within the E3 region in B19aT51 is indicated by an additional XhoI site within the E3 region, producing two fragments of 4.4 and 3.5 kb (Fig. 3C, lane 1). Cleavage and ligation removes the Tn, along with the XhoI site, yielding only one XhoI fragment of ∼7 kb (Fig. 3C, lane 2, asterisk). Insertion of the modified PCR-derived Tn oriented in the opposite direction reintroduces the XhoI site. Hence, after XhoI digestion of B19aT51T two Tn-containing fragments are generated (Fig. 3C, lane 3) that differ in size from those seen in B19aT51 due to the altered orientation of the Tn. Also, comparison of the HindIII patterns of B19aT51T (lane 5) and B19a (lane 6) clearly indicates the removal of the Tn sequence from fragment B after TnsABC* and ligase treatment. The Tn-encoded HindIII site resulting in the two Tn-containing fragments of B19aT51T is lost in B19a, and instead a wt-like 7.8-kb B fragment appears (lane 6). The same 7.8-kb HindIII B fragment is visible after release of the vector backbone by PacI cleavage, lending further support to the idea of B19a being a BAC containing the wild-type Ad19a genome (Fig. 3C, lanes 5 to 7). Finally, transfection of PacI-cleaved B19a DNA into 293 cells yielded viable virus (Ad19aB) whose HindIII restriction pattern was indistinguishable from that obtained for wt Ad19a DNA. Moreover, when tested, all biological features of this BAC-derived Ad19aB were identical to those of the original Ad19a strain (see Fig. 6A and B).
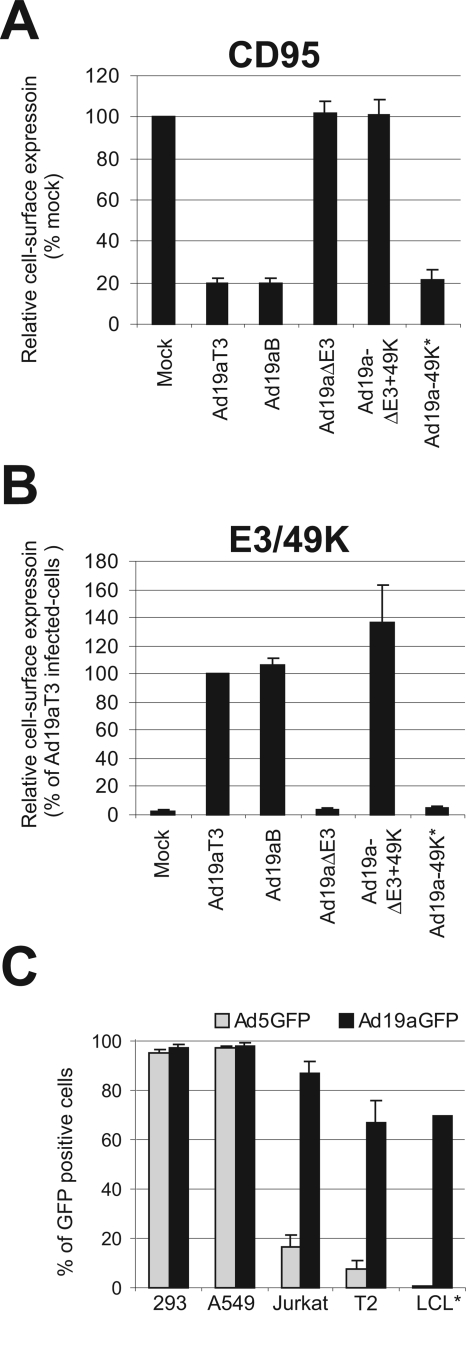
FIG. 6.
Phenotypes of various Ad19a mutant viruses generated with the procedure described in the text. (A) Down-regulation of CD95 (Fas) from the cell surface of infected A549 cells upon infection with plaque-purified wt Ad19a (Ad19aT3) or BAC-derived wt Ad19a (Ad19aB) as well as mutant Ad19a lacking E3 expression (Ad19aΔE3) or 49K expression (Ad19a49K*) or solely expressing 49K in the E3 region (Ad19aΔE3+49K), as determined by FACS analysis 21 h pi. The names of the corresponding viruses are indicated below the bar diagram. The mean value of fluorescence (MvF) deducted by examining that obtained after background staining with the secondary antibody alone was related to the MvF in mock-infected cells. The latter value was arbitrarily set to 100%. Bars depict the means compiled from four independent experiments. Error bars represent the means ± standard errors. (B) E3/49K cell surface expression as measured by FACS analysis with a MAb specific for 49K in A549 cells infected with the same viruses as described for panel A. The MvF deducted by that obtained after background staining with the secondary antibody alone was related to the MvF in wt Ad19aT3-infected cells. The latter was arbitrarily set to 100%. Bars depict the means compiled from at least three independent experiments. Error bars represent the means ± standard errors. (C) Comparison of the transduction capacity of GFP-expressing Ad19a and Ad5 vectors. Several lymphoid cell lines (Jurkat, T2, LCL) and, as a control, 293 cells were transduced with 25 PFU/cell Ad19aGFP (black bars) and Ad5GFP (gray bars). At 36 h pi the fraction of GFP-expressing cells was determined by FACS analysis. With the exception of the LCL data, which represent a single experiment, the bars represent the means of three experiments.
Tn-assisted mutagenesis of the Ad19a BAC (exposon mutagenesis).
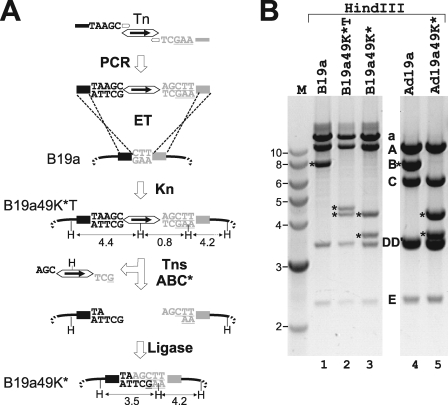
The data presented above indicate that the Tn7 ends can be used successfully as a cleavage-determining cis element and the Tn7 transposase-derived TnsABC* as a cleaving enzyme for in vitro DNA manipulation. Accordingly, it should be possible to utilize this system for the introduction of any mutant sequence flanking the Tns into any site of a recombinant DNA. Subsequent Tn excision by TnsABC* followed by DNA ligation should only leave the intended mutation in the Ad genome without retaining any unwanted operational sequence. Thus, precise and traceless introduction of mutations into Ad genomes should be possible. To test this idea, we introduced a mutation into the subgenus D-specific ORF E3/49K. This ORF codes for a highly glycosylated transmembrane protein that can be detected in the Golgi, early endosomes, and plasma membrane (8, 18, 70). On the basis of the location of the 49K gene in the E3 region and the prediction that at least one of the domains has an immunoglobulin-like fold we speculate that it might subvert the host immune system. However, to date its function remains unknown.
With the aim of eliminating 49K expression, 4 bp (TAAG) were inserted after the 11th codon (see Fig. S1 and Table S1 in the supplemental material), thereby generating a stop codon and a 1-base frame shift into the 49K ORF. First, a linear recombination fragment was produced by PCR using the Tn as a template (Fig. 4A). The upstream homology arm followed by the 5-base insertion (including the four additional bases plus the first base of the downstream homology) and the 22-base priming site specific to the left end of the Tn was incorporated into the forward primer. The reverse primer consists of the priming site on the Tn (30 bp) followed by the 2-base insertion and the downstream homology. The 2-bp insertion, together with the first base of the downstream homology, serves as a downstream copy of the 3-bp target duplication (Fig. 4A). The particular bases were chosen to provide a new HindIII site if the mutation was successful. ET recombination proficient-competent bacteria carrying B19a were transformed by this PCR product, and recombinants were plated out in the presence of Kn. Correct recombination is predicted to yield HindIII fragments of 4.4 kb, 4.2 kb, and 0.8 kb instead of the original 7.8-kb HindIII B fragment. These are generated by the HindIII sites flanking the E3 region, the one in the Tn and the one at the right end of the Tn newly created by the mutation. Indeed, recombinants with the expected restriction pattern (Fig. 4B, lanes 1 and 2) were isolated and the respective mutations were confirmed by sequencing (B19a49K*T).
FIG. 4.
Exposon mutagenesis. (A) Schematic drawing indicating the steps involved in “exposon mutagenesis.” As an example, a 4-bp insertion (TAAG) into the E3/49K gene is shown which was aimed at abrogating 49K expression by insertion of a stop codon and introduction of a reading frame shift. Simultaneously, a new HindIII site is introduced to conveniently monitor the success of the mutation. PCR was performed using a Tn template and ET primers 49KKOfor and 49KKOrev specific for the Tn ends (open arrows) and containing additional 40-base arms homologous to the upstream (black boxes) and downstream (gray boxes) viral target sequences (see Fig. S1 and Table S1 in the supplemental material for the exact sequences). In between these elements, the 5-base target repeat sequence (capital letters) is shown. For the upstream (left) primer this sequence includes the 4-base mutation (TAAG) and the first base (C) of the downstream homology (underlined gray capital letters). In the right primer, the complementary bases for the last two bases of the mutation were repeated (TC→AG), followed by the first three bases of the downstream homology arm (GAA). After ET recombination (ET) with wt B19a (the Ad19a BAC construct), Tn-containing recombinants (B19a49K*T) were selected based on kanamycin resistance. Treatment of the purified B19a49K*T BAC with TnsABC* yields compatible 5′ overhangs in the BAC backbone that can be recircularized by ligation. The resulting mutant B19a49K* contains only the designed 4-bp insertion and no operational sequences. HindIII sites (H) and the sizes (in kilobases) of the generated HindIII fragments are given. (B) Comparison of the HindIII restriction patterns of wt and mutant BACs (lanes 1 to 3) as well as wt Ad19a (lane 4) and reconstituted, BAC-derived mutant virus Ad19a49K* (lane 5). DNA from the bacteria and viruses indicated on the top was analyzed by HindIII digestion. Bands derived from fragment B are indicated (asterisks). Please note that the 0.8-kb fragment in lane 2 is not visible. After removal of the Tn sequences, only one additional HindIII site (created by the mutagenesis) is left and the 4.4-kb fragment is converted to 3.5 kb due to the loss of the Tn sequence (lane 3). Upon mutagenesis the normal B fragment of Ad19a is converted into two fragments (asterisks) of identical sizes, as seen in B19a49K* (lanes 4 and 5).
Subsequent treatment in vitro with TnsABC* and T4 DNA ligase should eliminate the Tn and yield B19a49K* containing the intended 4-bp insertion that creates a new HindIII site at the site of the mutation and therefore predicts 3.5-kb and 4.2-kb HindIII fragments. This pattern was confirmed by the restriction cut (Fig. 4B, lane 3). In addition, correct construction of both the Tn-containing intermediate B19a49K*T and the B19a49K* has been verified by DNA sequencing (see Fig. S1 in the supplemental material). On transfection of PacI-linearized B19a49K* DNA into 293 cells the mutant virus Ad19a49K* was successfully reconstituted, as shown by the presence of the same fragments in the viral DNA as in the B19a49K* DNA (Fig. 4B; compare lane 3 and 5). FACS analysis of A549 cells infected with the mutant virus Ad19a49K* (see Fig. 6) corroborated the selective loss of 49K expression, while all other E3 functions tested remained intact. The selective elimination of 49K expression was also confirmed by Western blotting (data not shown).
To assess the effect of the additional selection step on Tn-containing BACs, both normal DH10B cells and I-SceI-expressing DH10B cells were transformed with the reaction mixture and grown on Cm plates. Resulting colonies were analyzed by replica plating on Kn plates and by PCR using 49K-specific primers. The results show that the transposase-mediated cleavage was very efficient (see Table S2 in the supplemental material). More than 95% of the input DNA was cleaved, resulting in a dramatic reduction of Kn-resistant colony numbers after transformation of the TnsABC*-treated B19a49K*T compared to the results seen with untreated BAC. Of these colonies, 54% become Kn sensitive, and most of the Kn-sensitive clones carried the correct mutation, as analyzed by PCR (data not shown). Upon transformation of I-SceI-expressing bacteria with TnsABC*-T4 ligase-treated BAC DNA, 100% of the resulting colonies carried the correct genetic changes. I-CeuI treatment in vitro after ligation resulted in 96% efficiency but drastically reduced numbers of colonies (5.3%).
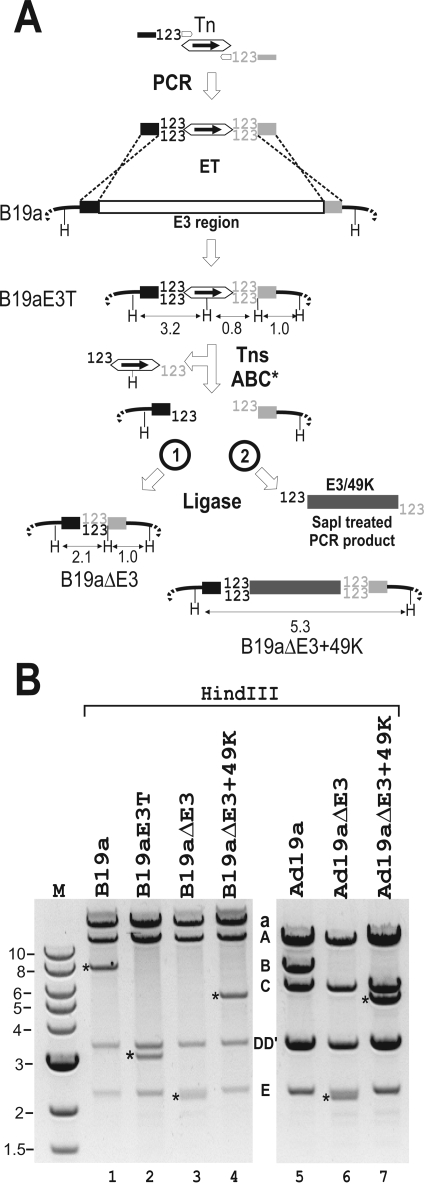
By modifying the above-described method it should be possible to introduce precise deletions into Ad BAC clones. In this case, the primers are designed such that the Tn element is flanked by 3-bp direct repeats (123 in Fig. 5) and the desired homologous sequences required for ET recombination. In the first step, the resulting PCR fragment is introduced into the BAC by ET recombination, replacing the region to be deleted. Subsequent transposase cleavage and ligation mediated by the 3-bp overhangs should allow the precise deletion of genes or nucleotides (Fig. 5A, process 1). This strategy was applied to delete the entire E3 coding region of Ad19a (4.5 kb), beginning with the start codon of the 12.1 ORF and extending to the last E3 ORF, 14.7K at the 3′ end (Fig. 2A). The observed HindIII restriction pattern of the DNA is consistent with the successful generation of intermediates and end products. The wt 7.8-kb fragment in B19a is replaced by a 3.2-kb fragment (and smaller fragments) in B19aE3T and by 2.1-kb and 1.0-kb (not visible) fragments in the B19aΔE3 end product (Fig. 5B, lanes 1 to 3). This same 2.1-kb fragment is visualized in HindIII-digested DNA of the reconstituted Ad19aΔE3 virus that otherwise exhibits a pattern identical to that seen with the wt except that the 7.8-kb HindIII B fragment is lost. Further analysis shows that Ad19aΔE3 is viable and does not express any of the E3 functions tested (Fig. 6).
FIG. 5.
Exposon mutagenesis for precise deletion and insertion of genes. (A) Schematic representation of Tn-assisted deletion (process 1, left) and gene insertion (process 2, right). The Tn-containing PCR-derived recombination fragment was designed to contain 40-bp homology arms (black and gray boxes) targeted to the beginning (5′ part) and end (3′ part) of the Ad19a E3 region marking the borders of the deletion and 3-bp (123) direct repeats introduced by the ET primers homologous to either Tn end. After ET recombination with wt B19a the coding region of Ad19a E3 (open box) was replaced by the Tn-containing PCR fragment, generating the Tn-containing intermediate B19aE3T that, on cleavage with TnsABC* and circularization via its compatible ends (process 1), yields the deletion mutant B19aΔE3. In a modification of the procedure, a new gene (E3/49K) was inserted (process 2) after treatment of a PCR-derived 49K protein-encoding insert (gray box) with SapI, generating compatible sticky ends with TnsABC*-treated B19aE3T. From the B19aΔE3+49K BAC clone an Ad19a mutant virus was reconstituted expressing only 49K as a single E3 gene. (B) BAC DNA extracted from B19a, B19aE3T, B19aΔE3, and B19aΔE3+49K was analyzed by HindIII digestion. Bands derived from fragment B are indicated by asterisks. Introduction of the Tn into the E3 region inserts two additional HindIII sites, one by the mutation and the other by the Tn. On HindIII digestion of B19aE3T a 3.2-kb fragment (asterisk in lane 2) and 1-kb and 0.8-kb fragments (not visible) are produced. Tn removal from B19aE3T results in an additional deletion (asterisk in lane 3; B19aΔE3). The 5.3-kb fragment (lane 4; B19aΔE3+49K) is consistent with the elimination of two HindIII sites and with the residual fragment B sequences being linked to the 49K expression cassette. HindIII-digested DNA extracted from wt Ad19a (lane 5) and the reconstituted recombinant viruses Ad19aΔ E3 (lane 6) and Ad19aΔE3+49K (lane 7) exhibit B-derived fragments identical in size to those seen in the corresponding BAC DNA (compare lanes 5 to 7 with lanes 1, 3, and 4).
Tn-assisted gene insertion.
The flexible design of Tn entry fragments also allows the insertion of larger DNA fragments or genes. The first steps are identical to those in the procedure described above, but during the ligation step the gene to be inserted is added. A prerequisite for successful insertion is that the gene contains sticky ends compatible with those created by transposase. This can easily be achieved by PCR using primer pairs with recognition sequences for restriction endonucleases that generate overhangs compatible with those of transposase, e.g., SapI. The TnsABC*-cleaved target BAC and the insert with compatible ends are ligated, and after I-SceI selection, mutants lacking the Tn are greatly enriched. This is illustrated here with two examples. In the first, we introduced an E3/49K expression cassette from pSG5-E3/49K in which E3/49K expression is under the control of the SV40 promoter (70). The entire 49K expression cassette of pSG5-E3/49K was amplified using primers that introduce two SapI cleavage sites at each end of the amplicon. Cleavage by SapI generates the desired 3-base overhangs compatible for insertion into the TnsABC*-treated B19aE3T (Fig. 5A, process 2). The resulting BAC clones were tested as described above. As shown in Fig. 5B, replacement of the Tn in B19aE3T by the 49K expression cassette (2.3 kb) eliminates two HindIII sites, resulting in a BAC clone with the 49K insert in a left-to-right orientation on a 5.3-kb fragment (lane 4; B19aΔE3+49K). The same characteristic 5.3-kb fragment is detected after cleavage of DNA from the corresponding, reconstituted recombinant Ad (Ad19aΔ E3+49K), demonstrating that the inserted gene is stable after several rounds of virus replication (Fig. 5B; compare lane 4 with lane 7).
Functional analysis of Ad19a mutants generated by exposon mutagenesis.
To assess the success and specificity of the introduced genetic alterations, we analyzed the phenotypes of the various Ad19a mutant viruses generated with the procedure described above. As the E3 region exhibits very complex splicing and previous mutations resulted in unintended secondary effects (19), it is crucial to analyze specific E3 functions in E3 mutant Ads. Ads remove several apoptosis receptors, including Fas (CD95), from the cell surface of infected cells to protect them from premature apoptosis (20, 41, 65, 71). Down-regulation of Fas from the cell surface requires the E3/10.4-14.5K proteins, also called RID (20, 65; for a review, see references 10 and 41). We have investigated the capacity of BAC-derived wt and mutant Ad19a to down-regulate CD95 (Fas) by FACS analysis (Fig. 6A). In wt Ad19a such as the plaque-purified Ad19aT3, the RID genes are expressed and, consequently, Fas cell surface levels are reduced to ∼20% compared to mock-infected cells (100%). A similar down-regulation is observed in cells infected with BAC-derived wt Ad19a (Ad19aB) or the Ad19a49K* mutant, in which expression of E3/49K (an E3 gene in the close vicinity of the RID genes) is specifically eliminated upon insertion of the 4-bp mutation (Fig. 4). This demonstrates that the intended elimination of E3/49K (70) expression did not affect the function of E3/RID and thus appears to be specific. By contrast, Ad19a viruses lacking all E3 genes (Ad19aΔ E3) or all but E3/49K (Ad19aΔE3+49K) are unable to modulate Fas from the cell surface, confirming for the first time that species D RID exhibits a functional activity similar to that seen with species C.
The generation of a 49K-specific MAb (M. Windheim., E. Kremmer, and H.-G. Burgert,, unpublished data) allowed us also to directly monitor the expression of 49K in wt and mutant Ad19a viruses, as measured by FACS analysis. A549 cells infected with plaque-purified wt Ad19a and BAC-derived wt Ad19a (Ad19aB) as well as mutant Ad19a solely expressing 49K in the E3 region (Ad19aΔE3+49K) synthesize 49K, whereas those infected with mutant viruses selectively lacking 49K expression (Ad19a49K*) or lacking E3 genes altogether (Ad19aΔE3) exhibit only background staining (Fig. 6B).
The above-described technology should also prove extremely useful for rapid exploration of the vector potential of various Ad serotypes for which only limited sequence data are available, as was the case with Ad19a. The genome could be cloned into BAC vectors by utilizing the ITR homologies, as demonstrated for Ad19a. To this end, we have generated an Ad19a vector expressing enhanced GFP. In analogy to the deletion of E3 (Fig. 5A), we have deleted the E1 region of Ad19a by introducing the Tn in this region by using B19aΔE3 as a target. In a second step, the Tn was replaced by an expression cassette encoding GFP under the control of the CMV immediate-early promoter and the SV40 enhancer (see Materials and Methods). A recombinant E1-negative Ad19a mutant virus expressing GFP, Ad19aΔE1GFPΔE3 (referred to here as Ad19aGFP), was viable in 293 cells, stably expressing Ad5 E1 genes. Interestingly, the Ad19a-derived GFP vector exhibited a transduction pattern remarkably different from that seen with the commonly used Ad5-derived gene therapy vector (Fig. 6C). This was revealed when different lymphoid cell lines were infected with Ad19aGFP or the corresponding Ad5GFP vector. At 36 h pi the fraction of GFP-expressing cells was quantitatively determined by FACS analysis. In contrast to the standard Ad5 vector (Fig. 6C), the Ad19a-derived vector efficiently transduced all lymphoid cell lines tested (Jurkat; T2; LCL) and C1R, Bristol8 (data not shown). Upon Ad19a transduction, 90% of Jurkat, 65% of T2, and 70% of LCL cells expressed GFP, whereas only 15%, 7%, and 2%, respectively, of the cells exhibited GFP expression after transduction with Ad5GFP. Correct titration of the vectors was confirmed by the equivalent susceptibility of 293 cells, which were used for titration of both types of vectors. Thus, Ad19aGFP seems to be superior to the Ad5GFP vector for transduction of lymphoid cells.
DISCUSSION
Although some species D Ads appear to have unique cell tropism and receptor usage characteristics and the entire sequence of one serotype (Ad17) from this species has been known for several years, no detailed description of a species D vector has been reported yet (58). We think that this might be due to the instability of their genome in E. coli. In our hands, attempts to maintain even a subgenomic fragment containing the Ad19a E3 region as standard high- and low-copy-number vectors in E. coli failed (data not shown). Since a number of unstable viral and cellular genomes have been successfully cloned as BACs in E. coli (9), we applied the BAC technology to construct and manipulate the Ad19a genome. We used homologous recombination, mediated by lambda phage red alpha, beta, and gam (ET recombination), to insert full-length Ad19a genomic DNA into a BAC vector that carries only the Ad19a ITRs. Although previous data showed that ET recombination is inefficient when repeat regions are targeted (75), we demonstrated in this study that a reasonable efficiency can be achieved by inserting a selectable Tn into the Ad genome in vitro prior to recombination. In contrast to the published recombinatorial construction of recombinant Ads (13), subcloning of large homology regions is not required for ITR-mediated ET cloning. Consequently, very small amounts of sequence information suffice to initiate cloning projects; hence, less-well-characterized serotypes and strains become amenable to genetic studies. Moreover, since small amounts of viral DNA are sufficient and even preparations from infected cells are suitable for Tn labeling, clinical isolates could be conserved by the presented approach without extensive tissue culture passage prior to cloning. After establishment of infectious Ad19a clones another round of ET recombination was applied to completely remove the cloning marker from the Ad19a-BAC. The resulting wt Ad19a genome was maintained in E. coli without any detectable changes, and the resulting reconstituted viruses obtained by transfection of 293 cells exhibited wt properties (Fig. 2, 3 and 6).
We also describe a novel, versatile method combining ET recombination and transposase manipulation to mutate BAC-cloned Ad genomes. This method is independent of restriction sites and leaves no operational sequences behind, thereby allowing traceless introduction of any genetic change into Ad genomes. In the first step, the genetic changes are introduced by ET recombination in the context of a Tn carrying a positive selection marker. In the second step, the Tn sequences are entirely removed by a transposase reaction in vitro. We coined this method “exposon mutagenesis” to emphasize the utilization of the transposase reaction for excision of the Tn-embeddedoperational sequences. Two-step recombinatorial mutagenesis methods have been previously described for manipulating mammalian genomic BACs (46, 74). In these approaches, the genetic changes are introduced along with a positive and a negative selection marker. The positive selection marker is first used to identify the intermediate recombinants, which is followed by a second round of recombination and negative selection to gain markerless mutants. However, viral BACs are particularly challenging objects for recombinatorial mutagenesis because the presence of repetitive sequences can be deleterious, especially when counter-selection is applied (69). Alternatively, the second step might be carried out without counterselection, but in this case extensive library screening is required to identify the right mutant in the nonselected pool.
Another disadvantage of most traceless recombinatorial mutagenesis approaches is that the manipulated genomes are exposed twice to an active recombination system, therefore doubling the risk of unwanted genetic events. By contrast, in our method one recombination event is sufficient to introduce the intended genetic change along with a marker that allows positive selection to preserve the genetic arrangement. In the second step, the marker is removed by in vitro treatment of the intermediate BAC with Tn7 transposase and T4 ligase, thus eliminating the need for a second recombination step. TnsABC*-mediated transposon removal is very efficient, and a potential background can easily be subtracted by simple replica plating to detect the loss of the Tn resistance marker. An additional advantage is that the same intermediate BAC can be used for the rapid generation of a large number of mutants in the same region or for the insertion of different transgenes at a predefined site. Therefore, exposon mutagenesis serves as a fast and simpler alternative to shuttle plasmid mutagenesis (43) and other techniques described so far. This is demonstrated here by the generation of several mutants in the Ad19a E3 region, the deletion of the entire E3 region, and the reinsertion of a single E3 gene, E3/49K, or its selective inactivation (Fig. 5 and 6). Thus, exposon mutagenesis is extremely useful for precise mutagenesis of E3 genes of Ad19a and for mutagenesis of Ad genomes in general. The latter was confirmed by successfully applying the technique for manipulation of the genomes of Ad2 and Ad5, both Ads of species C.
We have introduced point mutations and deletions in the E3/10.4K, E3/14.5K, E1B/19K, and E1B/55K genes (S. Obermeier, Z. Ruzsics, A. Hilgendorf, and H.-G. Burgert, data not shown) or have replaced the E1 region with a GFP expression cassette. Complex splicing is a hallmark of Ad transcription units, and deletion mutants often turned out to exhibit unintended secondary effects, e.g., affecting splicing of other E3 genes. Therefore, a method for introduction of precise and subtle changes is highly desirable. Principally, any Ad mutant can be generated by this method as long as the corresponding complementation is provided. Thus, exposon mutagenesis appears to be generally applicable and particularly useful for the functional analysis of genes, e.g., those involved in the EKC phenotype of species D Ads.
Using the above-described methodology, we have also generated a new basic Ad19a vector expressing GFP. This replication-deficient vector exhibits significantly higher transduction efficiency for human lymphoid cells (Fig. 6C) and primary human muscle cells (64) compared to conventional Ad5 vectors. This superior property appears to be due to two effects: (i) a higher uptake of Ad19a versus Ad5 particles (64) and (ii) enhanced expression of the GFP transgene. The latter effect can be demonstrated in those cell types (melanoma cells, breast cancer cells, etc.) in which the fractions of cells transduced by Ad5 and Ad19a vectors were similar or identical and yet GFP expression was three- to sixfold higher in Ad19a-transduced cells (data not shown). At present, the reasons for this increased transgene expression remain unclear. We speculate that the potency of either the E1 enhancers or other cis elements in the vicinity of the inserted transgene cassette may differ between the two Ad species. Alternatively, functions in trans that might act in a tissue- or cell type-specific manner may stimulate GFP expression in Ad19a vectors. This notion is supported by earlier data for Ad5 vectors indicating that E4 products can influence transgene expression (38). Whatever the underlying cause, the experiments point to unexpected differences between species C and D Ads. Exploitation of these differences may turn out to be advantageous for gene therapy or vaccination. For example, lower amounts of Ad19a vector might suffice to express similar levels of transgenes; hence, vector-mediated toxicity is likely to be reduced.
In contrast to the recently developed species B-based Ad vectors (24, 28, 53, 61, 62, 66), which propagate very poorly in 293 cells and typically require specific trans-complementing cell lines expressing at least the serotype-specific E1B/55K protein for preparation of high-titer E1-deleted vectors, the E1- and E3-deleted Ad19a-based vector can be propagated with essentially wt-like productivity in 293 cells. It has been shown that both the transactivation ability of the E1A gene products and the antiapoptotic activities of the E1B region are well conserved among human Ads (52). One possible explanation for the species-specific complementation is that other viral functions associated with E1 (and E4), such as late mRNA export, may be substantially different in species B compared to C and D. Alternatively, the relevant E1 function might be encoded by another gene locus in species D and, therefore, complementation might only be necessary for the conserved E1 functions. In any case, since propagation of the first-generation recombinant Ad19aGFP shown here can be performed by the same methods that are in use for Ad5-based vectors, establishment of new protocols is not required.
The recombinant Ad19aGFP vector transduced all human cell lines tested (this study) as well as primary muscle cells (64) and fibroblasts in vitro with high efficiency. This is consistent with the observation that EKC-associated Ads may use α(2-3) sialic acid-containing receptors present on the surface of most cell types (2, 3). In support of this notion, sialidase treatment of human myoblasts dramatically reduced transduction by Ad19aGFP but not that of Ad5GFP (64). However, further experiments are necessary to investigate the role of other receptor entities proposed for EKC-associated Ads (72). Taking these results together with our DC data (our DCs did not express CAR; data not shown), it is clear that in contrast to subgenus C Ads, Ad19a does not require CAR for infection. Thus, Ad19a, and possibly other Ads of species D, might be a valuable alternative to Ad5-based vectors that exhibit a limited targeting spectrum in different gene transfer applications (30, 32, 63). In particular, their ability to efficiently transduce DCs makes Ad19a vectors highly attractive for vaccination and immunotherapy. Further in vitro and in vivo experiments will be necessary to examine whether the increased efficacy of DC gene transfer also translates into a higher capacity of transduced DCs to stimulate a corresponding T-cell response. Our data obtained with lymphoid cell lines originating from B and T cells (Fig. 6 and data not shown) suggest that the increased transduction by Ad19aGFP compared to a corresponding Ad5 vector may extend to leukocytes in general (Fig. 6). Therefore, it is anticipated that vectors based on Ad19a should also be useful for cytotoxic therapy of leukemias and other lymphoid tumors.
Supplementary Material
Acknowledgments
This work was supported by a Mercia Spinner Pathfinder grant and grants from the Deutsche Forschungsgemeinschaft (BU 642-1 and SFB455) and the BBSRC (BB/D002877/1) to H.-G.B.
We thank M. Windheim for generously providing the pSG5-E3/49K plasmid and J. Bergelson for kindly providing the CAR-specific MAb. We are grateful to R. Magerstaedt for the initial help and advice to generate DCs. For critical reading of the manuscript, we thank J. Cox and S. Eldershaw.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org.
REFERENCES
- 1.Anderson, R. D., R. E. Haskell, H. Xia, B. J. Roessler, and B. L. Davidson. 2000. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7:1034-1038. [DOI] [PubMed] [Google Scholar]
- 2.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αV integrins. J. Virol. 74:7691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Berkner, K. L. 1988. Development of adenovirus vectors for the expression of heterologous genes. BioTechniques 6:616-629. [PubMed] [Google Scholar]
- 6.Bett, A. J., W. Haddara, L. Prevec, and F. L. Graham. 1994. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc. Natl. Acad. Sci. USA 91:8802-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biery, M. C., F. J. Stewart, A. E. Stellwagen, E. A. Raleigh, and N. L. Craig. 2000. A simple in vitro Tn7-based transposition system with low target site selectivity for genome and gene analysis. Nucleic Acids Res. 28:1067-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blusch, J. H., F. Deryckere, M. Windheim, Z. Ruzsics, N. Arnberg, T. Adrian, and H. G. Burgert. 2002. The novel early region 3 protein E3/49K is specifically expressed by adenoviruses of subgenus D: implications for epidemic keratoconjunctivitis and adenovirus evolution. Virology 296:94-106. [DOI] [PubMed] [Google Scholar]
- 9.Brune, W., M. Messerle, and U. H. Koszinowski. 2000. Forward with BACs: new tools for herpesvirus genomics. Trends Genet. 16:254-259. [DOI] [PubMed] [Google Scholar]
- 10.Burgert, H.-G., Z. Ruzsics, S. Obermeier, A. Hilgendorf, M. Windheim, and A. Elsing. 2002. Subversion of host defense mechanisms by adenoviruses. Curr. Top. Microbiol. Immunol. 269:274-319. [DOI] [PubMed] [Google Scholar]
- 11.Burgert, H. G., and J. H. Blusch. 2000. Immunomodulatory functions encoded by the E3 transcription unit of adenoviruses. Virus Genes 21:13-25. [PubMed] [Google Scholar]
- 12.Burmeister, W. P., D. Guilligay, S. Cusack, G. Wadell, and N. Arnberg. 2004. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 78:7727-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chartier, C., E. Degryse, M. Gantzer, A. Dieterle, A. Pavirani, and M. Mehtali. 1996. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70:4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig, N. L. 1996. Transposon Tn7. Curr. Top. Microbiol. Immunol. 204:27-48. [DOI] [PubMed] [Google Scholar]
- 15.Crouzet, J., L. Naudin, C. Orsini, E. Vigne, L. Ferrero, A. Le Roux, P. Benoit, M. Latta, C. Torrent, D. Branellec, P. Denefle, J. F. Mayaux, M. Perricaudet, and P. Yeh. 1997. Recombinational construction in Escherichia coli of infectious adenoviral genomes. Proc. Natl. Acad. Sci. USA 94:1414-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danthinne, X., and M. J. Imperiale. 2000. Production of first generation adenovirus vectors: a review. Gene Ther. 7:1707-1714. [DOI] [PubMed] [Google Scholar]
- 17.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. Wertheim-Van Dillen, G. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deryckere, F., and H. G. Burgert. 1996. Early region 3 of adenovirus type 19 (subgroup D) encodes an HLA-binding protein distinct from that of subgroups B and C. J. Virol. 70:2832-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitrov, T., P. Krajcsi, T. W. Hermiston, A. E. Tollefson, M. Hannink, and W. S. Wold. 1997. Adenovirus E3-10.4K/14.5K protein complex inhibits tumor necrosis factor-induced translocation of cytosolic phospholipase A2 to membranes. J. Virol. 71:2830-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsing, A., and H.-G. Burgert. 1998. The adenovirus E3/10.4K-14.5K proteins down-modulate the apoptosis receptor Fas/Apo-1 by inducing its internalization. Proc. Natl. Acad. Sci. USA 95:10072-10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francois, A., N. Eterradossi, B. Delmas, V. Payet, and P. Langlois. 2001. Construction of avian adenovirus CELO recombinants in cosmids. J. Virol. 75:5288-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 24.Gao, W., P. D. Robbins, and A. Gambotto. 2003. Human adenovirus type 35: nucleotide sequence and vector development. Gene Ther. 10:1941-1949. [DOI] [PubMed] [Google Scholar]
- 25.Gwinn, M., A. Stellwagen, N. Craig, J. Tomb, and H. Smith. 1997. In vitro Tn7 mutagenesis of Haemophilus influenzae Rd and characterization of the role of atpA in transformation. J. Bacteriol. 179:7315-7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgendorf, A., J. Lindberg, Z. Ruzsics, S. Honing, A. Elsing, M. Lofqvist, H. Engelmann, and H.-G. Burgert. 2003. Two distinct transport motifs in the adenovirus E3/10.4-14.5 proteins act in concert to down-modulate apoptosis receptors and the epidermal growth factor receptor. J. Biol. Chem. 278:51872-51884. [DOI] [PubMed] [Google Scholar]
- 28.Holterman, L., R. Vogels, R. van der Vlugt, M. Sieuwerts, J. Grimbergen, J. Kaspers, E. Geelen, E. van der Helm, A. Lemckert, G. Gillissen, S. Verhaagh, J. Custers, D. Zuijdgeest, B. Berkhout, M. Bakker, P. Quax, J. Goudsmit, and M. Havenga. 2004. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 78:13207-13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz, M. S. 2001. Adenoviruses, p. 2301-2326. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 30.Imperiale, M., and S. Kochanek. 2004. Adenovirus vectors: biology, design, and production. Curr. Top. Microbiol. Immunol. 273:335-357. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs, S. C., A. J. Davison, S. Carr, A. M. Bennett, R. Phillpotts, and G. W. G. Wilkinson. 2004. Characterization and manipulation of the human adenovirus 4 genome. J. Gen. Virol. 85:3361-3366. [DOI] [PubMed] [Google Scholar]
- 32.Kanerva, A., and A. Hemminki. 2004. Modified adenoviruses for cancer gene therapy. Int. J. Cancer 110:475-480. [DOI] [PubMed] [Google Scholar]
- 33.Ketner, G., F. Spencer, S. Tugendreich, C. Connelly, and P. Hieter. 1994. Efficient manipulation of the human adenovirus genome as an infectious yeast artificial chromosome clone. Proc. Natl. Acad. Sci. USA 91:6186-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, M., K. R. Zinn, B. G. Barnett, L. A. Sumerel, V. Krasnykh, D. T. Curiel, and J. T. Douglas. 2002. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur. J. Cancer 38:1917-1926. [DOI] [PubMed] [Google Scholar]
- 35.Li, Y., R.-C. Pong, J. M. Bergelson, M. C. Hall, A. I. Sagalowsky, C.-P. Tseng, Z. Wang, and J.-T. Hsieh. 1999. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 59:325-330. [PubMed] [Google Scholar]
- 36.Li, Y., and W. S. Wold. 2000. Identification and characterization of a 30K protein (Ad4E3-30K) encoded by the E3 region of human adenovirus type 4. Virology 273:127-138. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein, D., T. K., K. Doronin, A. Tollefson, and W. Wold. 2004. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 23:75-111. [DOI] [PubMed] [Google Scholar]
- 38.Lusky, M., L. Grave, A. Dieterle, D. Dreyer, M. Christ, C. Ziller, P. Furstenberger, J. Kintz, D. Ali Hadji, A. Pavirani, and M. Mehtali. 1999. Regulation of adenovirus-mediated transgene expression by the viral E4 gene products: requirement for E4 ORF3. J. Virol. 73:8308-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 79:14429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason, B. B., A. R. Davis, B. M. Brat, M. Chengalvala, M. D. Lubeck, G. Zandle, B. Kostek, S. Cholodofsky, S. Dheer, K. Molnar-Kimber, M. Satoshi, and P. P. Hung. 1990. Adenovirus vaccine vectors expressing hepatitis B surface antigen: importance of regulatory elements in the adenovirus major late intron. Virology 177:452-461. [DOI] [PubMed] [Google Scholar]
- 41.McNees, A. L., and L. R. Gooding. 2002. Adenoviral inhibitors of apoptotic cell death. Virus Res. 88:87-101. [DOI] [PubMed] [Google Scholar]
- 42.McVey, D., M. Zuber, D. Ettyreddy, D. E. Brough, and I. Kovesdi. 2002. Rapid construction of adenoviral vectors by lambda phage genetics. J. Virol. 76:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 94:14759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuguchi, H., M. A. Kay, and T. Hayakawa. 2001. Approaches for generating recombinant adenovirus vectors. Adv. Drug Deliv. Rev. 52:165-176. [DOI] [PubMed] [Google Scholar]
- 46.Muyrers, J. P., Y. Zhang, V. Benes, G. Testa, W. Ansorge, and A. F. Stewart. 2000. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 1:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng, P., R. J. Parks, D. T. Cummings, C. M. Evelegh, and F. L. Graham. 2000. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum. Gene Ther. 11:693-699. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen, T., J. Nery, S. Joseph, C. Rocha, G. Carney, K. Spindler, and L. Villarreal. 1999. Mouse adenovirus (MAV-1) expression in primary human endothelial cells and generation of a full-length infectious plasmid. Gene Ther. 6:1291-1297. [DOI] [PubMed] [Google Scholar]
- 49.Posfai, G., V. Kolisnychenko, Z. Bereczki, and F. R. Blattner. 1999. Markerless gene replacement in Escherichia coli stimulated by a double-strand break in the chromosome. Nucleic Acids Res. 27:4409-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posfai, G., M. Koob, H. Kirkpatrick, and F. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell, W. C. 2000. Update on adenovirus and its vectors. J. Gen. Virol. 81:2573-2604. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai, F., H. Mizuguchi, T. Yamaguchi, and T. Hayakawa. 2003. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol. Ther. 8:813-821. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Schuler, G., B. Schuler-Thurner, and R. M. Steinman. 2003. The use of dendritic cells in cancer immunotherapy. Curr. Opin. Immunol. 15:138-147. [DOI] [PubMed] [Google Scholar]
- 56.Sester, M., and H.-G. Burgert. 1994. Conserved cysteine residues within the E3/19K protein of adenovirus type 2 are essential for binding to major histocompatibility complex antigens. J. Virol. 68:5423-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shenk, T. E. 2001. Adenoviridae: the viruses and their replication, p. 2265-2300. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 58.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 59.Short, J. J., A. V. Pereboev, Y. Kawakami, C. Vasu, M. J. Holterman, and D. T. Curiel. 2004. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology 322:349-359. [DOI] [PubMed] [Google Scholar]
- 60.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sirena, D., Z. Ruzsics, W. Schaffner, U. F. Greber, and S. Hemmi. 2005. The nucleotide sequence and a first generation gene transfer vector of species B human adenovirus serotype 3. Virology 343:283-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stone, D., S. Ni, Z.-Y. Li, A. Gaggar, N. DiPaolo, Q. Feng, V. Sandig, and A. Lieber. 2005. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 79:5090-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatsis, N., and H. C. J. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thirion, C., H. Lochmuller, Z. Ruzsics, M. Boelhauve, C. Konig, C. Thedieck, S. Kutik, C. Geiger, S. Kochanek, C. Volpers, and H.-G. Burgert. 2006. Adenovirus vectors based on human adenovirus type 19a have high potential for human muscle-directed gene therapy. Hum. Gene Ther. 17:193-205. [DOI] [PubMed] [Google Scholar]
- 65.Tollefson, A. E., T. W. Hermiston, D. L. Lichtenstein, C. F. Colle, R. A. Tripp, T. Dimitrov, K. Toth, C. E. Wells, P. C. Doherty, and W. S. Wold. 1998. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature 392:726-730. [DOI] [PubMed] [Google Scholar]
- 66.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M.-P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wadell, G., and J. C. de Jong. 1980. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect. Immun. 27:292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner, M., A. Gutermann, J. Podlech, M. J. Reddehase, and U. H. Koszinowski.2002. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner, M., and U. H. Koszinowski. 2004. Mutagenesis of viral BACs with linear PCR fragments (ET recombination). Methods Mol. Biol. 256:257-268. [DOI] [PubMed] [Google Scholar]
- 70.Windheim, M., and H. G. Burgert. 2002. Characterization of E3/49K, a novel, highly glycosylated E3 protein of the epidemic keratoconjunctivitis-causing adenovirus type 19a. J. Virol. 76:755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Windheim, M., A. Hilgendorf, and H.-G. Burgert. 2004. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr. Top. Microbiol. Immunol. 273:29-85. [DOI] [PubMed] [Google Scholar]
- 72.Wu, E., S. A. Trauger, L. Pache, T.-M. Mullen, D. J. Von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, Y., and J. M. Bergelson. 2005. Adenovirus receptors. J. Virol. 79:12125-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, Y., F. Buchholz, J. P. Muyrers, and A. F. Stewart. 1998. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20:123-128. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, Y., J. P. Muyrers, G. Testa, and A. F. Stewart. 2000. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18:1314-1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.