Abstract
Studies using adherent cell lines have shown that glucose transporter-1 (GLUT-1) can function as a receptor for human T-cell leukemia virus type 1 (HTLV). In primary CD4+ T cells, heparan sulfate proteoglycans (HSPGs) are required for efficient entry of HTLV-1. Here, the roles of HSPGs and GLUT-1 in HTLV-1 and HTLV-2 Env-mediated binding and entry into primary T cells were studied. Examination of the cell surface of activated primary T cells revealed that CD4+ T cells, the primary target of HTLV-1, expressed significantly higher levels of HSPGs than CD8+ T cells. Conversely, CD8+ T cells, the primary target of HTLV-2, expressed GLUT-1 at dramatically higher levels than CD4+ T cells. Under these conditions, the HTLV-2 surface glycoprotein (SU) binding and viral entry were markedly higher on CD8+ T cells while HTLV-1 SU binding and viral entry were higher on CD4+ T cells. Binding studies with HTLV-1/HTLV-2 SU recombinants showed that preferential binding to CD4+ T cells expressing high levels of HSPGs mapped to the C-terminal portion of SU. Transfection studies revealed that overexpression of GLUT-1 in CD4+ T cells increased HTLV-2 entry, while expression of HSPGs on CD8+ T cells increased entry of HTLV-1. These studies demonstrate that HTLV-1 and HTLV-2 differ in their T-cell entry requirements and suggest that the differences in the in vitro cellular tropism for transformation and in vivo pathobiology of these viruses reflect different interactions between their Env proteins and molecules on CD4+ and CD8+ T cells involved in entry.
Human T-cell leukemia virus type 1 (HTLV-1) and type 2 (HTLV-2) are deltaretroviruses with similar genome structure and an overall nucleotide homology of approximately 70% (reviewed in reference 11). However, the two viruses differ in their pathobiology. HTLV-1 is the causal agent of adult T-cell leukemia and a progressive neurological disorder called HTLV-1-associated myelopathy/tropical spastic paraparesis (12, 34, 54). In contrast, HTLV-2 is essentially nonpathogenic, although a few cases of neurological disease in HTLV-2-infected individuals have been reported.
Entry of retroviruses into target cells involves interactions between the viral envelope (Env) glycoproteins, a surface glycoprotein (SU), and a transmembrane glycoprotein (TM), and specific cell surface molecules referred to as receptors. The SU protein is involved in receptor recognition, and the TM protein triggers the fusion of the viral and cellular membranes, allowing entry of viral particles. For some retroviruses such as ecotropic murine leukemia viruses, a single molecule is sufficient for attachment and entry; for others such as human immunodeficiency virus (HIV), multiple molecules are required (37).
Studies of viral interference indicate that HTLV-1, HTLV-2, and related simian viruses share a receptor (46, 47). Cells from a variety of species express molecules capable of supporting HTLV-1 Env-mediated fusion. Many, but not all, cell lines can be transduced by HTLV-1 Env-pseudotyped vectors and/or can fuse with HTLV Env-expressing cells. In contrast, all vertebrate lines tested specifically bind HTLV-1 SU, including cell lines previously shown not to support fusion by HTLV Env (15, 16, 18, 48). These observations indicated that there are differences between requirements for binding HTLV-1 Env proteins and for HTLV-1 Env-mediated fusion and entry.
More recently, two specific molecules have been demonstrated to be involved in HTLV binding and entry. Several years ago, glucose transporter 1 (GLUT-1) was shown to function as a receptor for HTLV. GLUT-1 was shown to specifically bind to a truncated soluble form of HTLV-1 and HTLV-2 SU proteins, and the level of GLUT-1 in target cells was shown to correlate with the titer of HTLV-2 Env-pseudotyped virus (28). Subsequently, overexpression of GLUT-1 in an HTLV-1-resistant cell line, MDBK, was shown to increase the titer of HTLV-1 and HTLV-2 Env-pseudotyped particles (8). As has been previously noted (36), these studies examined infection of adherent non-T-cell lines by HTLV Env-pseudotyped viruses; their relevance to the spread of HTLV viruses into their primary target cells, CD4+ and CD8+ T cells, has not been directly examined.
The efficient binding and entry of HTLV-1 also require heparan sulfate proteoglycan (HSPG), a type of glycosaminoglycan consisting of a protein core with heparan sulfate (HS) polysaccharide chains. Earlier studies demonstrated that enzymatic reduction of the cell surface levels of HSPGs reduced both the binding of soluble HTLV-1 SU and the titer of HTLV-1 Env-pseudotyped viruses in non-T-cell lines (39). More recently, we have reported that HSPGs also play a critical role in the binding and entry of HTLV-1 into CD4+ T cells (19). We observed that the majority of binding of both soluble HTLV-1 SU and HTLV-1 virions and the entry of HTLV-1 virions into established CD4+ T-cell lines and primary CD4+ cells involved interactions with HSPGs.
Although HTLV-1 and HTLV-2 Env can facilitate entry into many cell types in vitro, the tropism of HTLV in vivo is primarily limited to T lymphocytes. However, HTLV-1 and HTLV-2 differ in their T-cell tropism, both in vitro and in vivo. Adult T-cell leukemia is a malignancy of CD4+ T cells, and HTLV-1 has a preferential tropism for CD4+ T cells in individuals with neurological disease and asymptomatic patients (41). In HTLV-1-associated myelopathy/tropical spastic paraparesis patients, both CD4+ and CD8+ T cells serve as viral reservoirs (31). In contrast, HTLV-2 is found primarily in CD8+ T cells in infected individuals; in some individuals, infection of CD4+ T cells has also been observed (14, 23). In vitro studies of transformation following coculture of HTLV-producing cells with primary peripheral blood mononuclear cells have paralleled the in vivo observations. The majority of cells transformed by HTLV-1 are CD4+ T cells, while HTLV-2 preferentially transforms CD8+ T cells (33, 42, 43, 50, 52, 53).
Here, we examined whether the differences in the cellular tropism of HTLV-1 and HTLV-2 reflect differences in the cell surface expression of molecules involved in entry on CD4+ and CD8+ T cells. In contrast to HTLV-1, HSPGs do not play a significant role in the HTLV-2 Env-mediated binding and entry. Rather, the binding of HTLV-2 SU to primary T cells required significant cell surface levels of GLUT-1. Expression levels of HSPGs and GLUT-1 on primary CD4+ and CD8+ T cells were markedly different. CD4+ T cells generally expressed significantly higher levels of HSPGs than CD8+ T cells. Conversely, GLUT-1 is expressed at dramatically higher levels on CD8+ than on CD4+ T cells. Under these conditions, HTLV-2 entry was markedly higher in CD8+ T cells while HTLV-1 entry was higher on CD4+ T cells. Binding studies with HTLV-1/HTLV-2 SU recombinants revealed that preferential binding to CD4+ T cells mapped to the C-terminal portion of SU. Overexpression of GLUT-1 in CD4+ T cells increased HTLV-2 entry, while expression of HSPGs on CD8+ T cells increased entry of HTLV-1. These studies reveal that HTLV-1 and HTLV-2 differ in their entry requirements and suggest that the different pathobiology of HTLV-1 and HTLV-2 viruses reflects different interactions between their Env proteins and molecules on CD4+ and CD8+ T cells involved in entry.
MATERIALS AND METHODS
Cell culture and reagents.
MOLT4, SupT1, COS-7, CHO-K1 (HSPG positive; ATCC CCL-61), CHO-K1 2244 (HSPG negative; ATCC pgsD-677), and CHO-K1 2241 (low levels of proteoglycans; ATCC pgsB-618) cells were obtained from the American Type Culture Collection. HEK 293-T/17 is a highly transfectable subclone of a 293 line transformed with the simian virus 40 large T antigen (38). 729/ACH and 729/pH6neo, HTLV producer cell lines established by permanent transfection of proviral plasmids encoding HTLV-1 and HTLV-2, respectively, were a generous gift of P. Green (Ohio State University, Columbus, Ohio).
COS-7 and HEK 293-T/17 cells were maintained in Dulbecco's modified Eagle's medium (DMEM); CHO-K1, CHO-K1 2244, and CHO-K1 2241 in were maintained Dulbecco's modified Eagle's medium-Ham's F-12 medium (1:1); SupT1, Jurkat, and MOLT4 cells were maintained in RPMI 1640 medium; and 729/ACH and 729/pH6neo cells were maintained in Iscove modified Dulbecco medium. All of the media were supplemented with l-glutamine (2 mM), penicillin (100U/ml), streptomycin (100 ng/ml), and fetal calf serum (10%). T lymphocytes were isolated from either cord blood samples obtained during vaginal births at Frederick Memorial Hospital (Frederick, MD) or from leukopaks of peripheral blood collected according to NIH-approved Institutional Review Board protocols from healthy donors, as previously described (19). Cells were then enriched for CD4+ or CD8+ T cells, activated by 1 μg/ml phytohemagglutinin (PHA), and cultured in media containing interleukin-2 (IL-2), as previously described (19, 32).
The monoclonal antibody clone F58-10E4 (immunoglobulin M [IgM]) and HS lyase were obtained from Seikagaku Corp. The antibody that recognizes extracellular domains of human GLUT-1 (monoclonal antibody [MAb] 1418) was obtained from R&D Systems. Hydroxypropyl-β-cyclodextrin was purchased from Sigma.
Plasmids and transfections.
The plasmid encoding the soluble form of the HTLV-2 SU protein (HTSUII-IgG/pCMV-Env) was generated as follows. A DNA fragment encoding the HTLV-2 SU region was generated by PCR amplification, using the plasmid CMV-ENV-LTR-II (where CMV is cytomegalovirus and LTR is long terminal repeat) (17) as a template and the oligonucleotide primers 5′-CCTGAAAAAAGCTGCATGCCCAAG-3′ and 5′-CAGGACTAGTTCTAGAACGGCGGCGTCTTG-3′. The resultant DNA fragment was digested with SphI and SpeI and used to replace the SphI-SpeI fragment encoding the complete HTLV-1 SU (HTSU) region from HTSU-IgG/pCMV-Env (32). Four HTLV-1/HTLV-2 SU chimeras were made (see Fig. 5A). They are HTSU1/1/2-IgG/pCMV-Env, HTSU2/2/1-IgG/pCMV-Env, HTSU1/2/2-IgG/pCMV-Env, and HTSU2/1/1-IgG/pCMV-Env. DNA fragments (flanked by SphI and SpeI sites) encoding the appropriate amino acids were generated by PCR amplification of the SU coding regions of HTSU-IgG/pCMV-Env and HTSUII-IgG/pCMV-Env. The SphI-SpeI fragments were subcloned into the pCMV-Env backbone to generate the four constructs. The constructs were confirmed by sequencing. Soluble SU fusion proteins were generated from these plasmids, the plasmid encoding the HTLV-1 SU (HTSU-IgG/pCMV-Env), and the negative control avian leukosis and sarcoma virus subgroup A SU (55) following transfection of 293-T cells, as previously described (19). The amount of SU protein was determined using a rabbit IgG enzyme-linked immunosorbent assay, as previously described (18).
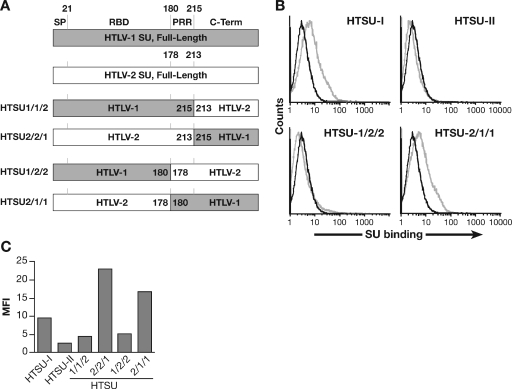
FIG. 5.
Differences in HTLV-1 and HTLV-2 binding to CD4+ T cells map to the C-terminal region of SU. (A) Schematic drawing of the SU coding regions of the vectors encoding parental and recombinant soluble HTLV SU. (B) CD4+ T cells, from adult peripheral blood, were activated for 3 days by PHA and IL-2. The binding of 100 ng of HTSU-IgG, HTSUII-IgG, HTSU1/2/2-IgG, HTSU2/1/1-IgG, and the control SUA was determined by flow cytometry. Black line, SUA-IgG; gray line, soluble HTLV SU. The mean fluorescence intensity values were as follows: for HTSU-IgG, 4.6; HTSUII-IgG, 0.5; HTSU1/2/2-IgG, 0.7; HTSU2/1/1-IgG, 3.6. (C) CD4+ T cells isolated from adult peripheral blood were activated with PHA and IL-2. The binding of 200 ng of HTSU-IgG, HTSUII-IgG, HTSU1/2/2-IgG, HTSU2/1/1-IgG, HTSU1/1/2-IgG, HTSU2/2/1-IgG, and the control SUA was determined by flow cytometry.
Construction of expression plasmids for hemagglutinin (HA)-GLUT-1 and HA-GLUT6-LL/AA (HA-GLUT-6 M) has been described previously (2, 25). The plasmid pcDNA3.1 was from Invitrogen. HA-syndecan-4 was a generous gift of Mike Simons (Dartmouth, Hanover, NH). For transfection, cells were washed once in phosphate-buffered saline (PBS), and 2 × 106 cells were resuspended in 100 μl of the specified electroporation buffer (Amaxa's Nucleofector solution V for MOLT4 and human T-cell solution for CD8+ T cells). Next, 3 μg of the plasmid was added, and the cells were transfected using Nucleofector I (Amaxa) according to the manufacturer's instructions. Transfections were performed in triplicate (a total of 6 ×106 cells/condition) and cultured in 2 ml of the medium at 37°C. Triplicates were pooled prior to analysis. For MOLT4, cells were split 1:3 on the day prior to the electroporation.
Flow cytometric analysis.
Specific binding of soluble HTLV SU proteins to target cells, the detection of cell surface expression of HSPG, and enzymatic removal of the HS chain of cell surface HSPGs were performed as recently described (17, 19).
To examine the level of cell surface expression of GLUT-1, 1 × 106 cells were spun down and resuspended in 200 μl of PBS-1% fetal calf serum-0.1% sodium azide (fluorescence-activated cell sorting [FACS] buffer). The cells were then incubated on ice for 30 min with 1 μg of either the anti-GLUT-1 antibody or an isotype control (mouse IgG2b). After being washed in FACS buffer, the cells were resuspended in 200 μl of FACS buffer and incubated on ice for 30 min with 1 μg of an anti-mouse IgG2b-conjugated antibody. After being washed in FACS buffer, the cells were resuspended in 350 μl of FACS buffer and immediately analyzed by flow cytometry. A total of 50,000 live cell events were measured on a FACScan (BD PharMingen) and analyzed using Flowjo software (Treestar).
Detection of cell surface GLUT by photoaffinity labeling.
CD4+ and CD8+ T cells, isolated from adult peripheral blood by negative selection, were activated as described above. A biotinylated photoaffinity label, 2-N-4-(1-azi-2, 2,2-trifluoroethyl)benzoyl-1,3-bis(d-glucose-4-yloxy)-2-propylamine (bis-glucose photolabel), was used to quantify the level of glucose transporters on the cell surface by using a modification of a previously described protocol (10, 45). After harvest, the cells were washed, 200 μl of 0.25 mM photolabel was added, and the cultures were immediately exposed to UV light six times for 30 s each time. After a washing step, the cells were resuspended in 500 μl of 2% Thesit detergent (Sigma) in PBS and incubated for 20 min at room temperature. After removal of the nuclei and cell debris, streptavidin beads (Pierce) were added to the samples, which were then incubated at 4°C overnight. Following centrifugation, the supernatant was transferred to another tube, and the protein was isolated and used to determine the level of intracellular GLUT-1. The streptavidin beads were washed and then resuspended in 50 μl of solubilization buffer to remove the photolabeled GLUT-1. Fifty micrograms of the protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% Tris-glycine gel (Invitrogen), transferred to Immobilon-P membrane (Millipore Corp.), and subjected to Western blotting analysis. The membranes were blocked with 3% bovine serum albumin (BSA) in Tris-buffered saline and hybridized at room temperature with a polyclonal antiserum directed against GLUT-1 (R&D) diluted 1:1,000 in 3% BSA in Tris-buffered saline. After 90 min, the blot was washed, incubated with I125-labeled protein A (0.2 mCi/ml) for 1 h at room temperature, washed again, and exposed to film. The blot was then scanned using a Fuji Phosphor Imager (FLA3000), and the bands were quantified using Image Gauge Software.
Retroviral vector transduction and HTLV virion internalization.
Pseudotyped retroviral vectors were generated and used to transduce the target cells as previously described (17). Briefly, target cells were incubated with 10-fold dilutions of supernatant containing the pseudotyped viruses, and negative control cultures (cells transduced with viral vectors with no Env) were included in each experiment. Four days later, 50,000 live cells were analyzed by flow cytometry for the expression of enhanced green fluorescent protein to determine the percentage of transduced cells. Titers were determined from the well with the lowest percentage that was at least 5% positive using the following formula: (% positive − % positive in negative control) × (number of cells in well on day of transduction). Titers were corrected to 1 ml. For all experiments, standard deviation was calculated.
To generate virus for the internalization assay, the HTLV-1 producer cell line 729/ACH and the HTLV-2 producer cell line 729/pH6neo were suspended at a concentration of 2 × 106 cells/ml. Sixteen hours later, the culture was centrifuged and the virus-containing supernatant was collected and filtered through a 0.45-μm-pore-size filter. The amount of virus present was quantified using a p19 (MA) enzyme-linked immunosorbent assay (Zeptometrix). Studies to examine the internalization of HTLV virions were performed as recently described (19).
RESULTS
Alteration of the cell surface of CD4+ T cells has a differential effect on HTLV-1 and HTLV-2 Env-mediated entry.
It has previously been reported that HTLV-1 and HTLV-2 share a receptor and that GLUT-1 can function as a receptor for both viruses. However, we have identified some substances that, when used to treat target cells, have a differential effect on HTLV-1 and HTLV-2 Env-mediated entry.
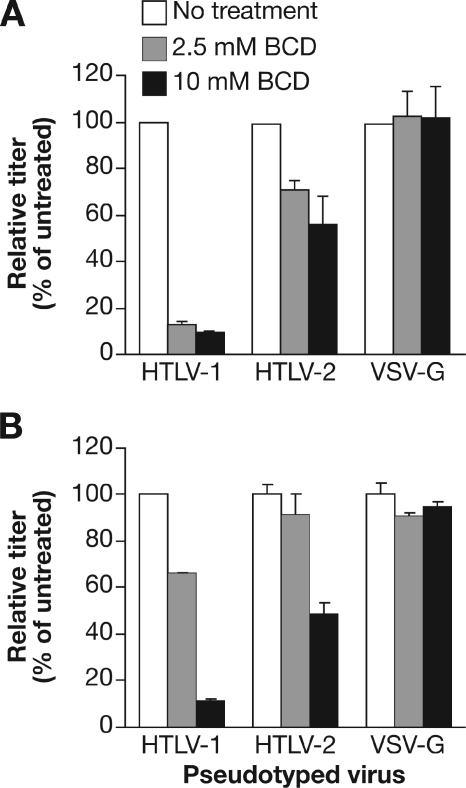
First, having observed that the disruption of lipid rafts decreases infection by HTLV-1 virions and the titer of HTLV-1 Env-pseudotyped viruses (51), we examined whether lipid raft disruption had a similar effect on HTLV-2 Env-mediated entry. Two CD4+ T-cell lines, SupT1 and Jurkat, were treated with hydroxypropyl-β-cyclodextrin (BCD), a substance that disrupts lipid rafts. As expected from previous studies, disruption of lipid rafts dramatically reduced the titer of HIV-based HTLV-1 Env-pseudotyped virions on both SupT1 (Fig. 1A) and Jurkat (Fig. 1B) cells. Disruption of lipid rafts has a less dramatic effect on reducing the HTLV-2 Env-pseudotyped virus. At 2.5 mM BCD, the HTLV-1 titer on SupT1 was reduced by 87%, while the HTLV-2 titer was reduced by 28%. For Jurkat, it was reduced by 34% for HTLV-1 and 9% for HTLV-2. The effect observed appeared to involve specific interactions between the Env proteins and the target cells, since entry of vesicular stomatitis virus G protein (VSV-G)-pseudotyped virus, which has previously been shown to be independent of lipid rafts, was not significantly affected by this treatment. Similar differences between HTLV-1 and HTLV-2 were observed following BCD treatment of adherent, non-T-cell lines (unpublished data).
FIG. 1.
Effect of lipid raft disruption on HTLV-1 Env- and HTLV-2 Env-mediated entry. HTLV-1 Env-, HTLV-2 Env-, or VSV-G-pseudotyped virus particles were generated as described in Materials and Methods. SupT1 cells (A) or Jurkat cells (B) were washed once in serum-free medium, resuspended at 5 × 106/ml in serum-free RPMI medium containing 0, 2.5, or 10 mM BCD and incubated at 37°C for 1 h. Cells were then washed three times with RPMI medium, resuspended in 0.5 ml of RPMI medium, and then mixed with an equal volume of the pseudotyped virus. The cells were transduced by spinoculation and then harvested 4 days later, and the titer was determined as described in Materials and Methods. Titers obtained in the absence of BCD were normalized to 100, and the relative titer was determined using the following formula: (titer with BCD/titer without BCD) × 100. The titer for each of the pseudotyped vectors in the absence of BCD was then determined. In SupT1 cells the titers in experiment 1 for each vector were the following: HTLV-1, 1.60 × 104; HTLV-2, 2.55 × 103; VSV-G, 5.54 × 107. In SupT1 cells the titers in experiment 2 were the following: HTLV-1, 5.95 × 104; HTLV-2, 1.95 × 103; and VSV-G, 1.06 × 107. In Jurkat cells, the titers were the following: HTLV-1, 5.62 × 103; HTLV-2, 1.15 × 103; and VSV-G, 4.33 × 106. For panel A, data shown are the average of two independent experiments; error bars represent standard deviation. For panel B, the titers were determined in duplicate; the error bars represent standard deviation. White bars, no BCD; gray bars, 2.5 mM BCD; black bars, 10 mM BCD. Data are from a representative experiment out of five (A) or three (B) performed.
HSPGs do not play a significant role in HTLV-2 Env-mediated binding and entry.
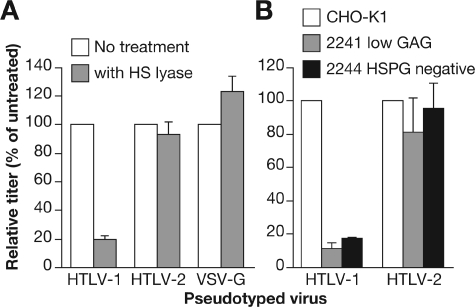
Having recently observed that the majority of binding of HTLV-1 virions on primary CD4+ T cells involves HSPGs (19), we wanted to investigate whether the differences in HTLV-1 and HTLV-2 Env-mediated entry might reflect differences in Env-HSPG interactions. Initially, the role of HSPG on HTLV-2 Env-mediated entry into non-T-cell lines was examined. Enzymatic removal of HS chains by HS lyase reduced the titer of HTLV-1 Env pseudotypes on COS cells by 82%, while the titer of the VSV-G-pseudotyped virus was slightly increased (Fig. 2A). In contrast, the titer of HTLV-2 Env pseudotypes was reduced by less than 7%. Similar studies were performed with cells carrying mutations in genes responsible for HSPG synthesis. HTLV-1 and HTLV-2 Env-pseudotyped virions were used to transduce parental CHO-K1 and two CHO-K1 cell lines carrying mutations in enzymes required for synthesis of HSPGs: CHO-K1 2241 (expresses low level of proteoglycans) and CHO-K1 2244 (negative for HSPGs). Similar to what was previously reported by other investigators (39), we found that the titer of HTLV-1 Env pseudotypes was significantly reduced in cell lines lacking HSPGs to 11.4% (CHO-K1 2241) and 17.6% (CHO-K1 2244) of the titer in the parental cell line (Fig. 2B, left). In contrast, the absence of HSPGs on the cell surface had a relatively modest effect on the titer of HTLV-2 Env-pseudotyped virions: the titers were 81.3% and 95.8% for CHO-K1 2241 and CHO-K1 2244 cells, respectively, of the titer in the CHO-K1 cell line (Fig. 2B, right). Thus, HSPGs do not play a significant role in HTLV-2 Env-mediated entry into these cell lines.
FIG. 2.
Effects of HSPG cell surface expression on HTLV-1 Env and HTLV-2 Env-mediated entry. (A) COS-7 cells (106) were suspended in 200 μl of HS lyase buffer (20 mM Tris, pH 7.4, 0.01% BSA, and 4 mM CaCl2) and then incubated for 2 h at 37°C with either 120 mU of HS lyase (gray bars) or left untreated (white bars). HTLV-1 Env-, HTLV-2 Env-, or VSV-G-pseudotyped virus particles were generated and used to transduce the COS-7 cells without spinoculation, and the titers were determined 3 days later. (B) HTLV-1 Env- and HTLV-2 Env-pseudotyped virus particles were used to transduce CHO-K1 cells (white bars), CHO-K1 2241 cells (gray bars), and CHO-K1 2244 cells (black bars) without spinoculation, and the titers were determined 3 days later. The data are the average of two independent experiments; error bars represent standard deviation. The titer for each of the pseudotyped virions on COS-7 cells in the absence of treatment (A) was as follows in experiment 1: HTLV-1, 6.8 × 103; HTLV-2, 5.4 × 104; VSV-G, 4.2 × 104. In experiment 2 the titers in COS-7 cells were as follows: HTLV-1, 5.9 × 104; HTLV-2, 3.7 × 104; VSV-G, 7.2 × 104. The titer for each of the pseudotyped vectors on the parental CHO-K1 cells (B) in experiment 1 were 4.4 × 103 for HTLV-1 and 4.0 × 103 for HTLV-2. In experiment 2 the titers in CHO-K1 cells were 7.8 × 104 for HTLV-1 and 5.7 × 104 for HTLV-2. Data shown are representative; an additional two (A) or three (B) experiments were performed.
HSPGs do not increase HTLV-2 binding and entry in CD4+ T cells.
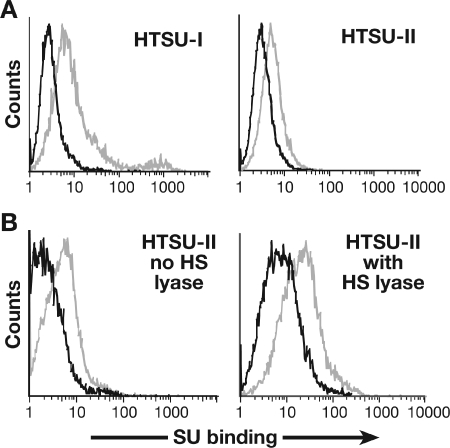
Having previously observed that removal of HSPG from CD4+ T cells eliminates the majority of binding of the HTLV-1 SU protein (19), we investigated the role of HSPGs in HTLV-2 SU binding. We generated a construct encoding a soluble form of the HTLV-2 SU (HTSUII-IgG) which is identical to the soluble HTSU-IgG we have generated previously (18), except that the coding region of HTLV-1 SU was replaced by that of the HTLV-2 SU protein. Initially, the level of binding of the soluble SU proteins on a CD4+ T-cell line (MOLT4) was measured by flow cytometry. The HTLV-2 SU bound specifically to the MOLT4 cells at a lower level than the HTLV-1 SU (Fig. 3A).
FIG. 3.
Effect of HSPGs on HTLV-2 SU binding on CD4+ T cells. (A) MOLT4 cells were incubated with 200 ng of either the soluble form of HTLV-1 SU (HTSU-IgG), the soluble form of HTLV-2 SU (HTSUII-IgG), or, as a negative control, a similar fusion protein (SUA-IgG) containing the SU protein from the avian retrovirus avian leukosis and sarcoma virus subgroup A. The amount of binding was determined by flow cytometry. The mean fluorescence intensity in this and all of the binding studies described was determined by subtracting the mean fluorescence intensity of the control (SUA-IgG) binding from the mean fluorescence intensity of specific (soluble HTLV SU) binding. Black line, SUA-IgG; gray line, HTSU-IgG or HTSUII-IgG. The mean fluorescence intensities of HTSU-IgG and HTSUII-IgG were 7.0 and 2.2, respectively. (B) CD4+ T cells, isolated from adult peripheral blood and activated for 5 days with anti-CD3/anti-CD28 antibody beads, were incubated with or without 10 mU of HS lyase. The left frame represents the binding of 200 ng of soluble HTLV-2 SU with no HS lyase, while the right frame represents the binding of soluble HTLV-2 SU with HS lyase. Black line, SUA-IgG; gray line, HTSUII-IgG. The mean fluorescence intensity of HTSUII-IgG incubated in buffer alone was 2.4, and for HTSUII-IgG treated with HS lyase, it was 12.2. Data are from a representative experiment out of two (A) or four (B) performed.
We next used this reagent to examine the role of HSPGs in the binding of HTLV-2 SU to activated CD4+ cells isolated from adult peripheral blood. In contrast to what has been previously reported for HTLV-1 SU, binding of HTLV-2 SU was not reduced when the HSPGs were removed by HS lyase (Fig. 3B). Treatment of CD4+ T cells with HS lyase reduced the level of the HSPGs by greater than 90% (data not shown).
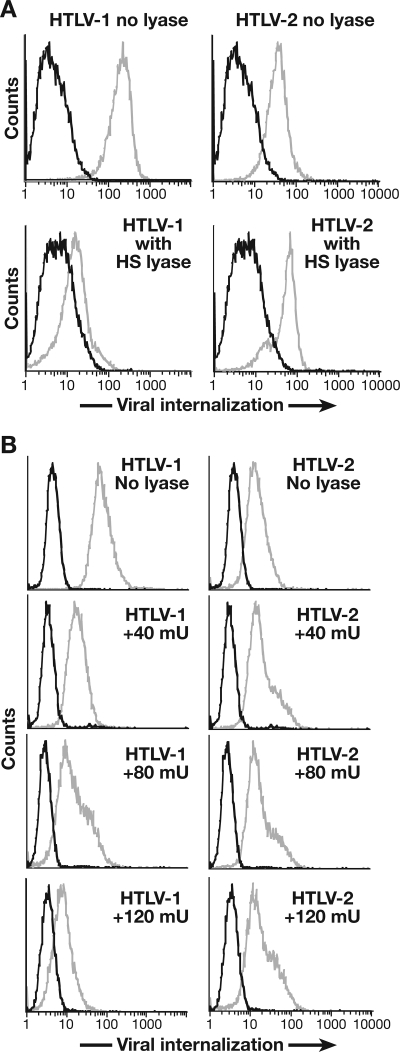
The role of HSPGs in the entry of HTLV-2 virions into CD4+ T cells was also examined. As one approach, purified CD4+ T lymphocytes isolated from adult peripheral blood and activated for 6 days were tested for viral internalization as described in Materials and Methods. As seen previously (19), enzymatic removal of HS chains from primary CD4+ T cells dramatically reduced internalization of HTLV-1 virions (by 96%) (Fig. 4A, left panels). In contrast, internalization of the HTLV-2 virions into these same cells was reduced by 4% (Fig. 4A, right panels). Staining with anti-HSPG antibodies confirmed that, following treatment, the level of HSPGs on the cell surface was not detectable (data not shown).
FIG. 4.
Effect of HSPGs on HTLV-2 entry into CD4+ T cells. (A) CD4+ T cells were isolated from adult peripheral blood lymphocytes, activated for 6 days with anti-CD3/anti-CD28 antibody beads, and then resuspended in HS lyase buffer and treated with 10 mU of HS lyase (bottom) or left untreated (top). The extent of viral internalization was determined 2 h after exposing the cells to 2 ng of either HTLV-1 (left) or HTLV-2 (right) as described in Materials and Methods. Black line, mouse IgG1 (isotype control); gray line, anti-HTLV MA (p19) antibody. The mean fluorescence intensity values were as follows: for HTLV-1 incubated in buffer alone, 174.5; for HTLV-1 treated with HS lyase, 7.2; for HTLV-2 incubated in buffer alone, 29.3; for HTLV-2 treated with HS lyase, 28.0. (B) SupT1 cells were resuspended in HS lyase buffer and incubated with 0, 40 mU, 80 mU, or 120 mU of HS lyase, as indicated, and the extent of internalization of 1 ng of virus was determined as in described for panel A. The mean fluorescence intensity values for HTLV-1 internalization were the follow ing under the specified conditions: incubated in buffer alone, 65.6; treated with 40 mU of HS lyase, 12.6; treated with 80 mU of HS lyase, 10.8; treated with 120 mU of HS lyase, 4.8. The mean fluorescence intensity values for HTLV-2 internalization were the following under the specified conditions: incubated in buffer alone, 65.6; treated with 40 mU of HS lyase, 12.6; treated with 80 mU of HS lyase, 10.8; treated with 120 mU of HS lyase, 4.8. The mean fluorescence intensity values for HTLV-2 internalization were the following under the specified conditions: incubated in buffer alone, 10.4; treated with 40 mU HS lyase, 16.4; treated with 80 mU HS lyase, 15.3; treated with 120 mU HS lyase, 14.9. Data are from a representative experiment out of six (A) or three (B) performed.
To further investigate the role of HSPGs on internalization of HTLV-2 virions, we treated the CD4+ T-cell line SupT1 with different concentrations of HS lyase. HS lyase treatment inhibited internalization of HTLV-1 virus in a dose-dependent manner (Fig. 4B, left panels). No decrease in HTLV-2 internalization was seen even at the highest concentrations of the enzyme (Fig. 4B, right panels). These results indicate that, unlike for HTLV-1, HSPGs are not critical for the efficient internalization of HTLV-2 into CD4+ T cells.
Differences in HTLV-1 and HTLV-2 binding to CD4+ T cells map to the C-terminal region of SU.
Next, we determined the region of SU responsible for efficient binding to activated CD4+ T cells. Studies of the organization of Env proteins of gammaretroviruses have shown that SU proteins consist of an N-terminal region which binds directly to the receptor (the receptor binding domain, or RBD), a short proline-rich “hinge” region (PRR), and a C-terminal region critical for fusion (1, 4, 5, 20, 24, 35). Later studies suggested that HTLV SU has a similar modular structure (21, 22, 29). Based on this organization, we generated four recombinants between HTLV-1 and HTLV-2 SU, producing chimeric proteins (Fig. 5A). The binding of one pair of recombinants in which the RBDs of the proteins were switched is shown in Fig. 5B. The SU containing the RBD from HTLV-2 and the PRR and C-terminal regions of HTLV-1 (HTSU-2/1/1) bound at high levels to activated CD4+ T cells, similar to that of the parental HTLV-1 SU. Conversely, the reciprocal SU (HTSU-1/2/2), like the parental HTLV-2 SU, bound at low levels to CD4+ T cells. Studies with a second pair of recombinant SU proteins revealed that the C-terminal 98 amino acids of HTLV-1 SU, outside both the RBD and the PRR, is sufficient to allow high levels of binding to CD4+ T cells (Fig. 5C). These results indicate that sequences in the C terminus of SU, outside the RBD, are responsible for the higher level of binding of HTLV-1 SU to CD4+ T cells.
Differential expression of HSPGs and different levels of HTLV-2 SU binding on CD4+ and CD8+ T cells.
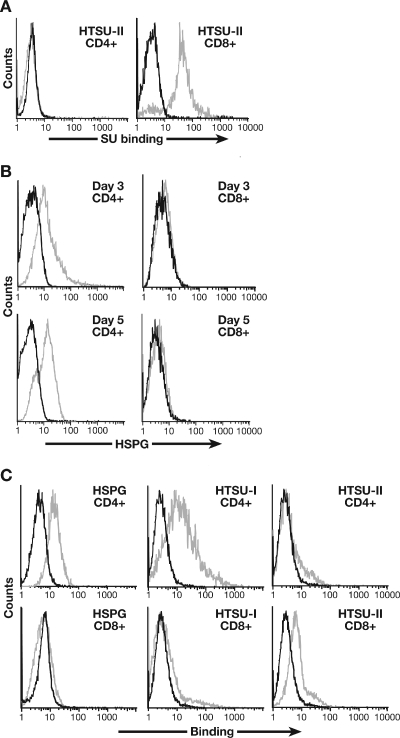
While HTLV-1 preferentially infects CD4+ T cells, HTLV-2 has a preferential tropism for CD8+ cells in vivo and in vitro. To compare the binding levels of HTLV-2 SU on primary T cells, purified populations of CD4+ and CD8+ T cells isolated from cord blood lymphocytes and activated for 5 days were assessed for the level of HTSUII-IgG binding. Under these conditions, the level of binding of HTLV-2 SU to the CD4+ T cells was below the level detectable in the assay (Fig. 6A, left). In contrast, the HTLV-2 bound at high levels to the CD8+ T cells (Fig. 6A, right).
FIG. 6.
Expression of HSPGs and the level of HTLV-2 SU binding on CD4+ and CD8+ T cells. (A) CD4+ and CD8+ T cells were isolated from cord blood lymphocytes by positive selection and activated by PHA and IL-2. Six days later, the level of binding of 200 ng of HTSUII-IgG was determined. Black line, SUA-IgG; gray line, HTSUII-IgG. The mean fluorescence intensity values were 0.2 for CD4+ and 21.9 for CD8+. (B) T lymphocytes were isolated from adult peripheral blood, separated into CD4+ and CD8+ populations, and activated by CD3/CD28 beads. Three and five days later, the cells were harvested and the level of HSPGs was determined. Black line, IgM isotype control; gray line, anti-HSPG antibody (F58-10E4). The mean fluorescence intensity values were 9.4 for CD4+ and 0.1 for CD8+ on day 3; on day 5 they were 7.8 for CD4+ and 0.4. for CD8+. (C) CD4+ and CD8+ T cells were isolated from cord blood lymphocytes and activated by PHA and IL-2, and the level of binding of 100 ng of SU protein was determined 16 h later. Left panels: black line, IgM isotype control; gray line, anti-HSPG antibody (F58-10E4). Middle and right panels: black line, SUA-IgG; gray line, HTSU-IgG (middle) or HTSUII-IgG (right). The mean fluorescence intensity values were as follows: HSPG/CD4+, 9.9; HSPG/CD8+, 0.83; HTSU/CD4+, 10.5; HTSU/CD8+, 1.0; HTSUII/CD4+, 1.1; HTSUII/CD8+, 4.0.
Since HSPGs do not play a major role in HTLV-2 SU binding (Fig. 3B), we examined whether the pattern of expression of HSPGs differed on activated CD4+ and CD8+ T cells. We recently observed that expression of HSPGs on CD4+ T cells peaked 3 to 5 days after activation (19). CD4+ and CD8+ T cells were isolated, activated, and assayed for the cell surface level of HSPGs by flow cytometry 3 and 5 days later. For CD4+ T cells, significant levels of HSPGs on the cell surface were observed at both time points (Fig. 6B, left graphs). In contrast, the level of HSPGs on the CD8+ T cells was very low (Fig. 6B, right graphs).
The different levels of HSPG expression on activated CD4+ and CD8+ T cells provided us the opportunity to examine the contributions of endogenous levels of HSPGs to HTLV SU binding. As one approach, the level of binding of HTLV-1 and HTLV-2 SU was compared. Cord blood CD4+ and CD8+ T cells were isolated, activated, and analyzed 16 h later. As expected, the CD4+ T cells expressed significant amounts of HSPG while CD8+ T cells expressed very low levels of HSPGs (Fig. 6C, left graphs). The binding of HTLV-1 SU paralleled the level of HSPGs; it was higher on the CD4+ T cells than on the CD8+ T cells (Fig. 6C, middle graphs). HTLV-2 binding does not correlate with the cell surface level of HSPGs: the level of binding was higher on CD8+ T cells than on the CD4+ T cells (Fig. 6C, right graphs).
Different levels of HTLV-1 and HTLV-2 entry reflect different levels of HSPGs and GLUT-1 on CD4+ and CD8+ T cells.
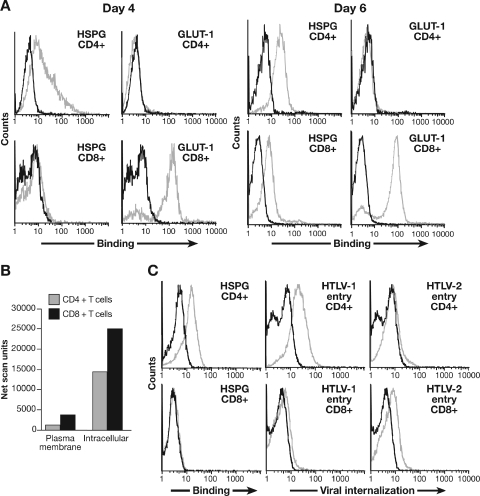
The expression of two molecules implicated in HTLV entry, HSPG and GLUT-1, was examined in parallel on samples of isolated CD4+ and CD8+ T cells from cord blood lymphocytes at 4 and 6 days following activation. Consistent with the results obtained with adult peripheral blood cells (Fig. 6A), expression of HSPGs 4 days after activation was high on CD4+ cells and low on CD8+ T cells (Fig. 7A, left graphs). At 6 days after activation, expression of HSPGs on CD4+ T cells was somewhat lower than on day 4 (Fig. 7A, top row); this is consistent with what we have previously reported (19). In contrast, HSPG expression on the CD8+ T cells was detectable, but lower than on CD4+ T cells, on day 6 (Fig. 7A, bottom row). The cell surface levels of GLUT-1 expression were high at both time points on CD8+ T cells (Fig. 7A, bottom row) and were either below the level of detection (day 4) or very low (day 6) on CD4+ T cells (Fig. 7A, top row).
FIG. 7.
Influence of endogenous cell surface levels of GLUT-1 and HSPGs on internalization of HTLV-1 and HTLV-2 virions into CD4+ and CD8+ T cells. (A) CD4+ and CD8+ T lymphocytes, isolated from adult peripheral blood, were analyzed for HSPG and GLUT-1 expression at 4 (left) and 6 (right) days after activation with anti-CD3/CD28 beads. Black line, isotype control; gray line, anti-HSPG antibody (F58-10E4) or anti-GLUT-1 (MAb 1418). The mean fluorescence intensity values on day 4 were as follows: HSPG/CD4+, 12.0; HSPG/CD8+, 0.2; GLUT-1/CD4+, −0.4; GLUT-1/CD8+, 60.4. Day 6 values were as follows: HSPG/CD4+, 15.9; HSPG/CD8+, 4.9; GLUT-1/CD4+, −0.2; GLUT-1/CD8+, 36.0. (B) Five days after activation, CD4+ and CD8+ T cells were exposed to UV light in the presence of a biotinylated photoaffinity label, lysed, processed, and analyzed as described in Materials and Methods. The level of membrane and intracellular GLUT-1 on the resulting Western blot was quantified. The net scan units were determined from the pixel density of bands on the scanned blot using the following formula: net scan units = total scan units of protein band − scan units of background (a nonradioactive portion of same column of the Western blot). (C) CD4+ and CD8+ T cells were harvested 2 days after activation. A portion of the samples was used to determine the cell surface levels of HSPG; the remainder was exposed to 1 ng of HTLV virions, and internalization levels were determined. Left panels: black line, IgM isotype control; gray line, anti-HSPG antibody (F58-10E4). Middle and right panels: black line, no virus; gray line, HTLV-1 (middle) or HTLV-2 (right). The mean fluorescence intensity values were as follows: HSPG/CD4+, 10.2; HSPG/CD8+, 0.2; HTLV-1 entry/CD4+, 15.7; HTLV-1 entry/CD8+, 1.1; HTLV-2 entry/CD4+, 0.7; HTLV-2 entry/CD8+, 6.0.
We have now examined expression of HSPGs and GLUT-1 on more than 25 samples of CD4+ and CD8+ T cells, isolated from both cord blood and adult peripheral blood, following activation with either CD3/CD28 beads or PHA and IL-2. Although there was genetic variability in the levels of expression in samples from different individuals, the pattern of expression following activation generally followed two patterns. At early times after activation (<4 days), HSPGs were at dramatically higher levels on CD4+ T cells than on CD8+ T cells. At later times (>5 days, depending on method of activation), significant levels of HSPGs were observed on some but not all CD8+ T cells. Similar results were obtained when the cell surface expression of a single member of the syndecan family of HSPGs on T cells was examined: syndecan-4 is expressed at much higher levels on CD4+ T cells than on CD8+ T cells (data not shown).
For GLUT-1, the expression level was consistently high on CD8+ cells and was consistently low or undetectable on CD4+ T cells, although some CD4+ T cells had low but detectable levels of GLUT-1. These results were confirmed using an independently derived anti-GLUT-1 antibody (data not shown). We also examined the cell surface expression of GLUT-1 on primary T cells using a different approach. Glucose transporter molecules present on the cell surface were photolabeled with a biotinylated probe that cross-links to glucose transporters after UV activation (13, 45). Following immunoprecipitation, the amount of GLUT-1 on the cell surface was determined by Western blotting with GLUT-1-specific antibodies. These studies confirmed that the level of GLUT-1 on the plasma membrane was higher on CD8+ than on CD4+ T cells and revealed that the intracellular levels of GLUT-1 were also higher in CD8+ than in CD4+ T cells (Fig. 7B).
The effect of different endogenous cell surface levels of HSPGs on virus internalization into primary T cells was also examined. Under conditions where CD4+ T cells had high levels of HSPGs and CD8+ T cells had low levels of HSPGs, HTLV-1 entry into CD4+ T cells was dramatically higher than in CD8+ T cells (Fig. 7C, left and middle graphs). In contrast, HTLV-2 entry was significantly higher in CD8+ than in CD4+ T cells (Fig. 7C, right graphs). These results suggest that different receptor complexes for HTLV-1 and HTLV-2 play a role in cell tropism of the viruses.
Altering the cell surface expression of components of the HTLV-1 and HTLV-2 receptor complexes alters cellular tropism of the viruses.
These studies indicate that differences between the levels of HTLV-1 and HTLV-2 SU binding and entry in T cells reflects differences in the cell surface expression of HSPGs and GLUT-1 on those cells. The level of HTLV-1 SU binding paralleled the level of HSPG, which generally was higher on CD4+ T cells. In contrast, HSPGs did not appear to increase the level of binding of HTLV-2 SU. Rather, a significant level of HTLV-2 SU binding appeared to require a threshold amount of GLUT-1 on the cell surface.
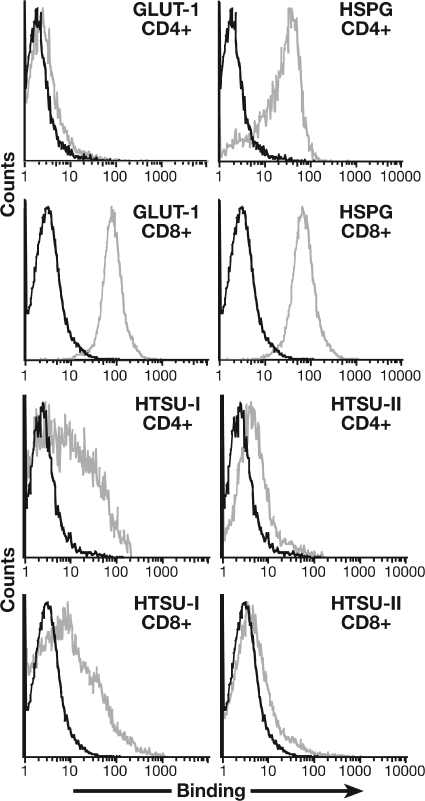
Although levels of HSPGs were nearly always higher on CD4+ T cells than on CD8+ T cells, we observed several samples at later times after activation in which high levels of HSPGs were expressed on the surface of both types of T cells. One such sample, harvested at 12 days after activation, is shown in Fig. 8. As expected, GLUT-1 was low but detectable on CD4+ and high on CD8+ T cells (Fig. 8, left graphs). Under these conditions, HTLV-1 SU bound at high levels to both CD4+ and CD8+ T cells (Fig. 8, left). HTLV-2 SU bound at lower levels to both types of cells (Fig. 8, right). The relatively low level of binding to CD8+ T cells likely reflects the high levels of HSPGs on these cells, which interfere with binding to GLUT-1.
FIG. 8.
Effect of high endogenous cell surface levels of HSPGs on CD8+ T cells on binding of HTLV-1 and HTLV-2 SU. CD4+ and CD8+ T cells were isolated from cord blood lymphocytes, activated by CD3/CD28 beads, and harvested 12 days later. Flow cytometry studies were performed as described in the legends of Fig. 7 (for HSPG and GLUT-1) and Fig. 6 (for HTSU-IgG and HTSUII-IgG). Black line, isotype control; gray line, anti-HSPG antibody (F58-10E4), anti-GLUT-1 (MAb 1418), HTSU-IgG, or HTSUII-IgG. The mean fluorescence intensity values were as follows: GLUT-1/CD4+, 0.73; GLUT-1/CD8+, 75.2; HSPG/CD4+, 18.5; HSPG/CD8+, 63.4; HTSU/CD4+, 6.4; HTSU/CD8+, 4.1; HTSUII/CD4+, 2.12; HTSUII/CD8+, 1.5.
One drawback of studies comparing binding and entry at different times following activation is that a number of other molecules might be differentially expressed on the cell surface. We directly examined whether overexpression of HSPGs in CD8+ T cells, under conditions where endogenous levels of HSPGs are low, would lead to increased HTLV-1 virion entry. A major source of HSPG on the cell surface is the syndecan family; of these, syndecan-4 is the most ubiquitously expressed. Recently, we have reported evidence that two members of this family, syndecan-2 and syndecan-4, are expressed on CD4+ T cells following activation (19). CD8+ T cells were transfected 3 days after activation with either a construct encoding syndecan-4 or a control plasmid (pcDNA). Two days later, the cells were exposed to HTLV-1 and HTLV-2 virions, and the amount of internalization was determined. As expected for CD8+ T cells early after activation, internalization levels on the cells transfected with the control plasmid were low for HTLV-1 and high for HTLV-2 (Fig. 9A, top row). Overexpression of syndecan-4 slightly decreased internalization of HTLV-2 (Fig. 9A, bottom right). In contrast, overexpression of syndecan-4 dramatically increased the level of entry of HTLV-1 into the CD8+ T cells (Fig. 9A, bottom left). Similar studies with syndecan-2 also increased entry into CD8+ T cells, although at a lower level than syndecan-4 (data not shown).
FIG. 9.
Effect of ectopic expression of HSPG in CD8+ T cells and GLUT-1 in CD4+ T cells on internalization of HTLV-1 and HTLV-2 virions. (A) Activated CD8+ T cells were transfected 3 days after activation either with a construct encoding syndecan-4, a type of HSPG, or a control plasmid (pcDNA). Two days later, the cells were exposed to 1 ng of HTLV-1 and HTLV-2 virions, and the amount of internalization was determined 2 h later. The mean fluorescence intensity values were as follows: HTLV-1 entry/pcDNA, 0.7; HTLV-1 entry/syndecan-4, 3.2; HTLV-2 entry/pcDNA, 2.6; HTLV-2 entry/syndecan-4, 1.67. (B) MOLT4 cells, a CD4+ T-cell line, were transfected with a GLUT-1-expressing plasmid (HA-GLUT-1) or a plasmid expressing a control glucose transporter (HA-GLUT-6 M). Three days later, they were exposed to 1 ng of either HTLV-1 or HTLV-2 virions, and the amount of internalization was determined 2 h later. Black lines, IgG1 isotype control; gray lines, anti-HTLV p19 (MA) antibody. The mean fluorescence intensity values were as follows: HTLV-1 entry/GLUT-6M, 2.5; HTLV-1 entry/GLUT-1, 3.7; HTLV-2 entry/GLUT-6M, −0.6; HTLV-2 entry/GLUT-1, 5.2.
We also examined whether increasing cell surface levels of GLUT-1 on a CD4+ T-cell line would increase HTLV-2 entry. These studies were performed using the CD4+ T-cell line MOLT4. The level of internalization of HTLV-2 into CD4+ T-cell lines, like primary T cells, is lower than the level of HTLV-1 internalization (Fig. 4B), and, like primary CD4+ cells, they express low levels of GLUT-1 on the cell surface (data not shown). MOLT 4 cells were transfected with either a GLUT-1-expressing plasmid (HA-GLUT-1) or, as a control, a plasmid encoding a mutant form of glucose transporter-6 (HA-GLUT-6 M) which is constitutively expressed on the cell surface. Three days later, they were exposed to HTLV-1 or HTLV-2 virions, and the extent of internalization was determined. The level of internalization of HTLV-2 was lower than HTLV-1 in the cells transfected with the control plasmid (note that the level of entry was below the negative control) (Fig. 9B, top row). Overexpression of GLUT-1 in the CD4+ T cells dramatically increased the level of internalization of HTLV-2 virions (Fig. 9B, bottom right). The amount of internalization of HTLV-1 was modestly increased in the cells expressing GLUT-1 (Fig. 9B, bottom left).
DISCUSSION
Early superinfection interference studies indicated that HTLV-1 and HTLV-2 share a receptor (46, 47), and recently GLUT-1 has been shown to function as a receptor for both viruses in adherent non-T-cell lines (8, 28). Here, we observed that the receptor complexes of HTLV-1 and HTLV-2 on T cells differ. While HTLV-1 requires HSPGs for efficient attachment and entry, HSPGs do not play a similar role for HTLV-2.
The observation that binding of HTLV-2 SU to primary T cells involves GLUT-1 is consistent with previous studies performed on the cell lines (28). However, the observation by our laboratory and others that HTLV-1 SU binds primarily to HSPGs (19, 39) is at odds with other studies that stated that both HTLV-1 and HTLV-2 SU bind directly to GLUT-1 (21, 22, 29). These latter studies also stated that the N-terminal 215 amino acids of the HTLV SU (RBD region) contain all the sequences needed for optimal binding. One difference between these studies was the reagents used. The studies showing binding to GLUT-1 were performed using a truncated form of HTLV-1 SU (H1RBD), containing only the RBD, while the studies showing HTLV-1 SU binding to HSPGs used full-length SU. Here, we observed that the ability of full-length HTLV-1 SU to bind at high levels to HSPG-positive CD4+ T cells mapped to regions outside the RBD (Fig. 5). This indicates that sequences important for HSPG binding, and possibly other molecule(s) involved in binding, are located in the C terminus of the HTLV-1 SU. This is similar to feline leukemia virus subgroup T, for which determinants for the receptor specificity have been shown to map outside the RBD (7).
The studies presented here demonstrate that HTLV-1 and HTLV-2 differ in their requirements for entry into CD4+ and CD8+ T cells. For HTLV-2, GLUT-1 appears to facilitate both binding and entry into T cells, favoring CD8+ T cells, which express high levels of GLUT-1 on the cell surface. The role of as yet unidentified additional molecules in HTLV-2 entry cannot be ruled out. For HTLV-1, the virus appears to bind to HSPGs at an early step in virus-T-cell interaction, favoring the activated, HSPG-rich CD4+ T cells. This interaction appears to be required for subsequent interactions of SU with GLUT-1 or other molecules, which in turn allows fusion and entry to occur. In this model, the differences in entry requirements between HTLV-1 and HTLV-2 would be analogous to the differences between CD4-dependent and CD4-independent primary isolates of HIV-2 and simian immunodeficiency virus, which differ in their requirements for a binding receptor but which share fusion receptors (40).
The precise role of HSPGs in HTLV-1 entry has not yet been defined. For many viruses, HSPGs play a role in the cellular attachment, either as a low-specificity attachment factor, as a specific binding receptor (26), or, as in the case of herpes simplex virus type 1, as a fusion receptor (49). Attachment factors are molecules that, while not required for entry, can dramatically increase the efficiency of viral entry by concentrating virus on the cell surface, thereby increasing the probability of interactions with the fusion receptors. It seems unlikely that HSPGs are solely functioning to increase HTLV-1 interactions with GLUT-1, since binding and entry into CD8+ cells were inefficient despite high levels of GLUT-1. With regard to HIV, although it was originally believed that HSPG was one of several attachment factors for HIV (30, 44), more recent studies have revealed that HSPG can play specific roles in HIV-1 transmission. In cells which are CD4 negative (brain endothelial cells) (3), HSPGs play a crucial role in HIV entry. Other studies have reported that HSPGs can function in trans to enhance viral spread (6). Significantly, a recent study revealed that a single amino acid in the V3 region of gp120 was responsible for the binding to HSPGs and that HIV-1 binds the HSPGs via a 6-O sulfation, indicating that HSPG-gp120 interactions are not the result of random interactions between basic residues and negative charges (9). We are currently attempting to define the specific role(s) of HSPGs in HTLV-1 infectivity.
It appears likely that least one additional molecule in addition to HSPG is capable of specifically binding HTLV-1 SU. Binding and entry of HTLV-1 can occur, although at a low level, in HSPG-negative cell lines (Fig. 2B) (39) and when HSPGs have been enzymatically removed from the surface of T cells (19, 39). We are currently investigating whether HTLV-1 binding with these other molecule(s) occurs as an alternate to HSPG binding or in concert with HSPG binding.
The observation that HTLV-1 uses HSPG and/or other molecules as binding receptor(s) does not necessarily contradict earlier studies indicating that the two viruses share a receptor. If both HTLV-1 and HTLV-2 use the same molecule as fusion receptor, it would be expected that Env-fusion receptor interactions in infected cells would prevent virus-cell membrane fusion for both viruses. This notion is supported by previous reports that expression of both HTLV-1 and HTLV-2 Env proteins alters glucose metabolism (28). In a recent study, two extracellular loops of GLUT-1 (extracellular loops 1 and 5) were shown to be required for infection but not involved in binding (27). The extracellular loop 6 of GLUT-1, in the context of another glucose transporter, was shown to be sufficient for HTLV-2 RBD binding (27). Those studies were performed using HTLV-2; it would be interesting to know whether the same or different regions of GLUT-1 are required for infection by HTLV-1.
A number of studies have shown that the majority of cells transformed by HTLV-1 are CD4+ T cells, while HTLV-2 preferentially transforms CD8+ T cells (33, 42, 43, 50, 52, 53). Since transformation of primary T cells by both HTLV-1 and HTLV-2 require the Tax protein (42, 43), it had been hypothesized that Tax was responsible for the differences between the transformation tropism of these two viruses. However, recent studies by Green and colleagues using recombinant HTLV-1/HTLV-2 viruses revealed that the transformation tropism did not map to Tax (53) or to the long terminal repeat (52). Rather, the in vitro transformation tropism maps to the Env protein: HTLV-2 with HTLV-1 Env preferentially transformed CD4+ cells, while HTLV-1 with HTLV-2 Env preferentially transformed CD8+ T cells (52). These observations indicate that HTLV-1 and HTLV-2 Env proteins interact differently with specific protein(s) in T lymphocytes. As the authors of that report suggest, these molecules could be involved in entry or in some postentry signaling pathway step that promotes transformation.
Here, we have observed that HSPGs and GLUT-1 are differentially expressed on CD4+ and CD8+ T cells, which, under most conditions, allows HTLV-1 to preferentially enter CD4+ T cells and HTLV-2 to preferentially enter CD8+ T cells. Ectopic expression of HSPG in CD8+ T cells and GLUT-1 in CD4+ T cells breaks down the tropism and allows both viruses to enter both cell types efficiently. These observations, along with those of Green and colleagues, suggest that the differences in the in vitro transformation tropism and the in vivo pathobiology of these viruses are due, at least in part, to differences in the receptor complexes that HTLV-1 and HTLV-2 use to bind and enter CD4+ and CD8+ T cells.
Acknowledgments
We thank Karen Yao, Norihiro Takenouchi, and Chris Grant for helpful suggestions and encouragement.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. K.F. was supported by the Japanese Society for the Promotion of Science.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors have no conflicting financial interests.
REFERENCES
- 1.Albritton, L. M., L. Tseng, D. Scadden, and J. M. Cunningham. 1989. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57:659-666. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hasani, H., D. R. Yver, and S. W. Cushman. 1999. Overexpression of the glucose transporter GLUT4 in adipose cells interferes with insulin-stimulated translocation. FEBS Lett. 460:338-342. [DOI] [PubMed] [Google Scholar]
- 3.Argyris, E. G., E. Acheampong, G. Nunnari, M. Mukhtar, K. J. Williams, and R. J. Pomerantz. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140-12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battini, J. L., O. Danos, and J. M. Heard. 1995. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 69:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini, J. L., J. M. Heard, and O. Danos. 1992. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J. Virol. 66:1468-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobardt, M. D., A. C. Saphire, H. C. Hung, X. Yu, B. Van der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, H. H., M. M. Anderson, F. C. Hankenson, L. Johnston, C. V. Kotwaliwale, and J. Overbaugh. 2006. Envelope determinants for dual-receptor specificity in feline leukemia virus subgroup A and T variants. J. Virol. 80:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coskun, A. K., and R. E. Sutton. 2005. Expression of glucose transporter 1 confers susceptibility to human T-cell leukemia virus envelope-mediated fusion. J. Virol. 79:4150-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Parseval, A., M. D. Bobardt, A. Chatterji, U. Chatterji, J. H. Elder, G. David, S. Zolla-Pazner, M. Farzan, T. H. Lee, and P. A. Gallay. 2005. A highly conserved arginine in gp120 governs HIV-1 binding to both syndecans and CCR5 via sulfated motifs. J. Biol. Chem. 280:39493-39504. [DOI] [PubMed] [Google Scholar]
- 10.Dudek, R. W., G. L. Dohm, G. D. Holman, S. W. Cushman, and C. M. Wilson. 1994. Glucose transporter localization in rat skeletal muscle. Autoradiographic study using ATB-[2-3H]BMPA photolabel. FEBS Lett. 339:205-208. [DOI] [PubMed] [Google Scholar]
- 11.Feuer, G., and P. L. Green. 2005. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 24:5996-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 2:407-410. [DOI] [PubMed] [Google Scholar]
- 13.Holman, G. D., I. J. Kozka, A. E. Clark, C. J. Flower, J. Saltis, A. D. Habberfield, I. A. Simpson, and S. W. Cushman. 1990. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J. Biol. Chem. 265:18172-18179. [PubMed] [Google Scholar]
- 14.Ijichi, S., M. B. Ramundo, H. Takahashi, and W. W. Hall. 1992. In vivo cellular tropism of human T cell leukemia virus type II (HTLV-II). J. Exp. Med. 176:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jassal, S. R., M. D. Lairmore, A. J. Leigh-Brown, and D. W. Brighty. 2001. Soluble recombinant HTLV-1 surface glycoprotein competitively inhibits syncytia formation and viral infection of cells. Virus Res. 78:17-34. [DOI] [PubMed] [Google Scholar]
- 16.Jassal, S. R., R. G. Pohler, and D. W. Brighty. 2001. Human T-cell leukemia virus type 1 receptor expression among syncytium-resistant cell lines revealed by a novel surface glycoprotein-immunoadhesin. J. Virol. 75:8317-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, K. S., S. Akel, C. Petrow-Sadowski, Y. Huang, D. C. Bertolette, and F. W. Ruscetti. 2005. Induction of human T cell leukemia virus type I receptors on quiescent naive T lymphocytes by TGF-beta. J. Immunol. 174:4262-4270. [DOI] [PubMed] [Google Scholar]
- 18.Jones, K. S., M. Nath, C. Petrow-Sadowski, A. C. Baines, M. Dambach, Y. Huang, and F. W. Ruscetti. 2002. Similar regulation of cell surface human T-cell leukemia virus type 1 (HTLV-1) surface binding proteins in cells highly and poorly transduced by HTLV-1-pseudotyped virions. J. Virol. 76:12723-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, K. S., C. Petrow-Sadowski, D. C. Bertolette, Y. Huang, and F. W. Ruscetti. 2005. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 79:12692-12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayman, S. C., H. Park, M. Saxon, and A. Pinter. 1999. The hypervariable domain of the murine leukemia virus surface protein tolerates large insertions and deletions, enabling development of a retroviral particle display system. J. Virol. 73:1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, F. J., N. Manel, E. N. Garrido, C. Valle, M. Sitbon, and J. L. Battini. 2004. HTLV-1 and -2 envelope SU subdomains and critical determinants in receptor binding. Retrovirology 1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, F. J., I. Seiliez, C. Denesvre, D. Lavillette, F. L. Cosset, and M. Sitbon. 2000. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 275:23417-23420. [DOI] [PubMed] [Google Scholar]
- 23.Lal, R. B., S. M. Owen, D. L. Rudolph, C. Dawson, and H. Prince. 1995. In vivo cellular tropism of human T-lymphotropic virus type II is not restricted to CD8+ cells. Virology 210:441-447. [DOI] [PubMed] [Google Scholar]
- 24.Lavillette, D., M. Maurice, C. Roche, S. J. Russell, M. Sitbon, and F. L. Cosset. 1998. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 72:9955-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisinski, I., A. Schurmann, H. G. Joost, S. W. Cushman, and H. Al-Hasani. 2001. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem. J. 358:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, J., and S. C. Thorp. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 22:1-25. [DOI] [PubMed] [Google Scholar]
- 27.Manel, N., J. L. Battini, and M. Sitbon. 2005. Human T cell leukemia virus envelope binding and virus entry are mediated by distinct domains of the glucose transporter GLUT1. J. Biol. Chem. 280:29025-29029. [DOI] [PubMed] [Google Scholar]
- 28.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 29.Manel, N., N. Taylor, S. Kinet, F. J. Kim, L. Swainson, M. Lavanya, J. L. Battini, and M. Sitbon. 2004. HTLV envelopes and their receptor GLUT1, the ubiquitous glucose transporter: a new vision on HTLV infection? Front. Biosci. 9:3218-3241. [DOI] [PubMed] [Google Scholar]
- 30.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai, M., M. B. Brennan, J. A. Sakai, C. A. Mora, and S. Jacobson. 2001. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 32.Nath, M. D., F. W. Ruscetti, C. Petrow-Sadowski, and K. S. Jones. 2003. Regulation of the cell-surface expression of an HTLV-I binding protein in human T cells during immune activation. Blood 101:3085-3092. [DOI] [PubMed] [Google Scholar]
- 33.Newbound, G. C., J. M. Andrews, J. P. O'Rourke, J. N. Brady, and M. D. Lairmore. 1996. Human T-cell lymphotropic virus type 1 Tax mediates enhanced transcription in CD4+ T lymphocytes. J. Virol. 70:2101-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet 1:1031-1032. [DOI] [PubMed] [Google Scholar]
- 35.Ott, D., and A. Rein. 1992. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J. Virol. 66:4632-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overbaugh, J. 2004. HTLV-1 sweet-talks its way into cells. Nat. Med. 10:20-21. [DOI] [PubMed] [Google Scholar]
- 37.Overbaugh, J., A. D. Miller, and M. V. Eiden. 2001. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol Rev. 65:371-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinon, J. D., P. J. Klasse, S. R. Jassal, S. Welson, J. Weber, D. W. Brighty, and Q. J. Sattentau. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 77:9922-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves, J. D., S. Hibbitts, G. Simmons, A. McKnight, J. M. Azevedo-Pereira, J. Moniz-Pereira, and P. R. Clapham. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J. Virol. 73:7795-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross, T. M., S. M. Pettiford, and P. L. Green. 1996. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 70:5194-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satoh, S., H. Nishimura, A. E. Clark, I. J. Kozka, S. J. Vannucci, I. A. Simpson, M. J. Quon, S. W. Cushman, and G. D. Holman. 1993. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 268:17820-17829. [PubMed] [Google Scholar]
- 46.Sommerfelt, M. A., and R. A. Weiss. 1990. Receptor interference groups of 20 retroviruses plating on human cells. Virology 176:58-69. [DOI] [PubMed] [Google Scholar]
- 47.Sommerfelt, M. A., B. P. Williams, P. R. Clapham, E. Solomon, P. N. Goodfellow, and R. A. Weiss. 1988. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science 242:1557-1559. [DOI] [PubMed] [Google Scholar]
- 48.Sutton, R. E., and D. R. Littman. 1996. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J. Virol. 70:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwari, V., C. Clement, M. B. Duncan, J. Chen, J. Liu, and D. Shukla. 2004. A role for 3-O-sulfated heparan sulfate in cell fusion induced by herpes simplex virus type 1. J. Gen. Virol. 85:805-809. [DOI] [PubMed] [Google Scholar]
- 50.Wang, T. G., J. Ye, M. D. Lairmore, and P. L. Green. 2000. In vitro cellular tropism of human T cell leukemia virus type 2. AIDS Res. Hum. Retrovir. 16:1661-1668. [DOI] [PubMed] [Google Scholar]
- 51.Wielgosz, M. M., D. A. Rauch, K. S. Jones, F. W. Ruscetti, and L. Ratner. 2005. Cholesterol dependence of HTLV-I infection. AIDS Res. Hum. Retrovir. 21:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie, L., and P. L. Green. 2005. Envelope is a major viral determinant of the distinct in vitro cellular transformation tropism of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 79:14536-14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye, J., L. Xie, and P. L. Green. 2003. Tax and overlapping rex sequences do not confer the distinct transformation tropisms of human T-cell leukemia virus types 1 and 2. J. Virol. 77:7728-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zingler, K., and J. A. Young. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]