Abstract
The pathways by which viruses enter cells are diverse, but in all cases, infection necessitates the transfer of the viral genome across a cellular membrane. Polyomavirus (Py) particles, after binding to glycolipid and glycoprotein receptors at the cell surface, are delivered to the lumen of the endoplasmic reticulum (ER). The nature and extent of virus disassembly in the ER, how the viral genome is transported to the cytosol and subsequently to the nucleus, and whether any cellular proteins are involved are not known. Here, we identify an ER-resident protein, Derlin-2, a factor implicated in the removal of misfolded proteins from the ER for cytosolic degradation, as a component of the machinery required for mouse Py to establish an infection. Inhibition of Derlin-2 function by expression of either a dominant-negative form of Derlin-2 or a short hairpin RNA that reduces Derlin-2 levels blocks Py infection by 50 to 75%. The block imposed by Derlin-2 inhibition occurs after the virus reaches the ER and can be bypassed by the introduction of Py DNA into the cytosol. These findings suggest a mode of Py entry that involves cytosolic access via the quality control machinery in the ER.
For members of the small, nonenveloped DNA viruses of the polyomavirus (Py) family, the manner by which the viral genome accesses the cytosol and ultimately the nucleus is only partially understood. The ∼5.3-kb viral DNA complexed with histones is packed within an icosahedral shell (diameter of 45 nm) consisting of 72 pentamers of the VP1 protein and an inner scaffold of 72 copies of the internal VP2/VP3 proteins (24). Murine Py VP1 binds to sialic acid residues of the GD1a and GT1b glycolipids and to glycoproteins at the cell surface, while the related simian virus 40 (SV40) VP1 binds to the GM1 ganglioside (24, 26). Shortly thereafter, these viruses are internalized via both caveolin-dependent (5, 17, 19, 21) and caveolin-independent (2, 6) pathways depending, in part, on the host cell. Ultimately, both viruses are transported to the endoplasmic reticulum (ER) (5, 6, 9, 14, 17, 19, 21). Following rearrangement or partial disassembly, an altered virus particle must then exit the ER and enter the nucleus. Results with SV40 indicate that the outer capsid shell of the virion is at least partially disassembled in the ER, exposing the internal VP2/3 proteins (17). While the nature of the subviral assembly that crosses the membrane is unknown, two different lines of evidence suggest that SV40 VP2/3 proteins accompany the viral genome across the membrane and contribute to infection: (i) injection of antibodies against SV40 VP2/3 into the cytosol inhibits infection (15), and (ii) the nuclear localization signal of VP2/3 is required for entry of SV40 into the nucleus (16). The ER-localized oxidoreductase ERp29 is capable of altering Py VP1 conformation, leading to increased hydrophobicity of the particle (13). Myristoylation of the internal capsid protein VP2 is also important but not absolutely essential for initiating infection (22). How the change in VP1 conformation and myristoylation of VP2 contribute to Py infection and what additional cellular factors might be involved in virus disassembly and exit from the ER are presently unknown.
The movement of proteins from the ER to the cytosol for proteasomal degradation is an essential step in a quality control pathway used by the cell to remove many misfolded polypeptides from the productive folding environment of the ER (27). Certain plant and bacterial toxins also utilize this pathway to access the cytosol (23). In human cytomegalovirus (HCMV)-infected cells, the HCMV-encoded glycoproteins US2 and US11 catalyze the movement of class I major histocompatibility complex (MHC) molecules from the ER into the cytosol (a process termed dislocation or retrotranslocation), where proteasomal degradation rapidly ensues (29, 30). The mammalian Derlin-1 protein is thus co-opted by HCMV US11 to achieve dislocation of class I MHC heavy chains to the cytosol, resulting in escape from immune detection (11, 32). Derlin-1 and its related mammalian family members (Derlin-2 and Derlin-3) are named for their similarity to Saccharomyces cerevisiae Der1p (10), a protein required for degradation of a subset of misfolded proteins. US11 interacts with class I MHC molecules and recruits them to the Derlin-1 polypeptide, part of a protein complex that includes the p97 AAA ATPase, which then moves the class I MHC heavy chain from the ER to the cytosol (11, 32). The US2 protein operates in a manner that is Derlin-1 independent (11), suggesting the existence of several distinct pathways that can accomplish dislocation. The pathways exemplified by US2- and US11-dependent degradation of class I MHC molecules are considered to be emblematic of the pathways via which misfolded proteins are cleared from the ER.
Derlin-2 is a close relative of Derlin-1 (approximately 30% identical) (11). Because of its homology to yeast Der1p, its structural similarity to Derlin-1, its similar intracellular location, as well as its interacting partners, it is likely that Derlin-2 is involved in some aspect of protein quality control and dislocation from the ER. Derlin-2 interacts with proteins heavily decorated with poly-glutathione S-transferase-ubiquitin (12), a result similar to that observed for Derlin-1 (32), which suggests a role for Derlin-2 in the elimination of misfolded polypeptides from the ER. A recent report indeed suggested that a reduction in the levels of Derlin-2 (or Derlin-3 but not Derlin-1) slows the degradation of a misfolded variant of α1-antitrypsin (18). Based on evidence that indicates the participation of Derlin-1 and other Derlin family members in the movement of misfolded proteins from the ER to the cytosol, we hypothesized that Derlins might be part of the conduit via which Py accesses the cytosol and establishes an infection. Here, we present evidence that Derlin-2, but not Derlin-1 or Derlin-3, is involved in the escape of Py virus from the ER and in establishing an infection.
MATERIALS AND METHODS
Antibodies, cell lines, and DNA constructs.
The following antibodies have been described previously: anti-Derlin-1, anti-Derlin-2 (11), anti-SEL1L (12), anti-T-cell receptor α (TCRα) (8), and anti-ribophorin I (3). The anti-protein disulfide isomerase (PDI) antibodies were generated within the Ploegh laboratory, and the anti-p97 antibodies were obtained from Research Diagnostics. The rat anti-polyomavirus large T antigen (LTAg) antibody was generated within the Benjamin laboratory. The 3T3 and C6 rat glioblastoma cell lines were cultured as described previously (5), as were HeLa and 293T cell lines (11). 3T3, C6, and HeLa cell lines stably expressing human Derlin proteins fused to green fluorescent protein (GFP) (DerlinGFP) (12) were made by retroviral transduction with the pMSCV-puro (Clontech) vector serving as the backbone. Constructs expressing short hairpin RNA (shRNA) for the knockdown of rat Derlin-1 and rat Derlin-2 were made using the pRETRO-SUPER (pRS) vector as described previously (12). The sequences used are as follows, with the numbers in parentheses indicating the nucleotide coordinates of the respective cDNAs, with 1 representing the adenosine of the start codon (the vector targeting GFP has been described previously [12]): TGGATATGCAGTTGCTGAT (nucleotides 347 to 365) (rat Derlin-1) and GGAGGCTAATCACCAATTTC (nucleotides 161 to 180) (rat Derlin-2).
Culture supernatants containing retroviruses were generated as described previously (11). Transduced HeLa, 3T3, and C6 cells were subsequently selected with 1 μg/ml, 2.5 μg/ml, and 6 μg/ml puromycin, respectively.
Viral infections and immunofluorescence microscopy.
Infections of 3T3 and C6 cells with the small plaque RA strain of Py, immunofluorescence microscopy, deconvolution, and analysis of colocalization were performed as described previously (5). C6 cells were plated onto 12-mm glass coverslips and grown to approximately 80% confluence at 37°C in a CO2 incubator for a minimum of 27 h in the presence of 30 μM GD1a. Multiplicities of infection were approximately 10 PFU/cell for 3T3 cells and several hundred PFU/cell for the less-infectible rat C6 cells (5). Virus was diluted in medium, cells and virus were incubated for 1 h at 37°C in a CO2 incubator, and virus was then removed by aspiration. Virus was allowed to replicate for 32 h at 37°C. Successful entry was assessed by nuclear expression of Py LTAg by immunofluorescence assay (see below). Data are presented as the percentages of nuclei that were LTAg positive in the treated sample relative to the percentages of nuclei that were LTAg positive in cells transduced with the control vector, which is calculated to be 100%. Values are presented as the means and standard deviations of triplicate samples where approximately 500 nuclei were counted per sample. Py genomic DNA was prepared as described previously (5) and was transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's specifications. At 32 h after transfection, cells were processed and examined for nuclear expression of Py LTAg as described below.
For analysis of Py colocalization, purified Texas Red-labeled Py (TRPy) particles were prepared as described previously (5) and added to Derlin-1GFP or Derlin-2GFP C6 cells at approximately 100 particles per cell. After the time of desired incubation, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA). Samples were permeabilized by treatment with either 0.1% Triton X-100 or phosphate-buffered saline containing 1% calf serum (Invitrogen) for examination of all antigens. Samples were incubated with primary antibody for 1 h at room temperature. Samples then were incubated with labeled secondary antibodies and incubated for 1 h at room temperature. For quantitation of LTAg staining, DAPI (4′,6′-diamidino-2-phenylindole) was included in the buffer containing the secondary antibody. The washed coverslips were mounted with Moviol, sealed with nail polish, and examined by standard fluorescence microscopy with a Nikon Eclipse TE300 microscope with an apochromatic Plan 60×/1.4 oil objective. For deconvolution microscopy, 8 to 12 random fields were selected. Data were collected with a Nikon Eclipse TE200 microscope with a an apochromatic Plan 60×/1.4 oil objective equipped with a DeltaVision optical sectioning system employing SoftWoRx software (Applied Precision, Inc., Issaquah, WA) with 0.2-μm-thick Z sections. Deconvolved Z sections were examined for colocalization of TRPy and the epitopes of interest using the SoftWoRx program. Approximately 2,000 particles were counted per time point per cell line. The data are shown as the means and standard deviations of the percentages of TRPy particles observed to colocalize with PDI or with Derlin-1GFP/Derlin-2GFP relative to the total number of TRPy particles. The selected images were saved as TIFF files and then imported and prepared using Adobe Photoshop 7.0.
Biochemical methods.
For assessment of ribophorin I 332 (RI332) and TCRα stability, one 10-cm dish of each DerlinGFP-transduced HeLa cell line was transfected with 5 μg of plasmid expressing either RI332 (3) (a kind gift from N. Erwin Ivessa) or TCRα (8) using FuGene6 (Roche) according to the manufacturer's specifications. Forty-eight hours posttransfection, the cells were processed for a pulse-chase experiment (20), followed by immunoprecipitations using the appropriate antibodies, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and fluorography. Coimmunoprecipitations and immunoblotting experiments were performed as described previously (12).
RESULTS
We first examined the biochemical properties of Derlin-2 in rat C6 glioblastoma cells, a cell line for which the intracellular trafficking pathways of Py have been well characterized (5). Rat Derlin-2 localizes to the ER membrane and forms a multisubunit complex with other proteins known to be involved in the dislocation of misfolded proteins from the ER. We observed coimmunoprecipitation of the p97 ATPase with both Derlin-1 and Derlin-2 in C6 cells, consistent with results using U373-MG cells (Fig. 1A and B) (12, 32). We also observed an association of Derlin-2 with the ER membrane-localized SEL1L-HRD1 ubiquitin ligase complex (12), while Derlin-1 associates weakly with this complex. Heterooligomerization of rat Derlin-1 and Derlin-2 is also readily observed in C6 cells, as was seen in the human cell lines HeLa and U373-MG (Fig. 1A) (12). The sequence conservation between Derlin-1 and Derlin-2 (11) as well as the roughly similar composition of protein complexes containing Derlin-1 and Derlin-2 suggest an analogous role for these two proteins in the dislocation from the ER to the cytosol. Recent results have shown the involvement of Derlin-2 and Derlin-3 in the degradation of α1-antitrypsin (18), consistent with our data.
FIG. 1.
Association of Derlin-2 with proteins involved in the removal of misfolded proteins from the ER. (A) The indicated cell lines were metabolically labeled for 16 h, lysates were prepared using digitonin as the detergent, and immunoprecipitations (IP) were performed using the antibodies indicated. Coimmunoprecipitating proteins were visualized via SDS-PAGE and fluorography, and the identities of the known coimmunoprecipitating proteins (determined by reimmunoprecipitation using specific antibodies) are indicated, as are nonspecifically associated polypeptides (*). SEL1L and p97 comigrate in the gel shown. rIgG, rabbit immunoglobulin G. (B) Immunoblot analysis of immunoprecipitations from equivalent amounts of lysates from C6 cells using the antibodies indicated. Ten percent of the input lysate was analyzed for comparison. The anti-human SEL1L antibody recognizes rat SEL1L in Western blots only weakly, accounting for the weak SEL1L signal in anti-Derlin-2 immunoprecipitates, which can be seen upon longer exposure.
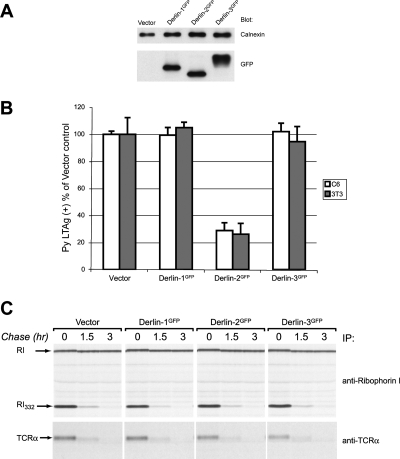
A GFP moiety attached to the C terminus of Derlin-1 (Derlin-1GFP) yields a dominant-negative Derlin-1 derivative that impairs US11- but not US2-mediated degradation of class I MHC heavy chains (11). To examine the possible role of all three Derlins in viral infection, we engineered similarly tagged versions of Derlin-2 and Derlin-3b (the longer isoform of Derlin-3, a protein approximately 70% identical to Derlin-2 and 30% identical to Derlin-1) (11, 18), with the expectation that the addition of a GFP domain would likewise interfere with processes specific to these other Derlin family members. Consistent with this idea, Derlin-2GFP inhibited neither US11- nor US2-mediated dislocation (11), and Derlin-3GFP had no effect on US11-mediated dislocation (B. N. Lilley, B. Mueller, and H. L. Ploegh, unpublished observations). Derlin-1GFP, Derlin-2GFP, or Derlin-3GFP was stably expressed in C6 rat glioblastoma cells at nearly equivalent levels, as determined by immunoblotting for GFP (Fig. 2A). Expression of the DerlinGFP constructs did not affect the growth of the cells under normal culture conditions (data not shown). We then examined the ability of Py to initiate infection in these cell lines by staining for large T antigen, the expression of which requires the successful delivery of the Py genome to the nucleus. No inhibition of infection relative to the control cell line was observed in cells that expressed Derlin-1GFP or Derlin-3GFP. In contrast, examination of cells that express Derlin-2GFP revealed a significant and highly reproducible reduction (71% ± 10%) in the number of LTAg-positive cells (Fig. 2B). Similar results were obtained using fully permissive mouse 3T3 cells that express the DerlinGFP constructs (Fig. 2B), indicating that the dominant-negative effect of Derlin-2GFP is not cell type or species specific in its role in Py infection.
FIG. 2.
Derlin-2GFP has a dominant-negative effect on Py infectivity but does not impair ER degradation. (A) Immunoblot of C6 DerlinGFP cells. Equivalent amounts of lysates from the indicated cell lines were separated by SDS-PAGE and then examined by immunoblotting with anti-calnexin (loading control) or anti-GFP. (B) 3T3 or C6 cells expressing the indicated constructs were infected with Py and stained for large T antigen. The percentage of positive cells reflects the number of LTAg-positive nuclei relative to the total number counted, with values normalized to cells transduced with the vector control as 100%. The error bars indicate standard deviations of the means. (C) HeLa cells expressing the indicated DerlinGFP constructs were transfected with RI332 (upper panel) or TCRα (lower panel). Cells were pulse labeled for 20 min and were chased for the indicated time points. Immunoprecipitations were performed using the appropriate antibodies, and samples were analyzed by SDS-PAGE and fluorography. Degradation of both substrate proteins proceeded at equivalent rates in all cell lines. Endogenous ribophorin I (RI) (top panel) was stable throughout the course of the experiment.
The expression of DerlinGFP constructs does not impair ER function, as indicated by the fact that the degradation of two well-characterized proteins that misfold in the ER, TCRα (8, 33) and RI332 (3), is not affected in cells that express any of the DerlinGFP constructs (Fig. 2C). Additionally, intoxication by Haemophilus ducreyi cytolethal distending toxin, a toxin known to traffic to the ER prior to entry into the cytosol, is not impaired by the expression of Derlin-1GFP or Derlin-2GFP (7). Expression of neither Derlin-1GFP nor Derlin-2GFP affects trafficking of the transferrin receptor through the secretory pathway (11).
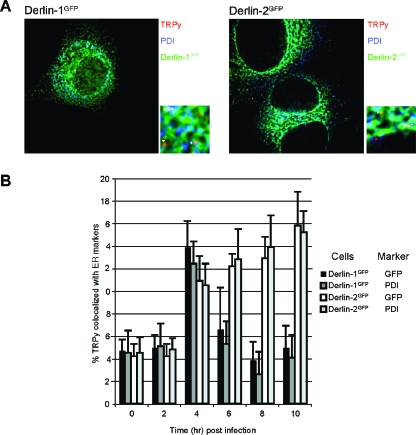
The reduction in the number of Py LTAg-positive cells that express Derlin-2GFP indicates a block to Py infection that occurs upstream of movement of the Py genome into the nucleus. Transfection of Py DNA into any of the DerlinGFP or control cell lines resulted in nearly identical numbers of Py LTAg-positive cells. The percentages of Py LTAg-positive cells are as follows: 100% ± 10% for C6 plus vector, 108% ± 11% for C6 plus Derlin-1GFP, 110% ± 8% for C6 plus Derlin-2GFP, and 106% ± 13% for C6 plus Derlin-3GFP (the percentage of positive cells reflects the number of LTAg-positive nuclei relative to the total number of nuclei counted, with values normalized to cells transduced with the vector control as 100%).The block to infection imposed by Derlin-2GFP thus occurs prior to the Py genome accessing the cytosol. Using immunofluorescence microscopy, we compared the transport of TRPy virions in cells that express the different DerlinGFP constructs. At 4 h after infection of C6 cells, roughly 15% of TRPy particles colocalized with the ER markers calreticulin (data not shown), PDI (Fig. 3A), and BiP (5). Expression of Derlin-2GFP did not affect the absolute number or percentage of virions that colocalized with PDI (Fig. 3B). Beginning at around 4 h postinfection, the number of TRPy particles that overlap with the ER marker declined in cells that express Derlin-1GFP, similar to data obtained with control C6 cells (data not shown) and similar to previously published data (5). The disappearance of TRPy puncta likely reflects diffusion within the ER of the Texas Red-labeled VP1 protein during the process of ER exit of the uncharacterized Py subviral assembly. Such diffusion would be consistent with the observation by immunoelectron microscopy of the VP1 protein remaining within the ER after SV40 infection (9). In contrast, cells that express Derlin-2GFP showed no decline in the percentage of TRPy particles in the ER colocalizing with PDI from 6 to 10 h postinfection (Fig. 3B). These results demonstrate that Py trafficking to the ER is not affected by Derlin-2GFP but rather remains trapped in the ER as a result of Derlin-2GFP expression.
FIG. 3.
Py virions traffic normally to the ER in cells that express Derlin-2GFP but are unable to escape from the ER. (A) Immunofluorescence of C6 cells expressing Derlin-1GFP (left panels) or Derlin-2GFP (right panels) after infection with TRPy (red). PDI is shown in blue, and the respective DerlinGFP proteins are shown in green. Z sections were examined for colocalizing particles and enlargements (×50) of the indicated areas are shown. Particles that colocalize with PDI are indicated by arrowheads with tails, and those that colocalize with DerlinGFP are indicated by arrowheads without tails. (C) Quantitation and time course of colocalization of TRPy particles with PDI or Derlin-1GFP/Derlin-2GFP. Cells expressing either Derlin-1GFP or Derlin-2GFP were infected with TRPy, and at the indicated time points, the cells were processed for immunofluorescence as described above (B). Z sections were examined for the numbers of virus particles colocalizing with either PDI or Derlin-1GFP/Derlin-2GFP (in the given cell line), as indicated to the right, relative to the total numbers of virus particles. The error bars indicate the standard deviations of the means from 6 to 12 different fields of cells. Cells expressing Derlin-2GFP exhibit prolonged colocalization of TRPy with both PDI and Derlin-2GFP well after such colocalization had diminished in cells that express Derlin-1GFP.
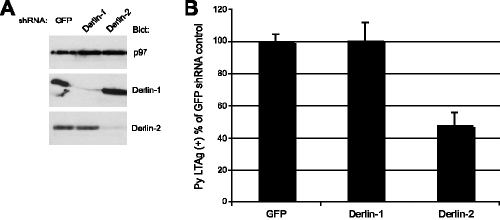
To further examine the effects of the loss of Derlin-2 function on Py infectivity, we generated shRNA vectors to reduce the expression of the rat Derlin-1 and Derlin-2 proteins in C6 cells. Using polyclonal antibodies raised against their cytoplasmic tails, we observed by immunoblotting a strong reduction in Derlin-1 protein levels and a modest but significant reduction in Derlin-2 levels in the cells that express the corresponding shRNA (Fig. 4A). Cells with reduced levels of Derlin-1 were infected as efficiently as cells expressing a control shRNA (Fig. 4B). In contrast, cells with reduced Derlin-2 levels showed a roughly 50% reduction in the number of T-antigen-positive cells (Fig. 3B). Up to 80% inhibition was observed in one experiment. The average reduction in infectibility correlated with the reduction in Derlin-2 protein levels, which was approximately 50 to 60%. Thus, Py infection is inhibited by two independent methods used to block Derlin-2 function.
FIG. 4.
shRNA directed against Derlin-2 but not Derlin-1 impairs Py infectivity. (A) Immunoblot analysis, using the antibodies shown, of equivalent microgram quantities of lysates from C6 cells expressing the indicated shRNA construct. Derlin-1 and Derlin-2 shRNA constructs specifically reduced the corresponding protein levels. p97 served as an internal loading control. (B) The same C6 cells as those in panel A were examined for Py infectivity by staining for large T antigen as described in the legend of Fig. 1. The data shown reflect the means and standard deviations of Py infectivity determined from multiple independent experiments.
DISCUSSION
Evidence is presented that Derlin-2, a protein involved in the dislocation of misfolded proteins from the ER, is a component of the host machinery required for Py infection. Results point to a role of this protein in mediating the translocation of the virus or subviral particle from the ER into the cytosol. Recent results have implicated the Derlins, including Derlin-2, in the clearance of misfolded proteins from the ER (11, 12, 18, 31, 32). Two different methods of inducing a Derlin-2 loss of function consistently diminished Py infectivity. Expression of the dominant-negative Derlin-2GFP did not have a general effect on ER function, indicating that nonspecific inhibition of ER functions does not occur in the cell lines examined and therefore would not account for the observed inhibition of Py infectivity. Furthermore, the effects on Py infection were highly specific to Derlin-2, even though both Derlin-1 and Derlin-3 are also involved in the catabolism of misfolded ER proteins and all three Derlins form complexes with a similar complement of factors (11, 12, 18, 31, 32).
Because the steps involving the alteration or partial disassembly of the virus particle leading to exit from the ER are not fully understood, it is difficult to pinpoint the precise step of the process in which Derlin-2 participates. Py particles traffic to the ER with normal efficiency and kinetics in cells that express Derlin-2GFP. However, virus continues to accumulate and remain within the ER of these cells for a prolonged time compared to control cells or cells expressing Derlin-1GFP. No inhibition was seen in cells expressing Derlin-2GFP following transfection with viral DNA. Taken together, these data strongly suggest that Derlin-2 plays a role in Py exit from the ER. While further experiments are required to define the mechanism by which Derlin-2 acts to promote Py escape, we note intriguing parallels between aspects of ER quality control and the properties of polyomaviral capsid proteins. Evidence reported previously for SV40 suggests that the VP2/3 proteins enter the cytosol (15, 16). The Py VP2 and VP3 proteins are intrinsically unstructured polypeptides, as assessed by protease sensitivity (1). We speculate that the loss or conformational alteration of VP1 within the ER (17), which for Py may involve the ERp29 oxidoreductase (13), could expose poorly structured regions of the internal VP2/3 proteins to Derlin-2 and associated proteins that recognize and eliminate misfolded cellular proteins from the ER (12, 18).
The altered or partially disassembled virus would represent a sizable particle, and the portal of exit and its dimensions remain unknown. The transfer of large particles across cellular membranes, however, is not without precedent: ∼10-nm colloidal gold particles coated with peroxisomal import sequences are readily transported across peroxisomal membranes (28). The extent to which prior disassembly of multisubunit substrates or unfolding of individual protein subunits is required during dislocation is not currently known, but complete unfolding may not be required for removal from the ER (4, 25). Derlin-1 and Derlin-2 are both part of multiprotein complexes involved in the clearing of the ER of misfolded proteins (11, 12, 18, 31, 32), and they are capable of handling at least partially folded substrates (4, 25).
The results presented here place Derlin-2 in a pathway required for Py infection at the level of exit from the ER. It now appears that the Derlin proteins are utilized by at least two families of viruses for different purposes: immune evasion in one case (HCMV US11 and Derlin-1) and viral entry in the other (Py and Derlin-2).
Acknowledgments
We thank N. Erwin Ivessa for reagents, Marie-Eve Paquet for technical assistance, and members of the Ploegh and Benjamin laboratories for helpful suggestions.
B.N.L. was supported by a Howard Hughes Medical Institute predoctoral fellowship. This work was supported by grants from the National Institutes of Health to T.L.B. (R01 CA-082395) and H.L.P.
REFERENCES
- 1.Chen, X. S., T. Stehle, and S. C. Harrison. 1998. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 17:3233-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Virgilio, M., H. Weninger, and N. E. Ivessa. 1998. Ubiquitination is required for the retro-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J. Biol. Chem. 273:9734-9743. [DOI] [PubMed] [Google Scholar]
- 4.Fiebiger, E., C. Story, H. L. Ploegh, and D. Tortorella. 2002. Visualization of the ER-to-cytosol dislocation reaction of a type I membrane protein. EMBO J. 21:1041-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert, J., and T. Benjamin. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 78:12259-12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra, L., K. Teter, B. N. Lilley, B. Stenerlow, R. K. Holmes, H. L. Ploegh, K. Sandvig, M. Thelestam, and T. Frisan. 2005. Cellular internalization of cytolethal distending toxin: a new end to a known pathway. Cell. Microbiol. 7:921-934. [DOI] [PubMed] [Google Scholar]
- 8.Huppa, J. B., and H. L. Ploegh. 1997. The alpha chain of the T cell antigen receptor is degraded in the cytosol. Immunity 7:113-122. [DOI] [PubMed] [Google Scholar]
- 9.Kartenbeck, J., H. Stukenbrok, and A. Helenius. 1989. Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol. 109:2721-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knop, M., A. Finger, T. Braun, K. Hellmuth, and D. H. Wolf. 1996. Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 15:753-763. [PMC free article] [PubMed] [Google Scholar]
- 11.Lilley, B. N., and H. L. Ploegh. 2004. A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429:834-840. [DOI] [PubMed] [Google Scholar]
- 12.Lilley, B. N., and H. L. Ploegh. 2005. Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. USA 102:14296-14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnuson, B., E. K. Rainey, T. Benjamin, M. Baryshev, S. Mkrtchian, and B. Tsai. 2005. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell 20:289-300. [DOI] [PubMed] [Google Scholar]
- 14.Mannova, P., and J. Forstova. 2003. Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport. J. Virol. 77:1672-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanishi, A., J. Clever, M. Yamada, P. P. Li, and H. Kasamatsu. 1996. Association with capsid proteins promotes nuclear targeting of simian virus 40 DNA. Proc. Natl. Acad. Sci. USA 93:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakanishi, A., D. Shum, H. Morioka, E. Otsuka, and H. Kasamatsu. 2002. Interaction of the Vp3 nuclear localization signal with the importin alpha 2/beta heterodimer directs nuclear entry of infecting simian virus 40. J. Virol. 76:9368-9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norkin, L. C., H. A. Anderson, S. A. Wolfrom, and A. Oppenheim. 2002. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 76:5156-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oda, Y., T. Okada, H. Yoshida, R. J. Kaufman, K. Nagata, and K. Mori. 2006. Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J. Cell Biol. 172:383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 20.Rehm, A., P. Stern, H. L. Ploegh, and D. Tortorella. 2001. Signal peptide cleavage of a type I membrane protein, HCMV US11, is dependent on its membrane anchor. EMBO J. 20:1573-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richterova, Z., D. Liebl, M. Horak, Z. Palkova, J. Stokrova, P. Hozak, J. Korb, and J. Forstova. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75:10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahli, R., R. Freund, T. Dubensky, R. Garcea, R. Bronson, and T. Benjamin. 1993. Defect in entry and altered pathogenicity of a polyoma virus mutant blocked in VP2 myristylation. Virology 192:142-153. [DOI] [PubMed] [Google Scholar]
- 23.Sandvig, K., and B. van Deurs. 2002. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 529:49-53. [DOI] [PubMed] [Google Scholar]
- 24.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369:160-163. [DOI] [PubMed] [Google Scholar]
- 25.Tirosh, B., M. H. Furman, D. Tortorella, and H. L. Ploegh. 2003. Protein unfolding is not a prerequisite for endoplasmic reticulum-to-cytosol dislocation. J. Biol. Chem. 278:6664-6672. [DOI] [PubMed] [Google Scholar]
- 26.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. A. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai, B., C. Rodighiero, W. I. Lencer, and T. A. Rapoport. 2001. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104:937-948. [DOI] [PubMed] [Google Scholar]
- 28.Walton, P. A., P. E. Hill, and S. Subramani. 1995. Import of stably folded proteins into peroxisomes. Mol. Biol. Cell 6:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 30.Wiertz, E. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 31.Ye, Y., Y. Shibata, M. Kikkert, S. van Voorden, E. Wiertz, and T. A. Rapoport. 2005. Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. USA 102:14132-14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye, Y., Y. Shibata, C. Yun, D. Ron, and T. A. Rapoport. 2004. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429:841-847. [DOI] [PubMed] [Google Scholar]
- 33.Yu, H., G. Kaung, S. Kobayashi, and R. R. Kopito. 1997. Cytosolic degradation of T-cell receptor alpha chains by the proteasome. J. Biol. Chem. 272:20800-20804. [DOI] [PubMed] [Google Scholar]