Abstract
Human immunodeficiency virus type 1 (HIV-1) assembly requires the converging of thousands of structural proteins on cellular membranes to form a tightly packed immature virion. The Gag polyprotein contains all of the determinants important for viral assembly and must move around in the cell in order to form particles. This work has focused on Gag mobility in order to provide more insights into the dynamics of particle assembly. Key to these studies was the use of several fluorescently labeled Gag derivatives. We used fluorescence recovery after photobleaching as well as photoactivation to determine Gag mobility. Upon expression, Gag can be localized diffusely in the cytoplasm, associated with the plasma membrane, or in virus-like particles (VLPs). Here we show that Gag VLPs are primarily localized in the plasma membrane and do not colocalize with CD63. We have shown using full-length Gag as well as truncation mutants fused to green fluorescent protein that Gag is highly mobile in live cells when it is not assembled into VLPs. Results also showed that this mobility is highly dependent upon cholesterol. When cholesterol is depleted from cells expressing Gag, mobility is significantly decreased. Once cholesterol was replenished, Gag mobility returned to wild-type levels. Taken together, results from these mobility studies suggest that Gag is highly mobile and that as the assembly process proceeds, mobility decreases. These studies also suggest that Gag assembly must occur in cholesterol-rich domains in the plasma membrane.
Human immunodeficiency virus type 1 (HIV-1) particle production involves a series of events that includes the association of viral proteins with the plasma membrane, incorporation of the RNA genome, clustering of thousands of Gag/Gag-Pol molecules, and subsequent release of the immature virion (for review, see references 11 and 13). The Pr55Gag precursor protein contains all of the structural determinants necessary for particle formation. Expression of Gag alone is necessary and sufficient to produce virus-like particles (VLPs) (43). It consists of the matrix (MA), capsid (CA), nucleocapsid (NC), and p6 regions. This retroviral precursor contains three major functional domains involved in particle assembly: the membrane association (M), interaction (I), and late (L) domains. The M domain in the HIV-1 Gag precursor is located within the matrix region, which is N-terminally myristoylated. The myristoyl group on the matrix domain is critical for membrane targeting and virus assembly (3, 14, 26). Biochemical analysis has shown that there is a highly basic region in the matrix domain spanning from amino acids 17 to 31 that is also required for membrane association (6, 34, 45, 49). Gag-Gag interaction through the I-domain plays a central role in the assembly process. While the HIV-1 I-domain has been mapped to the N-terminal region of the nucleocapsid domain (4, 41), there is also evidence that a dimerization domain within the C terminus of the capsid region plays a role in the assembly process (24, 31). The L-domain is located within the p6 region and facilitates budding (12).
Studies have shown that full-length Gag can be both membrane associated and localized in the cytoplasm (9). It is likely that this cytoplasmic pool has yet to associate with the membrane. The same has been seen in truncation mutants expressing only the MA and CA domains or just MA (32, 38). In all of these cases, mutating the myristoylation site in MA drastically reduces membrane association (17, 32). Also, pulse-chase experiments have provided evidence that newly synthesized Gag is not instantaneously membrane associated but becomes associated with the membrane over time (16). Where and how Gag assembly occurs is still unclear. Recently there has been conflicting evidence as to whether membrane binding is a prerequisite for Gag assembly. There are several groups that have used cell fractionation assays along with pulse-chase experiments and immunohistochemistry to support the idea that membrane association is necessary for the Gag-Gag interaction to occur (9, 24, 31, 44). However, other groups have shown evidence that at least some Gag multimerization can occur before membrane binding takes place (15, 20, 27, 38). Another aspect of Gag assembly appears to involve membrane microdomains or rafts (10, 16, 21). These observations that Gag displays an apparent preference to where it accumulates in the plasma membrane suggest that issues of how and where assembly occurs are not mutually exclusive but are quite dependent on each other.
A key aspect of assembly is the mobility of individual of Gag molecules, which must move in the membrane in order to form particles. Therefore, we have performed experiments to determine Gag mobility in living cells. Protein mobility has been used to determine protein dynamics in live cells. It has been used to show that some proteins are highly mobile whereas other proteins, such as histones, are not (25). Mobility can also provide insight into protein interactions in living cells. It has been shown that some protein interactions can alter or decrease protein diffusion. For example, glucocorticoid receptor mobility significantly decreases upon ligand binding (42). Interaction with cytoskeletal components can also limit a protein's ability to diffuse (19, 46). Analysis of protein mobility provides important insights into biochemical processes in the context of live cells.
Here we have characterized wild-type Gag-green fluorescent protein (GFP) as well as fluorescently tagged Gag mutants according to their mobility in living cells using fluorescence recovery after photobleaching (FRAP) and photoactivation. We have found that unassembled Gag-GFP is highly mobile in living cells. In contrast, Gag molecules assembled into VLPs are not mobile. We also found that cholesterol depletion causes a marked decrease in Gag-GFP mobility.
MATERIALS AND METHODS
Plasmid construction.
Figure 2 shows diagrams of the constructs used for this study. The Gag-GFP construct used in this report was a kind gift from Marilyn D. Resh (Memorial Sloan-Kettering Cancer Center, New York, NY) (17). Site-directed PCR mutagenesis was used in order to develop the MACA-GFP construct. Primers were designed to engineer a BamHI site at the 3′ end of the CA coding region of the Gag-GFP vector. The resulting PCR product was then digested to liberate the MACA coding region and ligate it back into the Gag-GFP EcoRI-BamHI site, thus replacing the gag gene with the maca gene. The MA-GFP construct was made by amplifying the ma gene from a pNL4-3 proviral construct with an EcoRI site at the 5′ end and a BamHI site at the 3′ end. The ma gene was then ligated into the pEGFP-N2 vector (Clontech) by digesting the EcoRI and BamHI sites in the multiple cloning site (MCS). The G2AGag-GFP mutant was produced by splicing by overlap extension PCR (18). Primers were designed to mutate the N-terminal glycine to an alanine. The resulting PCR product was cloned into the EcoRI and BamHI sites in the Gag-GFP construct, replacing the existing gag gene.
FIG. 2.
Diagram of Gag fusion proteins. All Gag derivatives were made as both GFP and PAGFP fusion proteins.
An empty PAGFP-C1 vector was a kind gift from Jennifer Lippincott-Schwartz (Bethesda, MD). PAGFP-N1 was constructed by replacing the AgeI-BsrGI fragment from the pEGFP-N1 vector (Clontech) with the AgeI-BsrGI fragment from PAGFP-C1, resulting in a vector with an MCS upstream of the GFP coding sequence. Gag-GFP was digested with BamHI and EcoRI, thereby liberating the Gag fragment, which was then ligated into the BamHI and EcoRI sites in the PAGFP-N1 MCS. MACA-PAGFP was made by liberating the MACA fragment by digesting with EcoRI and BamHI and ligating the fragment into the EcoRI and BamHI sites in the PAGFP-N1 MCS. The MA-PAGFP construct was made by amplifying the ma gene from the MA-GFP by PCR and ligating it into PAGFP-N1 by digesting both the amplified fragment and the empty vector with EcoRI and BamHI.
The sense-strand oligonucleotides for the PCR amplifications described above were as listed here. The primer used to insert a BamHI site at the 3′ end of the ca coding region was as follows: CATAAGGCAAGAGTTTTGGCGGATCCGGG. To amplify the ma gene from the pNL4-3 proviral construct, we used the following primer: GGTCAGCCAAAATTACTCAGAATTCTGCAGTCG. To construct the G2AGag-GFP mutant, the following primer was used: CGCGCGTCGACAGAGAGATGGCTGCGAGAGCGTCAGTATTAAG. In order to amplify the ma gene from the MAGFP vector, the following primers were used: AGGTCTATATAAGCAG and CGCTGAACTTGTGGCCGTT.
Cells and transfections.
HeLa cells were used in all experiments reported here. Cells were maintained in Dulbecco's modified Eagle medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine. Cells were seeded onto Delta T live cell microscopy dishes (Fisher) before transfecting. All transfections were done using Effectine transfection reagent (QIAGEN). For all experiments, cells were transfected with a total of 0.8 μg of plasmid DNA.
Immunofluorescence.
HeLa cells were transfected with Gag-GFP. After an 18-hour incubation, images were taken using an Olympus IX-70 inverted microscope and analyzed with Delta Vision software. After taking time-lapse images, cells were fixed with 3.7% formaldehyde in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (200 mM PIPES pH 6.8, 4 mM MgCl2, 2 mM EGTA). Cells were then stained for CD63 using an anti-CD63 monoclonal antibody (BD Pharmingen) and a Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.).
FRAP and photoactivation assays.
Cells were used for both FRAP and photoactivation experiments 18 h after transfection. Photobleaching experiments were done using a Zeiss LSM 510 confocal laser-scanning microscope. All experiments were done at 37°C. Pixel quantitation was done using the Zeiss LSM software. Photoactivation experiments were done using the MicroPoint Mosaic digital diaphragm system (Photonic Instruments, Inc., St. Charles, IL) mounted on an Olympus IX-70 inverted microscope. Pixel quantitation was made possible by using Slidebook 3.0 software (Intelligent Imaging Innovation, Inc.).
In our FRAP and photoactivation studies, two expressing cells are simultaneously imaged, with one analyzed by FRAP/photoactivation and the other to be used as a fluorescence control. The second cell is used as a control because although it is not exposed to the full strength of the laser as the bleached cells are, it is still scanned throughout the time course, thereby causing unavoidable photobleaching during the time course. For each time point analysis, the fluorescence intensity is calculated as a percentage of the fluorescence at time zero. Values for the bleached cells are normalized as percentages of the fluorescence intensity calculated for control cells. The values presented here are averages of several experiments, and the error bars represent the standard errors of the means.
In photoactivation experiments, due to the low background of PAGFP (a photoactivatable form of GFP), cells were cotransfected with free monomeric red fluorescent protein (mRFP) along with the photoactivatable protein in order to locate transfected cells. Regions of interest (ROIs) were selected and marked using the MicroPoint Mosaic software. In the figures shown below, the ROIs are marked with white boxes. Using a digital diaphragm these ROIs were activated and fluorescence intensities were recorded throughout the time lapse. For each experiment the ROI was subjected to the 413-nm light for 4 seconds. Immediately following activation, images were then taken approximately every 4 seconds for a total of 5 min. The relative intensities are calculated as percentages of the intensity at time zero and normalized to an area in a control cell. Each experiment was done six times, and data represent averages of these experiments. Photoactivation of unassembled Gag-PAGFP was repeated three times, and the averages are given below in Fig. 4.
FIG. 4.
Mobility of diffuse Gag. Gag-GFP-expressing cells were subjected to FRAP analysis 18 h after transfection. (A) The right-most image shows cells prior to bleaching, followed by the image taken immediately after bleaching. The photobleached area is located within the white box. Fluorescence recovery was monitored over time. (B) Gag-PAGFP-expressing cells were subjected to photoactivation. The right-most image shows a cell before photoactivation, followed by an image taken immediately after photoactivation. White box 1 represents activated ROI 1, while 2 and 3 are additional ROIs used for the analysis. Fluorescence within ROIs was followed over time. (C) Gag-GFP recovers to approximately 80% in approximately 3 min after photobleaching. (D) Gag-PAGFP reaches equilibrium in about 3 min after photoactivation. Error bars represent standard errors of the means.
Cholesterol depletion experiments.
Cells were seeded onto Delta T dishes and transfected as described above. At 18 h after transfection, cells were washed with phosphate-buffered saline (Cambrex). Medium was replaced with Dulbecco's modified Eagle medium supplemented with 10% delipidated fetal bovine serum. Methyl-β-cyclodextrin (MβCD; Sigma) at 10 mM was added to the medium, and cells were incubated for 1 h at 37°C. Before FRAP was done, cells were washed and medium was replaced with Dulbecco's modified Eagle's medium supplemented with delipidated fetal bovine serum. To replenish cells treated with MβCD, cells were washed with phosphate-buffered saline and medium was replaced with normal medium containing water-soluble cholesterol (Sigma) at a 10 mM concentration. Cells were incubated for 30 min at 37°C before being used for photobleaching experiments. Experiments were repeated six times.
RESULTS
FRAP and photoactivation.
In order to address the question of Gag mobility in live cells, we have utilized two techniques that require the use of fluorescently tagged proteins. The first is FRAP, which involves directing the laser of a confocal microscope to a specific region of interest in a cell expressing the fluorescent protein to be analyzed. The ROI is exposed to the maximum intensity of the laser, thereby permanently photobleaching the fluorescent signal. The mobility of the tagged protein is then measured by analyzing the recovery/return, or lack thereof, of fluorescent signal into the bleached ROI (for a review, see reference 8).
Until recently, techniques used to study protein mobility have been primarily limited to bleaching techniques. A new photoactivatable form of GFP, PAGFP, has been developed, allowing a complementary approach to study protein mobility (37). Upon expression, this protein displays weak fluorescence, but when excited with 413-nm light, fluorescence can be increased by up to 100-fold. A schematic representation of photoactivation is shown in Fig. 1A. After photoactivation, the movement of the fluorescent signal in the cell can be followed over time.
FIG. 1.
Photoactivation. (A) Schematic diagram of photoactivation. (B) HeLa cell expressing free PAGFP subjected to photoactivation. The right-most image shows the cell before photoactivation, followed by images of the cell immediately following photoactivation and at 60 seconds. White boxes indicate regions of interest. (C) Graphic representation of relative fluorescence intensity levels for two rounds of photoactivation of free PAGFP in a 60-second time period.
In photoactivation experiments, two ROIs are used to analyze mobility. The first ROI (ROI 1) represents the area of photoactivation, whereas the other (ROI 2) is chosen to measure the level of fluorescence in another region of the cell. Therefore, we can use the increase (or lack thereof) in signal within ROI 2 as an indication of protein mobility. If the observed protein is mobile, eventually the signals in both regions of interest will become equal. We refer to this time point as the point of equilibrium. If the signals do not reach a point of equilibrium, this suggests that a significant fraction of the protein is immobile. As an example, a HeLa cell expressing free PAGFP is shown in Fig. 1B. First, the ROIs are selected, followed by photoactivation. The cell in Fig. 1B was photoactivated several times (see movie S1 in the supplemental material), but here we only show two cycles of activation and an image of the cell at 60 seconds. The mobility curve for free PAGFP is given in Fig. 1C. After each photoactivation, a signal appears in ROI 1 and is lost over time. Concurrently, an increase of the fluorescent signal is observed in ROI 2. In the case of free PAGFP, the signal in the two ROIs reaches equilibrium about 28 seconds after the photoactivation.
Construct design for Gag fusion proteins used in FRAP and photoactivation studies.
Figure 2 shows a diagram of the fusion proteins used in this study. In order to define the potential role of the different Gag domains in mobility in living cells, several truncation mutants were used in addition to the full-length Gag construct. MACA-GFP and MACA-PAGFP contain only the matrix and capsid regions of Gag, eliminating the L and I domains responsible for interaction and budding. MA-GFP and MA-PAGFP contain only the matrix region, knocking out potential interaction through the CA domain. Myristoyl-deficient G2AGag-GFP and G2AGag-PAGFP were constructed to determine the difference in mobility between membrane- and non-membrane-associated Gag.
Mobility of Gag-GFP differs according to expression pattern.
Gag-GFP can exist in multiple states within a cell. The protein can be monomeric, within assembly intermediates, or within completely assembled VLPs. Here we define VLPs as bright point sources of fluorescence located in the plasma membrane. As reported by others, Gag can also accumulate in association with intracellular vesicles, such as multivesicular bodies (30, 40). In this case they could also be bright sources of fluorescence, looking much like VLPs. Intracellular vesicles are known to move rapidly in the cytoplasm as they travel along microtubule roadways (36). Time-lapse analysis of Gag-GFP-expressing cells revealed that a subset of the bright point sources did move rapidly within the cell. An example of this is shown in Fig. 3A, where a single point source was observed to move rapidly while the surrounding assemblies of GFP signal were relatively stationary (Fig. 3; see also movie S2 in the supplemental material). It was previously reported that CD63 is a marker for the intracellular vesicles containing Gag-GFP. Therefore, the cell observed in the time-lapse experiment shown in Fig. 3A was fixed at the end of the observation and stained for CD63. We found that only a subset of Gag-GFP point sources of light were associated with CD63 (red) (Fig. 3A and B). Figure 3C shows the area indicated by the white box from a 90° angle. This reveals that the majority of the Gag-GFP bright point sources associated with this cell are localized at the cell membrane, while CD63 is primarily located in the cytoplasm. Analysis of the extent of the three-dimensional overlap of Gag-GFP and CD63 signal is shown in the scatter plot in Fig. 3D and reveals that the signal overlap was only observed in a minority of the bright point sources of Gag-GFP. The Pearson's coefficient of correlation (r) was determined to be an average of 0.367 ± 0.046 for Gag-GFP and CD63, where an r value of 1 would represent complete colocalization. Taken together, these data suggest that the VLPs are mostly associated with the plasma membrane, while the CD63-positive vesicles are primarily located within the cytoplasmic regions. Further, the Gag-GFP-containing vesicles move rapidly, while the VLPs are stationary in the time scales of minutes used for our time-lapse analysis. Therefore, we will define the stationary membrane-associated bright point sources of Gag-GFP as VLPs for our analysis.
FIG. 3.
Gag-GFP localization. Gag-GFP-expressing cells were imaged to further define Gag-GFP punctae. (A) Images were taken of live Gag-GFP-expressing cells once every 30 s. White arrows indicate mobile point sources of light. The right-most image shows the complete path of this point source. The white box in panel A labeled with a * indicates the origin of these images. (B) Cells were fixed after the time lapse and stained for CD63 (red). (C) Gag-GFP and CD63 are not colocalized. (D) The area in the white box in panel A, shown from a 90° angle. The majority of puntae are localized at the cell membrane, while CD63 is located primarily in the cytoplasm. (E) Scatter plot showing the degree of overlap between green and red signals. The r value represents the Pearson's coefficient of correlation, for which an r value of 1 would represent complete colocalization.
In order to determine the mobility of full-length Gag-GFP in living cells, HeLa cells were transfected with Gag-GFP and analyzed for mobility after 18 h. During these experiments, cells expressing Gag-GFP were chosen on the basis of the Gag expression pattern at the time of analysis. First, cells displaying diffuse Gag-GFP localization were chosen and subjected to photobleaching. Figure 4A shows two cells both expressing Gag-GFP that displayed a mostly diffuse (unassembled) localization. Protein within the ROI was photobleached, and the signal was read within this region every 4 seconds. Within a 3-minute timeframe, the fluorescent signal returned to the region of interest, suggesting that diffusely expressed Gag-GFP is mobile during this phase of expression. Quantitation of these FRAP experiments is shown in Fig. 4C. The results show that Gag-GFP was photobleached to an average of about 30%. In just over 3 min, the signal recovered to an average of approximately 80% of the original signal, indicating that most of the Gag-GFP which had not assembled into apparent VLPs, was mobile in living cells.
We also used photoactivation to determine unassembled Gag mobility. In these experiments HeLa cells were transfected with Gag-PAGFP and subjected to photoactivation after 18 h. Due to the extremely low background fluorescence of PAGFP fusion proteins, cells were cotransfected with small amounts of unfused mRFP in order to identify cells transfected with PAGFP constructs. Cells were then sorted according to expression pattern following each experiment. Photoactivation demonstrated that unassembled Gag-PAGFP displayed similar mobility as in the FRAP experiments. The results from these experiments are shown in Fig. 4B and reveal that the GFP signal is almost undetectable until after photoactivation. The expression pattern is mostly cytoplasmic, with the exception of several VLPs. Throughout the time-lapse study, Gag-PAGFP moves throughout the cell as VLPs do not. Several of these time-lapse experiments were performed, and data were compiled and averaged.
The mobility curve for unassembled Gag-PAGFP is shown in Fig. 4D. After activation there was an average loss of relative fluorescence intensity over time, corresponding to the diffusion of activated Gag-PAGFP throughout the cell seen in Fig. 4B. There was also an almost immediate increase in relative fluorescence intensity in ROI 2, indicating that unassembled Gag-PAGFP is mobile in the cell. The two signals overlap in approximately 1 min. The immediate increase in fluorescence found in ROI 2 could also be due to the close proximity of the two ROIs. To address this question, a third ROI (ROI 3) was added to a region further away from ROI 1. The fluorescence within this region was recorded and analyzed. As expected, the relative fluorescence of ROI 3 increased at a slower rate than ROI 2. ROI 3 reached equilibrium with the other two regions of interests in approximately 2 min, providing further evidence for the idea that unassembled Gag-PAGFP is very mobile. The delay of increase in fluorescence in the third ROI is most likely due its distance from the activated region of interest. The decrease of fluorescence in the activated ROI and the increase in fluorescence in ROI 2 and ROI 3 all reached equilibrium at just over 2 min after photoactivation, suggesting that unassembled Gag-GFP is mobile; this is consistent with data from the corresponding FRAP experiments.
Cells expressing Gag-GFP, mostly assembled into VLPs, were also analyzed by FRAP. In these cells, the mobility profile is quite different. Cells expressing Gag-GFP assembled into VLPs did not display any recovery after photobleaching (Fig. 5A and C). This suggests that there is no exchange of Gag molecules between assembled and unassembled Gag molecules into a particular VLP, displaying an overall lack of molecular mobility, at least in this 5-minute time frame of observation.
FIG. 5.
Mobility of Gag VLPs. Gag-GFP-expressing cells were subjected to FRAP analysis 18 h after transfection. (A) The right-most image shows cells prior to bleaching, followed by the image taken immediately after bleaching. The photobleached area is located within the white box. Fluorescence recovery was monitored over time. (B) Gag-PAGFP-expressing cells were subjected to photoactivation. The right-most image shows a cell before photoactivation, followed by an image taken immediately after photoactivation. White box 1 indicates an activated ROI. Fluorescence within ROIs was followed over time. (C) Gag-GFP VLPs do not recover after photobleaching. (D) Gag-PAGFP VLPs are not mobile. The signals in ROIs 1 and 2 do not reach equilibrium.
Figure 5B shows a cell expressing Gag-PAGFP. After photoactivation, it was revealed that this particular cell had both VLPs and unassembled Gag-PAGFP. As the time-lapse progressed, the unassembled Gag-PAGFP diffused out of ROI 1 but the VLPs remained in the same general area. When quantified, it was shown that on average Gag-PAGFP VLPs are immobile within the cell. The slight decrease in fluorescence in ROI 1 and the slight increase in fluorescence in ROI 2 represents unassembled Gag-PAGFP present in the cell. However, these changes in fluorescence intensity between the two ROIs did not reach equilibrium after 5 min, suggesting a subset of the protein (the VLP) is immobile during the 5 min of analysis. These data are consistent with the results from FRAP studies which showed high mobility in unassembled Gag and negligible mobility in Gag assembled into VLPs.
Analysis of the mobility of Gag truncation mutations.
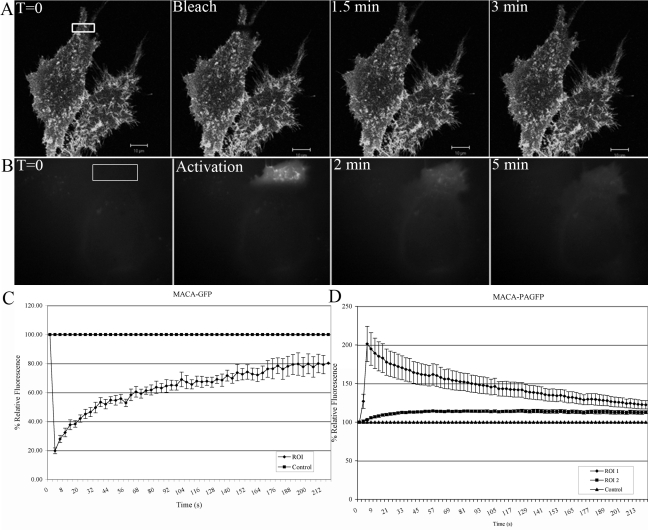
To gain insights into the mobility of potential intermediates in the assembly process, we analyzed Gag-GFP derivatives with carboxyl-terminal deletions removing domains involved in the assembly process. A MACA-GFP mutant lacking the NC (interaction) and p6 (late) domains, eliminating its ability to assemble into VLPs and bud from the plasma membrane, was first analyzed. MACA-GFP localized primarily in membrane structures resembling microvilli (Fig. 6A). After photobleaching, the fluorescent signal reached 80% in less than 3 min, resembling the recovery rate of unassembled Gag-GFP (Fig. 6B).
FIG. 6.
Mobility of the MACA mutant. Cells expressing MACA-GFP were subjected to photobleaching 18 h after transfection. (A) Upon expression, MACA localizes to membrane structures and recovers 3 min after photobleaching. (B) In cells expressing MACA-PAGFP and subjected to photoactivation, the signal moves away from the activated area, indicating mobility. (C) MACA-GFP recovers to 80% in 3.5 min after photobleaching. (D) MACA-PAGFP reaches equilibrium in about 3.5 min. Error bars represent standard errors of the means.
Photoactivation experiments with MACA-PAGFP gave similar results. After activation, MACA-PAGFP can be seen in membrane projections (Fig. 6B). As time progresses the signal gradually moves out of the ROI. Interestingly, Fig. 6D shows that the average fluorescence intensity of MACA-PAGFP decreases over time to an average minimum intensity of about 110% of the original fluorescence intensity. There was also an increase of intensity in ROI 2; however, in contrast to unassembled Gag-PAGFP, the decrease in fluorescence in ROI 1 and the increase in ROI 2 do not reach equilibrium until the end of the time lapse, a total of 4 min.
When MA-GFP is expressed, it also localizes primarily in the plasma membrane as well as membrane protrusions with some cytoplasmic localization (data not shown). FRAP analysis of cells expressing this fusion protein revealed that MA-GFP moved at a very rapid rate, recovering to an average of approximately 97% in 3 min (Fig. 7A). Photoactivation analysis also revealed that MA-PAGFP was highly mobile (Fig. 7B). This study reveals that in just over 30 seconds the MA-GFP fluorescence reaches equilibrium.
FIG. 7.
Mobility of additional Gag derivatives. (A) MA-GFP mobility in live cells. The signal recovers to approximately 97% 3 min after photobleaching. (B) MA-PAGFP is also highly mobile in the cell. The signals in both ROIs 1 and 2 reach equilibrium in less than 2 min. (C) Cytoplasmic Gag is mobile in the cell. G2AGag-GFP recovers to 80% after photobleaching in just over 2 min. (D) G2A-PAGFP is mobile. The signals in both ROIs reach equilibrium in about 2 min. Error bars represent standard errors of the means.
Gag mobility in the cytoplasm.
All truncation mutants used in these experiments are potentially associated with the plasma membrane through myristoylation. Therefore, to test Gag mobility in the absence of membrane association, we constructed a myristoyl-deficient Gag-GFP termed G2AGag-GFP. Live cells expressing G2AGag-GFP were subjected to FRAP analysis to determine their mobility (Fig. 7C). After photobleaching, G2AGag-GFP recovered to levels comparable to those of Gag-GFP in 2 min, revealing that Gag is highly mobile when localized in the cytoplasm. A photoactivatable G2AGag construct was also tested for mobility using photoactivation. As shown in Fig. 7D, the fluorescence in the cell reaches equilibrium in about 2 min, also suggesting a highly mobile protein. The photoactivation experiment confirmed that the G2AGag-PAGFP mobility is also comparable to that of Gag-GFP.
Analysis of mobility data.
When analyzing protein mobility, there are several key factors to consider. In order to properly compare the mobility of one protein to that of another, it is necessary to determine two equally important parameters: the mobile fraction, R, and the diffusion time, τD.
The mobile fraction, R, is defined as the fraction of fluorescent protein that can diffuse into the bleached region during the time course of the experiment. The mobile fraction can be determined by comparing the change in fluorescence as a result of photobleaching and the change in fluorescence from a time point just after bleaching to fluorescence at full recovery. Therefore, we define the mobile fraction as follows: R = (F∞ − F0)/(Fi − F0), where F∞ is the fluorescence after full recovery, F0 is the fluorescence immediately after photobleaching, and Fi is the fluorescence before photobleaching (23, 39). The closer R is to 1, the more mobile the protein of interest. The extent to which a fusion protein is bleached and the extent to which they recover can also provide great insight into the mobility of the protein. Here, extent of bleaching is defined as Fi − F0, and the extent of recovery is defined as F∞ − F0. The values used in these calculations are the fluorescence values resulting from normalizing to the control cells in each individual experiment. Table 1 lists R values as well as the extent of bleaching for each construct used in the FRAP experiments. Table 1 shows that as the fusion proteins get smaller in size, the more mobile they become, with MA having the largest mobile fraction. VLPs have the smallest R value. Also, the greater the mobile fraction, the more difficult the protein is to bleach. For example, MA-GFP was the most difficult to bleach, consistent with it having the largest mobile fraction.
TABLE 1.
FRAP and photobleaching analysis
| Construct | R | Extent of bleaching |
|---|---|---|
| dGag | 0.740 ± 0.13 | 71.96 ± 3.58 |
| Gag VLPs | 0.233 ± 0.04 | 78.03 ± 3.55 |
| G2AGag | 0.660 ± 0.05 | 69.19 ± 0.59 |
| MACA | 0.826 ± 0.07 | 80.21 ± 2.03 |
| MA | 0.914 ± 0.06 | 60.56 ± 2.41 |
While the definition of the mobile fraction is an informative parameter, it provides no information relating to the rate of movement. Diffusion time is defined as the time it takes the protein's fluorescent signal to reach half of its final level of intensity. As shown in Fig. 8A, and as expected, Gag-, MA-, and G2AGag-GFP fusion proteins all have similar τD values, with MACA having the largest. Gag-GFP VLPs had no value, reflecting a complete lack of mobility. The τD values are similar for FRAP and photoactivation. The G2AGag τD is equal in mobility to unassembled Gag and the truncation mutants. The τD value for free GFP has also been given in order to compare it with the other fusion proteins. It is by far the most mobile of all. Figure S3 in the supplemental material shows the mobility curves for free GFP as well as a farnesylated GFP (F-GFP), which is localized exclusively in the plasma membrane. The figure shows that free GFP moves too quickly to be bleached effectively. Alternatively, we used the time it takes to reach equilibrium of each derivative to compare the different Gag-GFP derivatives. Figure 8B shows that this method of analysis results in data consistent with τD values shown previously.
FIG. 8.
FRAP and photoactivation give similar diffusion times (τD). (A) Diffusion times for each construct used in the FRAP and photoactivation experiments. MACA constructs have the longest diffusion times, while free PAGFP has the shortest. Error bars represent standard errors of the means. (B) The time it takes for each photoactivatable Gag variant to reach the point of equilibrium is shown.
Gag-GFP mobility is cholesterol dependent.
Detergent-resistant membrane domains (DRMs) or lipid rafts have been suggested to be important for HIV-1 virus production (33). Previous studies have shown that when cholesterol is depleted from cells, there is a marked decrease in virus release into the supernatant. It has also been shown that Gag molecules are targeted to detergent-resistant membranes and that the I domain is necessary for this association to occur (10, 21). We performed FRAP experiments to determine if cholesterol, an important component of DRMs, was required for Gag mobility. Cells were transfected with Gag-GFP and, 18 h after transfection, were treated with MβCD for 1 hour and subjected to FRAP. Cells depleted of cholesterol could be bleached to an average relative fluorescence of about 40% and recovered to an average of about 60%. The untreated control was bleached to an average relative fluorescence of 48% and recovered to approximately 84% relative fluorescence (Fig. 9). Cholesterol was replenished in these cells by using water-soluble cholesterol. As a result, mobility was restored to levels displayed by the untreated cells, indicating that the decrease in Gag mobility caused by cholesterol depletion is reversible.
FIG. 9.
Effect of cholesterol on Gag-GFP mobility. Cells expressing Gag-GFP were treated with MβCD and subjected to FRAP. Treatment with MβCD decreased recovery to about 60% while untreated cells recovered to 80%. When cholesterol was replenished, recovery to approximately 80% was restored.
DISCUSSION
Thousands of Pr55Gag molecules must come together to form an immature virion in what is believed to be a complex multistep process. Insights into the process of assembly have come from a variety of methods, such as subcellular fractionation and electron microscopy and immunofluorescence, revealing that in most cell types, assembly takes place at the plasma membrane (20, 28, 47). Unfortunately, these methods use either disrupted or fixed cells, giving a static perspective of particle assembly. A key aspect of this assembly process is the mobility of Pr55Gag. To gain insights into the dynamic aspects of HIV assembly, we have utilized two approaches that allow the direct analysis of the mobility of Pr55Gag tagged with fluorescent protein derivatives in living cells: FRAP and photoactivation. Using these complementary approaches, we find that unassembled Pr55Gag is highly mobile in living cells, allowing individual Pr55Gag molecules to move from one end of a cell to the other on a time scale of tens of seconds. This rapid mobility potentially allows thousands of Pr55Gag molecules to come together to form a virion on a time scale of seconds.
It has been shown by others that Pr55Gag not assembled into VLPs or associated with intracellular vesicles can exist in assembly intermediates consisting of multiple molecules (22). Therefore, analysis of the mobility of membrane-associated and cytoplasmic Pr55Gag likely represents a mixed population of molecules in potentially different stages of assembly. To gain insights into the movement of potential intermediates of the assembly process, we have analyzed the mobility of deletion derivatives of Pr55Gag and a form of Gag that cannot be myristoylated and therefore will not interact efficiently with cellular membranes. We found that all derivatives except MACA have similar rates of mobility. However, they differ in R values, indicating different mobile fractions. Table 1 shows the trend in which R values (mobile phase) decrease as protein size increases. However, the small differences in size are unlikely to account for the observed differences. There is also a correlation in the potential to assemble and the mobile fraction for each derivative, with full-length Gag having the lowest R value and MA having the highest R value. Therefore, intermediates in the assembly process may become progressively immobile. There are two regions implicated in Gag-Gag interactions, CA and NC. Our results show that MACA-GFP and MACA-PAGFP truncation mutants are localized primarily in the plasma membrane, accumulating in structures resembling microvilli. MACA-GFP has an R value equidistant between that of diffusely expressed full-length Gag-GFP and MA-GFP.
Previous studies have shown that upon expression, G2AGag mutants do not form particles or associate with cellular membranes (17). Our results demonstrate that G2AGag-GFP has a similar mobile fraction compared to wild-type Gag-GFP (Table 1). Therefore, the rate of diffusion and the mobile fraction of G2AGag-GFP is comparable to that of wild-type Gag-GFP. This result was unexpected, since G2AGag-GFP, representative of cytoplasmic Gag, does not have the ability to either oligomerize or interact with membranes. This may suggest that Gag may be more free to move in the plasma membrane than in the cytoplasm or that this decreased mobile fraction of cytoplasmic Gag is a consequence of interactions with cellular factors in the cytosol that are inhibiting its ability to move. We favor the latter of the two.
In comparing data resulting from photoactivation to FRAP data, several similarities and differences became apparent. For example, diffusion times determined from FRAP data were very similar to point-of-equilibrium results from photoactivation experiments. An obvious difference is that it appears to be more difficult to detect subtleties in the immobile fraction when using photoactivation. This is evidenced by the observation that in almost all cases a point of equilibrium was reached throughout the cell in photoactivation experiments. In contrast, in FRAP experiments mobility is evaluated by protein diffusion into a small area. Therefore, it seems that photoactivation is more useful in determining movement throughout the cell, while FRAP is more accurate for detecting an immobile fraction.
Our data show that cholesterol depletion decreases the mobility of unassembled Gag-GFP. At first glance, this may seem counterintuitive, but a decrease in protein diffusion by cholesterol extraction is a familiar theme in the field of biophysics. As mentioned previously, cholesterol is a critical component of DRMs or lipid rafts (7). When cholesterol is extracted from the plasma membrane, lipid rafts are disrupted. There is a plethora of literature available implicating the role of membrane rafts in HIV-1 particle formation, virus production, and virus infectivity (5, 10, 29, 33). Recent studies have shown that major histocompatibility complex (MHC) class II complex mobility in the plasma membrane decreases upon cholesterol depletion (48). This effect on MHC II mobility was restored by cholesterol replenishment. Cholesterol depletion also had an adverse affect on the mobilities of the epidermal growth factor receptor and human epidermal growth factor receptor 2 (35). The reason for these effects on protein mobility seems to be that when cholesterol is depleted, it disrupts an important complex formed between cholesterol and phospholipids in the plasma membrane (36). This causes the formation of solid-like phospholipid domains that can lead to confinement of membrane proteins, thereby decreasing their mobilities. It has also been suggested that cholesterol depletion destabilizes lipid tails within the plasma membrane (1). These results may explain the defect in virus production upon cholesterol depletion in previous reports (11, 39). A decrease in the mobility of Gag could decrease the rate of intramolecular interactions necessary for assembling a particle. Also, the results presented in this report suggest and support the idea that Gag is targeted to highly specific domains on the plasma membrane.
Somehow, up to 5,000 molecules of Gag must come together to form a tightly packed particle (2). We have used FRAP and photoactivation to gain insight into how quickly Gag can move within the membrane to assemble. Our data reveal that Gag is present in mobile and immobile fractions and that the mobile fractions can move rather quickly. The rapid mobility of Gag in the membrane suggests that assembly may be a rapid process which can happen within seconds.
Supplementary Material
Acknowledgments
We thank Marilyn Resh for providing Pr55GFP and Jenniffer Lippincott-Schwartz for PAGFP. We also thank Mei Ling Chen and the UIC core facility for technical assistance and Andrea Anderson for her role in the free PAGFP analysis.
This work was supported by National Institutes of Health grant R01 AI52051 to T.J.H. T.J.H. is an Elizabeth Glaser Scientist.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Benninger, R. K., B. Onfelt, M. A. Neil, D. M. Davis, and P. M. French. 2005. Fluorescence imaging of two-photon linear dichroism: cholesterol depletion disrupts molecular orientation in cell membranes. Biophys. J. 88:609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs, J. A., M. N. Simon, I. Gross, H. G. Krausslich, S. D. Fuller, V. M. Vogt, and M. C. Johnson. 2004. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 11:672-675. [DOI] [PubMed] [Google Scholar]
- 3.Bryant, M., and L. Ratner. 1990. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. USA 87:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burniston, M. T., A. Cimarelli, J. Colgan, S. P. Curtis, and J. Luban. 1999. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J. Virol. 73:8527-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 22:217-227. [DOI] [PubMed] [Google Scholar]
- 6.Conte, M. R., and S. Matthews. 1998. Retroviral matrix proteins: a structural perspective. Virology 246:191-198. [DOI] [PubMed] [Google Scholar]
- 7.Crane, J. M., and L. K. Tamm. 2004. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 86:2965-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day, R. N. 2005. Imaging protein behavior inside the living cell. Mol. Cell. Endocrinol. 230:1-6. [DOI] [PubMed] [Google Scholar]
- 9.Derdowski, A., L. Ding, and P. Spearman. 2004. A novel fluorescence resonance energy transfer assay demonstrates that the human immunodeficiency virus type 1 Pr55Gag I domain mediates Gag-Gag interactions. J. Virol. 78:1230-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, L., A. Derdowski, J. J. Wang, and P. Spearman. 2003. Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts. J. Virol. 77:1916-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed, E. O. 2001. HIV-1 replication. Somat. Cell Mol. Genet. 26:13-33. [DOI] [PubMed] [Google Scholar]
- 12.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez, C., and T. J. Hope. 2005. The ins and outs of HIV replication. Cell. Microbiol. 7:621-626. [DOI] [PubMed] [Google Scholar]
- 14.Gottlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, X., A. Roldan, J. Hu, M. A. Wainberg, and C. Liang. 2005. Mutation of the SP1 sequence impairs both multimerization and membrane-binding activities of human immunodeficiency virus type 1 Gag. J. Virol. 79:1803-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halwani, R., A. Khorchid, S. Cen, and L. Kleiman. 2003. Rapid localization of Gag/GagPol complexes to detergent-resistant membrane during the assembly of human immunodeficiency virus type 1. J. Virol. 77:3973-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 19.Kao, H. P., J. R. Abney, and A. S. Verkman. 1993. Determinants of the translational mobility of a small solute in cell cytoplasm. J. Cell Biol. 120:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, Y. M., B. Liu, and X. F. Yu. 1999. Formation of virus assembly intermediate complexes in the cytoplasm by wild-type and assembly-defective mutant human immunodeficiency virus type 1 and their association with membranes. J. Virol. 73:5654-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingappa, J. R., R. L. Hill, M. L. Wong, and R. S. Hegde. 1997. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J. Cell Biol. 136:567-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippincott-Schwartz, J., E. Snapp, and A. Kenworthy. 2001. Studying protein dynamics in living cells. Nat. Rev. Mol. Cell Biol. 2:444-456. [DOI] [PubMed] [Google Scholar]
- 24.Melamed, D., M. Mark-Danieli, M. Kenan-Eichler, O. Kraus, A. Castiel, N. Laham, T. Pupko, F. Glaser, N. Ben-Tal, and E. Bacharach. 2004. The conserved carboxy terminus of the capsid domain of human immunodeficiency virus type 1 Gag protein is important for virion assembly and release. J. Virol. 78:9675-9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misteli, T. 2001. Protein dynamics: implications for nuclear architecture and gene expression. Science 291:843-847. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa, Y., S. Hinata, H. Tomoda, T. Goto, M. Nakai, C. Aizawa, H. Tanaka, and S. Omura. 1996. Complete inhibition of human immunodeficiency virus Gag myristoylation is necessary for inhibition of particle budding. J. Biol. Chem. 271:2868-2873. [DOI] [PubMed] [Google Scholar]
- 27.Morikawa, Y., D. J. Hockley, M. V. Nermut, and I. M. Jones. 2000. Roles of matrix, p2, and N-terminal myristoylation in human immunodeficiency virus type 1 Gag assembly. J. Virol. 74:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nermut, M. V., W. H. Zhang, G. Francis, F. Ciampor, Y. Morikawa, and I. M. Jones. 2003. Time course of Gag protein assembly in HIV-1-infected cells: a study by immunoelectron microscopy. Virology 305:219-227. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nydegger, S., M. Foti, A. Derdowski, P. Spearman, and M. Thali. 2003. HIV-1 egress is gated through late endosomal membranes. Traffic 4:902-910. [DOI] [PubMed] [Google Scholar]
- 31.Ono, A., D. Demirov, and E. O. Freed. 2000. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J. Virol. 74:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono, A., J. M. Orenstein, and E. O. Freed. 2000. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 74:2855-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orr, G., D. Hu, S. Ozcelik, L. K. Opresko, H. S. Wiley, and S. D. Colson. 2005. Cholesterol dictates the freedom of EGF receptors and HER2 in the plane of the membrane. Biophys. J. 89:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandit, S. A., D. Bostick, and M. L. Berkowitz. 2004. Complexation of phosphatidylcholine lipids with cholesterol. Biophys. J. 86:1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson, G. H., and J. Lippincott-Schwartz. 2004. Selective photolabeling of proteins using photoactivatable GFP. Methods 32:445-450. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Caballero, D., T. Hatziioannou, J. Martin-Serrano, and P. D. Bieniasz. 2004. Human immunodeficiency virus type 1 matrix inhibits and confers cooperativity on Gag precursor-membrane interactions. J. Virol. 78:9560-9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reits, E. A., and J. J. Neefjes. 2001. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 3:E145-E147. [DOI] [PubMed] [Google Scholar]
- 40.Resh, M. D. 2005. Intracellular trafficking of HIV-1 Gag: how Gag interacts with cell membranes and makes viral particles. AIDS Rev. 7:84-91. [PubMed] [Google Scholar]
- 41.Sandefur, S., R. M. Smith, V. Varthakavi, and P. Spearman. 2000. Mapping and characterization of the N-terminal I domain of human immunodeficiency virus type 1 Pr55Gag. J. Virol. 74:7238-7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaaf, M. J., and J. A. Cidlowski. 2003. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol. Cell. Biol. 23:1922-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shioda, T., and H. Shibuta. 1990. Production of human immunodeficiency virus (HIV)-like particles from cells infected with recombinant vaccinia viruses carrying the gag gene of HIV. Virology 175:139-148. [DOI] [PubMed] [Google Scholar]
- 44.Spearman, P., R. Horton, L. Ratner, and I. Kuli-Zade. 1997. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 71:6582-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spearman, P., J. J. Wang, N. Vander Heyden, and L. Ratner. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaminathan, R., S. Bicknese, N. Periasamy, and A. S. Verkman. 1996. Cytoplasmic viscosity near the cell plasma membrane: translational diffusion of a small fluorescent solute measured by total internal reflection-fluorescence photobleaching recovery. Biophys. J. 71:1140-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tritel, M., and M. D. Resh. 2000. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J. Virol. 74:5845-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrljic, M., S. Y. Nishimura, W. E. Moerner, and H. M. McConnell. 2005. Cholesterol depletion suppresses the translational diffusion of class II major histocompatibility complex proteins in the plasma membrane. Biophys. J. 88:334-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.