Abstract
Acquired human immunodeficiency virus type 1(HIV-1) resistance to the fusion inhibitor enfuvirtide (ENF) is primarily associated with mutations within the highly conserved first heptad repeat (HR1) region of gp41. Viral env sequences, however, are remarkably variable, and the envelope genetic background could have an important impact on optimal expression of HR1 mutations. We have examined the genetic evolution of env sequences, ENF susceptibility, and Env replicative capacity in patients failing ENF treatment. Sequential plasma-derived virus populations, obtained from six patients initiating ENF treatment as part of a salvage therapy, were studied using a recombinant phenotypic assay evaluating the entire gp120 and the gp41 ectodomains. Regardless of major differences in the baseline ENF susceptibilities, viral populations with similar phenotypic ENF resistance (50% inhibitory concentration, >3,000 ng/ml) were selected under treatment in four of six patients. As expected, in all patients ENF-resistant viruses harbored one or more HR1 mutations (positions 36, 38, and 43). Interestingly, in five patients the emergence of resistance mutations was not associated with reduced Env replicative capacity. Phylogenetic analysis of env sequences in sequential samples from two patients showed that the HR1 mutations had emerged in the context of env quasi-species that were different from those prevalent at baseline. Thus, the envelope genetic context appears to play a critical role in the selection of HR1 mutations and the expression of ENF resistance, thereby conditioning the evolution of HIV-1 under fusion inhibitor selective pressure.
Considerable effort is currently being devoted to the development of antiviral agents able to prevent human immunodeficiency virus type 1 (HIV-1) entry into target cells. The HIV-1 entry process is mediated by the trimeric viral envelope glycoproteins (Env) exposed at the surface of the virion (45). The HIV-1 entry is a multistep process (11, 14, 45). The sequential interaction of the surface subunit, gp120, with CD4 and a chemokine receptor (CCR5 or CXCR4) exposed on the cell membrane triggers conformational changes in the ectodomain of the transmembrane subunit, gp41, which ultimately lead to fusion between the viral and host cell membranes. Like most of the retroviral transmembrane proteins, the ectodomain of gp41 contains an N-terminal fusion peptide followed by two heptad repeat domains (HR1 and HR2), which are connected by a non-helical loop region of 25 to 30 amino acids. Membrane fusion is currently thought to result from the insertion of the fusion peptide into the cellular membrane, and the formation of a six-helix bundle in which the central trimeric HR1 coiled-coil forms three hydrophobic grooves onto which three HR2 domains pack in reverse orientation (4, 5, 40, 43).
Although several HIV-1 entry inhibitors have been evaluated in clinical trials and have shown promising prospects for therapy (1, 28), the only entry inhibitor licensed to date is the fusion inhibitor enfuvirtide (ENF; also called T20), which has shown potent antiviral activity in patients, resulting in sustained viral load reduction when used in combination with an optimized background regimen of protease and/or reverse transcriptase inhibitors (21-23). Similar to results with other antiviral agents, however, ENF-resistant HIV-1 variants may emerge under the selective pressure of ENF (26, 32, 39, 42) whenever the treatment fails to completely suppress viral replication in vivo. Understanding the determinants of, and the constraints to, the development of resistance to ENF is essential for the optimization of the clinical use of this new class of inhibitors.
ENF is a 36-amino-acid peptide derived from the sequence of HR2 (24, 44). ENF binding to HR1 is thought to compete with the folding of the HR2 domain onto HR1, thus preventing Env-mediated membrane fusion (1, 5, 17). Accordingly, viral variants selected both in vitro and in vivo in the presence of ENF carry resistance mutations in the HR1 domain (35, 42). Mutations in HR2 have also been described in virus from treated patients and are thought to exert a compensatory role through reinforcement of the interaction between HR1 and HR2 in the context of a mutated HR1 (2, 25, 46).
The molecular target of ENF, the HR1 domain, is highly conserved among HIV-1 isolates (10, 37), and ENF resistance mutations are not seen in untreated patients. However, we along with others have shown that baseline susceptibility to ENF is quite variable among viruses from different patients (9, 20, 39), and variations in domains of Env other than HR1 are thought to account for these differences in baseline susceptibility (8, 15, 34). In particular, susceptibility to fusion inhibitors appears to be dependent on the affinity of gp120 for the coreceptors as well as on coreceptor density at the cell surface, both of which factors affect fusion kinetics (34). A faster fusion process will reduce the length of time during which the ENF molecular target is exposed, thus reducing the virus sensitivity to this inhibitor (28, 30, 34). We thus hypothesized that in patients failing ENF treatment, the biological properties of Env, and therefore the whole envelope genetic background, could influence the selection of ENF resistance mutations, thereby conditioning the emergence of ENF-resistant viruses.
In this study, we retrospectively evaluated the genotypic and phenotypic Env properties associated with the emergence of resistance to ENF in six patients who received the drug as part of a salvage regimen. Although baseline viruses differed in their susceptibility to ENF, viral resistance to ENF attained similar levels in four of the six patients, suggesting that a threshold level of resistance needs to be reached in vivo. Genotypically resistant variants were observed within weeks after starting ENF treatment, while phenotypic resistance, at the virus population level, developed with variable kinetics in different individuals. In contrast to viruses selected by protease and reverse transcriptase inhibitors, ENF-resistant viruses from five of these six patients did not show reduced replicative capacities, suggesting that the env quasi-species that emerged under ENF treatment provided a favorable genetic background for the expression of resistance mutations. Phylogenetic analysis of env sequences from plasma virus populations at sequential time points supported this hypothesis, since ENF resistance mutations emerged in viral quasi-species that were not dominant at baseline.
MATERIALS AND METHODS
Patients.
The six patients included in this study had received ENF administered subcutaneously (90 mg twice daily) for at least 12 weeks (range, 12 to 50 weeks) in addition to a background antiretroviral regimen that included protease inhibitors and nucleoside and nonnucleoside reverse transcriptase inhibitors. All patients were clinically classified as category C with CD4+ T-cell counts of <200 cells/μl of blood at the beginning of treatment including ENF (3). Three of the six patients (patients 2, 4, and 5) underwent a treatment interruption prior to initiation of ENF treatment for 80, 35, and 12 weeks, respectively. Patients were monitored at the Hôpital Saint-Antoine (Paris, France). Sequential plasma samples were obtained before, during, and after treatment including ENF. Plasma HIV-1 viral load measurements were performed using the Amplicor HIV-1 monitor test (Roche Molecular Systems, Inc.), with a detection limit of 50 HIV-1 RNA copies/ml. CD4+ T cells were quantified by flow cytometry analysis using a FACScan (BD Biosciences). The genotypes of protease and reverse transcriptase plasma HIV-1 RNA were determined as described in the PCR and sequencing procedures of the French Agence Nationale de Recherche sur le SIDA (ANRS; National Agency for AIDS Research) AC11 Resistance group (http://www.hivfrenchresistance.org/).
Extraction and amplification of plasma viral RNA.
Extraction and amplification of viral RNA by reverse transcriptase-PCR (RT-PCR) was carried out as previously described (20). Briefly, RNA was extracted from frozen sequential plasma samples using the QIAmp viral RNA purification protocol (QIAGEN). The envelope gene was amplified by RT-PCR by using the following primers: E00, 5′-TAGAAAAGAGCAGAAGACAGTGGCAATGA-3′ (nucleotides [nt] 6197 to 6224 of pNL4-3), and E01−, 5′-CCAGTCCCCCCTTTTCTTTTAAAA-3′ (nt 9054 to 9078). An aliquot of the RT-PCR product was then used in a nested PCR with the following primers: E10, 5′-GTGGGTCACAGTCTATTATGGGGT-3′ (nt 6322 to 6345), and FuB, 5′-GGTGGTAGCTGAAGAGGCACAGG-3′ (nt 8500 to 8522). This approach allows the amplification of a 2,200-bp fragment that spans the gp120 and the extracellular domain of gp41. PCR products were verified by agarose gel electrophoresis and column purified (QIAGEN) prior to transfection and sequencing. To prevent sampling bias, three independent RT-PCRs were carried out for each plasma sample, and products were pooled together and used for phenotypic and genotypic analysis.
Cell lines.
The human cell lines 293-T and U373MG-CD4 stably expressing either CCR5 and/or CXCR4 (19) were propagated in Dulbecco's modified Eagle's medium (Gibco) supplemented with 60 μg/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum. The U373MG-CD4 cell line expressing both CCR5 and CXCR4, here referred to as U373MG-dual, was obtained after transfection of the U373MG-CD4-CCR5 cell line with a CXCR4 expression vector also expressing blasticidin resistance. To obtain this cell line, the human CXCR4 cDNA from the previously described RcCMV (31) was cloned as a BamHI-NotI fragment in pcDNA6/V5-His (Invitrogen). Individual drug-resistant cell clones were screened for their sensitivity to infection with the HIV-1 NL4-3 strain, and a permissive clone was maintained in culture in the presence of 3 μg of blasticidin per ml. CD4, CXCR4, and CCR5 expression levels at the cell surface of the target cells were regularly monitored. All the U373MG-CD4 cell line derivatives contain the Escherichia coli-galactosidase indicator gene (lacZ) under transcriptional control of the HIV-1 long terminal repeat, allowing the quantification of HIV-1 infectivity through a β-galactosidase activity.
Production of recombinant virus.
We have developed and previously described a recombinant virus assay that permits the assessment of viral susceptibility to entry inhibitors (20). Briefly, the pNL4-3 molecular clone was modified by deleting the region of the envelope gene encoding gp120 and the ectodomain of gp41 (positions 6480 to 8263) and replacing it with a linker that contains a unique MluI restriction site (vector 43-Δenv). Recombinant viruses were produced by cotransfection of 293-T cells with 8 μg of MluI-linearized 43-Δenv vector and 0.8 μg of the PCR product obtained from plasma virus samples, which encompasses the deleted region and carries short overlaps on each side of the deletion that allow homologous recombination. This procedure leads to the production of recombinant virus particles expressing the gp120 and gp41 ectodomain of patient origin and the cytoplasmic domain of gp41 from pNL4.3. Theoretically, the chimeric nature of gp41 could affect Env function to some extent. For example, a perturbation of Env fusogenicity and antibody binding has been documented for viruses in which the cytoplasmic domain of gp41 was artificially shortened (12, 13). Replacement of the cytoplasmic domain, however, is expected to have a milder impact on Env functions than its truncation. On the other hand, the fact that the cytoplasmic tail of gp41 derives from NL4.3 guarantees the functional compatibility between Gag and Env in recombinant virus particles. Culture supernatants were collected 40 h after transfection and centrifuged for 10 min at 1,800 rpm to remove cell debris. HIV-1 p24 antigen production was quantified for each viral stock by enzyme-linked immunosorbent assay (Innogenetics/Ingen).
To verify that the recombinant virus populations produced by transfection of producer cells carried the same sets of mutations as the input RT-PCR product, we compared the sequence of the RT-PCR product used for transfection of producer cells to the sequence of proviral DNA obtained after infection of MT4R5 target cells. Target cells were infected using the spinoculation procedure to prevent the selective loss of poorly infectious variants from the recombinant virus population. The comparison was carried out on the ectodomain of gp41 for all time points of the follow-up of patients 1, 2, and 5 (for a total of 18 sequences, representing 3107 amino acid positions) (data not shown). In addition, the entire gp120 sequence from the follow-up of patient 1 was analyzed (seven sequences, corresponding to 3402 residues). Overall, the vast majority of the 6,509 residues analyzed here was identical between RT-PCR and proviral DNA samples. A total of 14 residues differed, representing a concordance rate of 99.78%.
Determination of recombinant virus susceptibility to ENF.
U373MG-dual target cells were seeded into 96-well plates 2 days before infection at a density of 2,000 cells/well. Infection with 100 μl of fresh viral suspension was carried out in triplicate, in the absence or the presence of increasing concentrations of ENF (from 5 to 15,000 ng/ml) (T20; American Peptide Company, Inc.). Forty hours after infection, cells were lysed using 100 μl/well of lysis buffer (5 mM MgCl2 and 0.1% NP-40 in 1× phosphate-buffered saline [PBS]), and 100 μl/well of chromogenic substrate (6 mM chlorophenol red-β-d-galactopyranoside; CPRG [Roche]) in lysis buffer was added. The optical density (OD) was measured after 4 to 8 h of incubation at 37°C. The concentrations of ENF inhibiting 50% of virus infectivity (IC50 values) were calculated by using the median-effect equation (6).
Evaluation of the efficiency of viral envelope to mediate HIV-1 infection in the absence of ENF (Env replicative capacity).
The U373MG-dual target cells seeded as previously described, were infected with serial dilutions of fresh viral supernatant normalized by p24 antigen content in the absence of ENF. For each virus input, β-galactosidase activity resulting from transactivation of the long terminal repeat-lacZ reporter cassette in target cells was measured as OD units. A linear regression was calculated for each virus by plotting the OD values as a function of the p24 virus input (data not shown). Different viruses were characterized by different slopes. For each patient, the virus from the latest time point available before the beginning of the ENF treatment was used as reference (and was attributed a replicative capacity of 100%). The Env replicative capacity of viruses obtained during and after treatment including ENF was expressed as percentage of the reference virus, by comparing the slopes of the corresponding linear regressions.
Sequencing and phylogenetic analysis of the gp41 ectodomain.
For the six patients, 700 bp of the RT-PCR products derived from sequential plasma samples encoding the ectodomain of gp41 were sequenced by using the sense primer, designated ecto-primer: 5′-CCCGGCTGGTTTTGCGATT-3′ (nt 6871 to 6889). For patients 1 and 2, the entire gp120 sequence of consecutive virus populations was determined. In addition, for phylogenetic analysis, the 2,200-bp RT-PCR products derived from the sequential plasma samples of patients 1 and 2 were TA-cloned into pCR2.1-TOPO (Invitrogen). To isolate clones, individual colonies were cultured in Luria-Bertani medium, and plasmid DNAs were purified by using a QIAprep Spin Miniprep kit (QIAGEN). For each time point, the genotype of 6 to 17 independent clones (with the exception of a pre-ENF therapy sample from patient 2) was determined by sequencing a 700-bp fragment of the envelope gene encoding the entire ectodomain of gp41 using the ecto-primer. Nucleotide sequences were aligned by the CLUSTAL W program (41). Phylogenetic analysis was performed by using the DNA maximum-likelihood method in the Phylogeny Inference Package (PHYLIP, version 3.5); the gp41 ectodomain nucleotide sequences of the clones and unrooted trees were drawn by using TreeView (version 1.6.1) (29). To test the probability that a resistance mutation would occur on one or more occasions, phylogenetic distances were estimated by the neighbor-joining method using the PAUP* 4.0 (Phylogenetic Analysis Using Parsimony and other methods) program (36). The nucleotide position of the codons implicated in resistance mutation was weighted in order to consider that all genotypes with a given resistance mutation were constrained to be a monophyletic group. The constrained tree and the best estimated tree without constraints were compared by using the Kishino-Hasegawa test (16). If the null hypothesis was rejected (P < 0.05), a double occurrence of the mutation was considered more likely.
Statistical analysis.
Results of ENF resistance and Env replicative capacity are expressed as the mean ± standard error of the mean. Viral populations obtained from each patient at different time points during resistance development were compared for their IC50 values and their Env replicative capacity by using parametric one-way analysis of variance. Posttest comparisons were made using a Bonferroni posttest for selected time points.
Nucleotide accession numbers.
The sequences reported in this paper have been deposited in the GenBank/EMBL/DDBJ databases under accession numbers DQ863719 to DQ863939. They correspond to sequences of gp41 ectodomain (patients 1 to 6) and gp120 (patients 1 and 2) of plasma virus populations over time, and to gp41 ectodomain nucleotide sequences of clones derived from patients 1 and 2.
RESULTS
Evolution of plasma viral load and CD4+ T-cell counts under treatment.
To evaluate the virological response to treatment including ENF, viral load (VL) in plasma samples was monitored over time. In parallel, CD4+ T-cell counts were measured. In the six patients, the median plasma VL before ENF treatment was 5.3 log10 RNA copies/ml of plasma (range, 4.7 log10 to 5.7 log10). The median CD4+ T-cell counts was of 14.5 cells/μl (range, 4 to 29), and the genotype of the viral populations harbored a median of 5.5 (range, 5 to 7) resistance mutations to nucleoside reverse transcriptase inhibitors, 1.5 (range, 0 to 3) to nonnucleoside reverse transcriptase inhibitors, and 10 (range, 2 to 13) to protease inhibitors. On the basis of the genotype, the median number of drugs presumed to be effective in addition to ENF was 1.5 (range, 1 to 4) according to the French ANRS resistance algorithm (http://www.hivfrenchresistance.org/) (data not shown).
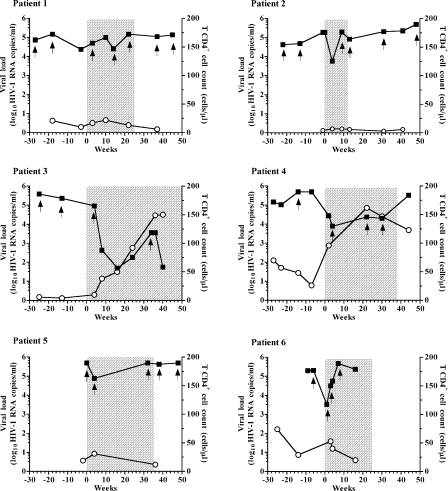
The evolution of plasma VL and CD4+ T-cell counts was followed for up to 80 weeks (Fig. 1). All participants exhibited a plasma HIV-1 load reduction after the beginning of the treatment including ENF, ranging from −0.47 to −3.67 log10 RNA copies/ml (median, −1.65 log10). For two of the six patients (patients 1 and 5), the reduction in plasma VL did not exceed 1 log10, leading to withdrawal of ENF from their therapeutic regimen at weeks 25 and 35, respectively. Three other patients had an initial decrease in VL, but this response was transient (from 1 to 4 weeks), and ENF treatment was interrupted after 24 to 38 weeks. Patient 3, who had a ΔVL of −3.67 log10 RNA copies/ml after 16 weeks of treatment including ENF and a rise in CD4+ T cells from 4 to 146 cells/μl, continued the ENF therapy, despite a transient viral rebound (at week 34). For the six patients, the level of plasma VL reduction did not correlate with the number of presumed effective molecules used in the regimen in addition to ENF (r = 0.2390; P = 0.7) and was not significantly different in patients who did or did not undergo a treatment interruption before the therapy including ENF (unpaired t test, P = 0,1).
FIG. 1.
Evaluation of plasma HIV-1 load (log10 RNA copies/ml) (filled squares) and CD4+ T-cell counts (cells/μl) (open circles) before, during, and after the treatment including ENF for the six patients studied. HIV-1 RNA levels were measured by a quantitative RT-PCR assay, and CD4+ T-cell counts were measured by flow-activated cytometric assay. The chronology of the follow-up is indicated in weeks on the x axis. The beginning of the treatment including ENF is referred to as week 0, and the sampling times preceding ENF introduction are indicated with negative values. Plasma samples analyzed by phenotypic assay and by sequencing are marked in the graph with arrows. The time of treatment including ENF is shaded.
Baseline susceptibility to ENF.
We along with others have previously shown that the level of susceptibility to ENF is quite variable in ENF-naïve patients (9, 20, 32, 39). As a consequence, an understanding of the evolution of phenotypic ENF resistance requires the determination of pretherapy susceptibility to ENF. For each patient, the evaluation of the baseline ENF susceptibility of plasma virus populations was performed from one or two plasma samples obtained prior to ENF treatment by using a recombinant virus assay (20).
The IC50 values of viruses derived from the same patient sampled at an interval of 9 to 12 weeks before initiation of the ENF-based salvage treatment (patients 1, 2, and 3) showed little variation in ENF susceptibility (<1.3-fold) (Fig. 2), suggesting that susceptibility to ENF was stable over time in the absence of ENF selection. However, baseline ENF IC50 values were quite variable in different patients, ranging from 56 to 896 ng/ml. These IC50 values were in the range of previously reported data for ENF-naïve patients, obtained either with recombinant assay systems or by using virus isolates recovered from cultures of peripheral blood mononuclear cells (9, 20, 32, 39).
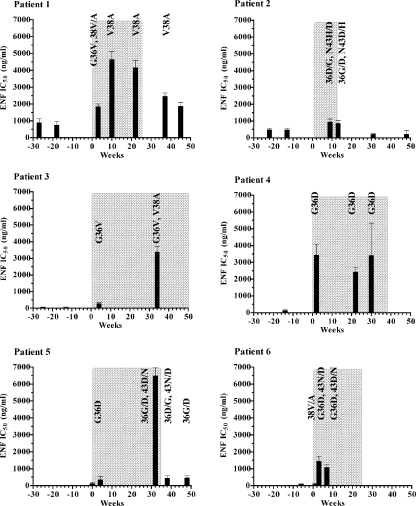
FIG. 2.
Evolution of phenotypic ENF susceptibility. ENF susceptibility of recombinant viruses expressing envelope proteins of plasma virus populations sampled at different time points for each patient was determined in a single-cycle assay. Columns represent mean IC50 values to ENF of at least three independent experiments (bars represent standard errors). The chronology of the follow-up is indicated on the x axis in weeks, and the time of treatment including ENF is shaded. HR1 resistance mutations harbored by each plasma virus population are indicated.
At baseline, none of the six viral populations carried any of the HR1 mutations located between amino acids 36 and 45 of the gp41 ectodomain that have been associated with ENF resistance (35, 39, 42). All viruses showed the subtype B consensus sequence in this region, 36-GIVQQQNNLL-45, with the exception of the viral population of patient 2, which was characterized by a mixture of amino acids Q and H in position 39. A previous study showed that the substitution Q39H is a natural polymorphism shared by about 1 to 2% of HIV-1-infected patients and does not affect the susceptibility to ENF (27).
Evolution of genotypic and phenotypic resistance to ENF during treatment.
We analyzed and compared the number and position of ENF resistance mutations that emerged in the virus populations of treated patients (Tables 1 and 2) and their impact on the level of phenotypic resistance over time (Fig. 2). For each time point, three independent RT-PCRs were performed and pooled together. The sequencing reactions and Env phenotypic analyses were conducted using the same pooled PCR products.
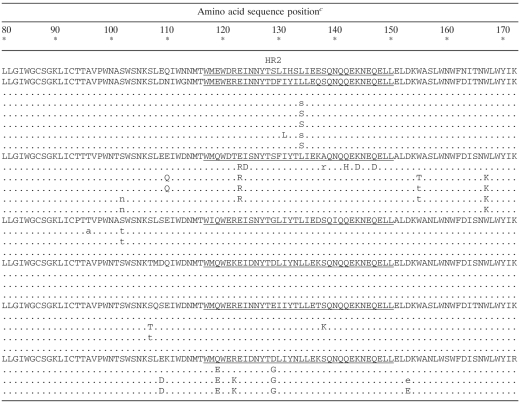
TABLE 1.
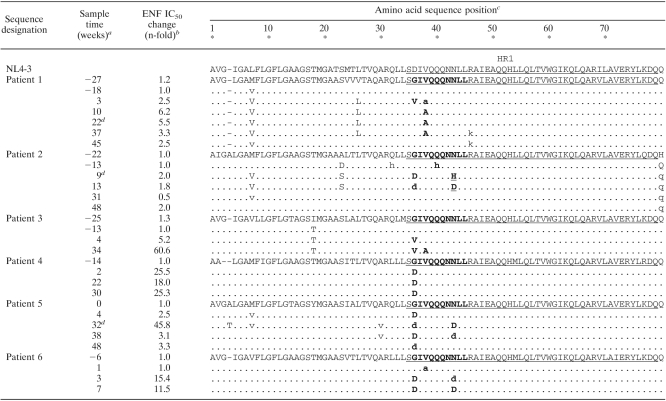
Sequence alignments of gp41 ectodomain (amino acids 1 to 172) of plasma virus populations over time
The sample times analyzed for each patient are indicated in reference to the beginning of the treatment including ENF, at week 0.
For each patient, ENF IC50 the relative change reflects the increase (n-fold) in the mean ENF IC50 tested with each plasma sample obtained during and after treatment including ENF relative to the mean ENF IC50 of the pre-ENF therapy viruses obtained at the latest time point available before the beginning of the ENF treatment.
NL4-3 numbering. The HR1 domain (amino acids 35 to 79) and the HR2 regions (amino acids 117 to 150) of the gp41 ectodomain are underlined. Boldface indicates residues of the HR1 domain of the gp41 ectodomain (positions 36 to 45) previously shown to be associated with HIV-1 resistance to ENF. For a given position, a lowercase letter indicates that the amino acid variant was present in a minority proportion in association with the wild-type (baseline) amino acid. For patient 2, at position 43, the populations harbored a mixture of mutant amino acids in which a histidine (H) residue dominated over an aspartic acid residue (D) at week 9 (designated as H); however, aspartic acid residue (D) was the most frequent residue at this position four weeks later. Dashes correspond to amino acid insertion or deletion..
ENF treatment was stopped at weeks 25, 12, and 35 for patients 1, 2, and 5, respectively.
Regardless of baseline ENF susceptibility, the ENF IC50 values measured under ENF treatment reached a level of >3,000 ng/ml in four of six patients. This increase of ENF resistance could be reached either very rapidly after the beginning of the ENF treatment (patient 4) or progressively (patients 1, 3, and 5). The rapid development of ENF resistance was associated with the detection of one or two mutations in the HR1 domain of gp41 (Table 1). For patient 4, the only substitution observed was G36D. This change, which was detected at the second week of ENF treatment, was maintained throughout treatment and was associated with a high level of resistance (IC50, 3,442 ng/ml). In contrast, in patients 3 and 5, the presence of a single substitution in position 36 of the HR1 domain of gp41 (G36V or G36D) 4 weeks after the beginning of the treatment was associated with a suboptimal level of ENF resistance (<500 ng/ml). Several weeks later however, additional HR1 mutations appeared in the virus populations of these two patients: V38A in patient 3 and N43D in patient 5, together with a marked increase in ENF IC50 (greater than 3,000 ng/ml). In patient 1, a different evolution profile was observed. At 3 weeks after the start of ENF treatment, the prevalent resistance mutation was G36V, associated with a mixture of amino acids V and A in position 38. At weeks 10 and 22, the only persisting mutation was V38A. For these four patients, the change in resistance measured between baseline and time points during course of ENF treatment was statistically significant (P < 0.05 for patient 4; P < 0.0001 for patients 1, 3, and 5).
For patients 2 and 6, the increases in ENF resistance were modest, since IC50 values remained lower than 1,500 ng/ml after 9 and 7 weeks of ENF treatment, respectively. The viral population derived from patient 6 at 1 week after the beginning of ENF treatment harbored a mixture of amino acids in position 38 of the HR1 domain and was not associated with an increase in ENF resistance. At weeks 3 and 7, the viral populations from this patient carried two mutations associated with ENF resistance, G36D and N43D, and were characterized by an IC50 close to 1,500 ng/ml. A single plasma sample was available for patient 2 during the ENF treatment (week 9), for which the viral population, carrying mutations in two positions [G36D and N43(H/D)], was characterized by an IC50 of about 1,000 ng/ml.
Progressive loss of resistance mutations after interruption of ENF treatment.
For three patients (1, 2 and 5), we analyzed the evolution of ENF resistance mutations and of phenotypic resistance in plasma viral populations up to 36 weeks after ENF interruption (Fig. 2 and Table 1). For patient 1, ENF IC50 values decreased significantly from 4,167 to 2,471 ng/ml (P < 0.01) 12 weeks after ENF interruption (week 37), while the viral population still harbored the V38A mutation. Strikingly at week 45, the IC50 value was still relatively high (1,872 ng/ml), despite the loss of detection of the V38A mutation. For patient 2, the viral population present 1 week after the end of the ENF treatment was characterized by an ENF susceptibility comparable to that of the previous time point (IC50 of <1,500 ng/ml) and harbored the mutations (G36D and N43D/H) present during ENF treatment. Nineteen and 36 weeks after the end of ENF treatment, the IC50 values were 219 and 230 ng/ml, respectively, and the corresponding viral populations carried a wild-type genotype in the HR1 domain of gp41. For patient 5, a rapid decrease in ENF IC50 (from 6,509 to 448 ng/ml) was observed 3 weeks after ENF interruption (P < 0.001). This decrease in ENF resistance occurred despite the persistence of mutations G36D and N43D, the latter as part of a mixture with wild type. Ten weeks later, only mutation G36D persisted, while the IC50 did not notably change.
Evolution of Env replicative capacity before, during, and after treatment including ENF.
The selection of drug resistance mutations in protease and reverse transcriptase is often accompanied by reductions in enzyme efficiency, resulting in decreased virus replicative capacity in the absence of inhibitor (7, 33). To evaluate the impact of ENF resistance on HIV-1 replicative capacity, we measured the infectivity of viruses carrying patient-derived env sequences in a single-cycle assay (Env replicative capacity). Samples obtained before, during, and after ENF treatment were analyzed for each patient. Serial dilutions of recombinant virus-containing supernatants were used to infect U373MG-dual target cells in the absence of ENF. For each patient, Env replicative capacity was expressed as a percentage of the value measured for the corresponding pre-ENF therapy viral population (Fig. 3).
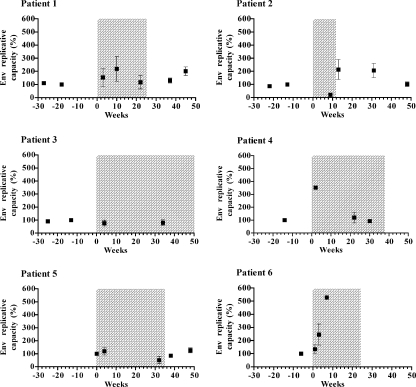
FIG. 3.
Evolution of the Env replicative capacity. The Env replicative capacity of recombinant viruses expressing the primary envelope proteins from plasma samples obtained during and after treatment including ENF was measured in a single-cycle assay. For each patient, Env replicative capacities are expressed as a percentage of that of pre-ENF therapy viruses, obtained at the latest time point available before the beginning of the ENF treatment. Squares represent means of Env replicative capacities of at least three independent infection experiments (standard errors are indicated). The time of treatment including ENF is shaded.
For patients 1, 2, and 3, the Env replicative capacity of samples obtained before ENF treatment appeared to be stable over time. The changes in Env replicative capacity during and after ENF treatment followed several distinct pathways in different patients. For three of the six patients (1, 3, and 5) for whom ENF resistance development was progressive, Env replicative capacity did not vary significantly over time (P = 0.4, P = 0.8, and P = 0.1, respectively). For patient 2, Env replicative capacity decreased from 100% to 20% under ENF treatment (P < 0.001), increased after ENF interruption to reach about 200% at weeks 13 and 31 (P < 0.001), and finally went back to baseline level at week 48. For patients 4 and 6, Env replicative capacity significantly increased under ENF treatment. This increase was transient for patient 4, for whom Env replicative capacity returned to values equivalent to baseline level at weeks 22 and 30 (120% and 93%, respectively).
Phylogenetic analysis of viral env quasi-species.
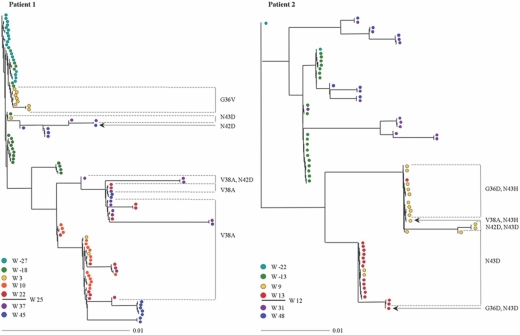
To study the evolution of envelope genotypes during viral escape to ENF treatment, we sequenced and analyzed the gp41 ectodomain from clones derived from patients 1 and 2 over time. These two patients were selected for this study because samples were available at two time points before ENF treatment and during and after treatment cessation, allowing a comprehensive analysis of genotypic changes. The bulk PCR products amplified from plasma samples were subcloned, and 6 to 17 independent clones were sequenced at each time point analyzed (with the exception of week −22 for patient 2, for which only two clones were analyzed). All the clones differed in their nucleotide sequence. Trees were constructed by using DNA maximum-likelihood method (Fig. 4).
FIG. 4.
Genotypic evolution of the gp41 ectodomain in patients 1 and 2. At the bottom of each panel, the different time points analyzed before, during, and after the treatment including ENF are indicated and identified with a specific color. The week from which the treatment including ENF was stopped is also indicated (at weeks 25 and 12 for patients 1 and 2, respectively). At the indicated times, RNA was extracted from plasma, and the envelope gene was amplified by RT-PCR and cloned, and the gp41 ectodomain was sequenced. The phylogenetic trees obtained by DNAPARS are shown. The presence of ENF resistance mutations located between residues 36 to 45 of the HR1 domain is indicated.
For patient 1, 3 weeks after the beginning of ENF treatment, viral variants carrying three different ENF resistance mutations were observed. The majority of the variants (8/11) harbored the G36V resistance mutation, one variant had the N43D mutation, and the two others had the V38A mutation. Viral population at this time point was characterized by a suboptimal level of resistance (IC50 of 1,853 ng/ml). All viral variants present at weeks 10 and 22 harbored the V38A mutation in the HR1 domain. The ENF IC50 values for the virus populations at these time points were greater than to 4,000 ng/ml. The tree topology suggested that these variants were phylogenetically related to the two V38A-carrying variants, present at week 3. Accordingly, the Kishino-Hasegawa test was consistent with the interpretation that the resistance V38A mutation occurred on one occasion in a monophylogenetic group (P = 0.3). At week 37, 12 weeks after the interruption of ENF treatment, most envelope sequences still carried the V38A mutation, sometimes in association with the N42D mutation. At week 45, all sequences carried a wild-type genotype between amino acids 36 to 45, with the notable exception of a variant that conserved the N42D mutation. The tree topology suggested that the envelope sequences present after the interruption of ENF treatment emerged both from pre-ENF treatment quasi-species and from the monophylogenetic group carrying the V38A mutation that appeared during ENF treatment. Thus, while most viruses did not express ENF resistance mutations, the populations represented a mixture of viral species similar to those present prior to the treatment and variants derived from the distinct population that had developed ENF resistance.
For patient 2, at 9 weeks after the beginning of ENF treatment, a variety of variants expressing four different resistance genotypes were present: the N43D resistance mutation in the HR1 domain of gp41 in combination with the N42D mutation (2/16 clones), the N43D mutation alone (4 clones), the N43H mutation in combination with the G36D mutation (9 clones), and the N43H mutation associated with the V38A mutations (1 clone). At week 13, only 1 week after ENF had been stopped, all viruses continued to express resistance mutations, and two of the genotypes identified at week 9 were still present: 15 of the 16 variants sequenced harbored the N43D mutation (one of them in combination with the G36D mutation); the other variant carried the N43H substitution in association with the G36D mutation. Not surprisingly considering the topology of the tree, the Kishino-Hasegawa test did not support the conclusion that resistance mutations G36D and N43D had occurred on more than one occasion (P = 0.4 and P = 0.3, respectively). At weeks 31 and 48, all the env quasi-species harbored a wild-type genotype phylogenetically related to baseline sequences.
DISCUSSION
In this study we evaluated the development of resistance to ENF in six patients who harbored virus populations characterized by different baseline susceptibility to ENF and who received ENF as part of a salvage therapy regimen. By characterizing the evolution of genotypic and phenotypic ENF resistance and by measuring the effect of resistance on Env replicative capacity, several interesting patterns emerged. First, viruses carrying HR1 resistance mutations appeared rapidly in all patients, and the development of strong resistance did not appear to be influenced by the level of baseline resistance. Second, the extent that a given mutation in HR1 conferred resistance to ENF was found to be highly variable depending on the envelope sequence in which it developed. Third, the Env replicative capacity of viruses carrying resistance mutations that emerged during treatment was often equivalent or superior to that of viruses present prior to treatment. Moreover, in both patients for whom phylogenetic studies were performed, the viral populations carrying resistance mutations that emerged following treatment were not closely related to the population present prior to treatment. Taken together, these findings indicate that the genetic context in which ENF resistance mutations arise can influence in several distinct ways the likelihood that such mutants will emerge as a dominant population during treatment.
Kinetics of the evolution of resistance.
Resistance mutations were readily detected in all patients at the first time point analyzed following treatment, and several competing genotypes were identified in some patients. These findings show that ENF exerted a strong selective pressure and suggest that viruses carrying resistance mutations may have preexisted in minority env quasi-species in these patients. Once high-level resistance was attained (i.e., IC50 of >3,000 ng/ml), no further increases in the IC50 values were generally observed, but this level of resistance could be attained either rapidly or progressively. Analysis of these initial time points showed that baseline susceptibility did not appear to influence either the level of phenotypic resistance achieved or the kinetics of the development of resistance. Thus, patients with high pretreatment susceptibility to ENF could develop high levels of resistance following treatment (e.g., patient 4) more quickly than patients with lower baseline susceptibility (e.g., patients 1 and 2). Our findings are consistent with preliminary data from clinical trials in which differences in the baseline susceptibility were found to have no significant impact on the virological response to a salvage treatment regimen in which ENF was included (38). These findings are not surprising in view of our observation that the envelopes of resistant variants that emerged from patients 1 and 2 were phylogenetically distinct from those present prior to treatment. In these two patients, the dominant baseline population, regardless of its susceptibility to ENF, did not provide the proper genetic background for optimal expression of HR1 resistance mutations. Therefore, its phenotypic resistance properties could not be predictive of the likelihood or kinetics of emergence of full ENF resistance.
Similar to the kinetics of the emergence of resistance, the kinetics of the loss of phenotypic resistance following the interruption of ENF treatment was variable in different patients. For patients 1 and 5, who reached high-level resistance under ENF treatment, two consecutive samples obtained after withdrawal of ENF from the treatment regimen were analyzed. In patient 1, the decrease in the ENF resistance level was gradual, while in patient 5 ENF resistance was no longer detectable within 3 weeks of stopping ENF. Two independent, but nonexclusive phenomena may be responsible for this difference. On the one hand, the virus populations from these two patients had very different baseline ENF susceptibility levels (relatively high IC50 for patient 1 and low IC50 for patient 5). As a consequence, replacement of the resistant virus population by pre-ENF-therapy variants is expected to result in a more gradual reduction of IC50 for patient 1 than for patient 5. On the other hand, highly resistant viral variants from patient 5 had a reduction in Env replicative capacity, in contrast to resistant variants harbored by patient 1. In this setting, ENF withdrawal may be expected to lead to a more rapid replacement of the unfit resistant virus population in patient 5 than in patient 1.
Envelope context and ENF resistance.
The envelope context in which HR1 mutations occurred had a strong impact on phenotypic resistance. Thus, mutations at position 36 that emerged in the initial plasma samples of patients 1, 4, and 5 were associated with moderate, very high, and negligible phenotypic resistance, respectively. Strikingly, patients 4 and 5 had similar baseline resistance levels, but the emergence of variants carrying the G36D mutation was associated with either high resistance (IC50 of >3,000 ng/ml; patient 4) or only moderate resistance (IC50 of 300 ng/ml; patient 5). Similarly, mutants carrying the V38A mutation were associated with a high level of ENF resistance in patients 1 and 3 but emerged only transiently in patient 6 and were associated with low ENF resistance. Previous studies have also reported data showing that the impact of HR1 mutations on the level of ENF resistance is context dependent (39, 46), but the mechanism explaining this phenomena remains to be identified. It is noteworthy, however, that in cases where ENF resistance mutations produced only suboptimal resistance, Env replicative capacity was usually maintained, suggesting that the impact of resistance mutations on viral resistance and viral fitness was not tightly linked (see below). The identification of viral sequences that determine whether a given context is favorable or unfavorable for the expression of high resistance by ENF resistance mutations may prove to be a difficult task, given that multiple discontinuous envelope domains participate in the entry process and can modulate phenotypic resistance to ENF (8, 15, 34).
Envelope context and Env replicative capacity.
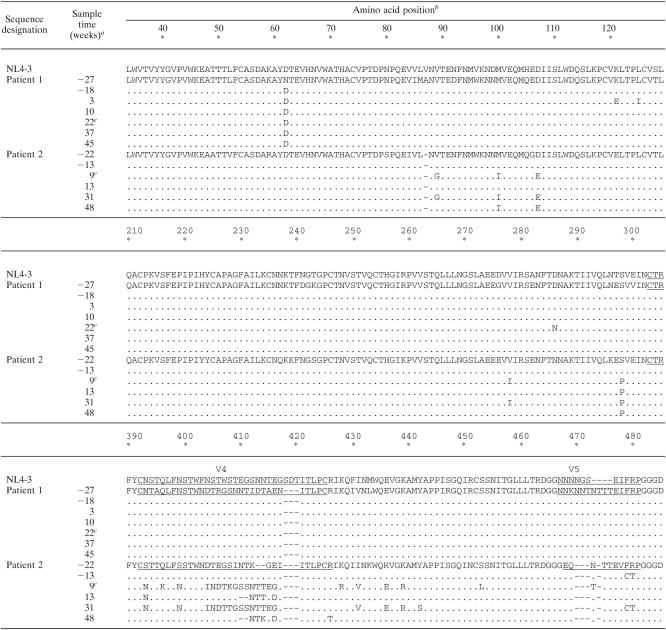
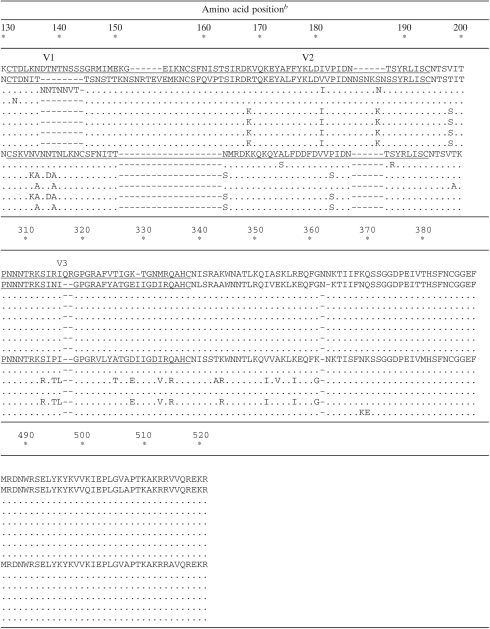
An important observation in our study was the finding that no significant reduction of Env replicative capacity was observed for resistant viruses that emerged in five of the six patients studied here, and indeed for two of these patients Env replicative capacity of resistant viruses was actually higher than that of viruses present prior to ENF treatment. This finding is in apparent contrast with conclusions from two previous studies, which reported that the introduction of ENF resistance mutations impairs Env replicative capacity. This discrepancy may be more apparent than real. Prior studies have examined the impact of ENF resistance mutations introduced by site-directed mutagenesis or in recombinant viruses in which only a portion of the ectodomain of gp41 was patient derived (25, 26). In our study, Env replicative capacity was evaluated for recombinant viruses in which the entire gp120 and the extracellular domain of gp41 were patient derived, and resistance mutations emerged in vivo, allowing the selection of the most favorable combination of gp120 and gp41. Numerous studies have demonstrated that viral entry requires cooperative interactions between gp120 and gp41. Thus, these observations are consistent with the possibility that the impact of modifications in the sequence of HR1 is modulated by the sequence of the accompanying gp120 and imply that the context in which ENF resistance mutations occur can affect not only the level of resistance obtained but also viral replicative capacity. Accordingly, longitudinal analysis of env sequences from patients 1 and 2 (Tables 1 and 2) showed that ENF treatment resulted in the replacement of both gp41 and gp120 dominant sequences in the plasma virus population. In particular, for patient 1, the initial emergence of G36V in gp41 at week 3 was accompanied by the selection of a gp120 sequence characterized by a shorter V1 domain compared to baseline sequence (week −18), among other changes. Interestingly the same deletion in V1 was present in the plasma population at week −27, suggesting that under ENF selective pressure, resistance mutations emerged in a preexisting minority env context. This shorter V1 domain was present in the dominant gp120 sequence at all subsequent time points, including those after ENF withdrawal. The replacement of the dominant gp120 sequence may help explaining the residual ENF resistance observed at the latest time points after ENF interruption and reversion of HR1 resistance mutations. Similarly, for patient 2, a novel gp120 (and gp41) sequence characterized the first time point during ENF treatment. This sequence was present also after ENF interruption (week 31). In this case, the loss of HR1 mutations in the new env context may be responsible for the higher replicative capacity, compared to baseline, of post-ENF virus populations.
It may seem counter-intuitive that the in vitro Env replicative capacity of resistant viruses could be higher than that of the dominant virus population at baseline. It is important to emphasize, however, that in vivo the replicative capacity of viruses with different envelopes depends on numerous factors, including the affinity of the envelope glycoproteins for receptors, the efficiency of fusion, their tendency to induce cellular cytotoxicity, and their sensitivity to neutralizing antibodies. Current evidence suggests that improvements in one of these parameters may have a negative impact on others. For example, laboratory-adapted HIV strains generally have a higher Env replicative capacity measured in vitro than primary viral isolates but show increased sensitivity to neutralizing antibodies (18, 33). In this context, the selection of Env complexes with more efficient entry process may participate to ENF resistance, either by reducing the time during which the ENF target sequence is exposed or by reducing the number of interactions between the viral and cellular proteins required to allow membrane fusion. Despite the good or even superior Env replicative capacity of resistant viruses, our findings indicate that the in vivo “fitness” of these viruses in the absence of drug was reduced compared to that of pretreatment viruses, because discontinuation of ENF led to the disappearance of ENF resistance mutations; in cases where resistant viruses were shown to belong to genetically distinct viral populations, these populations were progressively replaced by viruses more closely related to those present prior to therapy. Thus, maintenance or improvement of Env replicative capacity in the resistant viruses must have been associated with concessions in other parameters affecting overall fitness, but further studies are required to identify their nature. For example, the evaluation of the sensitivity of ENF-resistant viruses to neutralization by autologous antibodies would be of interest.
Overall our study shows that evolution of strong viral resistance to ENF appears to be constrained by the context in which HR1 resistance mutations appear for two reasons. First, the extent that mutations promote ENF resistance is variable in different contexts. Second, the Env replicative capacity of resistant viruses that emerge in vivo is usually as good as or even better than that of viruses present prior to treatment. Our findings suggest that the dominant envelope genotype at baseline, regardless of its innate sensitivity to ENF, may generally not provide an optimal context for the expression of resistance mutations. In this case, resistance appears in an apparently novel genetic context, likely to be present at baseline as part of minority viral populations.
TABLE 1.
Continued
TABLE 2.
Sequence alignments of gp120 (amino acids 34 to 521) of plasma virus populations derived from patients 1 and 2 over time
The sample times analyzed for each patient are indicated in reference to the beginning of the treatment including ENF, at week 0.
NL4-3 numbering. The five hypervariable regions of gp120 (V1 to V5) are underlined. Dashes correspond to amino acid insertion or deletion.
ENF treatment was stopped at weeks 25 and 12 for patients 1 and 2, respectively.
TABLE 2.
Continued
Acknowledgments
This work was supported in part by the French ANRS. A.G. is a fellow of the ANRS.
REFERENCES
- 1.Baldwin, C. E., R. W. Sanders, and B. Berkhout. 2003. Inhibiting HIV-1 entry with fusion inhibitors. Curr. Med. Chem. 10:1633-1642. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, C. E., R. W. Sanders, Y. Deng, S. Jurriaans, J. M. Lange, M. Lu, and B. Berkhout. 2004. Emergence of a drug-dependent human immunodeficiency virus type 1 variant during therapy with the T20 fusion inhibitor. J. Virol. 78:12428-12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1993. From the Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 269:729-730. [PubMed] [Google Scholar]
- 4.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. H., T. J. Matthews, C. B. McDanal, D. P. Bolognesi, and M. L. Greenberg. 1995. A molecular clasp in the human immunodeficiency virus (HIV) type 1 TM protein determines the anti-HIV activity of gp41 derivatives: implication for viral fusion. J. Virol. 69:3771-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou, T.-C. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-102. In T.-C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, San Diego, Calif.
- 7.Clavel, F., and A. J. Hance. 2004. HIV drug resistance. N. Engl. J. Med. 350:1023-1035. [DOI] [PubMed] [Google Scholar]
- 8.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, X. Wu, W. A. O'Brien, L. Ratner, J. C. Kappes, G. M. Shaw, and E. Hunter. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, Z. Zhang, W. A. O'Brien, L. Ratner, G. M. Shaw, and E. Hunter. 2001. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. J. Virol. 75:8605-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, X. N., Y. Xiao, M. P. Dierich, and Y. H. Chen. 2001. N and C domains of HIV-1 gp41: mutation, structure and functions. Immunol. Lett. 75:215-220. [DOI] [PubMed] [Google Scholar]
- 11.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 12.Edwards, T. G., S. Wyss, J. D. Reeves, S. Zolla-Pazner, J. A. Hoxie, R. W. Doms, and F. Baribaud. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J. Virol. 76:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabuzda, D. H., A. Lever, E. Terwilliger, and J. Sodroski. 1992. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 66:3306-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo, S. A., C. M. Finnegan, M. Viard, Y. Raviv, A. Dimitrov, S. S. Rawat, A. Puri, S. Durell, and R. Blumenthal. 2003. The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 1614:36-50. [DOI] [PubMed] [Google Scholar]
- 15.Heil, M. L., J. M. Decker, J. N. Sfakianos, G. M. Shaw, E. Hunter, and C. A. Derdeyn. 2004. Determinants of human immunodeficiency virus type 1 baseline susceptibility to the fusion inhibitors enfuvirtide and T-649 reside outside the peptide interaction site. J. Virol. 78:7582-7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishino, H., and M. Hasegawa. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J. Mol. Evol. 29:170-179. [DOI] [PubMed] [Google Scholar]
- 17.Kliger, Y., S. A. Gallo, S. G. Peisajovich, I. Munoz-Barroso, S. Avkin, R. Blumenthal, and Y. Shai. 2001. Mode of action of an antiviral peptide from HIV-1. Inhibition at a post-lipid mixing stage. J. Biol. Chem. 276:1391-1397. [DOI] [PubMed] [Google Scholar]
- 18.Labrijn, A. F., and W. H. I. Parren. 1999. Neutralizing epitopes of HIV-1, p. 18-34. In C. Kuiken, B. Foley, B. H. Hahn, P. Marx, F. E. McCutchan, J. Mellors, et al. (ed.), HIV sequence compendium 1999. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N. Mex.
- 19.Labrosse, B., A. Brelot, N. Heveker, N. Sol, D. Schols, E. De Clercq, and M. Alizon. 1998. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J. Virol. 72:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrosse, B., J. L. Labernardiere, E. Dam, V. Trouplin, K. Skrabal, F. Clavel, and F. Mammano. 2003. Baseline susceptibility of primary human immunodeficiency virus type 1 to entry inhibitors. J. Virol. 77:1610-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalezari, J. P., E. DeJesus, D. W. Northfelt, G. Richmond, P. Wolfe, R. Haubrich, D. Henry, W. Powderly, S. Becker, M. Thompson, F. Valentine, D. Wright, M. Carlson, S. Riddler, F. F. Haas, R. DeMasi, P. R. Sista, M. Salgo, and J. Delehanty. 2003. A controlled phase II trial assessing three doses of enfuvirtide (T-20) in combination with abacavir, amprenavir, ritonavir and efavirenz in non-nucleoside reverse transcriptase inhibitor-naive HIV-infected adults. Antivir. Ther. 8:279-287. [PubMed] [Google Scholar]
- 22.Lalezari, J. P., J. J. Eron, M. Carlson, C. Cohen, E. DeJesus, R. C. Arduino, J. E. Gallant, P. Volberding, R. L. Murphy, F. Valentine, E. L. Nelson, P. R. Sista, A. Dusek, and J. M. Kilby. 2003. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. Aids 17:691-698. [DOI] [PubMed] [Google Scholar]
- 23.Lalezari, J. P., K. Henry, M. O'Hearn, J. S. Montaner, P. J. Piliero, B. Trottier, S. Walmsley, C. Cohen, D. R. Kuritzkes, J. J. Eron, Jr., J. Chung, R. DeMasi, L. Donatacci, C. Drobnes, J. Delehanty, and M. Salgo. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 24.Lawless, M. K., S. Barney, K. I. Guthrie, T. B. Bucy, S. R. Petteway, Jr., and G. Merutka. 1996. HIV-1 membrane fusion mechanism: structural studies of the interactions between biologically active peptides from gp41. Biochemistry 35:13697-13708. [DOI] [PubMed] [Google Scholar]
- 25.Lu, J., P. Sista, F. Giguel, M. Greenberg, and D. R. Kuritzkes. 2004. Relative replicative fitness of human immunodeficiency virus type 1 mutants resistant to enfuvirtide (T-20). J. Virol. 78:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzo, S., A. Castagna, A. Monachetti, H. Hasson, A. Danise, E. Carini, P. Bagnarelli, A. Lazzarin, and M. Clementi. 2004. Genotype and phenotype patterns of human immunodeficiency virus type 1 resistance to enfuvirtide during long-term treatment. Antimicrob. Agents Chemother. 48:3253-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mink, M., M. Greenberg, L. Mosier, S. Janumpalli, D. Davison, L. Jin, T. Melby, P. Sista, D. Lambert, N. Cammack, M. Salgo, and T. Matthews. 2002. Impact of HIV-1 gp41 amino acid substitutions (positions 36-45) on susceptibility to T-20 (enfuvirtide) in vitro: analysis of primary virus isolates recovered from patients during chronic enfuvirtide treatment and site-directed mutants in NL4-3, abstr. 22. Antivir. Ther. 7(Suppl. 1):S24. [Google Scholar]
- 28.Moore, J. P., and R. W. Doms. 2003. The entry of entry inhibitors: a fusion of science and medicine. Proc. Natl. Acad. Sci. USA 100:10598-10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 30.Platt, E. J., J. P. Durnin, and D. Kabat. 2005. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J. Virol. 79:4347-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pleskoff, O., N. Sol, B. Labrosse, and M. Alizon. 1997. Human immunodeficiency virus strains differ in their ability to infect CD4+ cells expressing the rat homolog of CXCR-4 (fusin). J. Virol. 71:3259-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poveda, E., B. Rodes, J. L. Labernardiere, J. M. Benito, C. Toro, J. Gonzalez-Lahoz, J. L. Faudon, F. Clavel, J. Schapiro, and V. Soriano. 2004. Evolution of genotypic and phenotypic resistance to enfuvirtide in HIV-infected patients experiencing prolonged virologic failure. J. Med. Virol. 74:21-28. [DOI] [PubMed] [Google Scholar]
- 33.Quinones-Mateu, M. E., and E. J. Arts. 2001. HIV-1 fitness: implications for drug resistance, disease progression, and global epidemic evolution, p. 134-170. In C. Kuiken, B. Foley, B. H. Hahn, P. Marx, F. E. McCutchan, J. Mellors, et al. (ed.), HIV sequence compendium 2001. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N. Mex.
- 34.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimsky, L. T., D. C. Shugars, and T. J. Matthews. 1998. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 37.Sanders, R. W., B. Korber, M. Lu, B. Berkhout, and J. P. Moore. 2002. Mutational analyses and natural variability of the gp41 ectodomain, p. 43-68. In C. Kuiken, B. Foley, B. H. Hahn, P. Marx, F. E. McCutchan, J. Mellors, et al. (ed.) HIV sequence compendium 2002. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N. Mex.
- 38.Sista, P., T. Melby, M. L. Greenberg, R. DeMasi, D. Kuritzkes, M. Nelson, C. Petropoulos, M. Salgo, N. Cammack, and T. J. Matthews. 2003. Subgroup analysis of baseline susceptibility and early virological response to enfuvirtide in the combined TORO studies. Antivir. Ther. 8:S60. [Google Scholar]
- 39.Sista, P. R., T. Melby, D. Davison, L. Jin, S. Mosier, M. Mink, E. L. Nelson, R. DeMasi, N. Cammack, M. P. Salgo, T. J. Matthews, and M. L. Greenberg. 2004. Characterization of determinants of genotypic and phenotypic resistance to enfuvirtide in baseline and on-treatment HIV-1 isolates. AIDS 18:1787-1794. [DOI] [PubMed] [Google Scholar]
- 40.Tan, K., J. Liu, J. Wang, S. Shen, and M. Lu. 1997. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. USA 94:12303-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 44.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 46.Xu, L., A. Pozniak, A. Wildfire, S. A. Stanfield-Oakley, S. M. Mosier, D. Ratcliffe, J. Workman, A. Joall, R. Myers, E. Smit, P. A. Cane, M. L. Greenberg, and D. Pillay. 2005. Emergence and evolution of enfuvirtide resistance following long-term therapy involves heptad repeat 2 mutations within gp41. Antimicrob. Agents Chemother. 49:1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]