Abstract
Antimicrobial peptides are found in a number of body compartments and are secreted at mucosal surfaces, where they form part of the innate immune system. Many of these small peptides have a broad spectrum of inhibitory activity against bacteria, fungi, parasites, and viruses. Generally, the peptide's mode of action is binding and disruption of membranes due to its amphipathic properties. Histatin 5 is a salivary peptide that inhibits Candida albicans, an opportunistic fungus that causes oropharyngeal candidiasis in a majority of human immunodeficiency virus type 1 (HIV-1)-infected patients progressing towards AIDS. Previously, we increased the fungicidal properties of histatin 5 by replacing amino acids in the active domain of histatin 5 (Dh-5) (A. L. Ruissen, J. Groenink, E. J. Helmerhorst, E. Walgreen-Weterings, W. van’t Hof, E. C. Veerman, and A. V. Nieuw Amerongen, Biochem. J. 356:361-368, 2001). In the current study, we tested the anti-HIV-1 activity of Dh-5 and its derivatives. Although Dh-5 inhibited HIV-1 replication, none of the peptide variants were more effective in this respect. In contrast, one of the derivatives, Dhvar2, significantly increased HIV-1 replication by promoting the envelope-mediated cell entry process. Most likely, Dhvar2 affects membranes, thereby facilitating fusion of viral and cellular membranes. This study shows that modification of antimicrobial peptides in order to improve their activity against a pathogen may have unpredictable and unwanted side effects on other pathogens.
Antimicrobial peptides have been identified in all species investigated, where they form part of the innate and possibly adaptive immune system. Many of these peptides have a broad spectrum of activity against bacteria, fungi, parasites, and viruses. Several different types of antimicrobial peptides have been found in humans (37, 42). Although very heterogeneous in amino acid sequence, they share some characteristics, like a low molecular mass (1 to 5 kDa), a positively charged domain of 10 to 25 amino acids, and the tendency to form amphipathic structures (28, 37). Generally, the peptide's mode of action is binding and disruption of membranes, but some studies indicate that these peptides also act intracellularly (12, 13, 31).
Three modes of action of antimicrobial peptides have been described with respect to viruses and human immunodeficiency virus type 1 (HIV-1) in particular: direct virolysis, inhibition of transcription from the long terminal repeat (LTR) promoter, and block of cell entry by binding to cell surface receptors (5, 23, 39, 43). In this study, we investigated the anti-HIV-1 activity of derivatives of histatin 5, an antimicrobial peptide present in human saliva. Histatins form a group of electrophoretically distinct histidine-rich polypeptides with microbicidal activity that are present in human parotid and submandibular gland secretions (35). Histatin 5 is active against the yeast Candida albicans, an opportunistic fungus that causes oropharyngeal candidiasis in a majority of HIV-1-infected patients (6). Internalization of histatin 5 and subsequent targeting of the mitochondria leads to release of vital components and cell death of the yeast (7). To increase the activity of this peptide, amino acids in the active domain (Dh-5) of histatin 5 have been substituted. The resulting peptides (Dhvar2 to Dhvar5; Table 1) have previously been shown to possess increased fungicidal activity, and all but one (Dhvar5) are more amphipathic than Dh-5 (14, 25). In the present study, we tested the anti-HIV-1 activity of Dh-5 and its derivatives. Dh-5 inhibited HIV-1 replication, but none of the derivatives was a more efficient inhibitor. Surprisingly, one of the peptides (Dhvar2) dramatically increased HIV-1 replication by promoting the envelope (Env)-mediated entry process. Dhvar2 probably destabilizes membranes and thereby facilitates fusion of viral and cellular membranes. This study shows that the development of more potent antimicrobial agents against certain pathogens like Candida may have unexpected and dangerous side effects if they are used in the context of other pathogens like HIV-1.
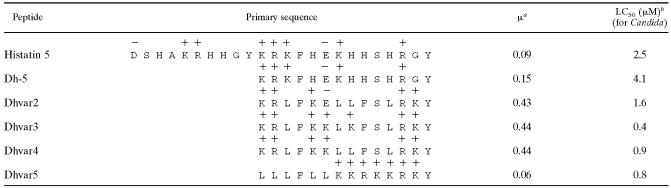
TABLE 1.
Amino acid sequencesc
Calculated mean hydrophobic moment, which is a measure for amphipathicity, of the peptide in α-helical onformation (14).
The active domain (Dh-5) of salivary peptide histatin 5 was modified in order to increase the antifungal activity. Charges at neutral pH are depicted above the amino acids.
MATERIALS AND METHODS
Peptide synthesis and purification.
The active domain of histatin 5 (Dh-5, KRKFHEKHHSHRGY) and variants (Dhvar2, KRLFKELLFSLRKY; Dhvar3, KRLFKKLKFSLRKY; Dhvar4, KRLFKKLLFSLRKY; Dhvar5, LLLFLLKKRKKRKY) were synthesized by F-moc chemistry with a MilliGen 9050 peptide synthesizer (MilliGen/Biosearch, Bedford, MA) and purified to at least 95% by reverse-phase high-performance liquid chromatography (RP-HPLC; Jasco Corporation, Tokyo, Japan). The authenticity of the peptides was confirmed by ion trap mass spectrometry with an LCQ Deca XP (Thermo Finnigan, San Jose, CA). During synthesis, Dh-5 and Dhvar2 were labeled with fluorescein isothiocyanate (FITC) for FACScan analysis as described previously (7). Labeling was achieved by addition of 25 μl FITC (1 mg/ml) in dimethyl sulfoxide to 1 ml of a peptide solution (0.66 mM) in water, adjusted to pH 9.7 with Na2CO3. After overnight incubation at 4°C in the dark, residual FITC was inactivated by incubation with 50 μl of 1 M NH4Cl for 2 h. The fluorescein-peptide conjugates were stored in aliquots at −20°C until use. LuSIV or SupT1 T cells were incubated for 30 min at 4°C with labeled peptides at a final concentration of 40 μM. Cells were then analyzed by a fluorescent-activated cell sorter (FACS) (FACScan; BD Biosciences, San Jose, CA).
Cells, viruses, and lentiviral vectors.
The PM1 and SupT1 T-cell lines were cultured in RPMI medium 1640 (Life Technologies) supplemented with 10% fetal calf serum (FCS), 2 mM sodium pyruvate, 10 mM HEPES, 2 mM l-glutamine, penicillin (100 U/ml) (Sigma-Aldrich, St. Louis, MO), and streptomycin (100 μg/ml) (Invitrogen, Breda, The Netherlands). The LuSIV cell with an integrated LTR-luciferase reporter construct has been described previously (24). Cells were maintained in the same medium as the PM1 and SupT1 cells but with 300 μg/ml hygromycin B to maintain the luciferase genetic construct. Human embryonic kidney (HEK) 293T adherent cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% FCS, 2 mM sodium pyruvate, 10 mM HEPES, 2 mM l-glutamine, penicillin, and streptomycin. HIV-1 was produced by electroporation of PM1 T cells with 5 μg of the molecular clone of T-tropic HIV-1 LAI. The virus-containing supernatant was harvested 5 days posttransfection and was filtered and stored at −80°C. The concentration of virus was determined by CA-p24 enzyme-linked immunosorbent assay (ELISA). The full-length simian immunodeficiency virus (SIV)-mac239 clone (11) was kindly provided by Y. Guan and M. A. Wainberg (McGill University AIDS Centre, Montreal, Canada). The virus was produced by electroporation of CEMx174 cells (26). Lentiviral vectors with CXCR4-using Env of the HIV-1 HXB2 strain or vesicular stomatitis virus glycoprotein (VSV-G) were produced as follows. 293T cells (2.2 × 106) were seeded in a T25 flask the day prior to transfection. The next day, medium was replaced with 2.2 ml medium without antibiotics. Subsequently, 16 μl Lipofectamine 2000 and 1.5 ml Optimem (Invitrogen) was used to transfect lentiviral vector plasmid pRRLcpptpgkgfppreSsin (29) (2.4 μg) with packaging plasmids pSYNGP (1.5 μg) (a kind gift of S. Kingsman; Oxford Biomedia, Oxford, United Kingdom) (16), RSV-rev (0.6 μg), and pVSV-G (0.8 μg) (8, 44) or pSV7D plasmid encoding HXB2 gp160 (0.8 μg). The pSV7D gp160 plasmid was a kind gift of J. Binley (Torrey Pines Institute for Molecular Sciences, La Jolla, CA). Medium was replaced the next day with 4 ml of fresh medium. On day 3, medium was harvested in the morning and replaced with fresh medium. This procedure was repeated in the evening and the next day. Cellular debris from the lentiviral vector-containing medium was removed by low-speed centrifugation, and supernatant was stored at 4°C. On the fourth day, supernatants were pooled and concentrated using an Amicon Ultra concentrator (molecular weight cutoff, 100 kDa; Millipore Corporation, Bedford, MA).
Replication assays.
Single-cycle replication was assayed with LuSIV cells: 4 ng CA-p24 HIV-1 was preincubated at 0, 3.1, 12.5, 50, or 200 μM Dh-5 or Dhvar2 to Dhvar5 in a final volume of 50 μl at 37°C. After 30 min, 50 × 103 LuSIV cells were added (200 μl), and luciferase production was determined 24 h later, as previously described (10). The final concentration of the peptides was 0.6, 2.5, 10, or 40 μM, respectively. Fusion inhibitor T20 was added at different time points before or after infection, as indicated in the text (end concentration, 1, 10, 100, 500, or 1,000 ng/ml). Preincubation of different mixtures of HIV-1, LuSIV, and Dhvar2 was performed as follows. Equal volumes (80 μl) of HIV-1, LuSIV cells, and Dhvar2 were preincubated in pairs at 37°C. All components were mixed after 30 min and cocultured for 24 h, followed by luciferase measurement. Single-cycle replication assays with lentiviral vectors were performed with concentrated supernatant containing the lentiviral vector with HIV-1 Env or VSV-G, which was preincubated with 200 μM Dh-5, Dhvar2, or Dhvar4 in 250 μl. After 30 min, 500 × 103 T cells were added (LuSIV, PM1, or SupT1), followed by coculture for 3 days in a final volume of 1 ml (50 μM final peptide concentration). At day 3, cells were harvested, fixated in 4% paraformaldehyde, and analyzed by FACS for the expression of green fluorescent protein (GFP) (FACScan; BD Biosciences). Replication assay with PM1 cells was initiated with 0.1 or 1 ng CA-p24 HIV-1, which was preincubated with 200 μM Dhvar2 or mock treated for 30 min at 37°C, followed by coculture with 80 × 103 PM1 cells in a final volume of 250 μl (final concentration of Dhvar2, 40 μM). Viral replication was followed by CA-p24 ELISA of the supernatant.
gp120 ELISA.
ELISAs were performed as described previously (21, 22). LAI gp120 (100 ng/ml) was captured onto the solid phase using antibody D7324 to the C5 region (Aalto Bio Reagents, Dublin, Ireland). Bound gp120 was detected with purified immunoglobulin (Ig) from pooled serum of HIV-1-infected individuals (HIVIg), CD4-IgG2 (1), or one of the monoclonal antibodies 17b (with our without soluble CD4 [sCD4] [17]), 2G12 (27, 34), and IgG1b12 (4). Competition experiments were performed at half-maximal binding concentrations of the respective reagents. These were 3 (HIVIg), 0.15 (CD4-IgG2), 0.02 (17b plus sCD4), 0.1 (17b alone), 0.2 (2G12), and 0.02 (IgGb12) μg/ml. LAI gp120, CD4-IgG2, and sCD4 were kindly donated by M. Franti and W. Olson (Progenics Pharmaceuticals, Inc., Tarrytown, NY). 17b and HIVIg were a kind gift of J. Binley (Torrey Pines Institute for Molecular Sciences, La Jolla, CA), and IgG1b12 was provided by D. R. Burton (The Scripps Research Institute, La Jolla, CA). 2G12 from H. Katinger was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program.
Peptide depletion assay.
PM1 T cells were infected with HIV-1, cells were spun down at peak infection (1020 ng CA-p24/ml), and virus-containing supernatant was used to infect fresh PM1 cells. Supernatant with virus (990 ng CA-p24/ml) was harvested 40 h later and spun down in the ultracentrifuge for 2 h at 33,000 × g. Virus was resuspended in phosphate-buffered saline (PBS) (final concentration, 950 ng CA-p24/ml) and incubated with Dh-5 or Dhvar2 (40 μM) for 30 min at 37°C. Alternatively, 2.5 × 106 LuSIV, PM1, or SupT1 cells were incubated in 1 ml PBS with Dh-5 or Dhvar2. Cells and virus were subsequently removed by centrifugation (cells, 400 × g; virus, 33,000 × g), and the remaining concentration of peptide in the supernatant was determined by capillary zone electrophoresis (CZE) and RP-HPLC. CZE separations were conducted on a Biofocus 2000 instrument (Bio-Rad, Hercules, CA) equipped with an uncoated fused silica capillary of 24 cm and 50 μm internal diameter (ID), essentially according to the instructions of the manufacturer. Briefly, the capillary was rinsed for 60 s with the low-pH Bio-Rad CZE phosphate buffer (0.1 M phosphate, supplemented with polymer modifier). Appropriate dilutions of the supernatants were mixed with 10-times-diluted Bio-Rad CZE phosphate buffer, containing 40 μM imidazole as an internal standard. Samples were injected by pressure injection at 20 lb/in2/s. Separation was performed at a voltage of 15 kV (cathode at the detector site) and a temperature of 20°C. On-line UV detection of the samples was accomplished at 200 nm. Run time of the separation was 10 min. RP-HPLC separations were performed by HPLC (Jasco, Tokyo, Japan) on a reversed-phase C18 column (Vydac 218TP54; 24 cm and 4.6 mm ID; 5 μm particle size) developed with a linear gradient of acetonitrile containing 0.1% trifluoroacetic acid. On-line UV detection of the samples was accomplished at 214 nm. Run time of the separation was 20 min. Relative peptide concentrations in the samples were determined based on the relevant peak areas.
RESULTS
Dh-5 inhibits, but derivatives enhance, HIV-1 replication.
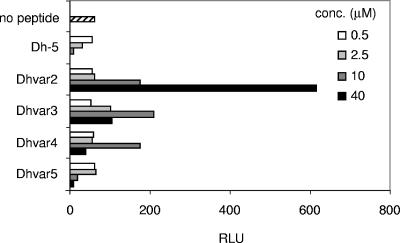
Amino acids in the active domain (Dh-5) of salivary peptide histatin 5 have previously been replaced to increase the fungicidal activity of this peptide against Candida albicans (Table 1). Dhvar2 to Dhvar4 are more amphipathic, while Dhvar5 is less amphipathic than Dh-5 (14, 25). Because Candida is associated with immune-compromised patients like HIV-1-infected persons progressing towards AIDS (6), we wanted to explore the antiviral properties of Dh-5. We furthermore investigated whether the variant peptides displayed enhanced antiviral activity, which was expected due to their increased antifungal properties. We incubated HIV-1 with these peptides and tested viral infectivity in a single-cycle replication assay with LuSIV reporter cells. These reporter cells contain the firefly luciferase gene downstream of the LTR promoter, resulting in Tat-mediated luciferase expression, which is a measure of infectivity (24). Dh-5 was able to reduce the infectivity of HIV-1, but modification of Dh-5 did not enhance the anti-HIV-1 properties of this peptide: none of the peptide variants decreased luciferase production more than Dh-5 (Fig. 1). Surprisingly, the Dhvar2, Dhvar3, and Dhvar4 peptides with increased amphipathicity stimulated HIV-1 infection. In the absence of HIV-1, none of these peptides influenced the background luciferase levels of the LuSIV cells, excluding that the peptides trigger signaling pathways in the cell that lead to LTR activation and luciferase production (data not shown). Dhvar2 showed the highest enhancing effect by increasing luciferase production 10-fold, on average.
FIG. 1.
Modified antimicrobial peptides with improved antifungal properties enhance HIV-1 infectivity. The active domain (Dh-5) of salivary peptide histatin 5 was modified in order to increase the antifungal activity. Dh-5 and derivatives were tested for their anti-HIV-1 activity in a single-cycle replication assay. HIV-1 was incubated with the peptides and subsequently cocultured with LuSIV cells. These reporter cells express luciferase after HIV-1 infection, which was detected 24 h later. RLU, relative light units; conc., concentration.
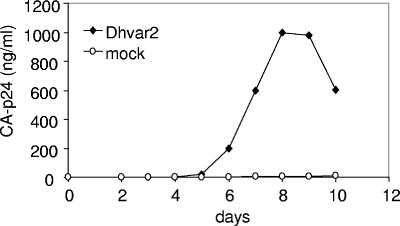
Dhvar2 also stimulated HIV-1 replication in T cells over a prolonged period of time. PM1 T cells were infected with Dhvar2-treated or mock-incubated HIV-1, and viral replication was followed by CA-p24 ELISA on the supernatant. The results are very obvious when a noninfectious dose of HIV-1 was used, in which case Dhvar2 rescued HIV-1 replication (Fig. 2). When a high viral input was used, we found no stimulation by Dhvar2 in comparison to the mock-treated sample (results not shown). Cell toxicity was observed at higher concentrations of Dhvar2 (>60 μM), which obviously decreased viral replication (data not shown).
FIG. 2.
Dhvar2 can rescue HIV-1 replication at low viral inputs. A noninfectious HIV-1 dose of 0.4 ng CA-p24/ml was mock treated or incubated with Dhvar2, followed by coculture with PM1 T cells. Viral replication was subsequently followed by measuring CA-p24 production in the supernatant by ELISA.
Dhvar2 binds to cells.
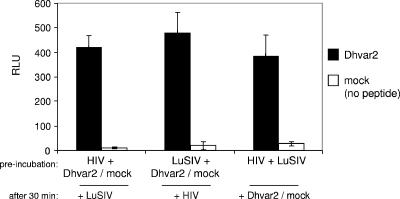
To elucidate the mechanism by which Dhvar2 enhances HIV-1 infection, we preincubated the peptide (or medium as a mock treatment) either with HIV-1 or the LuSIV cells for 30 min at 37°C. Both treatments yielded the approximately 10-fold enhancement (Fig. 3). Preincubation of virus and cells prior to addition of Dhvar2 yielded the same level of stimulation. This experiment therefore does not identify the target for Dhvar2 stimulation, but it does show that the peptide has a broad time span in which it can stimulate HIV-1 infection.
FIG. 3.
Dhvar2 preincubation experiments. (Black bars) HIV-1, LuSIV, and Dhvar2 were preincubated in different pairs, as indicated on the x axis. After 30 min, all were incubated together for an additional 24 h. (White bars) The same experiment was also performed with medium instead of Dhvar2 (mock treatment). Luciferase was measured after 24 h. Error bars represent standard deviations. RLU, relative light units.
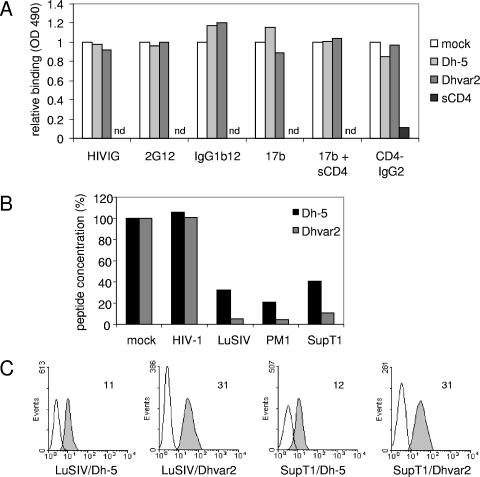
We next investigated whether Dhvar2 (or Dh-5 as a control) affects the binding of a broad range of reagents to the HIV-1 envelope (Env) gp120 protein in a gp120 ELISA (Fig. 4A). We focused on reagents that bind to the CD4 and coreceptor binding sites, since binding to these sites might provide a plausible mechanism for stimulation of infectivity by Dhvar2. Purified Ig from HIV-1-infected individuals (HIVIg) was used as well as several monoclonal antibodies. 2G12 binds to a glycan-dependent epitope on the outer domain of gp120 (27), IgGb12 targets the CD4 binding site (4), and 17b is directed to a CD4-induced site overlapping the coreceptor binding site (17). 17b was therefore tested with and without soluble CD4 (sCD4). Furthermore, we tested CD4-IgG2, which is a tetrameric CD4-based molecule that is directed to the CD4 binding site (1). Increasing amounts of peptide were allowed to compete with these respective reagents for binding to gp120, which was captured on an ELISA plate. The reagents were used at the predetermined half-maximal binding concentrations. CD4-IgG2 binding was also performed in the presence of sCD4 to provide a control for competition efficiency. Although we observed efficient inhibition of CD4-IgG2 binding to gp120 by sCD4, we did not observe an effect of Dh-5 or Dhvar2 on binding of the reagents to gp120 (Fig. 4A). We tested a wide range of concentrations of the peptides (0, 3.7, 11.1, 33.3, and 100 μM), and 100 μM is twice the concentration that stimulates virus infectivity. Even this amount of peptide did not influence binding of the various reagents to gp120, indicating that the peptide's mode of action does not involve binding to the CD4 or coreceptor binding sites on gp120.
FIG. 4.
Dhvar2 binds T cells. (A) gp120 ELISA. Increasing amounts of peptide (Dh-5 or Dhvar2) were allowed to compete with several reagents for binding to captured gp120. The reagents (HIVIg, 2G12, IgGb12, 17b [with or without sCD4], and CD4-IgG2) target the CD4 or coreceptor binding sites on gp120 and were used at their predetermined half-maximal binding capacity. Competition of sCD4 with CD4-IgG2 was performed in parallel to provide a control for competition efficiency. Results obtained with the highest concentration (100 μM) of peptide are depicted. nd, not done. (B) Peptide depletion assay. HIV-1 or cells (LuSIV, PM1, SupT1) were added to a stock of Dh-5 or Dhvar2 in PBS. After incubation for 1 h, virus and cells were spun down, and the remaining concentration of peptide was determined. (C) FACS analysis. Dh-5 and Dhvar2 were labeled with FITC. After incubation with LuSIV or SupT1 T cells and subsequent washing, cells were analyzed by FACS. The mean fluorescence intensity is indicated. OD, optical density.
To further identify the target of Dhvar2, we tested whether Dhvar2 or Dh-5 could be depleted from a PBS solution by virus or T cells. High concentrations of virus (950 ng CA-p24/ml) did not deplete the peptides from the solution by virus centrifugation. In contrast, T cells (LuSIV, PM1, or SupT1) could deplete both peptides from the solution (Fig. 4B). The amount of Dh-5 that remained in solution was, on average, five times higher than that of Dhvar2, indicating that Dhvar2 binds more efficiently to T cells than Dh-5. This observation was confirmed with FITC-labeled peptides that can be detected by FACS analysis. The amount of FITC-labeled Dhvar2 that bound to LuSIV or SupT1 T cells was, on average, three times higher than the amount of Dh-5 (Fig. 4C). Combined, these experiments suggest that the target of Dhvar2 is the T cell rather than the virus.
Stimulation by Dhvar2 is dependent on HIV-1 Env.
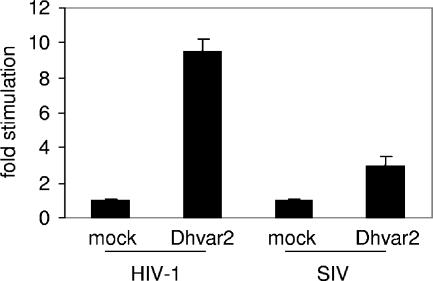
To investigate whether the stimulatory properties of Dhvar2 are also applicable to other viruses, we studied whether the peptide could enhance LuSIV infection by CCR5-using SIV-mac239. Although not as efficient as the stimulation observed with CXCR4-using HIV-1, Dhvar2 enhanced SIV infection by a factor of 3.5 (Fig. 5).
FIG. 5.
Dhvar2 enhances infectivity of both HIV-1 and SIV. LuSIV cells were infected with HIV-1 or SIV-mac239 in the presence of 40 μM Dhvar2. Luciferase was determined after 24 h. Error bars represent standard deviations.
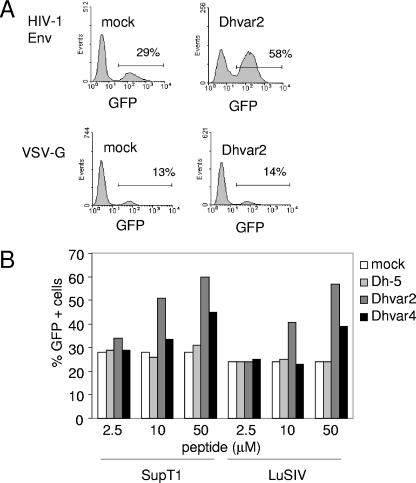
To study whether Dhvar2 stimulates viruses that use other entry mechanisms, we compared a lentiviral vector that is pseudotyped with either the vesicular stomatitis virus glycoprotein (VSV-G) or HIV-1 Env. We used a standard lentiviral vector with the green fluorescent protein (GFP) reporter gene (29). After entry of this pseudovirus into the cell and subsequent reverse transcription, GFP will be produced, which can be detected by FACS 3 days later. Dhvar2 could elevate the transduction efficiency of the HIV-1 Env-based vector twofold (Fig. 6A, upper row). In contrast, the percentage of GFP-positive SupT1 T cells was not elevated by Dhvar2 when the vector was bearing VSV-G (Fig. 6A, second row). This result clearly demonstrates that the enhancing effect of Dhvar2 is dependent on the HIV-1 Env protein and excludes that the peptide affects other steps of the retroviral replication cycle shared by both vectors, which includes reverse transcription, integration, transcription, and translation. The FACS results with SupT1 T cells are quantified in Fig. 6B. Similar results were obtained when the vector was used to infect LuSIV cells (Fig. 6B). As a control, we included Dh-5 and Dhvar4. No stimulation was observed with Dh-5, and a slight stimulation was observed with Dhvar4.
FIG. 6.
Stimulation by Dhvar2 is dependent on HIV-1 Env. (A) SupT1 T cells were infected with a lentiviral vector in the presence of Dhvar2. The vector with the GFP gene was pseudotyped with either HIV-1 Env or VSV-G. After 3 days, the percentage of GFP-positive cells was determined by FACS. Representative FACS plots are shown, with the percentage of GFP-positive cells indicated. (B) SupT1 or LuSIV cells were infected with the lentiviral vector bearing HIV-1 Env, which was mock treated or incubated with Dh-5, Dhvar2, or Dhvar4. Three days later, the percentage of GFP-positive cells was determined.
Dhvar2 promotes HIV-1 Env-mediated entry.
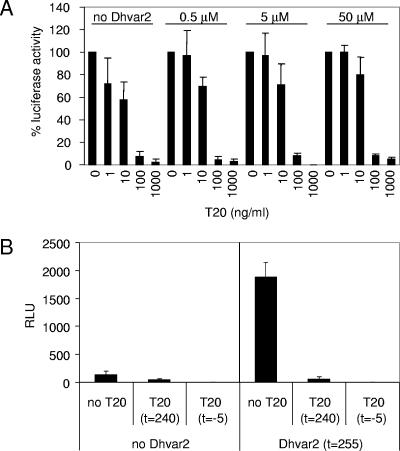
VSV and HIV-1 use different routes of cellular entry: VSV enters through endocytosis, whereas HIV-1 enters through receptor-mediated fusion of viral and cellular membranes (19, 20). The experiment using VSV-G and HIV-1 Env-pseudotyped lentiviral vectors indicates that the effect of Dhvar2 is HIV-1 Env dependent (Fig. 6). From these results, one cannot discriminate between effects on Env-mediated membrane fusion or postentry steps preceding reverse transcription (e.g., uncoating). To make this distinction, we employed the entry inhibitor T20. This peptide stabilizes a transient structural Env intermediate, thereby blocking subsequent conformational changes and the fusion of viral and cellular membranes (2, 9). We first investigated whether Dhvar2 interferes with the inhibitory properties of T20 by titrating both peptides on LuSIV cells that were subsequently infected with HIV-1. Addition of Dhvar2 did not alter the inhibition profile of T20 (Fig. 7A). This result demonstrates that T20 inhibition is dominant over Dhvar2 stimulation, justifying further usage of T20 in these assays. We next infected LuSIV cells with HIV-1 and added T20 after 240 min to stop the ongoing infection process. Compared to the control infection, T20 partially reduced the luciferase production, demonstrating that a substantial amount of virus, but not all of it, had already entered the cells within 240 min (Fig. 7B, left panel). As a control, T20 was added 5 min before HIV-1 infection (t = −5 min), which totally abolished luciferase production. When Dhvar2 was added at t = 255 min, this expectedly led to a considerable increase in luciferase production. However, this stimulation was not observed when T20 was supplied 15 min prior to Dhvar2 addition (Fig. 7B, right panel). If Dhvar2 stimulated a postentry step, one would expect an increase in luciferase production despite the presence of T20, because this drug inhibits new entry events but not virus that has already been fused with the cell at the time of T20 addition. We therefore conclude that Dhvar2 promotes the Env-mediated entry step.
FIG. 7.
Dhvar2 promotes HIV-1 Env-mediated cell entry. (A) The 90% inhibitory concentration of fusion inhibitor T20 is unaffected by Dhvar2. LuSIV cells were infected with HIV-1 in the presence of varying concentrations of Dhvar2 and T20. Luciferase production was determined 24 h later. (B) In the left panel, LuSIV cells were HIV-1 infected, followed by T20 addition 240 min later to stop the ongoing infection process. In the right panel, Dhvar2 was added at t = 255 min, with or without prior (t = 240 min) T20 application. As a control, T20 was added 5 min prior to HIV-1 infection. Error bars represent standard deviations. RLU, relative light units.
DISCUSSION
Vaginal, rectal, or oral transmission of HIV-1 involves crossing of mucosal tissue, a process that is not yet entirely understood (30, 40). One of the complicating factors for the virus is antimicrobial peptides that are secreted at mucosal surfaces as part of the innate immune system. Their use as candidate forms of prophylaxis against HIV-1 has therefore been proposed (5, 36, 38). There are several studies on antimicrobial peptides and inhibition of HIV-1 replication, and their mode of action is diverse, including disruption of the viral membrane, inhibition of reverse transcription, or prevention of viral entry (5, 18, 23, 32, 33, 39, 43). Previously, we reported that human saliva contains several compounds that inhibit HIV-1 at different stages of the replication cycle (3). We have now investigated the active domain of one salivary peptide (histatin 5) and derivatives thereof. Amino acids in the active domain (Dh-5) were substituted, yielding peptide variants (Dhvar2 to Dhvar5) with increased fungicidal activity (14, 25). We report that Dh-5 decreases the infectivity of HIV-1 in a single-cycle replication assay. Unexpectedly, none of the peptide variants were more potent antivirals. Interestingly, the Dhvar2 variant significantly stimulated HIV-1 up to 30-fold in the single-cycle replication assay.
We found that Dhvar2 binds to T cells and that it enhances Env-mediated viral entry. Stimulation was observed with CCR5-using SIV, CXCR4-using HIV-1, and pseudotyped viruses, but not when this pseudovirus was bearing the VSV-G protein. VSV and HIV-1 have different routes of entry: VSV enters a cell through endocytosis, whereas HIV-1 enters through receptor-mediated fusion of viral and cellular membranes (19, 20). In a pulse-chase experiment with the T20 entry inhibitor, we could demonstrate that Dhvar2 acts early at the entry step (Fig. 7B). Membrane interaction is a very general property of antimicrobial peptides (37). Possibly, Dhvar2 facilitates fusion of the viral and cellular membrane due to the fact that this amphipathic peptide inserts in membranes and weakens the structure of the lipid bilayer. We observed a strong correlation between amphipathicity and the stimulatory or inhibitory properties of the different peptides (Table 1). We therefore do not think that Dhvar2 has a specific protein-based receptor on T cells or the virus.
The difference between Dh-5 and the variants is the predicted 3-dimensional α-helical conformation: Dhvar2, Dhvar3, and Dhvar4 have a more pronounced separation between the hydrophilic and hydrophobic amino acids than Dh-5, leading to an optimal amphipathicity in the α-helical conformation (Table 1). While in the context of Candida the derivatives have an increased fungicidal activity, this apparently does not apply to the HIV-1-human cell interaction. At the concentrations used in our study, Dhvar2 seems to affect the membrane such that Env-mediated membrane fusion and subsequent viral entry is promoted. This also applies to Dhvar3 and Dhvar4 to a lesser extent.
Interestingly, one of the Dh-5 variants (Dhvar5) shows sequence similarity to the C terminus of a recently described papillomavirus capsid protein (15). This peptide mediates escape of viral genome degradation by destabilizing the membrane of the endocytic compartment. Remarkably, Dhvar5 is the only Dh-5 variant that did not stimulate HIV-1 infection. Furthermore, Dhvar5 is the only variant that is less amphipathic than Dh-5. These results demonstrate that the stimulatory properties of a peptide on viruses is context dependent and therefore difficult to predict. This papillomavirus study, combined with our results, suggests that minor membrane destabilization may be beneficial to a broad range of viruses.
Recently, another study showed that antimicrobial peptides with comparable bactericidal effects have differential effects on HIV-1 replication. One peptide, cryptdin 3 (Crp3), stimulated HIV-1 in an unidentified step preceding reverse transcription (33). Crp3 is a member of the α-defensin family and has been described to form anion-selective pores in mammalian cell membranes (41). It is therefore possible that the mode of action is comparable to that of Dhvar2. We are the first to show that peptides with minor amino acid substitutions can have an unexpected reverse effect on HIV-1 replication.
Our findings are relevant for the design and clinical application of antimicrobial peptides. Candida albicans is the predominant species of yeast isolated from patients with oral candidiasis, which is a frequent symptom of HIV-1 infection and a criterion for staging and progression of AIDS (6). Treating candidiasis patients with the peptide variants with increased antifungal activity seems tempting but would be unadvisable and potentially dangerous if the patient is HIV-1 infected. In the absence of HIV-1 infection, people who receive treatment for oral candidiasis with the variant peptides should be aware of the increased susceptibility to HIV-1 infection.
In conclusion, our study shows that modification of antimicrobial peptides in order to improve their activity may have unwanted and potentially dangerous side effects on other pathogens. Since these effects are difficult to predict, this should be considered in the development of antimicrobial peptides for clinical application.
Acknowledgments
This research was funded by grant 7008 from Aids Fonds Netherlands (B.B.) and a fellowship from the Anton Meelmeijer foundation and NWO/CW-VENI (R.W.S.).
We thank the following persons for providing us with valuable reagents: J. E. Clements for the LuSIV cells; J. Seppen for the lentiviral vector system; Y. Guan and M. A. Wainberg for the SIV-mac239 clone; J. Binley for the pSV7D plasmids, HIVIg, and 17b antibody; D. R. Burton for the IgG1b12 antibody; M. Franti and W. Olson for LAI gp120, CD4-IgG2, and sCD4; S. Kingsman for the pSYNGP packaging plasmid; and finally, the 2G12 antibody from H. Katinger was obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
REFERENCES
- 1.Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M. C. Gauduin, R. A. Koup, and J. S. McDougal. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 11:533-539. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, C. E., R. W. Sanders, and B. Berkhout. 2003. Inhibiting HIV-1 entry with fusion inhibitors. Curr. Med. Chem. 10:1633-1642. [DOI] [PubMed] [Google Scholar]
- 3.Bolscher, J. G., K. Nazmi, L. J. Ran, F. A. van Engelenburg, H. Schuitemaker, E. C. Veerman, and A. V. Nieuw Amerongen. 2002. Inhibition of HIV-1 IIIB and clinical isolates by human parotid, submandibular, sublingual and palatine saliva. Eur. J. Oral Sci. 110:149-156. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, and P. L. Nara. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 5.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Repentigny, L., D. Lewandowski, and P. Jolicoeur. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin. Microbiol. Rev. 17:729-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Hertog, A. L., J. van Marle, H. A. van Veen, W. van't Hof, J. G. Bolscher, E. C. Veerman, and A. V. Nieuw Amerongen. 2005. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 388:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dull, T., R. Zufferey, M. Kelly, R. J. Mandel, M. Nguyen, D. Trono, and L. Naldini. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72:8463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 10.Groot, F., T. B. Geijtenbeek, R. W. Sanders, C. E. Baldwin, M. Sanchez-Hernandez, R. Floris, Y. van Kooyk, E. C. de Jong, and B. Berkhout. 2005. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN-gp120 interaction. J. Virol. 79:3009-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, Y., J. B. Whitney, K. Diallo, and M. A. Wainberg. 2000. Leader sequences downstream of the primer binding site are important for efficient replication of simian immunodeficiency virus. J. Virol. 74:8854-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. Oomen, E. C. Veerman, A. V. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 13.Helmerhorst, E. J., R. F. Troxler, and F. G. Oppenheim. 2001. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 98:14637-14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmerhorst, E. J., W. van't Hof, E. C. Veerman, I. Simoons-Smit, and A. V. Nieuw Amerongen. 1997. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 326:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamper, N., P. M. Day, T. Nowak, H. C. Selinka, L. Florin, J. Bolscher, L. Hilbig, J. T. Schiller, and M. Sapp. 2006. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J. Virol. 80:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotsopoulou, E., V. N. Kim, A. J. Kingsman, S. M. Kingsman, and K. A. Mitrophanous. 2000. A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 gag-pol gene. J. Virol. 74:4839-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorin, C., H. Saidi, A. Belaid, A. Zairi, F. Baleux, H. Hocini, L. Belec, K. Hani, and F. Tangy. 2005. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 334:264-275. [DOI] [PubMed] [Google Scholar]
- 19.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1982. Pathway of vesicular stomatitis virus entry leading to infection. J. Mol. Biol. 156:609-631. [DOI] [PubMed] [Google Scholar]
- 21.Moore, J. P., Q. J. Sattentau, R. Wyatt, and J. Sodroski. 1994. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 68:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson, W. E., Jr., B. McDougall, D. Tran, and M. E. Selsted. 1998. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 63:94-100. [DOI] [PubMed] [Google Scholar]
- 24.Roos, J. W., M. F. Maughan, Z. Liao, J. E. Hildreth, and J. E. Clements. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307-315. [DOI] [PubMed] [Google Scholar]
- 25.Ruissen, A. L., J. Groenink, E. J. Helmerhorst, E. Walgreen-Weterings, W. van't Hof, E. C. Veerman, and A. V. Nieuw Amerongen. 2001. Effects of histatin 5 and derived peptides on Candida albicans. Biochem. J. 356:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salter, R. D., D. N. Howell, and P. Cresswell. 1985. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 21:235-246. [DOI] [PubMed] [Google Scholar]
- 27.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, M. G., and R. E. Hancock. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407-431. [PubMed] [Google Scholar]
- 29.Seppen, J., M. Rijnberg, M. P. Cooreman, and R. P. Oude Elferink. 2002. Lentiviral vectors for efficient transduction of isolated primary quiescent hepatocytes. J. Hepatol. 36:459-465. [DOI] [PubMed] [Google Scholar]
- 30.Shugars, D. C., and S. M. Wahl. 1998. The role of the oral environment in HIV-1 transmission. J. Am. Dent. Assoc. 129:851-858. [DOI] [PubMed] [Google Scholar]
- 31.Skerlavaj, B., D. Romeo, and R. Gennaro. 1990. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect. Immun. 58:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, L., C. M. Finnegan, T. Kish-Catalone, R. Blumenthal, P. Garzino-Demo, G. M. Terra Maggiore, S. Berrone, C. Kleinman, Z. Wu, S. Abdelwahab, W. Lu, and A. Garzino-Demo. 2005. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J. Virol. 79:14318-14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanabe, H., A. J. Ouellette, M. J. Cocco, and W. E. Robinson, Jr. 2004. Differential effects on human immunodeficiency virus type 1 replication by alpha-defensins with comparable bactericidal activities. J. Virol. 78:11622-11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troxler, R. F., G. D. Offner, T. Xu, J. C. VanderSpek, and F. G. Oppenheim. 1990. Structural relationship between human salivary histatins. J. Dent. Res. 69:2-6. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, C. C., P. Emau, Y. Jiang, B. Tian, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2003. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 19:535-541. [DOI] [PubMed] [Google Scholar]
- 37.van't Hof, W., E. C. Veerman, E. J. Helmerhorst, and A. V. Amerongen. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597-619. [DOI] [PubMed] [Google Scholar]
- 38.Van Compernolle, S. E., R. J. Taylor, K. Oswald-Richter, J. Jiang, B. E. Youree, J. H. Bowie, M. J. Tyler, J. M. Conlon, D. Wade, C. Aiken, T. S. Dermody, V. N. KewalRamani, L. A. Rollins-Smith, and D. Unutmaz. 2005. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J. Virol. 79:11598-11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wachinger, M., A. Kleinschmidt, D. Winder, N. von Pechmann, A. Ludvigsen, M. Neumann, R. Holle, B. Salmons, V. Erfle, and R. Brack-Werner. 1998. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 79:731-740. [DOI] [PubMed] [Google Scholar]
- 40.Younai, F. S. 2001. Oral HIV transmission. J. Calif. Dent. Assoc. 29:142-148. [PubMed] [Google Scholar]
- 41.Yue, G., D. Merlin, M. E. Selsted, W. I. Lencer, J. L. Madara, and D. C. Eaton. 2002. Cryptdin 3 forms anion selective channels in cytoplasmic membranes of human embryonic kidney cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G757-G765. [DOI] [PubMed] [Google Scholar]
- 42.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, L., W. Yu, T. He, J. Yu, R. E. Caffrey, E. A. Dalmasso, S. Fu, T. Pham, J. Mei, J. J. Ho, W. Zhang, P. Lopez, and D. D. Ho. 2002. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298:995-1000. [DOI] [PubMed] [Google Scholar]
- 44.Zufferey, R., T. Dull, R. J. Mandel, A. Bukovsky, D. Quiroz, L. Naldini, and D. Trono. 1998. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 72:9873-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]