Abstract
Human cytomegalovirus (HCMV) is the most common cause of congenital infections in developed countries, with an incidence varying between 0.5 and 2.2% and consequences varying from asymptomatic infection to lethal conditions for the fetus. Infants that are asymptomatic at birth may still develop neurological sequelae, such as hearing loss and mental retardation, at a later age. Infection of neural stem and precursor cells by HCMV and consequent disruption of the proliferation, differentiation, and/or migration of these cells may be the primary mechanism underlying the development of brain abnormalities. In the present investigation, we demonstrate that human neural precursor cells (NPCs) are permissive for HCMV infection, by both the laboratory strain Towne and the clinical isolate TB40, resulting in 55% and 72% inhibition of induced differentiation of human NPCs into neurons, respectively, when infection occurred at the onset of differentiation. This repression of neuronal differentiation required active viral replication and involved the expression of late HCMV gene products. This capacity of HCMV to prevent neuronal differentiation declined within 24 h after initiation of differentiation. Furthermore, the rate of cell proliferation in infected cultures was attenuated. Surprisingly, HCMV-infected cells exhibited an elevated frequency of apoptosis at 7 days following the onset of differentiation, at which time approximately 50% of the cells were apoptotic at a multiplicity of infection of 10. These findings indicate that HCMV has the capacity to reduce the ability of human NPCs to differentiate into neurons, which may offer one explanation for the abnormalities in brain development associated with congenital HCMV infection.
Approximately 70 to 100% of the healthy adult population is seropositive for human cytomegalovirus (HCMV) (1, 18), a member of the herpesvirus family. Lifelong latency is established after a primary HCMV infection, and the virus has developed a number of strategies for evading immune recognition (53). HCMV is an opportunistic pathogen, causing significant morbidity and mortality primarily in immunocompromised patients. Furthermore, transmission of HCMV to the fetus is the most common type of intrauterine infection (23, 60), with a worldwide incidence of 0.5 to 2.2% (2, 23, 55). The fetus can be infected during all trimesters, in connection with either a primary or recurrent infection in the pregnant women (38, 55). A congenital HCMV infection may be asymptomatic, but can also lead to a congenital syndrome characterized by, e.g., microcephaly (the most prominent manifestation of brain disorder), hearing loss, mental retardation, periventricular calcification, hydrocephalus, and/or microgyria (4-6, 12, 23).
Approximately 5 to 10% of the infants infected exhibit symptoms at birth, and an additional 10 to 15% will develop neurological sequelae, such as mental retardation and hearing loss, at a later age (13, 41, 42). The neuropathogenesis of these brain disorders has not yet been fully elucidated. However, in light of the fact that HCMV-infected cells are located predominately in the ventricular and subventricular zones (SVZ) (6, 43), this virus may disrupt the functions of neural stem cells present in these regions.
CMV is a species-specific virus, which entails obvious difficulties in investigating its pathogenesis in humans. Therefore, most of the research in this field has employed murine CMV (MCMV) as a model to study the neuropathogenesis of CMV infection. Mouse stem cells are permissive for infection by MCMV, which has been shown to inhibit the self-renewal, differentiation, and proliferation of murine neuronal stem cells in vitro (29). In addition, such infection in the developing mouse brain disturbs neuronal migration and induces loss of neuronal cells (50), possibly by promoting apoptosis in noninfected cells (30). However, even though apoptosis has been found to be blocked in primary neuronal cultures infected with MCMV (30), infection of both astrocytes and neurons in vitro enhances the frequency of cell death (57).
Recent progress in the isolation and culture of human neural precursor cells (NPCs) in vitro offers new possibilities for characterizing the effects of HCMV on the functions of these cells. Human neural precursor cells can be expanded as neurospheres, giving rise to both phenotypic neuronal and glial cells following differentiation in vitro. Although astrocytes (31, 33), neurons (35), the neuronal cell line HCN-A1 (45), and an oligodendrocytic cell line (54) can all be infected with HCMV, only astrocytes and neurons appear to be fully permissive for such infection. In mixed cultures of the latter two types of cells, HCMV seems to preferentially infect astrocytes (33, 57). Recently, Cheeran et al. showed that human NPCs are susceptible to infection with HCMV (11). Infection of NPCs or differentiated cells with HCMV is associated with impaired cell proliferation (11, 35) and an increased frequency of cell death (33, 35, 57) that may or may not be due to apoptosis (33, 35). Furthermore, HCMV infection of differentiated cells is associated with cell-cell reinfection (36). Infection of neuroepithelial stem cells with HCMV, in contrast to the case with MCMV, does not affect differentiation (35, 36), although it does enhance cell death (36). In the present investigation, we utilized forebrain tissues from fetuses aborted electively as a unique source of human neural stem and progenitor cells, appropriate for characterizing the consequences of HCMV infection. We demonstrate that HCMV inhibits the induction of neuronal differentiation and induces apoptosis in infected cells, observations that may shed some light on the pathogenesis of developmental brain disorders associated with congenital HCMV infection.
MATERIALS AND METHODS
Forebrain tissue from human fetuses aborted during the first trimester.
After obtaining written informed consent from the pregnant women and approval by the Regional Human Ethics Committee, Stockholm, Sweden, forebrain tissue was collected from human fetuses aborted electively during weeks 5 to 12 of gestation. The gestational age of the tissue was determined from the size of the fetus and by examination of anatomical landmarks in accordance with England's atlas (16). The subcortical forebrain was dissected under sterile conditions in Dulbecco's modified Eagle's medium (DMEM)-F-12 medium (Invitrogen, Life Technologies) and subsequently processed for the establishment of neurosphere cultures or for the induction of differentiation as described below.
Establishment of neurosphere cultures from human forebrain tissue.
The samples of human tissues were dissociated mechanically into single-cell suspensions by employing a glass-Teflon homogenizer, following which neurospheres were established and propagated by a slight modification of the procedure developed by Carpenter et al. (8). In brief, 20 ml of cells (10 × 104 to 15 × 104/ml) was cultured in 75-ml noncoated flasks (Nunc) suspended in DMEM-F-12 (1:1; Invitrogen Life Technologies) supplemented with 0.6% glucose, 5 mM HEPES, heparin (2 μg/ml; Sigma), N2 (1% [vol/vol], Life Technologies), epidermal growth factor (EGF; 20 ng/ml [R&D Systems]), basic fibroblast growth factor (bFGF; 20 ng/ml [R&D Systems]), and ciliary neurotrophic factor (CNTF; 10 ng/ml [R&D Systems]), which resulted in the formation of free-floating neurospheres. These cells were maintained at 37°C in an atmosphere of 5% CO2 and 95% O2, with fresh medium added twice each week. The neurospheres were harvested every 7 to 10 days (depending on the rate of growth of the individual culture) using TrypLE in accordance with the manufacturer's instructions (Invitrogen, Life Technologies) and thereafter recultured in fresh medium as described above. In all cases, the medium in which neurospheres were cultured was free from antibiotics. Herein, we refer to cultured human neural cells grown as free-floating neurospheres as NPCs.
Differentiation of NPCs into neuroblasts in vitro.
The neurospheres of human cells obtained as described above were subsequently dissociated using TrypLE (again, as described above) and thereafter suspended (60,000 cells/ml) in neurobasal medium supplemented with B27 (both procured from Invitrogen Life Technologies) and seeded (1 ml/well) on poly-d-lysine (0.1 mg/ml; Sigma) circular glass coverslips (13 mm in diameter) in 24-well plates (Nunc). The cells were then allowed to differentiate for 7 days prior to fixation and the performance of immunocytochemistry. Different strains and doses of HCMV were added to the culture medium either at the time of initiation of differentiation or 1 to 3 days later. The differentiation in neurobasal medium promotes the cells to differentiate primarily to neurons and not to astrocytes (less than 1% glial fibrillary acidic protein [GFAP]-positive cells in our cultures).
Strains of HCMV.
Cells were infected with either the HCMV laboratory strain Towne or the clinical strain TB40 at a multiplicity of infection (MOI) of 0.1 to 10 and fixed 7 days postinfection (dpi). The viral strain Towne was propagated as follows. Cell-free viral stocks were prepared from supernatants of human lung fibroblast cell cultures, frozen, and stored until use at −70°C. TB40 was propagated in human umbilical vein endothelial cells (HUVECs) with EGM2 medium. HUVECs were used until passage 7. Virus titers were determined by plaque assays as described previously (61).
Flow cytometric analysis.
A fluorescence-activated cell sorter (FACSort; Becton Dickinson) was used to analyze NPCs prepared from human forebrain and cultured in vitro for the intracellular expression of nestin, prior to differentiation. For this purpose, approximately 1 × 105 to 2 × 105 cells were fixed with the Cytofix solution of the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions, prior to incubation on ice for 30 min with antibodies against nestin (diluted 1:3,000; Chemicon). The cells were then washed with phosphate-buffered saline (PBS) and subsequently incubated with fluorescein isothiocyanate-conjugated rabbit anti-mouse antibody (2 μl/tube; Dakopatts) for 30 min, followed by a final rinse in PBS. Nonspecific fluorescence was determined using equal volumes of cells incubated with the corresponding mouse monoclonal antibody.
Cells were also analyzed for the expression of CD133 prior to and after differentiation, by incubating cells with CD133-specific antibody (1:20; Miltenyi Biotec) according to the manufacturer's instructions or with the corresponding isotype control (Dakopatts). Finally, the specific fluorescence of the cells was analyzed in a flow cytometer (FACSort; Becton Dickinson), using CELLQuest software (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.).
PI staining for measurement of DNA loss.
NPCs were differentiated into neurons in the presence or absence of HCMV (TB40), in neurobasal medium. Cells were harvested 7 days later using TrypLE (as described above) and fixed in 70% ethanol for at least 30 min at 4°C. Ethanol was removed by centrifugation, and cells were washed twice with PBS. Thereafter, cells were treated with RNase (100 μg/ml) (to ensure DNA labeling only) before being stained with propidium iodide (PI; 50 μg/ml). The DNA loss (hypodiploidy) was assessed by flow cytometry analysis using a FACScan flow cytometer (FACSort; Becton Dickinson).
Immunocytochemical evaluation.
The differentiated cells on the glass coverslips were first fixed with buffered 4% paraformaldehyde, pH 7.4, for 10 min at room temperature and thereafter were preincubated with 1.5% normal goat serum for 30 min prior to incubation with the primary antibodies. The primary monoclonal antibodies employed were directed against the following antigens: nestin (diluted 1:100; Chemicon), the proliferating cell nuclear antigen (PCNA; diluted 1:20 [Santa Cruz Biotechnology, Inc.]), microtubule-associated protein 2 (MAP2; diluted 1:25 [Abcam]), immediate-early (IE) protein (diluted 1:200[Argene Parc Technologique, Delta Sud, France]), pp65 (diluted 1:100; Argene Parc Technologique), or gB (diluted 1:100; 7-17). The primary rabbit polyclonal antibodies used were raised against β-tubulin III (diluted 1:1,200; Berkeley Antibody Company), active caspase 3 (diluted 1:500; BD Biosciences), and PCNA (diluted 1:20; Santa Cruz Biotechnology, Inc.). These primary antibodies were diluted in 0.1 M PBS containing 0.3% Triton X-100 as indicated above, prior to incubation with the cells for 2 h at room temperature or overnight at +4°C, followed by three rinses in PBS.
Visualization of bound primary antibodies was achieved by incubation for 60 min at room temperature with secondary antibodies conjugated to Alexa Fluor 488 (diluted 1:900; Molecular Probes) or Cy3 (diluted 1:1,800; Jackson Immunoresearch Laboratories, Inc.) followed by a final rinse in PBS. In addition, the cell nuclei were stained with DRAQ-5 (diluted 1:1,000; Alexis Corporation) or Hoechst stain (1 μg/ml) prior to mounting the coverslips with FluorSave reagent (Calbiochem) or PVA-DABCO [9.6% polyvinylalcohol, 24% glycerol, and 2.5% 1,4-diazabicyclo (2.2.2) octane in 67 mM Tris-Cl, pH 8.0]. Negative control slides were processed in the same manner, but omitting the primary antibodies. The images were examined in a fluorescence microscope (Axiophot; Zeiss) and documented using a charge-coupled device camera (ORCA-ER, C4742-95; Hamamatsu) together with the Openlab software for Macintosh (Improvision). The numbers and/or percentages of cells expressing the different antigens analyzed were calculated by counting three fields per glass (magnification, ×20).
Proliferation assay: thymidine incorporation.
Cells were seeded onto poly-d-lysine-coated 96-well plates, (15,000 cells/well) in a differentiation-promoting medium (neurobasal) and infected at the time of seeding. At different time points (1 to 7 days later), the cells were pulsed with 1 μCi [methyl-3H]thymidine (Amersham, Life Science) for 16 h before they were harvested onto filters using a plate harvester (Harvester 996; Tomtec, Hamden, Conn.) according to the manufacturer's instructions and counted in an automated counter (1450 MicroBeta Trilux; Wallac AB, Bromma, Sweden).
Treatment with Foscavir.
Following infection with strain Towne by incubation for 2 h at an MOI of 1 and 10, cells were exposed to Foscavir (0.5 mM foscarnet sodium; Astra Zeneca, Södertälje, Sweden) for 7 days. Thereafter, these cells were fixed as described above and stained for the expression of immediate-early (IE) and late (gB) HCMV genes, β-tubulin III, PCNA, and active caspase 3 as described above. As a control for the toxicity of Foscavir, uninfected cells were also exposed to this compound.
Evaluation of the effect of medium from virus-infected cells. (i) Differentiation in the presence of medium from virus-infected cells.
Following 7 days in cultures, the media from uninfected and HCMV-infected cells were collected, cleared by centrifugation, filtered to remove virus particles, and then added to fresh cultures of uninfected cells at the time of initiation of the differentiation. Cells were fixed 7 days later and stained for the expression of β-tubulin III, active caspase 3, and IE HCMV antigens.
(ii) Virus production by infected cells.
After 7 dpi, the medium from infected cells was collected, cleared by centrifugation, and stored at −70°C. After thawing and dilution (1:2), this medium was used to infect fibroblasts (MRC-5), which were subsequently stained for the presence of IE HCMV antigen.
Statistical analysis.
Data are expressed as mean ± standard deviation (SD). A paired Student's t test was used to determine statistically significant differences (P < 0.05).
RESULTS
NPCs are permissive for HCMV infection.
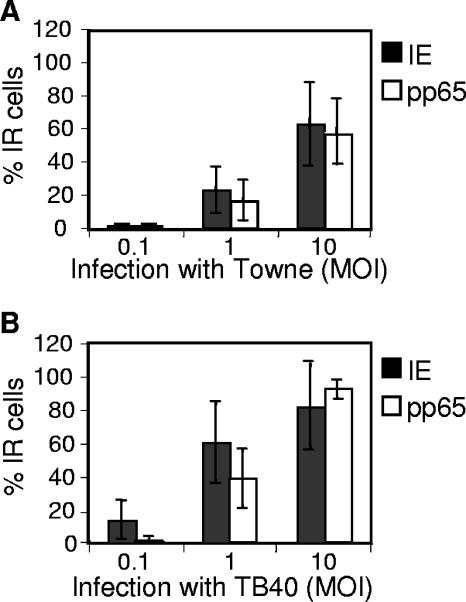
In order to evaluate their susceptibility to infection by the Towne and TB40 strains of HCMV, cells were harvested 7 dpi and stained for the presence of early (IE) and late (gB or pp65) HCMV proteins. At an MOI of 0.1, IE immunoreactive (IR) cells were mainly demonstrated in cultures infected with TB40 (14% ± 12%, n = 5), as compared to cells infected with Towne (1% ± 1%, n = 3) (Fig. 1). However, at an MOI of 1 or 10, IE-positive cells were present both in cell cultures infected by the Towne strain (23% ± 14% and 63% ± 25% for MOIs of 1 and 10, respectively) or TB40 (61% ± 24% and 82% ± 26% for MOIs of 1 and 10, respectively) strains (Fig. 1). These NPCs also expressed gB and pp65, demonstrating that both early and late viral gene transcription occurs in infected cells: i.e., the cells are permissive for the infection (Fig. 1). In order to assess the production of virus by infected NPCs, the medium from such cells was collected at 7 dpi and used to culture fibroblasts, which are fully permissive to infection by HCMV. Following 7 days of such exposure to medium from cells infected with an MOI of 10, <5% of the fibroblasts expressed HCMV proteins, while none of these cells expressed such proteins when the initial infection was performed with an MOI of 0.1 or 1 (data not shown). Thus, although NPCs are completely permissive for infection, few virus particles appear to be released.
FIG. 1.
NPCs are permissive to HCMV infection. The susceptibility of NPCs to infection with either the Towne or TB40 strains of HCMV was analyzed at 7 dpi by staining with antibodies directed towards HCMV proteins. The mean percentage ± SD of cells staining positively (IR) for early (IE) and late (pp65) gene products is shown. n = at least 3 independent experiments.
HCMV prevents the induction of neuronal differentiation.
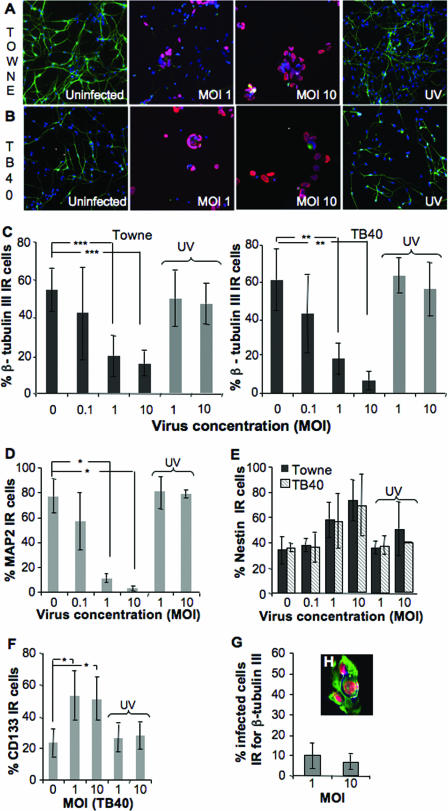
We infected NPCs at the same time differentiation was induced on coated wells in neurobasal medium, and cells were analyzed for the expression of β-tubulin III 7 days later. Prior to differentiation, the majority of these cells express nestin (92% ± 3%) and CD133 (73% ± 15%), markers widely used for identifying NPCs, and this expression is reduced as differentiation occurs. Infection with the laboratory strain Towne significantly inhibited this neuronal differentiation, as demonstrated by decreased expression of β-tubulin III, at MOIs of 1 (55%) and 10 (75%), but not with an MOI of 0.1 (Fig. 2A and C). This inhibition of the maturation was dependent on active replication of the virus, since cells infected with virus that had been inactivated with UV light, and thus did not replicate, matured and expressed β-tubulin III at levels similar to those detected in uninfected cells (Fig. 2A and C). To verify that the inhibition of the induced neuronal differentiation was also manifested by a clinical isolate, NPCs were infected with TB40, which was found to prevent induction of neuronal differentiation in a similar manner (Fig. 2B and C). Infection with this strain at an MOI of 1 resulted in 75% inhibition, a value that was elevated to 85% inhibition at an MOI of 10. In this case, even an MOI of 0.1 reduced the number of cells expressing β-tubulin III, although not to an extent that was statistically significant (Fig. 2C). To certify that HCMV prevented NPCs from differentiating into neurons and not only altered the expression of β-tubulin III, cells were also stained for the neural marker MAP2. Indeed, the expression of this marker was also reduced in HCMV-infected cultures, as compared to uninfected cell cultures (Fig. 2D). In addition, the very early stage of neuronal differentiation includes cellular processes that are lacking in infected cultures (Fig. 2A and B). Moreover, the ability of both virus strains (TB40 and Towne) to block differentiation was independent of the number of passages, since even after 14 passages, infection attenuated the number of cells expressing β-tubulin III and MAP2 (data not shown). Clearly, HCMV can prevent induction of the differentiation of human NPCs into neurons.
FIG. 2.
HCMV inhibits the induction of the differentiation of NPCs into neurons. Differentiation of NPCs into neurons was initiated in the presence or absence of different concentration of the HCMV (MOIs of 0.1 to 10; or UV-inactivated HCMV, MOIs of 1 and 10) Towne or TB40 strain. Cells were fixed at 7 dpi and stained for β-tubulin III (green), IE protein (red), and Hoechst strain (blue, nuclear staining); the samples are depicted in panels A (Towne) and B (TB40). The mean percentage ± SD of the cells staining positively for β-tubulin III is presented in panel C (n = 11 for Towne and n = 5 for TB40). The expression of MAP2 in uninfected or HCMV (TB40)-infected cultures 7 days after the initiation of differentiation is shown in panel D (n = 3, mean value ± SD). The expression of nestin and CD133 in uninfected cell cultures and in cultures infected with HCMV was analyzed, and the mean percentages ± SD of cells expressing nestin (n = 5 for Towne and n = 4 for TB40) and CD133 (n = 4 for TB40) are shown in panels E and F, respectively. The percentage of infected cells (IE positive) that were IR for β-tubulin III is shown in panel G (Towne, n = 10). Panel H shows one representative example of infected cells stained for IE protein (red), nestin (green), and Hoechst stain (blue, nuclear staining). Statistical analysis was performed with Student's paired t test. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
We also analyzed the phenotype of the cells present in the infected cell cultures: i.e., we examined whether infected NPCs remained immature. In the undifferentiated cultures, a majority of the cells stained positively for both nestin and CD133 (data not shown), and this was also the case in cultures infected with Towne (MOIs of 1 and 10) (Fig. 2E and F, n = 5) or TB40 (Fig. 2E and F, n = 4). While only a small fraction (<10%) of the cells infected with Towne or TB40 (data not shown) expressed β-tubulin III (Fig. 2G), the vast majority of the infected cells remained nestin positive (Fig. 2H), which suggest that a majority of the infected cells appeared to remain undifferentiated.
The products of late HCMV genes are responsible for inhibition of neuronal differentiation.
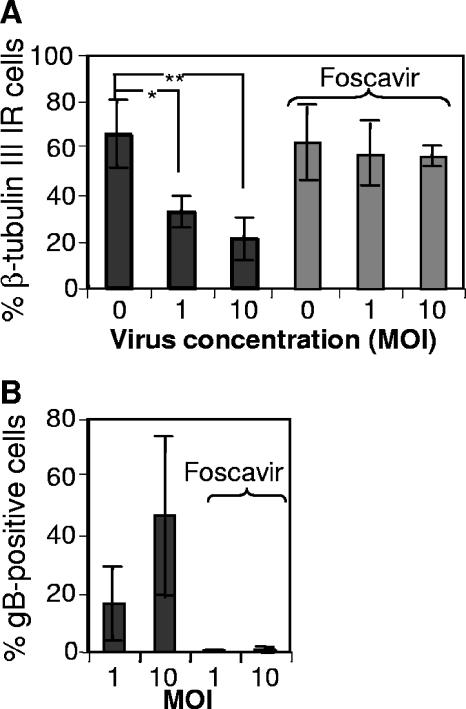
Since active virus replication was required to block neuronal maturation, we examined in greater detail whether products of early or late HCMV genes were responsible for this prevention by treating cells (both uninfected and infected) with Foscavir (0.5 mM), a selective inhibitor of late HCMV gene transcription. We confirmed that Foscavir treatment was functioning as expected by demonstrating, through analysis of gB, that in the presence of this drug only a very small percentage (0 to 1%) of the cells in infected cultures expressed gB (Fig. 3B). The lack of effect of Foscavir on neuronal differentiation was apparent from the fact that similar percentages of uninfected cells expressed β-tubulin III in the absence and presence of this drug (66% ± 15% versus 63% ± 15%, respectively; Fig. 3). The reduction in the number of cells staining positively for β-tubulin III when infected with Towne at MOIs of 1 and 10 (33% ± 7% and 21% ± 9%, respectively) was prevented by Foscavir (57 ± 13 and 57 ± 4, respectively) (Fig. 3; n = 4). Thus, the products of late HCMV genes appear to be responsible for the inhibition of neuronal differentiation.
FIG. 3.
The products of late HCMV genes are responsible for inhibition of neuronal differentiation. Differentiation of NPCs into neurons was induced in the presence or absence of Towne (at MOIs of 1 and 10) and with and without exposure to Foscavir (0.5 mM), which arrests late HCMV gene transcription. The presence of neurons was demonstrated by staining for β-tubulin III 7 dpi, and the mean percentages ± SD of cells with expression are shown in panel A (n = 4). The presence of gB (a product of late HCMV gene expression) in virus-infected cell cultures in the absence or presence of Foscavir is depicted in panel B (mean values ± SD, n = 4). Statistical analysis was performed employing Student's paired t test. **, P ≤ 0.01; *, P ≤ 0.05.
It is noteworthy that an MOI of 1 resulted in a 55% inhibition of the number of cells expressing β-tubulin, although only a minority of the cells (approximately 20%) were infected. Therefore, we investigated whether infected cells were releasing a soluble factor, encoded by either the host or viral genome that could cause such inhibition. For this purpose, the medium from uninfected and HCMV-infected cell cultures was collected, cleared by centrifugation, and added to uninfected cells at the time of initiation of differentiation. However, the ability to differentiate into neurons was not influenced by these media (data not shown), suggesting that a release of soluble factors is not involved in the inhibition observed.
Infected cell cultures exhibit an attenuated ability to proliferate.
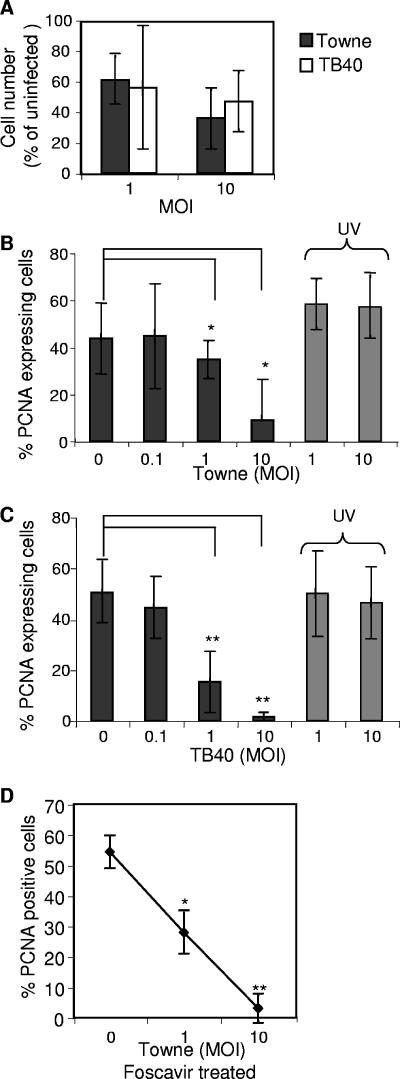
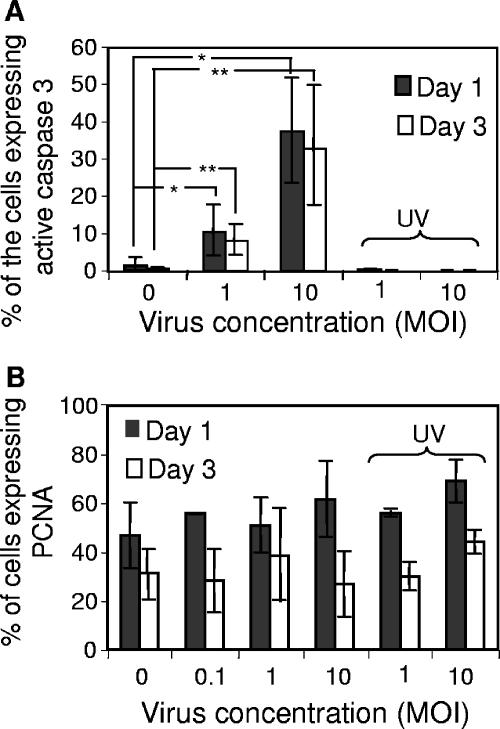
The reduction in the total cell number observed in infected cultures (Fig. 4A), in comparison to uninfected cultures, could be a consequence of inhibition of cell proliferation and/or induction of cell death. In order to determine whether infection by HCMV influences cell proliferation in our system, as has been observed in HCMV-infected fibroblasts (34) and in human NPCs (11), cells were stained for PCNA. Infection of NPCs with the laboratory strain Towne resulted in significantly attenuated proliferation (Fig. 4B) at an MOI as low as 1 (33% reduction; P < 0.05). Interestingly, infection with the clinical strain TB40 displayed an ever more pronounced effect (Fig. 4C), with a 70% reduction in the expression of PCNA at an MOI of 1. This difference probably reflects the difference in the infection level between the two strains (Fig. 1). The effect on cell proliferation was also analyzed by [3H]thymdine incorporation, which demonstrated a significantly reduced cell growth in infected cell cultures (data not shown). This negative effect on the proliferation was dependent on active virus replication (Fig. 4B and C), but not on expression of late viral genes, since cells treated with Foscavir (0.5 mM) also displayed decreased expression of PCNA (Fig. 4D). Thus, HCMV appears to inhibit progression of the cell cycle, which suggests a possible explanation for the reduced cell number present in infected cultures.
FIG. 4.
HCMV infection reduces the ability of NPCs to proliferate. The numbers of cells observed in infected versus uninfected cultures are presented in panel A. In order to determine whether HCMV interferes with the cell proliferation, the number of cells expressing PCNA, a proliferation marker, was analyzed, as the mean percentage ± SD following infection with Towne (B; n = 5) or TB40 (C; n = 4) as well as with UV-inactivated virus (B and C). The percentage of PCNA-expressing cells in Foscavir (0.5 mM)-treated cultures, with or without HCMV infection (Towne), is shown in panel D (n = 3, mean value ± SD). Statistical analysis was performed employing Student's paired t test. **, P ≤ 0.01; *, P ≤ 0.05.
HCMV evokes apoptosis of infected cells.
HCMV infection causes characteristic morphological alterations in cells, including swelling and formation of lobular nuclei. In most cell types, HCMV infection has been shown to block apoptosis, involving several different mechanisms and HCMV proteins (19, 47, 52, 63). However, there are discrepancies in the reports regarding the ability of the virus to cause apoptosis in NPC/neural stem cells.
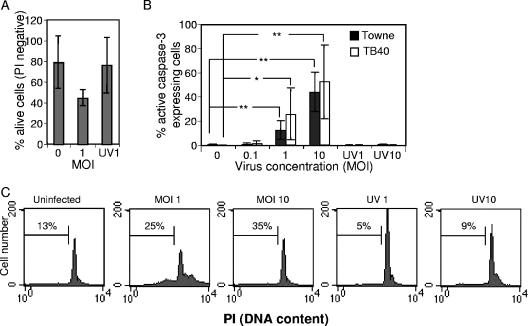
Uninfected and HCMV-infected cells were investigated for the presence of cell death by staining of the unfixed cells with PI and analysis using flow cytometry at 7 dpi. In infected cultures, an increased cell death was demonstrated (Fig. 5A [data only shown for TB40-infected cells]). To further characterize whether this increased cell death was due to necrosis or apoptosis, the results of immunofluorescence analysis of active caspase 3 staining, a marker for apoptosis (Fig. 5B), and flow cytometry analysis of nuclear DNA loss (hypodiploidy) (Fig. 5C), a typical feature of apoptosis, were assessed 7 days after the onset of differentiation, in the presence or absence of HCMV. Infection of our cells with Towne or TB40 induced apoptosis, since there was an enhanced level of active caspase 3 (Fig. 5B), as well as a higher percentage of hypodiploidic cells (Fig. 5C). However, increased apoptotic activity was not observed in infected cells treated with Foscavir (0.5 mM), which is known to block late, but not early, viral gene transcription (data not shown). Furthermore, the induced cell death observed in infected cultures was not mediated by soluble factors, host or virus encoded, since medium from uninfected and HCMV-infected cell cultures added to uninfected cells at the time of initiation of differentiation did not result in apoptosis (data not shown). Since MCMV has been found to induce apoptosis in even uninfected cells in infected animals, we stained our cells for both active caspase 3 and IE protein, to determine whether such a phenomenon was also occurring here. The majority (70%) of the apoptotic cells in our cultures were infected, demonstrating clearly that it was primarily the infected cells that underwent apoptosis.
FIG. 5.
HCMV infection evokes apoptosis of infected cells. The percentage of living cells (PI negative) was measured by flow cytometry at 7 days after the onset of differentiation in uninfected, HCMV (TB40)-infected, or UV-HCMV-infected cells (A; n = 3, mean value ± SD). Induction of apoptosis was examined by staining uninfected and HCMV-infected cells at 7 dpi for active caspase 3 (B; the mean percentages ± SD for Towne [n = 7] or TB40 [n = 5]) or by flow cytometry analysis of DNA loss by PI staining (C; numbers indicate the percentage of hypodiploid cells from one representative experiment of four for TB40). Statistical analysis was performed employing Student's paired t test. **, P ≤ 0.01; *, P ≤ 0.05.
Inhibition of neuronal differentiation by HCMV occurs early in the differentiation process.
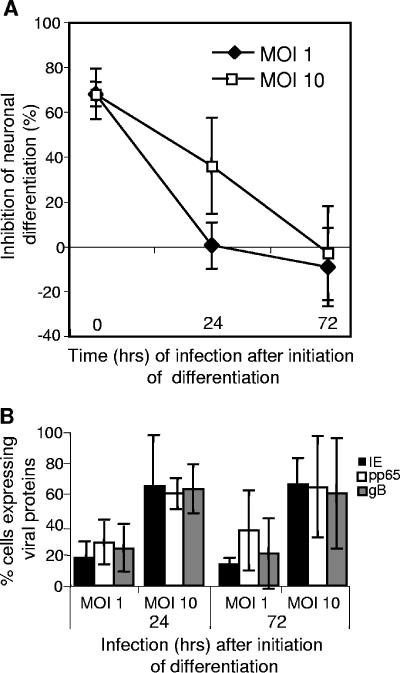
To examine whether HCMV can influence the differentiation of cells already committed to differentiate into neurons, we infected NPCs with Towne at different time points (24 to 72 h) following initiation of the differentiation. All cells were fixed and analyzed for phenotypic markers and the expression of viral antigens at 7 dpi. Neuronal differentiation of these cells was not affected if they were infected with Towne at an MOI of 1, at 24 or 72 h following stimulation (Fig. 6A). However, at an MOI of 10, a reduction in the percentage of cells expressing β-tubulin III (Fig. 6A) was present after 24 h, but not after 72 h (Fig. 6A). In order to be certain that this lack of inhibition was not simply due to an absence of or attenuated HCMV infection, cells were also stained for virus-specific proteins. Clearly the cells were infected at similar levels (compare Fig. 6B with Fig. 1C), even when the virus was added at later time points. Thus, the ability of HCMV to interfere with the maturation is limited to the initial 24-h period following stimulation.
FIG. 6.
Inhibition of the neuronal differentiation by HCMV occurs early in the differentiation process. NPCs were infected with Towne at the onset of differentiation or 24 to 72 h later and fixed after 7 days of incubation for analysis of the expression of β-tubulin III. The mean percentage inhibition ± SD (n ≥ 5) is depicted in panel A, and cell susceptibility to infection is depicted in panel B (mean ± SD, n ≥ 5).
Infection with HCMV 1 or 3 days following initiation of differentiation still induced apoptosis, but no longer influenced cell growth.
Since infection with HCMV at the same time as initiation of differentiation attenuated the rate of cell proliferation and simultaneously enhanced the frequency of apoptosis, we examined whether infection 1 or 3 days later exerted similar effects. Interestingly, infection at these time points following initiation of differentiation did not influence cell proliferation (Fig. 7B), but still elevated the frequency of apoptosis (Fig. 7A), which was dependent on active virus replication. Thus, interference by HCMV with these different cellular functions was affected differentially by the state of cell differentiation.
FIG. 7.
Infection with HCMV 1 or 3 days following initiation of differentiation still induced apoptosis. NPCs infected with Towne or UV-inactivated Towne 1 or 3 days following initiation of differentiation were examined for the expression of active caspase 3 (A), a marker for apoptosis, and PCNA (B), a marker for proliferation. The values shown are mean percentages ± SD (n ≥ 4). Statistical analysis was performed employing Student's paired t test. **, P ≤ 0.01; *, P ≤ 0.05.
DISCUSSION
Although HCMV is the most common cause of congenital infection, the mechanisms underlying pathogenic responses to such infection have not yet been fully characterized. In light of the fact that HCMV is known to interfere with various cellular functions (14, 20, 39, 40, 56), the abnormalities in brain development associated with a congenital infection most likely involve viral interference with the functions of NPCs. This hypothesis is in agreement with the observation that HCMV tends to preferentially infect the ventricular zone and SVZ of the fetal brain (6, 43), which is where undifferentiated neuroepithelial cells, including neural stem and progenitor cells, are primarily located (15, 27). An enhanced understanding of how HCMV interferes with the function of NPCs is also of considerable interest in connection with future possibilities for neural repair by transplantation of these cells (7-9, 17). Clinical HCMV infection occurs very frequently during the period following transplantation of hematopoietic stem cells and organs. A susceptibility of human neural stem cells to malfunctions caused by HCMV, which is indicated by the pronounced incidence of neurological symptoms associated with congenital HCMV infection, might present a serious obstacle to neural transplantation, especially since most individuals carry a latent HCMV infection.
In the present investigation, we have demonstrated that HCMV inhibits the induced differentiation of NPCs into neurons. Infected cells express nestin and CD133, markers for undifferentiated cells/NPCs, implying that these cells remain undifferentiated. In contrast, infection with nonreplicating virus does not block the maturation of NPCs into neurons.
Inhibition of the maturation of NPCs into neurons by HCMV appears to be mediated by late viral gene products, since treatment with Foscavir prevents this inhibition. Interestingly, although only a minority (approximately 20%) of the cells was infected with the virus at an MOI of 1, much more pronounced inhibition of maturation was observed. One obvious possible explanation for this apparent discrepancy is that the inhibition is mediated by the production and release of a soluble factor(s), encoded either by the host or viral genome. Indeed, the interference of HCMV with the functions of, e.g., dendritic and endothelial cells, is known to be mediated by soluble factors (48, 62). However, in the presence of medium collected from infected cell cultures, NPCs were found here to differentiate in a normal manner, suggesting that soluble factors are not involved. However, it is still possible that soluble factors requiring close cell-to-cell contact or factors that are rapidly degraded or removed might be involved.
Previously, HCMV has been shown to inhibit the induced differentiation of both macrophages and dendritic cells through a direct contact with these cells (21, 22). This observation suggests that HCMV can induce the same phenomenon in different types of cells via different mechanisms and/or viral genes. Furthermore, the ability of the virus to inhibit induced cell maturation was shown here to be limited to cells not yet committed to differentiate. Thus, viral inhibition of neuronal differentiation is attenuated 24 h after initiation of differentiation and disappears completely after 3 days. This observation is in agreement with earlier reports that the ability of HCMV to interfere with differentiation into macrophages and dendritic cells is limited to immature cells (21, 22).
Neuropathological studies have revealed that HCMV can infect a wide variety of cells in the central nervous system, including neuroepithelial precursor cells and neuronal, glial, and endothelial cells. However, in vitro studies have revealed conflicting data regarding the virus' cellular tropism, and it has been suggested that the susceptibility of cells to HCMV infection is at least partially dependent on their state of differentiation (46). Whereas primary cultures of differentiated human astrocytes support productive replication of HCMV (10, 33, 37), the susceptibility of immortalized astrocytic cell lines to infection by this virus varies from complete resistance to partial expression of viral genes (46). Moreover, there are similar reports concerning neurons. While the ability of the neuronal HCN-1A cell line to support HCMV replication is reported to be elevated in connection with differentiation (45), cultures of highly enriched and purified mature neuronal cultures from the fetal cortex do not support viral growth or exhibit any morphological changes following viral infection (33, 45). In the present study, NPCs and cells that had already begun to differentiate for 1 or 3 days were both permissive for infection and exhibited cytopathic effect, at similar levels, which is in concordance with previous reported data (11). These discrepancies between the findings of different groups of researcher may be due to the use of different concentrations of virus, different viral strains, cells of different origins, and/or different culture conditions.
Here, we discovered that NPCs are more susceptible to infection by TB40 (a clinical strain of HCMV) than by Towne (a laboratory strain). Surprisingly, although the infected cells stained positively for both early and late gene products, very few infectious virus particles were detectable in the supernatants. Productive HCMV infection accompanied by a block in release of virus can occur with HCMV-infected macrophages (25, 59), possibly reflecting disruption of the organized tubulin network in infected cells. Even without release of virus particles, HCMV-infected cells can transfer virus to other cells by direct cell interaction (35, 59), which is most likely what happens with NPCs in culture.
The reduction in the number of cells present in our infected cultures is in agreement with previous findings (11, 30) and can be due to an inhibition of the cell cycle progression and/or increased cell death. The ability of HCMV to interfere with cell cycle progression in infected fibroblasts has been well characterized (26, 29, 34). In our experiments, HCMV infection reduced the proliferative activity of infected cells, more so in the case of the clinical isolate TB40 than the laboratory strain Towne, possibly due to the higher level of infection by the former (61% versus 23% IE protein-expressing cells at an MOI of 1, respectively). This negative influence on proliferation was only exerted when the cells were infected at the onset of differentiation, suggesting once again that the ability of the virus to interfere with cellular functions depends on the state of differentiation of the cell. Since NPCs and neurons exhibit similar susceptibilities to HCMV infection, the divergent effects on growth are not due to different degrees of infection. Moreover, the reduced proliferative capacity was dependent on transcription of early viral gene product(s).
Furthermore, HCMV is known to block apoptosis in infected fibroblasts (19, 47, 52, 63), and the virus was recently also shown to inhibit apoptosis in human NPCs (11). In contrast, we here describe a clearly opposite observation, since the infected cells in our study displayed an augmented frequency of apoptosis, regardless of whether the cells were infected at the onset of differentiation or at later time points. This discrepancy between our observations and those of Cheeran et al. (11), who also examined immature cells obtained from fetal brains, most likely depends on the differences in tissue origin, culture conditions, and viral strains used in the different experiments. In the present study, we examined NPCs obtained from the forebrain and cultured these cells as free-floating neurospheres in the presence of the growth factors CNTF, EGF, and FGF. In contrast, Cheeran et al. examined cells from the whole central nervous system (CNS; except for the spinal cord and the brainstem), and the cells examined were grown as adherent cells in the presence of EGF and FGF.
Neural stem cells grown either as neurospheres or an adherent monolayer are described to consist of different cell populations. While neurospheres consist of multipotent neural stem cells and cells in different stages of differentiation, cells grown as a monolayer mainly consist of neural precursor cells and less multipotent neural stem cells (3). Since current evidence suggest that neural stem cells grown as neurospheres consist of different cell populations as compared to adherent monolayers of these cells, we have most likely examined very different cell populations. In support of this hypothesis, neurospheres appear to consist of multipotent neural stem cells as well as neural precursor cells in different stages of differentiation, but cells grown in monolayers mainly consist of less multipotent neural stem cells and neural precursor cells (3). Therefore, we may have examined a more immature cell population that responded differently to HCMV infection. Since we also have used different viral strains of HCMV, strain variability, which is well known in the field, may also explain the differences in the divergent results obtained. Furthermore, other different cellular properties have also been described in cells obtained from different regions of the CNS (24).
Apoptosis was not observed in infected cultures treated with Foscavir, indicating that the induction of apoptosis of infected cells is caused by viral late gene expression or transcription and/or may also be due to the inhibition of differentiation. In addition, soluble factors, virus or host encoded, were not responsible for the induced apoptotic activity that we observed in infected cells. Furthermore, the HCMV protein US28 was recently found to be proapoptotic (44), but the interaction between pro- and antiapoptotic viral proteins in HCMV-infected cells has not been fully examined. Infection of astrocytes with HCMV is associated with increased cell death, but whether this phenomenon is due to enhanced apoptotic activity remains controversial (33, 35). Moreover, MCMV induces apoptosis in uninfected neuronal cells in vivo (30), but not in in vitro culture (30). In addition, HCMV has been reported to induce apoptosis in hematopoietic progenitor MO7 cells (51). Clearly the capacity of HCMV to block or even induce apoptosis in infected cells may be dependent on the stage of differentiation, cells of different origins and/or different culture conditions, and the virus strains involved.
As mentioned, microcephaly and polymicrogyria are the most prominent features of brain disorders associated with congenital CMV infection in humans (5, 6, 43). One hypothesis is that these brain disorders originate from the disturbance of cellular processes within the ventricular region, in which stem cells are proliferating, as well as from disruption of the movement of these cells away from the ventricular zone, their developmental commitment termination to the neuronal or glial lineage, and the subsequent migration of the latter cells. This hypothesis is supported by the observation that cells in the ventricular and SVZ regions are the primary targets for the virus in connection with congenital HCMV infection (43). The impairment of migration (50) and reduction in self-renewal and differentiation of murine neuronal stem cells caused by MCMV (29) also strengthen this theory. In addition, placental MCMV infection in mice results in the development of microcephaly (32) and preferential infection of cells in the ventricular zone and SVZ (28, 29, 32, 49, 58). In summary, we have shown in this study that HCMV not only prevents the neuronal differentiation, but also induces apoptosis and arrested cell growth in these cells. Further studies are required in order to elucidate the underlying mechanisms and clinical consequences of these effects.
Acknowledgments
This study was supported financially by grants from the Craig Hospital, Denver, Colo.; the Stockholms Sjukhems Foundation; the Swedish Research Council (14X-06555, K2004-16X-12615-07A); the Swedish Society of Medicine (2003-633); the Tobias Foundation (28/03 and 29/04); the Swedish Heart and Lung Foundation (200441486); the Swedish Children's Cancer Research Foundation (200441486); and the Swedish Cancer Foundation (57/2006).
REFERENCES
- 1.Ahlfors, K. 1984. IgG antibodies to cytomegalovirus in a normal urban Swedish population. Scand. J. Infect. Dis. 16:335-337. [DOI] [PubMed] [Google Scholar]
- 2.Ahlfors, K., S. A. Ivarsson, S. Harris, L. Svanberg, R. Holmqvist, B. Lernmark, and G. Theander. 1984. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand. J. Infect. Dis. 16:129-137. [DOI] [PubMed] [Google Scholar]
- 3.Alexanian, A. R., M. J. Crowe, and S. N. Kurpad. 2006. Efficient differentiation and integration of lineage-restricted neural precursors in the traumatically injured adult cat spinal cord. J. Neurosci. Methods 150:41-46. [DOI] [PubMed] [Google Scholar]
- 4.Bale, J. F., Jr. 1984. Human cytomegalovirus infection and disorders of the nervous system. Arch. Neurol. 41:310-320. [DOI] [PubMed] [Google Scholar]
- 5.Bale, J. F., Jr., P. F. Bray, and W. E. Bell. 1985. Neuroradiographic abnormalities in congenital cytomegalovirus infection. Pediatr. Neurol. 1:42-47. [DOI] [PubMed] [Google Scholar]
- 6.Becroft, D. M. 1981. Prenatal cytomegalovirus infection: epidemiology, pathology and pathogenesis. Perspect. Pediatr. Pathol. 6:203-241. [PubMed] [Google Scholar]
- 7.Buc-Caron, M. H. 1995. Neuroepithelial progenitor cells explanted from human fetal brain proliferate and differentiate in vitro. Neurobiol. Dis. 2:37-47. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter, M. K., X. Cui, Z. Y. Hu, J. Jackson, S. Sherman, A. Seiger, and L. U. Wahlberg. 1999. In vitro expansion of a multipotent population of human neural progenitor cells. Exp. Neurol. 158:265-278. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers-Redman, R. M., T. Priestley, J. A. Kemp, and A. Fine. 1997. In vitro propagation and inducible differentiation of multipotential progenitor cells from human fetal brain. Neuroscience 76:1121-1128. [DOI] [PubMed] [Google Scholar]
- 10.Cheeran, M. C., S. Hu, G. Gekker, and J. R. Lokensgard. 2000. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J. Immunol. 164:926-933. [DOI] [PubMed] [Google Scholar]
- 11.Cheeran, M. C., S. Hu, H. T. Ni, W. Sheng, J. M. Palmquist, P. K. Peterson, and J. R. Lokensgard. 2005. Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J. Neurosci. Res. 82:839-850. [DOI] [PubMed] [Google Scholar]
- 12.Cinque, P., R. Marenzi, and D. Ceresa. 1997. Cytomegalovirus infections of the nervous system. Intervirology 40:85-97. [DOI] [PubMed] [Google Scholar]
- 13.Conboy, T. J., R. F. Pass, S. Stagno, W. J. Britt, C. A. Alford, C. E. McFarland, and T. J. Boll. 1986. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics 77:801-806. [PubMed] [Google Scholar]
- 14.Craigen, J. L., K. L. Yong, N. J. Jordan, L. P. MacCormac, J. Westwick, A. N. Akbar, and J. E. Grundy. 1997. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology 92:138-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doetsch, F., I. Caille, D. A. Lim, J. M. Garcia-Verdugo, and A. Alvarez-Buylla. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703-716. [DOI] [PubMed] [Google Scholar]
- 16.England, M. 1990. A colour atlas of life before birth. University of Leicester, Leicester, United Kingdom.
- 17.Flax, J. D., S. Aurora, C. Yang, C. Simonin, A. M. Wills, L. L. Billinghurst, M. Jendoubi, R. L. Sidman, J. H. Wolfe, S. U. Kim, and E. Y. Snyder. 1998. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat. Biotechnol. 16:1033-1039. [DOI] [PubMed] [Google Scholar]
- 18.Forbes, B. A. 1989. Acquisition of cytomegalovirus infection: an update. Clin. Microbiol. Rev. 2:204-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gredmark, S., W. B. Britt, X. Xie, L. Lindbom, and C. Söderberg-Naucler. 2004. Human cytomegalovirus induces inhibition of macrophage differentiation by binding to human aminopeptidase N/CD13. J. Immunol. 173:4897-4907. [DOI] [PubMed] [Google Scholar]
- 21.Gredmark, S., and C. Söderberg-Nauclér. 2003. Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J. Virol. 77:10943-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gredmark, S., T. Tilburgs, and C. Söderberg-Nauclér. 2004. Human cytomegalovirus inhibits cytokine-induced macrophage differentiation. J. Virol. 78:10378-10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho, M. 1991. Congenital and perinatal human cytomegalovirus infection, p. 205-277. In M. Ho (ed.), Cytomegalovirus: biology and infection. Plenum Press, New York, N.Y.
- 24.Horiguchi, S., J. Takahashi, Y. Kishi, A. Morizane, Y. Okamoto, M. Koyanagi, M. Tsuji, K. Tashiro, T. Honjo, S. Fujii, and N. Hashimoto. 2004. Neural precursor cells derived from human embryonic brain retain regional specificity. J. Neurosci. Res. 75:817-824. [DOI] [PubMed] [Google Scholar]
- 25.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalejta, R. F., and T. Shenk. 2002. Manipulation of the cell cycle by human cytomegalovirus. Front. Biosci. 7:d295-d306. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko, Y., S. Sakakibara, T. Imai, A. Suzuki, Y. Nakamura, K. Sawamoto, Y. Ogawa, Y. Toyama, T. Miyata, and H. Okano. 2000. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev. Neurosci. 22:139-153. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki, H., I. Kosugi, Y. Arai, and Y. Tsutsui. 2002. The amount of immature glial cells in organotypic brain slices determines the susceptibility to murine cytomegalovirus infection. Lab. Investig. 82:1347-1358. [DOI] [PubMed] [Google Scholar]
- 29.Kosugi, I., Y. Shinmura, H. Kawasaki, Y. Arai, R. Y. Li, S. Baba, and Y. Tsutsui. 2000. Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab. Investig. 80:1373-1383. [DOI] [PubMed] [Google Scholar]
- 30.Kosugi, I., Y. Shinmura, R. Y. Li, S. Aiba-Masago, S. Baba, K. Miura, and Y. Tsutsui. 1998. Murine cytomegalovirus induces apoptosis in non-infected cells of the developing mouse brain and blocks apoptosis in primary neuronal culture. Acta Neuropathol. (Berlin) 96:239-247. [DOI] [PubMed] [Google Scholar]
- 31.Lecointe, D., C. Hery, N. Janabi, E. Dussaix, and M. Tardieu. 1999. Differences in kinetics of human cytomegalovirus cell-free viral release after in vitro infection of human microglial cells, astrocytes and monocyte-derived macrophages. J. Neurovirol. 5:308-313. [DOI] [PubMed] [Google Scholar]
- 32.Li, R. Y., and Y. Tsutsui. 2000. Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 62:79-85. [DOI] [PubMed] [Google Scholar]
- 33.Lokensgard, J. R., M. C. Cheeran, G. Gekker, S. Hu, C. C. Chao, and P. K. Peterson. 1999. Human cytomegalovirus replication and modulation of apoptosis in astrocytes. J. Hum. Virol. 2:91-101. [PubMed] [Google Scholar]
- 34.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy, M., D. Auger, and S. R. Whittemore. 2000. Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J. Hum. Virol. 3:215-228. [PubMed] [Google Scholar]
- 36.McCarthy, M., L. Resnick, F. Taub, R. V. Stewart, and R. D. Dix. 1991. Infection of human neural cell aggregate cultures with a clinical isolate of cytomegalovirus. J. Neuropathol. Exp. Neurol. 50:441-450. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy, M., C. Wood, L. Fedoseyeva, and S. R. Whittemore. 1995. Media components influence viral gene expression assays in human fetal astrocyte cultures. J. Neurovirol. 1:275-285. [DOI] [PubMed] [Google Scholar]
- 38.Natali, A., P. Valcavi, M. C. Medici, E. Dieci, S. Montali, and C. Chezzi. 1997. Cytomegalovirus infection in an Italian population: antibody prevalence, virus excretion and maternal transmission. New Microbiol. 20:123-133. [PubMed] [Google Scholar]
- 39.Odeberg, J., H. Browne, S. Metkar, C. J. Froelich, L. Brandén, D. Cosman, and C. Söderberg-Nauclér. 2003. The human cytomegalovirus protein UL16 mediates increased resistance to natural killer cell cytotoxicity through resistance to cytolytic proteins. J. Virol. 77:4539-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odeberg, J., B. Plachter, L. Branden, and C. Söderberg-Naucler. 2003. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 101:4870-4877. [DOI] [PubMed] [Google Scholar]
- 41.Pass, R. F., S. Stagno, G. J. Myers, and C. A. Alford. 1980. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758-762. [PubMed] [Google Scholar]
- 42.Peckham, C. S. 1991. Cytomegalovirus infection: congenital and neonatal disease. Scand. J. Infect. Dis. Suppl. 80:82-87. [PubMed] [Google Scholar]
- 43.Perlman, J. M., and C. Argyle. 1992. Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann. Neurol. 31:64-68. [DOI] [PubMed] [Google Scholar]
- 44.Pleskoff, O., P. Casarosa, L. Verneuil, F. Ainoun, P. Beisser, M. Smit, R. Leurs, P. Schneider, S. Michelson, and J. C. Ameisen. 2005. The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. FEBS J. 272:4163-4177. [DOI] [PubMed] [Google Scholar]
- 45.Poland, S. D., L. L. Bambrick, G. A. Dekaban, and G. P. Rice. 1994. The extent of human cytomegalovirus replication in primary neurons is dependent on host cell differentiation. J. Infect. Dis. 170:1267-1271. [DOI] [PubMed] [Google Scholar]
- 46.Poland, S. D., P. Costello, G. A. Dekaban, and G. P. Rice. 1990. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J. Infect. Dis. 162:1252-1262. [DOI] [PubMed] [Google Scholar]
- 47.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 85:3555-3567. [DOI] [PubMed] [Google Scholar]
- 48.Senechal, B., A. M. Boruchov, J. L. Reagan, D. N. Hart, and J. W. Young. 2004. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood 103:4207-4215. [DOI] [PubMed] [Google Scholar]
- 49.Shinmura, Y., S. Aiba-Masago, I. Kosugi, R. Y. Li, S. Baba, and Y. Tsutsui. 1997. Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Am. J. Pathol. 151:1331-1340. [PMC free article] [PubMed] [Google Scholar]
- 50.Shinmura, Y., I. Kosugi, S. Aiba-Masago, S. Baba, L. R. Yong, and Y. Tsutsui. 1997. Disordered migration and loss of virus-infected neuronal cells in developing mouse brains infected with murine cytomegalovirus. Acta Neuropathol. (Berlin) 93:551-557. [DOI] [PubMed] [Google Scholar]
- 51.Sindre, H., H. Rollag, M. K. Olafsen, M. Degre, and K. Hestdal. 2000. Human cytomegalovirus induces apoptosis in the hematopoietic cell line MO7e. APMIS 108:223-230. [DOI] [PubMed] [Google Scholar]
- 52.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 98:7829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Söderberg-Naucler, C., and J. Y. Nelson. 1999. Human cytomegalovirus latency and reactivation—a delicate balance between the virus and its host's immune system. Intervirology 42:314-321. [DOI] [PubMed] [Google Scholar]
- 54.Spiller, O. B., L. K. Borysiewicz, and B. P. Morgan. 1997. Development of a model for cytomegalovirus infection of oligodendrocytes. J. Gen. Virol. 78:3349-3356. [DOI] [PubMed] [Google Scholar]
- 55.Stagno, S., R. F. Pass, G. Cloud, W. J. Britt, R. E. Henderson, P. D. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 56.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 57.Van Den Pol, A. N., E. Mocarski, N. Saederup, J. Vieira, and T. J. Meier. 1999. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J. Neurosci. 19:10948-10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Den Pol, A. N., J. Vieira, D. D. Spencer, and J. G. Santarelli. 2000. Mouse cytomegalovirus in developing brain tissue: analysis of 11 species with GFP-expressing recombinant virus. J. Comp. Neurol 427:559-580. [DOI] [PubMed] [Google Scholar]
- 59.Waldman, W. J., D. A. Knight, E. H. Huang, and D. D. Sedmak. 1995. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J. Infect. Dis. 171:263-272. [DOI] [PubMed] [Google Scholar]
- 60.Weller, T. H. 1971. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N. Engl. J. Med. 285:267-274. [DOI] [PubMed] [Google Scholar]
- 61.Wentworth, B. B., and L. French. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253-258. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto-Tabata, T., S. McDonagh, H.-T. Chang, S. Fisher, and L. Pereira. 2004. Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J. Virol. 78:2831-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]