Abstract
Platelets can engulf human immunodeficiency virus type 1 (HIV-1), and a significant amount of HIV-1 in the blood of infected individuals is associated with these cells. However, it is unclear how platelets capture HIV-1 and whether platelet-associated virus remains infectious. DC-SIGN and other lectins contribute to capture of HIV-1 by dendritic cells (DCs) and facilitate HIV-1 spread in DC/T-cell cocultures. Here, we show that platelets express both the C-type lectin-like receptor 2 (CLEC-2) and low levels of DC-SIGN. CLEC-2 bound to HIV-1, irrespective of the presence of the viral envelope protein, and facilitated HIV-1 capture by platelets. However, a substantial fraction of the HIV-1 binding activity of platelets was dependent on DC-SIGN. A combination of DC-SIGN and CLEC-2 inhibitors strongly reduced HIV-1 association with platelets, indicating that these lectins are required for efficient HIV-1 binding to platelets. Captured HIV-1 was maintained in an infectious state over several days, suggesting that HIV-1 can escape degradation by platelets and might use these cells to promote its spread. Our results identify CLEC-2 as a novel HIV-1 attachment factor and provide evidence that platelets capture and transfer infectious HIV-1 via DC-SIGN and CLEC-2, thereby possibly facilitating HIV-1 dissemination in infected patients.
Binding of human immunodeficiency virus type 1 (HIV-1) to cell surface factors other than the viral receptor CD4 and a chemokine coreceptor does not allow viral entry but can profoundly impact viral infectivity, particularly when the viral receptors are expressed at low levels (14, 57). The interaction of HIV-1 with cellular factors that promote viral attachment (termed attachment factors) can increase HIV-1 infection of the cells expressing these factors (termed infection in cis) or can augment infection of adjacent cells (termed infection in trans or transmission). Attachment factors promote HIV-1 infection most likely by concentrating viruses on the cell surface (14, 38, 57), thereby increasing the chance that the viral envelope protein (Env) can engage CD4 and a coreceptor, presented both in cis and in trans. Additionally, binding to certain attachment factor-expressing cells can conserve viral infectivity over several days, and during this time virus can be transmitted to susceptible cells (14).
The concept of HIV-1 capture by attachment factors, conservation of viral infectivity, and subsequent transfer of bound virions to permissive cells has initially been proposed for dendritic cells (DCs) expressing the lectin dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) (17). Thus, it was shown that capture of HIV-1 by DCs profoundly augments HIV-1 infectivity for T cells (7, 8), a mechanism proposed to facilitate HIV-1 spread (8, 17), and DC-SIGN, which binds to the HIV-1 Env (17), was found to be involved in the HIV-1 association with DCs (1, 3, 17, 23, 31, 33, 62). Initial reports suggested that DC-SIGN might capture and internalize HIV-1 into a low-pH endosomal compartment where infectivity is preserved (28). Subsequently, the intracellularly stored viruses could be transferred to T cells, which requires the formation of an infectious synapse—a microenvironment formed at the interface between DC and T cell that serves as a conduit for HIV-1 transfer (25, 34). The importance of DC-SIGN-mediated HIV-1 transport into a low-pH compartment for virus transfer to T cells has recently been challenged (37). Also, evidence that the proposed conservation of HIV-1 infectivity might actually be due to productive infection of transmitting cells has been reported (37, 56). Nevertheless, it is undisputed that DC-SIGN captures HIV-1 and augments viral infectivity, at least in some cell culture systems, and an association between polymorphisms in the DC-SIGN gene and risk of HIV-1 infection has been documented (31, 33). Therefore, DC-SIGN constitutes a potential target for therapeutic or preventive intervention.
Despite the contribution of DC-SIGN to HIV-1 transmission by DCs, other attachment factors are also involved in this process (20, 55, 60). A role for the lectins mannose receptor and langerin, which are expressed on DC subsets, has been suggested previously (55). However, evidence that these lectins facilitate DC binding to infectious HIV-1 is lacking and the factors responsible remain to be identified. Notably, several CD4-negative cell types, including platelets and red blood cells, can bind and transmit HIV-1 (39, 40), suggesting that cells other than DCs might also express HIV-1 attachment factors.
Attachment of HIV-1 to platelets might be particularly efficient, since these cells retain a substantial fraction of HIV-1 present in whole-blood preparations from infected patients (29). Binding of HIV-1 to platelets can result in virus uptake (63, 64), which could explain the efficient HIV-1 retention by these cells. The fate of HIV-1 engulfed by platelets is at present unclear. Since different compartments within platelets harbor either intact or degraded HIV-1 (6), it is conceivable that platelets either facilitate viral dissemination or decrease the number of infectious HIV-1 particles in the bloodstream (6, 63). Moreover, HIV-1 interactions with platelets might contribute to thrombocytopenia, which is defined by less than 15 × 104 platelets/μl on at least two consecutive counts and is a relatively common condition among HIV-1 patients (35, 49). Analysis of the mechanisms of HIV-1 attachment to platelets might therefore reveal important aspects of the role of platelets in HIV-1 infection.
Here, we show that C-type lectin-like receptor 2 (CLEC-2) is expressed on platelets and contributes to HIV-1 capture by these cells. However, a substantial fraction of the HIV-1 binding activity of platelets was found to be due to DC-SIGN, which is expressed on platelets at low levels. HIV-1 attached to platelets remained infectious over a prolonged time, indicating that platelets might facilitate HIV-1 dissemination via the bloodstream.
MATERIALS AND METHODS
Plasmid construction.
The CLEC-2 coding sequence was reverse transcriptase PCR amplified from purified mRNA obtained from human liver tissue and cloned into pcDNA3.1 (Invitrogen). The amino acid sequence encoded by the CLEC-2 construct is identical to that specified in GenBank entry AF124841.
Cell culture.
Jurkat, DAMI, HEL, and B-THP cell lines and CEMx174 R5 cells were maintained in RPMI 1640 medium supplemented with fetal calf serum (FCS) and antibiotics. CEMx174 R5 cells express endogenous CXCR4 and exogenous CCR5 and express luciferase and green fluorescent protein under control of the HIV promoter (22). 293T, 293T-REx, Huh-7, Hep-2, HFF, Vero, COS-7, HOS, HeLa, MCR5, and U373 cells were cultured in Dulbecco's modified Eagle's medium or minimal essential medium (HFF) supplemented with FCS and antibiotics.
Preparation of platelets, PBMCs, and DCs.
For preparation of platelets, blood samples were collected in vials containing EDTA and centrifuged at 200 × g at room temperature. The upper platelet-rich plasma was collected and centrifuged at 1,200 × g for 20 min at room temperature. The pellet was then washed in phosphate-buffered saline and the platelet count determined. Alternatively, platelets were obtained from platelet concentrates prepared for administration to human patients. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by use of Ficoll gradient centrifugation, resuspended in RPMI medium supplemented with 10% FCS and antibiotics, and either stimulated with phytohemagglutinin at 5 μg/ml and interleukin-2 (IL-2) at 100 U/ml for 3 days or maintained in medium for 3 days. DCs were derived from monocytes by treatment with 800 U/ml granulocyte-macrophage colony-stimulating factor (Wyeth) and 250 U/ml IL-4 (Strathmann), as previously described (46). DCs were matured by a 48-h incubation in medium supplemented with 200 U/ml IL-1β (Strathmann), 1 μg/ml prostaglandin E2 (Sigma), 10 ng/ml tumor necrosis factor alpha (Strathmann), 40 U/ml granulocyte-macrophage colony-stimulating factor, and 250 U/ml IL-4. The identity of the cells was confirmed by fluorescence-activated cell sorter analyses of the surface markers CD83, CD86, and major histocompatibility complex class II.
Viruses.
Plasmid HIV-1 NL4-3 Luc encodes a replication-competent variant of the laboratory-adapted CXCR4-tropic molecular HIV-1 clone NL4-3, in which the nef gene has been replaced by luciferase (42). Plasmid pNL4-3-Luc-R−E− (13) is an NL4-3 Luc-related construct in which the env and vpr genes were inactivated. NL4-3 Luc and NL4-3-Luc-R−E− virus stocks were generated by CaPO4 transfection of 293T cells as described previously (50). Alternatively, NL4-3 Luc was amplified in CEMx174 R5 cells or PBMCs. The primary HIV-1 isolates 92/BR/020, 93/BR/020, and 92/HT/596 (obtained via the AIDS Research and Reference Reagent Program) were amplified in CEMx174 R5 cells. All virus stocks were sterile filtered by employing filters of 0.4 μm pore size, aliquoted, and stored at −80°C.
HIV-1 binding and transmission.
Platelets, B-THP cell lines, or transiently transfected 293T cells were resuspended in medium supplemented with 10% FCS. Cells (1 × 105 for platelets or 3 × 104 for B-THP or 293T cells) were incubated with 5 ng or 10 ng of replication-competent HIV-1 NL4-3 Luc reporter virus or primary HIV-1 isolates for 3 h at 37°C, and unbound virus was subsequently removed by washing. In order to determine transmission, the cells were then cocultivated with CEMx174 R5 target cells, which express luciferase under control of the viral promoter, and luciferase activities in cell lysates were determined 3 days after cocultivation by employing a commercially available kit (Promega). Alternatively, the cells were lysed in 1% Triton X-100, and HIV-1 binding was assessed by quantification of the p24 content in the cellular lysates with a p24 antigen capture enzyme-linked immunosorbent assay (Murex). For inhibition of HIV-1 transmission, platelets were preincubated for 1 h at 37°C with goat serum specific for the CLEC-2 ectodomain (R&D Systems) and/or monoclonal antibody (MAb) 526 (DC-SIGN/R specific) (R&D Systems) or control MAbs or sera at a final concentration of 10 μg/ml. Alternatively, platelets were preincubated with different dilutions of the mannose polymer mannan (Sigma) or control carbohydrates.
Flow cytometric analysis for CLEC-2 and DC-SIGN expression.
Cell surface expression of CLEC-2 and DC-SIGN on platelets was detected using anti-CLEC-2 MAb 13B10 or 13H11 and anti-DC-SIGN MAb 507 (DC-SIGN specific) in combination with Alexa Fluor 647-labeled secondary antibody (Invitrogen). Expression of CLEC-2 on cell lines and primary cells was determined identically; however, a phycoerythrin-coupled secondary antibody was employed for detection (Vector). Isotype-matched MAbs were included as negative controls. Staining was analyzed by flow cytometry employing a FACScan cytometer (Becton Dickinson).
Immunostaining.
CLEC-2 transfected and untransfected B-THP cell pellets were fixed in formalin, paraffin wax embedded, and immunostained as described for tissues. Anonymized sections of liver and bone marrow were obtained from the Department of Histopathology, John Radcliffe Hospital, Oxford, United Kingdom, with local Research Ethics Committee approval and were immunostained as described previously (44) with mouse MAb DC28, mouse MAb anti-CD61 (clone Y2/51; Dako), or goat polyclonal anti-CLEC-2 (R&D Systems) and detected using standard staining kits from Dako and Vector Laboratories. Images were taken at room temperature with an Olympus BX40 microscope equipped with a 40×/0.75 0.17 lens and a Nikon Coolpix 950 digital camera. Images were analyzed with Adobe Photoshop version 7 software.
RESULTS
Platelets and megakaryocytes express CLEC-2.
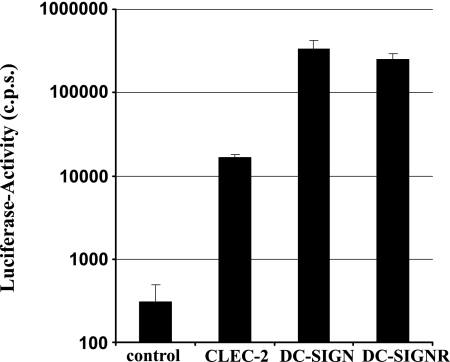
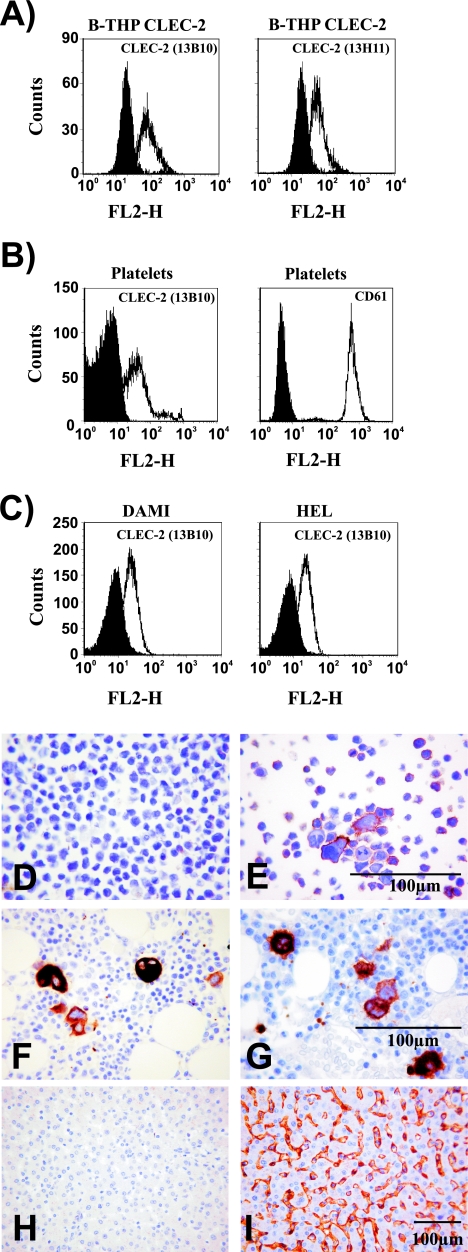
Attachment factors other than DC-SIGN can contribute to HIV-1 interactions with DCs and possibly other cell types (40, 55). In order to identify novel HIV-1 attachment factors, we tested several lectins for their ability to transmit HIV-1 to susceptible cells. Analysis of HIV-1 transmission by 293T cells transiently expressing lectins revealed that cells expressing CLEC-2 transmit HIV-1 to cocultivated T cells, albeit with lower efficiency than DC-SIGN or the related protein DC-SIGNR/L-SIGN (Fig. 1), indicating that CLEC-2, similarly to DC-SIGN, might function as an HIV-1 attachment factor. Expression of CLEC-2 mRNA in bone marrow, DCs, and liver has been documented previously (12), and in a recent study we found CLEC-2 protein on platelets (54). However, CLEC-2 protein expression has not been analyzed systematically. In order to assess whether CLEC-2 is expressed on cell types involved in HIV-1 infection, we immunized mice with purified CLEC-2 ectodomain and generated two MAbs as described previously (4). Staining with both MAbs detected CLEC-2 on the surface of stably transfected B-THP cells (a Raji-derived B-cell line [61]) (Fig. 2A) and 293T-REx cells, the latter expressing CLEC-2 in a doxycycline-inducible fashion (data not shown). In contrast, no staining was observed with B-THP cells expressing DC-SIGN or DC-SIGNR (data not shown), indicating that these MAbs are adequate tools to study CLEC-2 expression. Flow cytometric analyses of a panel of cell lines and primary cells, including immature and mature DCs, yielded no evidence for appreciable endogenous CLEC-2 expression (Table 1). However, CLEC-2 was readily detectable on the surface of platelets (Fig. 2B) and megakaryocytic cell lines (Fig. 2C). The identity and purity of the platelet preparations analyzed were confirmed by staining for CD61 (Fig. 2B), a marker for platelets and megakaryocytes. CLEC-2 expression was further investigated by staining of tissue sections with a goat serum raised against the CLEC-2 ectodomain. The specificity of the serum was confirmed by staining of B-THP CLEC-2 cells (Fig. 2D and E). In agreement with the expression of CLEC-2 on megakaryocytic cell lines and the previously reported CLEC-2 mRNA expression in bone marrow and liver (12), megakaryocytes (Fig. 2F and G) and liver sinusoidal endothelial cells (LSECs) (Fig. 2H and I) were positive for CLEC-2. Thus, megakaryocytes and LSECs, which are both permissive to HIV-1 infection (11, 53, 59), express CLEC-2, and HIV-1 might employ this lectin to promote its attachment to these cell types. Moreover, CLEC-2 is present on the surface of platelets and might contribute to the previously documented HIV-1 uptake by these cells (63).
FIG. 1.
CLEC-2 facilitates HIV-1 transmission by 293T cells. The indicated lectins and a control vector were transiently expressed in 293T cells, and the cells were incubated with NL4-3 Luc, washed, and cocultivated with CEMx174 R5 target cells. Luciferase activities in cell lysates were determined 3 days after cocultivation. A representative experiment performed in triplicate is shown, and error bars indicate standard deviations. Similar results were obtained in two independent experiments. c.p.s., counts per second.
FIG. 2.
Platelets and megakaryocytes express CLEC-2. (A) B-THP CLEC-2 cells were stained with anti-CLEC-2 antibodies (white) or isotype-matched control antibodies (black) and analyzed by flow cytometry. Similar results were obtained in three independent experiments. (B) Flow cytometric analyses of CLEC-2 (white, left panel) and CD61 (white, right panel) expression on platelets. Staining with isotype-matched control antibodies is shown in black. The results were confirmed in five independent experiments with platelet preparations from different donors. Identical results were obtained upon staining with antibody 13H11. (C) The megakaryocytic cell lines DAMI and HEL were stained with anti-CLEC-2 (white) or isotype-matched control antibody (black) and analyzed by flow cytometry. Similar results were obtained in two independent experiments. FL2-H, fluorescence in channel 2-height. (D to I) Sections of human tissue or B-THP CLEC-2 cells were formalin fixed, paraffin embedded, and immunostained (brown) for CLEC-2 or CD61 or immunostained with omission of the primary antibody as a control. B-THP CLEC-2 cells were control stained (D) or stained with CLEC-2-specific antiserum (E). Bone marrow was stained for CD61 (F) or CLEC-2 (G). Liver sections were control stained (H) or stained for CLEC-2 (I).
TABLE 1.
CLEC-2 expression in a panel of cell lines and primary cells
| Cell line or type | CLEC-2 expressiona |
|---|---|
| Cell lines | |
| Huh-7 | − |
| Hep-2 | − |
| Vero | − |
| COS-7 | − |
| 293T | − |
| 293T-REx | − |
| 293T-REx CLEC-2 | + |
| Jurkat | − |
| CEMx174 R5 | − |
| B-THP | − |
| B-THP CLEC-2 | + |
| HOS | − |
| HeLa | − |
| HFF | − |
| MCR5 | − |
| U373 | − |
| DAMI | + |
| HEL | + |
| Primary cells | |
| Immature DCs | − |
| Mature DCs | − |
| Unstimulated PBMCs | − |
| Stimulated PBMCs | − |
| Platelets | + |
+, positive; −, negative.
CLEC-2 binds and transmits HIV-1.
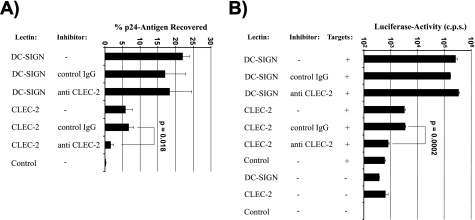
We next analyzed the CLEC-2 interaction with HIV-1 by employing stably transfected B-THP cell lines, since B-THP cells mediate robust DC-SIGN-dependent HIV transmission (61). Indeed, DC-SIGN-expressing B-THP cells bound HIV-1 efficiently, recovering about 22% of the input virus (Fig. 3A). CLEC-2-expressing cells also captured HIV-1, albeit less efficiently than DC-SIGN B-THP cells (about 6% of input virus recovered). Importantly, HIV-1 capture by CLEC-2 B-THP cells was specific, since antiserum against CLEC-2 strongly diminished HIV-1 binding to CLEC-2 B-THP cells but had no appreciable impact on virus capture by DC-SIGN B-THP cells (Fig. 3A). Capture of HIV-1 by attachment factor-expressing cells does not necessarily result in efficient transmission of virus to susceptible cells (4, 43). Therefore, we investigated whether HIV-1 bound by CLEC-2 can be transferred to permissive cells. DC-SIGN-expressing B-THP cells transmitted HIV-1 over 400 times more efficiently than control cells, and transmission was not blocked by CLEC-2-specific antiserum (Fig. 3B). In contrast, the B-THP cell line expressing CLEC-2 transferred HIV-1 to target cells about fivefold more efficiently than control cells and transmission could be blocked by CLEC-2-specific antiserum. Since previous reports suggest that residual permissiveness of B-THP DC-SIGN cells to HIV-1 infection might confound results obtained in transmission experiments (37), we repeated the experiment with CLEC-2-expressing 293T-REx cell lines. These cells transmitted HIV-1 in a CLEC-2-dependent manner and with an efficiency similar to that of CLEC-2 B-THP cells but were nonpermissive to infection (data not shown), confirming that CLEC-2 can function as an attachment factor that promotes capture of infectious HIV-1.
FIG. 3.
CLEC-2 binds HIV-1, and bound virus is infectious for adjacent target cells. (A) B-THP cell lines were incubated with the indicated inhibitors and pulsed with p24-normalized HIV-1 NL4-3 Luc. Unbound virus was removed, cells were lysed, and the amount of p24 antigen in lysates was determined. The amount of virus recovered is expressed as a percentage of the input virus. A representative experiment is shown. Similar results were obtained in three independent experiments. (B) The experiment was conducted as described for panel A. However, after removal of unbound virus, platelets were cocultivated with CEMx174 R5 target cells (+) and luciferase activities in cell lysates determined 3 days after cocultivation. A representative experiment is shown. Results were confirmed in three independent experiments. Error bars represent standard deviations. P values were determined using a two-sided dependent-sample t test. IgG, immunoglobulin G; c.p.s., counts per second.
CLEC-2 binds HIV-1 irrespective of the presence of the viral Env protein.
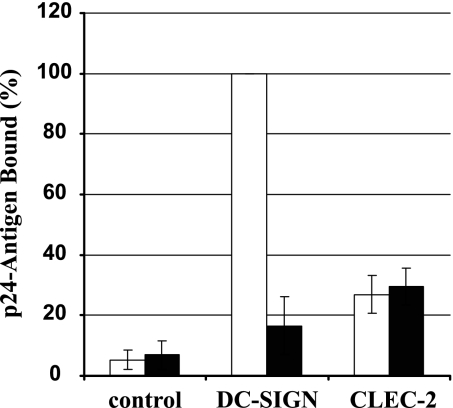
DC-SIGN binds to the HIV-1 Env protein in a carbohydrate-dependent fashion and facilitates transmission of bound virions to target cells (2, 58). We examined whether CLEC-2-dependent HIV-1 capture also requires the presence of the viral Env protein. To this end, lectin-expressing B-THP cell lines were incubated with p24-normalized HIV-1 with or without Env protein (Fig. 4). DC-SIGN-positive cells captured Env-containing HIV-1 with high efficiency, while binding of Env-deficient HIV-1 was close to background. In contrast, CLEC-2-expressing cells bound both Env-positive and Env-negative viruses with about fivefold-higher efficiency than control cells (Fig. 4), suggesting that CLEC-2 might interact with a cellular factor incorporated into the HIV-1 envelope and not with the viral Env protein. Indeed, DC-SIGN- but not CLEC-2-expressing B-THP cells bound to soluble HIV-1 Env, further underlining that CLEC-2 might not interact directly with HIV-1 Env (data not shown). However, CLEC-2 did not increase infection of 293T cells by HIV-1 reporter viruses bearing heterologous viral glycoproteins (data not shown), suggesting that CLEC-2 might not generally augment infectivity of HIV-1-derived viruses but might specifically promote HIV-1 transmission to target cells. The molecular mechanisms governing this process await further investigation.
FIG. 4.
Specificity of the HIV-1 interaction with CLEC-2. The indicated B-THP cell lines were incubated with p24-normalized HIV-1 NL4-3 Luc harboring Env (white bars) or Env-deficient, bald NL4-3-Luc-R−E− (black bars), washed, and lysed and the p24 content in lysates quantified. The efficiency of p24 binding is shown relative to capture of Env bearing NL4-3 Luc by B-THP DC-SIGN cells, which was set as 100%. The results represent the averages of three independent experiments performed with different virus stocks, and error bars indicate standard errors of the means.
DC-SIGN and CLEC-2 mediate HIV-1 binding and transfer by platelets.
Platelets can internalize HIV-1 (63, 64), and Polybrene-mediated attachment of HIV-1 to platelets increases viral infectivity (48), suggesting that HIV-1 association with platelets might modulate viral spread in peripheral blood cells. We therefore investigated whether platelets can retain infectious HIV-1 and whether CLEC-2 contributes to the HIV-1 interaction with platelets. When platelets were incubated with HIV-1 and unbound virus was removed, the virus-pulsed cells bound and transmitted HIV-1 to cocultivated susceptible cells with high efficiency and in a partly CLEC-2-dependent manner (Fig. 5A). Importantly, comparable CLEC-2-dependent HIV-1 transmission was observed with virus generated in 293T cells (Fig. 5A), CEMx174 R5 cells (a B/T-cell hybrid) (Fig. 5B, left panel), or PBMCs (Fig. 5B, right panel), the last constituting primary HIV-1 target cells, suggesting that CLEC-2 can facilitate platelet interactions with HIV-1 generated in T cells of infected patients. The capture of the laboratory-adapted, CXCR4-tropic HIV-1 strain NL4-3 by platelets was about 50% dependent on CLEC-2 (Fig. 5A and B). However, primary HIV-1 isolates differed in their levels of dependence on CLEC-2 for attachment to platelets. Indeed, 20 to 30% of the binding of isolates 92/BR/020 (subtype B) and 93/BR/020 (subtype F) (both viruses are CCR5-tropic) was mediated by CLEC-2, while capture of 92/HT/596 (R5X4-tropic) seemed to be independent of CLEC-2 (Fig. 5C). Thus, CLEC-2 on platelets interacts with HIV-1 in an isolate-dependent manner, possibly due to differential incorporation of cellular factors into the viral envelope, and promotes capture of infectious HIV-1 by these cells.
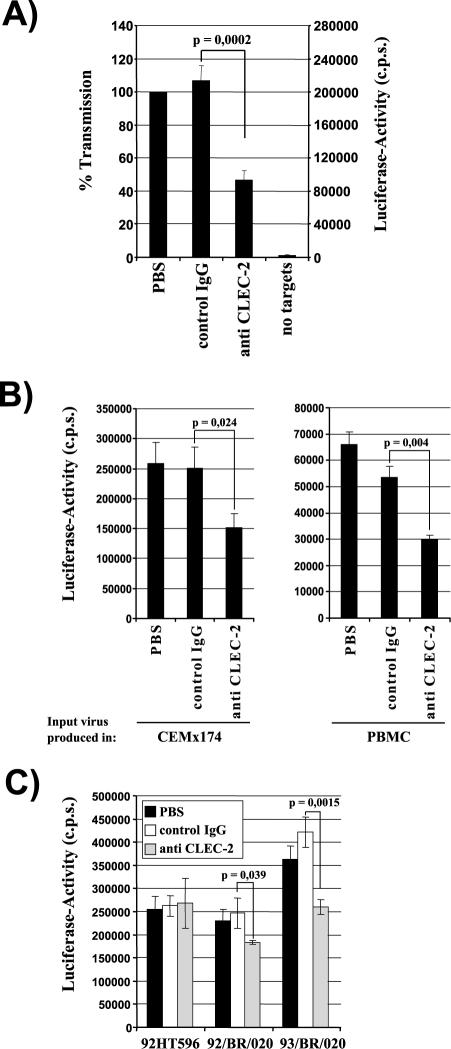
FIG. 5.
CLEC-2 contributes to HIV-1 capture by platelets. (A) Platelets were incubated with phosphate-buffered saline (PBS), control serum, or anti-CLEC-2 serum and pulsed with HIV-1 NL4-3 Luc generated in 293T cells. After unbound virus was removed, CEMx174 R5 target cells were added and luciferase activities in culture lysates determined 3 days after cocultivation. The averages of seven independent experiments are shown. Error bars indicate standard errors of the means. (B) The transmission experiment was performed as described for panel A, but NL4-3 Luc generated in CEMx174 R5 cells (left panel) or PBMCs (right panel) was employed. The results of representative experiments performed in triplicate are presented, and error bars indicate standard deviations. Similar results were obtained in an independent experiment. (C) HIV-1 capture was assessed as described for panel A, using the indicated primary HIV-1 isolates generated in CEMx174 R5 cells. A representative experiment is shown. The results were confirmed in an independent experiment with a different platelet preparation. Error bars indicate standard deviations. P values were determined using a two-sided (A) independent- or (B and C) dependent-sample t test. IgG, immunoglobulin G; c.p.s., counts per second.
Since only a portion of the HIV-1 capture activity by platelets seemed to be CLEC-2 dependent, we asked whether other lectins contribute to the HIV-1 interaction with platelets. Preincubation with the mannose polymer mannan substantially reduced HIV-1 capture by platelets, while glucose or fucose had no effect (Fig. 6A), suggesting that the HIV-1 interaction with these cells is to a large degree mediated by mannose-specific lectins. Therefore, we investigated whether the mannose-specific lectin DC-SIGN is expressed on platelets and promotes HIV-1 interaction with these cells. Immunostaining of tissue sections revealed that megakaryocytes (Fig. 7A and B) and some platelets (Fig. 7C and D) express DC-SIGN, and low levels of DC-SIGN were also detected on the surface of platelet preparations by flow cytometry (Fig. 7E). When retention of HIV-1 NL4-3 by platelets and subsequent transfer to the permissive cell line CEMx174 R5 were analyzed, antibodies directed against DC-SIGN diminished HIV-1 capture by platelets by about 80%, and addition of antiserum directed against CLEC-2 further reduced HIV-1 capture close to background levels (Fig. 6B), indicating that DC-SIGN and, to a lesser degree, CLEC-2 can be sufficient to mediate the HIV-1 interaction with platelets.
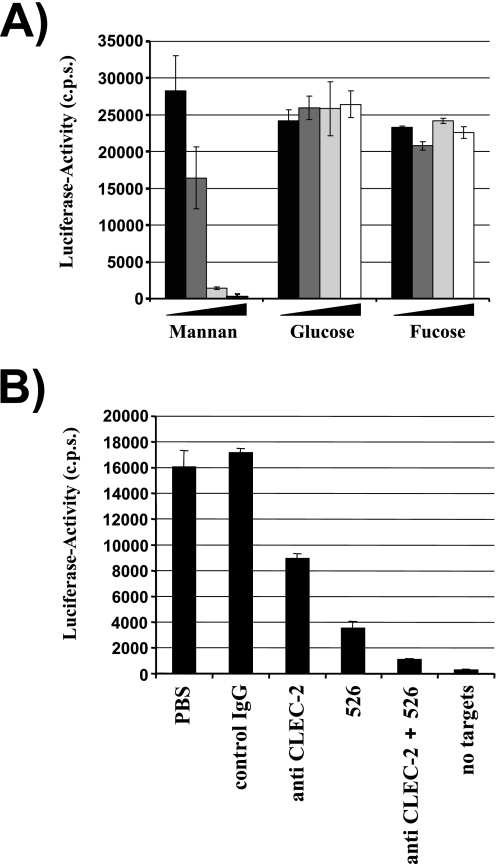
FIG. 6.
A substantial fraction of the HIV-1 capture activity of platelets is dependent on DC-SIGN. (A) The capture assay was carried out as described in the legend for Fig. 5. However, platelets were incubated with rising concentrations of mannan, glucose, or fucose before virus was added. The following carbohydrate concentrations were employed: 0 μg/ml (black bars), 5 μg/ml (dark-gray bars), 10 μg/ml (light-gray bars), and 20 μg/ml (white bars). Similar results were obtained in an independent experiment. (B) HIV-1 capture by platelets was assessed as described for panel A. However, cells were incubated with the indicated inhibitors (MAb 526 blocks ligand binding to DC-SIGN) before addition of virus. Similar results were obtained in three independent experiments. Error bars indicate standard deviations. PBS, phosphate-buffered saline; IgG, immunoglobulin G; c.p.s., counts per second.
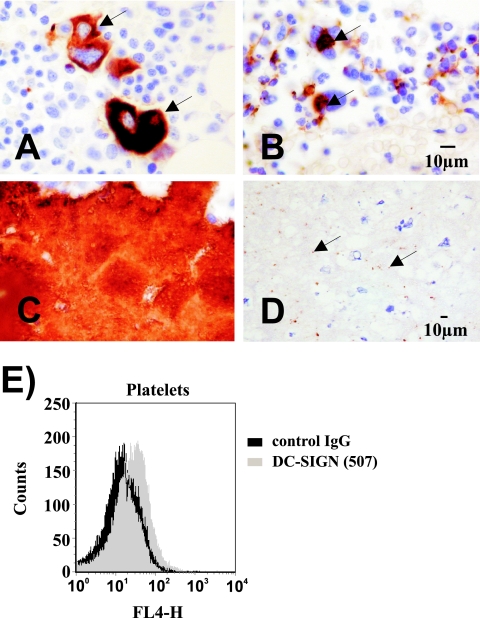
FIG. 7.
Megakaryocytes (arrows in panels A and B) and platelets (C and D) express DC-SIGN. (A to D) Sections of human bone marrow were immunostained (using the methodology described in the legend for Fig. 2) with anti-DC-SIGN (B and D) or anti-CD61 (A and C). DC-SIGN stains megakaryocytes (B, arrows) and a subpopulation of platelets (D, arrows). (E) Platelets were stained with the indicated anti-DC-SIGN MAb, and staining was analyzed by flow cytometry. Similar results were obtained in three independent experiments with different platelet preparations. IgG, immunoglobulin G; FL4-H, fluorescence in channel 4-height.
Finally, we asked whether the association of HIV-1 with platelets augments and/or conserves viral infectivity. Platelets failed to augment HIV-1 infection of cocultured target cells when virus, platelets, and target cells were mixed simultaneously (Fig. 8, 0 h). However, when virus was first incubated with platelets over 24 or 48 h and CEMx174 R5 target cells were added thereafter, moderate conservation of infectivity was observed (Fig. 8). A combination of antibodies against DC-SIGN and CLEC-2 substantially diminished preservation of infectivity, suggesting that HIV-1 attachment to these factors is important for HIV-1 stabilization by platelets. Thus, platelets can capture HIV-1 via DC-SIGN and CLEC-2 and maintain bound virus in an infectious state, a function that might augment HIV-1 dissemination via the bloodstream.
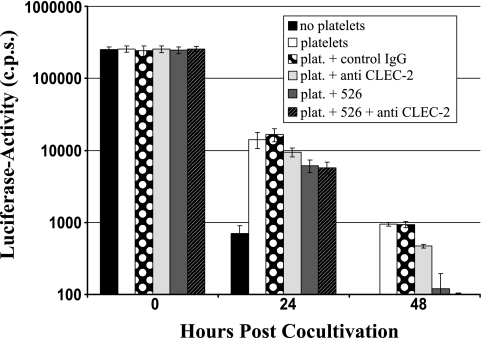
FIG. 8.
Platelets conserve HIV-1 infectivity in a DC-SIGN- and CLEC-2-dependent manner. HIV-1 NL4-3 Luc was incubated with platelets, cell-free medium (no platelets), or platelets (plat.) preincubated with the indicated inhibitors (MAb 526 blocks ligand binding to DC-SIGN). Subsequently, CEMx174 R5 target cells were added at the indicated time points and luciferase activities in cell lysates determined 3 days after cocultivation. The results are representative of three independent experiments. Error bars indicate standard deviations. IgG, immunoglobulin G; c.p.s., counts per second.
DISCUSSION
Evidence that platelets retain an appreciable fraction of blood-borne HIV-1 has been reported previously (29). The association of HIV-1 with platelets might facilitate viral spread via the bloodstream and might contribute to the development of thrombocytopenia, which frequently afflicts HIV-1/AIDS patients (49). Despite its potentially important impact on HIV-1 pathogenesis, the interaction of HIV-1 with platelets has been characterized incompletely on the molecular level. We show that platelets can employ the lectins CLEC-2 and DC-SIGN to capture HIV-1 and that bound virus remains infectious for at least 2 to 3 days (Fig. 8 and data not shown), a sufficient time frame to allow efficient platelet-mediated HIV-1 dissemination via the bloodstream.
DC-SIGN is a C-type (calcium-dependent) lectin that recognizes adequately spaced mannose and fucose groups displayed by endogenous and exogenous ligands, like the heavily glycosylated HIV-1 Env protein (21, 58). DC-SIGN is expressed mainly by DCs in the anogenital mucosa and in lymphoid tissue (16, 18, 24), albeit recent reports propose that DC-SIGN-positive cells in lymphoid tissue exhibit characteristics of macrophages (19, 27). Moreover, DC-SIGN is found on certain types of tissue macrophages (51, 52). Our results, confirmed by a recent study (6), indicate that megakaryocytes and platelets also express DC-SIGN (Fig. 7) and that DC-SIGN is important for binding of platelets to HIV-1 (Fig. 6 and 8). Whether platelets capture other pathogens known to bind to DC-SIGN remains to be determined. Since it has been suggested that hepatitis C virus, which is recognized by DC-SIGN (15, 32, 45), attaches to platelets (47), the molecular analysis of this interaction seems to be of particular interest.
Six cysteine residues in the ligand binding domain of CLEC-2 are conserved among known C-type lectin-like molecules (12). However, CLEC-2 lacks the amino acid motif known to mediate calcium complexation and carbohydrate binding by C-type lectins (12). Therefore, the structures recognized by CLEC-2 on the cellular surface and on HIV-1 particles await clarification. Our observation that CLEC-2 binds HIV-1 irrespective of the presence of the viral Env protein suggests that CLEC-2 mediates HIV-1 attachment to platelets by recognizing a cellular factor incorporated into the HIV-1 envelope upon viral budding from infected cells. This is not without precedence, since several cellular molecules are known to be incorporated into the viral envelope and to modulate HIV-1 infection by interacting with their cognate ligands on the surface of HIV-1-exposed cells (10, 57). In this regard, it is important to note that comparable CLEC-2-dependent HIV-1 transmission was observed with virus produced in the kidney-derived cell line 293T, the T/B-cell hybrid CEMx174, or primary T cells (Fig. 5A and B), indicating that the putative CLEC-2 ligand might be broadly expressed and that CLEC-2 on platelets can most likely capture HIV-1 generated in T cells of the peripheral blood of infected patients. Nevertheless, primary HIV-1 isolates exhibited differential CLEC-2 dependence for binding to platelets, suggesting that incorporation of the putative CLEC-2 ligand into the viral envelope might depend on the viral background. Indeed, it has been shown previously that HIV-1 strains grown in the same cell type incorporate host cell-derived HLA-DR with profoundly different efficiencies (9). Thus, examination of CLEC-2 binding of a broad variety of primary HIV-1 isolates and laboratory-adapted viruses will be required to further address this question. Apart from efficient CLEC-2 expression on the surface of platelets and megakaryocytes, CLEC-2 protein was also detected in LSECs (Fig. 2H and I). This cell type, which shares certain features with DCs, can efficiently take up soluble antigens from the bloodstream and can cross-present antigen to CD8 T cells, thereby inducing tolerance (30). It is conceivable that CLEC-2 contributes to antigen uptake by LSECs. Additionally, CLEC-2 might promote HIV-1 infection of LSECs, which are permissive to HIV-1 (53).
It has been hypothesized that upon mucosal transmission HIV-1 could be taken up by DCs in a DC-SIGN-dependent manner (17) and/or could infect DCs (56). DCs containing infectious HIV-1 could subsequently migrate into lymphoid tissue and transmit virus to adjacent T cells (17). However, analysis of the DC-SIGN promoter revealed that certain polymorphisms modulate the risk for parenterally acquired HIV-1 infection, while an association between polymorphisms and risk for mucosal HIV-1 infection was not observed (33). Therefore, DC-SIGN seems to impact HIV-1 spread mainly once the virus has reached the bloodstream, while passage of HIV-1 through the genital mucosa might be DC-SIGN independent. Such a model would be in agreement with efficient DC-SIGN-dependent HIV-1 capture and subsequent dissemination of the virus by platelets, which are abundant in peripheral blood. HIV-1 capture by platelets in the blood of infected individuals could in part also be due to CLEC-2, and it remains to be determined whether the CLEC-2 gene is polymorphic and whether polymorphisms alter the risk of HIV-1 infection.
Platelets are anucleate cell fragments produced by megakaryocytes and play a pivotal role in thrombosis and hemostasis (41). Reduced platelet counts are commonly found in the peripheral blood of HIV-1-infected individuals, and it is believed that several factors contribute to thrombocytopenia in HIV-1 infection (49). Thus, HIV-1 patients can generate antibodies that trigger platelet lysis (36), and association of immune complexes with platelets can also promote platelet destruction (5). Moreover, megakaryocytes express CD4 and the HIV-1 coreceptors CXCR4 and CCR5 and are susceptible to HIV-1 infection (49), which might reduce platelet production. Attachment factor engagement might facilitate HIV-1 infection of megakaryocytes. However, our analysis of bone marrow sections revealed intracellular but no unequivocal surface expression of DC-SIGN on megakaryocytes, and it remains to be determined if DC-SIGN contributes to HIV-1 infection of these cells. In contrast, CLEC-2 was detected on the surface of megakaryocytes in bone marrow (Fig. 2G) as well as on megakaryocytic cell lines and contributed to HIV-1 capture by the latter cells (data not shown). Therefore, CLEC-2 might modulate infection of megakaryocytes in HIV-1 patients and might thereby contribute to thrombocytopenia.
HIV-1 capture by DCs involves internalization of virions into intracellular vesicles and subsequent trafficking of HIV-1 to contact areas between DCs and T cells, so-called infectious synapses (25, 34). Infectious synapses are characterized by accumulation of virions on the DC side and concentration of CD4 and chemokine coreceptor on the T-cell side and thus provide a suitable microenvironment for efficient HIV-1 transfer (26). Platelets can also take up HIV-1, and intact virus is found in endocytic vesicles located relatively close to the cellular membrane (6, 63). It is thus conceivable that HIV-1-pulsed platelets, similarly to DCs, release virus upon contact with certain cell types and thereby allow HIV-1 transmission to susceptible cells. While DC-SIGN can endocytose antigens and promote HIV-1 uptake by platelets (6), endocytic HIV-1 uptake by CLEC-2 seems less likely. In fact, CLEC-2 is a novel type of signaling receptor which induces platelet activation in response to the snake toxin rhodocytin via a signaling pathway that involves Src kinases and leads to activation of Syk and phospholipase C γ2 (54). Whether CLEC-2 signaling plays a role in the interaction with HIV-1 is currently unclear. However, our preliminary results indicate that deletion of cytoplasmic amino acid residues critical for CLEC-2 signaling does not interfere with HIV-1 transfer by cell lines (data not shown), suggesting that signaling might be dispensable for CLEC-2-dependent HIV-1 transmission.
In summary, CLEC-2 is a novel HIV-1 attachment factor which promotes virus capture by cell lines and platelets. The binding of HIV-1 to platelets is mediated by both CLEC-2 and DC-SIGN, and platelet-associated virus is infectious for a prolonged time, suggesting that platelets might promote HIV-1 spread in infected individuals.
Acknowledgments
Some of the reagents used in this study were obtained via the AIDS Research and Reference Reagent Program. We thank Robert W. Doms, B. Fleckenstein, and K. von der Mark for support, N. Landau for CEMx174 R5 cells, B. Schmidt for virus stocks, the Department of Transfusion Medicine for platelet preparations, and N. Finze for the p24 enzyme-linked immunosorbent assay.
This work was supported by grants from the DFG (Center grant no. 466) (to H.H., T.G., A.M., M.G., J.E., A.S., and S.P.), Graduiertenkolleg 1071 (to C.C.), the Medical Research Fund, Oxford University (to E.J.S. and P.S.), and MEXT (no. 16790533 to K.S.-I.). S.P.W. is a BHF chair, and G.L.F. holds a BHF studentship.
REFERENCES
- 1.Arrighi, J. F., M. Pion, M. Wiznerowicz, T. B. Geijtenbeek, E. Garcia, S. Abraham, F. Leuba, V. Dutoit, O. Ducrey-Rundquist, Y. van Kooyk, D. Trono, and V. Piguet. 2004. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 78:10848-10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baribaud, F., R. W. Doms, and S. Pöhlmann. 2002. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets 6:423-431. [DOI] [PubMed] [Google Scholar]
- 3.Baribaud, F., S. Pöhlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baribaud, F., S. Pöhlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettaieb, A., P. Fromont, F. Louache, E. Oksenhendler, W. Vainchenker, N. Duedari, and P. Bierling. 1992. Presence of cross-reactive antibody between human immunodeficiency virus (HIV) and platelet glycoproteins in HIV-related immune thrombocytopenic purpura. Blood 80:162-169. [PubMed] [Google Scholar]
- 6.Boukour, S., J. M. Masse, L. Benit, A. Dubart-Kupperschmitt, and E. M. Cramer. 2006. Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. J. Thromb. Haemost. 4:426-435.. [DOI] [PubMed] [Google Scholar]
- 7.Cameron, P. U., U. Forsum, H. Teppler, A. Granelli-Piperno, and R. M. Steinman. 1992. During HIV-1 infection most blood dendritic cells are not productively infected and can induce allogeneic CD4+ T cells clonal expansion. Clin. Exp. Immunol. 88:226-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 9.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 10.Cantin, R., S. Methot, and M. J. Tremblay. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79:6577-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelucci, C., M. Federico, R. Guerriero, G. Mattia, I. Casella, E. Pelosi, U. Testa, G. Mariani, H. J. Hassan, and C. Peschle. 1998. Productive human immunodeficiency virus-1 infection of purified megakaryocytic progenitors/precursors and maturing megakaryocytes. Blood 91:1225-1234. [PubMed] [Google Scholar]
- 12.Colonna, M., J. Samaridis, and L. Angman. 2000. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur. J. Immunol. 30:697-704. [DOI] [PubMed] [Google Scholar]
- 13.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 14.Gallay, P. 2004. Syndecans and HIV-1 pathogenesis. Microbes Infect. 6:617-622. [DOI] [PubMed] [Google Scholar]
- 15.Gardner, J. P., R. J. Durso, R. R. Arrigale, G. P. Donovan, P. J. Maddon, T. Dragic, and W. C. Olson. 2003. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc. Natl. Acad. Sci. USA 100:4498-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., D. J. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353-357. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., A. Pritsker, M. Pack, I. Shimeliovich, J. F. Arrighi, C. G. Park, C. Trumpfheller, V. Piguet, T. M. Moran, and R. M. Steinman. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, Y., H. Feinberg, E. Conroy, D. A. Mitchell, R. Alvarez, O. Blixt, M. E. Taylor, W. I. Weis, and K. Drickamer. 2004. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 11:591-598. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, M., J. M. Harouse, A. Gettie, C. Buckner, J. Blanchard, and C. Cheng-Mayer. 2003. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J. Virol. 77:989-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson, B., F. Baribaud, S. Pöhlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolly, C., and Q. J. Sattentau. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643-650. [DOI] [PubMed] [Google Scholar]
- 27.Krutzik, S. R., B. Tan, H. Li, M. T. Ochoa, P. T. Liu, S. E. Sharfstein, T. G. Graeber, P. A. Sieling, Y. J. Liu, T. H. Rea, B. R. Bloom, and R. L. Modlin. 2005. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 29.Lee, T. H., R. R. Stromberg, J. W. Heitman, L. Sawyer, C. V. Hanson, and M. P. Busch. 1998. Distribution of HIV type 1 (HIV-1) in blood components: detection and significance of high levels of HIV-1 associated with platelets. Transfusion 38:580-588. [DOI] [PubMed] [Google Scholar]
- 30.Limmer, A., J. Ohl, C. Kurts, H. G. Ljunggren, Y. Reiss, M. Groettrup, F. Momburg, B. Arnold, and P. A. Knolle. 2000. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6:1348-1354. [DOI] [PubMed] [Google Scholar]
- 31.Liu, H., Y. Hwangbo, S. Holte, J. Lee, C. Wang, N. Kaupp, H. Zhu, C. Celum, L. Corey, M. J. McElrath, and T. Zhu. 2004. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J. Infect. Dis. 190:1055-1058. [DOI] [PubMed] [Google Scholar]
- 32.Lozach, P.-Y., H. Lortat-Jacob, A. de Lacroix de Lavalette, I. Staropoli, S. Foung, A. Amara, C. Houlès, F. Fieschi, O. Schwartz, J.-L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 278:20358-20366. [DOI] [PubMed] [Google Scholar]
- 33.Martin, M. P., M. M. Lederman, H. B. Hutcheson, J. J. Goedert, G. W. Nelson, Y. van Kooyk, R. Detels, S. Buchbinder, K. Hoots, D. Vlahov, S. J. O'Brien, and M. Carrington. 2004. Association of DC-SIGN promoter polymorphism with increased risk for parenteral, but not mucosal, acquisition of human immunodeficiency virus type 1 infection. J. Virol. 78:14053-14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 35.Miguez-Burbano, M. J., J. Jackson, Jr., and S. Hadrigan. 2005. Thrombocytopenia in HIV disease: clinical relevance, physiopathology and management. Curr. Med. Chem. Cardiovasc. Hematol. Agents 3:365-376. [DOI] [PubMed] [Google Scholar]
- 36.Nardi, M., S. Tomlinson, M. A. Greco, and S. Karpatkin. 2001. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell 106:551-561. [DOI] [PubMed] [Google Scholar]
- 37.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olinger, G. G., M. Saifuddin, M. L. Hart, and G. T. Spear. 2002. Cellular factors influence the binding of HIV type 1 to cells. AIDS Res. Hum. Retrovir. 18:259-267. [DOI] [PubMed] [Google Scholar]
- 40.Olinger, G. G., M. Saifuddin, and G. T. Spear. 2000. CD4-negative cells bind human immunodeficiency virus type 1 and efficiently transfer virus to T cells. J. Virol. 74:8550-8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Packham, M. A. 1994. Role of platelets in thrombosis and hemostasis. Can. J. Physiol. Pharmacol. 72:278-284. [DOI] [PubMed] [Google Scholar]
- 42.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pöhlmann, S., G. J. Leslie, T. G. Edwards, T. Macfarlan, J. D. Reeves, K. Hiebenthal-Millow, F. Kirchhoff, F. Baribaud, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus: virus binding and transfer are dissociable functions. J. Virol. 75:10523-10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pöhlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pöhlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prechtel, A. T., N. M. Turza, D. J. Kobelt, J. I. Eisemann, R. S. Coffin, Y. McGrath, C. Hacker, X. Ju, M. Zenke, and A. Steinkasserer. 2005. Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J. Gen. Virol. 86:1645-1657. [DOI] [PubMed] [Google Scholar]
- 47.Pugliese, A., L. Gennero, M. Cutufia, M. Enrietto, E. Morra, P. Pescarmona, and A. Ponzetto. 2004. HCV infective virions can be carried by human platelets. Cell Biochem. Funct. 22:353-358. [DOI] [PubMed] [Google Scholar]
- 48.Rusert, P., M. Fischer, B. Joos, C. Leemann, H. Kuster, M. Flepp, S. Bonhoeffer, H. F. Gunthard, and A. Trkola. 2004. Quantification of infectious HIV-1 plasma viral load using a boosted in vitro infection protocol. Virology 326:113-129. [DOI] [PubMed] [Google Scholar]
- 49.Scaradavou, A. 2002. HIV-related thrombocytopenia. Blood Rev. 16:73-76. [DOI] [PubMed] [Google Scholar]
- 50.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pöhlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 51.Soilleux, E. J., L. S. Morris, B. Lee, S. Pöhlmann, J. Trowsdale, R. W. Doms, and N. Coleman. 2001. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 195:586-592. [DOI] [PubMed] [Google Scholar]
- 52.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445-457. [PubMed] [Google Scholar]
- 53.Steffan, A. M., M. E. Lafon, J. L. Gendrault, C. Schweitzer, C. Royer, D. Jaeck, J. P. Arnaud, M. P. Schmitt, A. M. Aubertin, and A. Kirn. 1992. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 89:1582-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki-Inoue, K., G. L. J. Fuller, A. García, J. A. Eble, S. Pöhlmann, O. Inoue, T. K. Gartner, S. C. Hughan, A. C. Pearce, G. D. Laing, R. D. G. Theakston, E. Schweighoffer, N. Zitzmann, T. Morita, V. L. J. Tybulewicz, Y. Ozaki, and S. P. Watson. 2006. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 107:542-549. [DOI] [PubMed] [Google Scholar]
- 55.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pöhlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 56.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 57.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 58.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 59.Voulgaropoulou, F., S. E. Pontow, and L. Ratner. 2000. Productive infection of CD34+-cell-derived megakaryocytes by X4 and R5 HIV-1 isolates. Virology 269:78-85. [DOI] [PubMed] [Google Scholar]
- 60.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]
- 62.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youssefian, T., A. Drouin, J. M. Masse, J. Guichard, and E. M. Cramer. 2002. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 99:4021-4029. [DOI] [PubMed] [Google Scholar]
- 64.Zucker-Franklin, D., S. Seremetis, and Z. Y. Zheng. 1990. Internalization of human immunodeficiency virus type I and other retroviruses by megakaryocytes and platelets. Blood 75:1920-1923. [PubMed] [Google Scholar]