Abstract
The human polyomavirus JC virus (JCV) is the causative agent of the fatal demyelinating disease progressive multifocal leukoencephalopathy (PML), which is commonly seen in AIDS patients. The bicistronic viral RNA, which is transcribed at the late phase of infection, is responsible for expressing the viral capsid proteins and a small regulatory protein, agnoprotein. Immunohistochemical analysis of brain tissue from subjects with AIDS/PML revealed colocalization of the human immunodeficiency virus type 1 (HIV-1) transactivator, Tat, and JCV agnoprotein in nucleus and cytoplasm of “bizarre” astrocytes. In accord with this observation, we detected the copresence of agnoprotein and Tat in human astrocytes upon infection with JCV and HIV-1 or in astrocytic cells expressing these proteins after transfection. Interestingly, results from infection of human astrocytes with HIV-1 and JCV showed a decrease in the level of HIV-1 replication in cells that are coinfected with JCV. Conversely, a slight increase in the level of JCV replication was observed in the presence of HIV-1. The copresence of JCV and HIV-1 in astrocytes prompted us to investigate the possible cross-interaction of agnoprotein with Tat and its impact on HIV-1 gene transcription. Our results demonstrate that agnoprotein through its N-terminal domain associates with Tat and the interaction causes the suppression of Tat-mediated enhancement of HIV-1 promoter activity in these cells. Results from RNA and protein binding assays showed that agnoprotein can inhibit the association of Tat with its target RNA sequence, TAR, and with cyclin T1. Furthermore, agnoprotein is able to interfere with cross-interaction of Tat with the p65 subunit of NF-κB and Sp1, whose functions are critical for Tat activation of the long terminal repeat. These observations unravel a new pathway for the molecular interaction of these two viruses in biologically relevant cells in the brains of AIDS/PML patients.
Human immunodeficiency virus type 1 (HIV-1) invasion of the central nervous system (CNS) induces a variety of clinical abnormalities, including dementia, ataxia, and memory loss (51). Histologically, brain tissue samples from patients with HIV encephalopathy exhibit astrogliosis, cerebral vasculitis, neuronal loss, myelin pallor, formation of multinucleated giant cells, and apoptosis (50). Progressive multifocal leukoencephalopathy (PML) represents one of the most common complications of HIV-1 infection (5). PML is a subacute demyelinating disease that results from the cytolytic destruction of oligodendrocytes, the myelin-producing cells of the CNS, by the human neurotropic polyomavirus, JC virus (JCV) (68). PML is most often encountered in patients with immunocompromised conditions that alter T-cell-mediated immunity (68). At present, one of the most frequent underlying diseases in PML patients is AIDS. In fact, PML is now considered as an AIDS-defining illness (6), and there is a disproportionate incidence of PML in HIV-1-infected individuals, as it affects 4 to 8% of AIDS patients (5). The significantly higher incidence of PML in AIDS patients than in other immunosuppressed individuals has suggested that the presence of HIV-1 in the brain may directly or indirectly contribute to the pathogenesis of PML. Evidence for a direct role of HIV-1 in JCV activation comes from several studies showing up-regulation of the JCV late promoter by the HIV-1-encoded regulatory protein, Tat (12-14, 59).
Activation of the JCV late gene leads to the production of viral capsid proteins which eventually form virions and lyse the infected cells. In addition, the late region of JCV encodes a small, highly basic protein known as agnoprotein (35). Several studies have shown that agnoprotein, which is conserved among the members of the polyomavirus family, has a critical role in the regulation of viral gene expression and replication (35, 46, 52-56). Furthermore, agnoprotein has the ability to modulate certain important host cell functions, including cell cycle progression and DNA repair (20, 21). In addition to oligodendrocytes, JCV replicates in astrocytes in cell culture, and expression of its proteins in astrocytes of PML patients, with or without AIDS, has been attributed to an abnormal feature of these cells, so-called “bizarre” astrocytes. With respect to HIV-1, unlike its productive replication in microglia and resident macrophages in brain, astrocytes provide a poor host for viral replication (9, 39). Regardless, limited expression of the HIV-1 regulatory and envelope proteins has been detected in astrocytes of patients with CNS disorders (1, 57, 60, 65, 66). Thus, it is evident that in the brains of AIDS patients with PML, astroglial cells can serve as a unique site where both viruses may coexist during the course of the disease. With this notion and in light of earlier observations demonstrating cross-communication between JCV and HIV-1 through HIV-1 Tat protein, we designed experiments to investigate whether agnoprotein of JCV has the capacity to physically and functionally interact with HIV-1 Tat.
MATERIALS AND METHODS
Cell culture.
Primary human fetal astrocytes were prepared according to a modified procedure based on the methods of Cole and de Vellis (16) and Yong and Antel (79). Astrocytes were plated (2 × 105 in 60-mm dishes) and maintained in regular growth medium (Dulbecco's modified Eagle medium-F-12 supplemented with 15% fetal bovine serum). U-87MG (ATTC HTB14), a human glioblastoma cell line, was grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum. NIH 3T3 cells expressing JCV agnoprotein (agnopositive) and control agnonegative NIH 3T3 cell lines (20) and the HeLa cell line, containing an integrated HIV-1 long terminal repeat (LTR) with a chloramphenicol acetyltransferase gene have been described previously (73).
Transfection.
Primary astrocytes were transfected using FuGENE 6 transfection reagent (Roche, Inc., Indianapolis, IN). U-87MG cells and HL3T1 cells were transfected using the calcium phosphate precipitation method (30).
Plasmids.
pCMV-Tat; pGST-Tat expression vectors for deletion mutants 1-86, 1-72, 20-72, and 50-72; and reporter constructs based on pGL3-basic vector (Promega Corp., Madison, WI) were used. The plasmid containing full-length (−450 to +80) HIV LTR and its mutant variant with no TAR sequence (+3 to +80) were previously described (58). The HIVNL4-3GFPVpr construct was kindly provided by B. E. Sawaya and V. Planelles. The cyan fluorescent protein (CFP)-Tat construct has been described previously (19). pTR(AAV)-agnoprotein was generated by PCR amplification of the agnogene using forward (5′-TATGCGGCCGCTAATACGACTCACTATAGG-3′) and reverse (5′-TAGAATAGGGCCCTCTAGATGCATGCTCGA-3′) primers followed by enzymatic digestion of the PCR product by NotI endonuclease and subcloning of the resulting fragment into NotI-digested pTR-UF5 plasmid. pCMV-agnoprotein and its deletion mutants, pGEX1λT-agnoprotein and its deletion mutants, and pYFP-agnoprotein were previously described (20).
Antibodies.
Rabbit polyclonal antibody against JCV agnoprotein was previously described (24). Anti-HIV-1 Tat (R705) was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Anti-cyclin T1 (T-18), anti-Cdk9 (C-20), anti-p65 (F-6), and anti-Sp1 (PEP 2) antibodies were purchased from Santa Cruz Biotechnology. Anti-lamin A was from Cell signaling. Anti-α-tubulin, clone B512, was obtained from Sigma-Aldrich. Goat anti-rabbit immunoglobulin G (IgG)-phycoerythrin (PE)-conjugated secondary antibody was from Imgenex (San Diego, CA).
Coinfection.
For immunostaining, primary cultures of human fetal astrocytes in the log phase of growth were infected with the JR-FL strain of HIV-1 as follows. In all, 50 ng of p24-containing virus stock was added to every 1 × 106 cells. Cells were incubated with virus stock in a small volume of serum-free medium for 2 h at 37°C. The cells were then washed twice with phosphate-buffered saline (PBS), and fresh medium was added (Dulbecco's modified Eagle's medium-F-12 supplemented with 15% fetal bovine serum). After 1 day, the same cells were infected with the Turbo (Mad1/SVEdelta) strain of JCV at a multiplicity of infection of 1. Cells were incubated with virus stock in serum-free medium for 2 h at 37°C. The cells were then washed twice with PBS, and fresh medium was added. For flow cytometric analysis, human primary culture of astrocytes was transduced with HIV-1(NL4-3) green fluorescent protein (GFP)-Vpr (72). After 24 h, cells were infected with Mad-1/SVEdelta JCV at a multiplicity of infection of 1.0 (67). In parallel, control uninfected cells or cells infected with JCV or HIV-1 alone were prepared at day 7.
Preparation of cellular protein extracts.
For preparation of whole-cell extract, cells were lysed for 30 min on ice in LB1 buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100) containing 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 0.2 mM Na-orthovanadate. Cell debris was pelleted by centrifugation at 14,000 rpm for 15 min at 4°C. The supernatant was assayed for protein content by Bradford analysis (Bio-Rad) and was either used immediately or stored at −80°C. Nuclear and cytoplasmic fractions were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Rockford, IL). Prior to extract preparation, cells were counted and an amount of extract equivalent to the same number of cells was loaded in each lane for Western blot analysis. For luciferase assays, experiments were performed with 5 × 106 cells in 60-mm dishes. Cells were harvested 36 h posttransfection, and protein extracts were used to examine the level of luciferase activity with the Promega assay kit (Madison, WI).
In vitro translation.
Agnoprotein and Tat were synthesized in vitro and radiolabeled with [35S]methionine using the TNT T7 Quick Coupled transcription/translation system (Promega).
Expression and purification of recombinant GST fusion proteins.
One hundred milliliters of overnight cultures of Escherichia coli (DH5α), transformed with pGST-Tat or pGEX1λT-agnoprotein and their respective deletion mutant plasmids, was diluted in fresh Luria-Bertani medium broth supplemented with ampicillin. Cultures were induced with 0.4 M isopropyl-β-d-thiogalactopyranoside (IPTG) at an optical density at 600 nm of 0.5 and were incubated for an additional 2 h at 37°C. Cells were collected by centrifugation and resuspended in 10 ml of lysis buffer containing 20 mM Tris (pH 8.0), 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40 supplemented with 1 mM phenylmethylsulfonyl fluoride, 2 mM lysozyme, and 0.6 mM leupeptin. After sonication, lysates were cleared by centrifugation at 12,000 × g and incubated with 300 ml of glutathione-Sepharose beads overnight at 4°C. Glutathione S-transferase (GST) fusion proteins were purified by three cycles of washing and centrifugation with 10 ml of lysis buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining.
In vitro protein-protein interactions (GST pull-down assay) and Western blotting.
GST pull-down assays were performed as we have previously described (20, 21). For in vitro binding assays, either 8 μl of 35S-labeled in vitro-translated agnoprotein or Tat or 250 μg of whole-cell protein lysate prepared from U-87MG or NIH 3T3 cells with or without agnoprotein was used. These were incubated with 15 μg of GST or fusion proteins GST-Tat 1-86, GST-Tat 1-72, GST-Tat 20-72, and GST-Tat 50-72 or GST-agnoprotein 1-71, agnoprotein 1-54, agnoprotein 1-36, agnoprotein 18-71, agnoprotein 37-71, and agnoprotein 18-54. These proteins were coupled to glutathione Sepharose beads in 300 μl of HNTG buffer (2 0 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 10% glycerol) containing 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 0.2 mM Na-orthovanadate for 2 h at 4°C. After incubation, the beads were pelleted and washed five times with 1 ml of HNTG buffer. The bound proteins were eluted with Laemmli sample buffer, heated to 95°C for 10 min, and separated by SDS-PAGE. Agnoprotein and Tat were detected by either fluorography or immunoblot analysis with antiagnoprotein or anti-Tat antibodies, respectively. For detection of Cdk9, p65, and Sp1 in GST pull-down assays, Western blots with appropriate antibodies were used. For Western blots with total cell protein, 50 μg of protein was run on SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with antibody. Bound antibody was detected using the ECL enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL) according to the manufacturer's recommendations.
Coimmunoprecipitation.
HL3T1 cells were transfected with plasmids expressing Tat or agnoprotein alone or in combination. Total protein extract was prepared and 250 μg was incubated with an anti-cyclin T1 antibody in 500 μl of HNTG buffer overnight at 4°C. Immunocomplexes were precipitated by the addition of protein A-Sepharose beads, washed four times with rocking at 4°C in 1 ml of HNTG buffer, and resolved by SDS-PAGE followed by Western blotting using an anti-Tat antibody. Radiograms were analyzed by the Quantity One program (Molecular Imager FX; Bio-Rad) to determine the intensity of bands.
Flow cytometric analysis.
Cells were harvested, rinsed with PBS, and fixed in suspension in 1% methanol-free formaldehyde in PBS on ice for 20 min. Cells were then resuspended in 73% ethanol for at least 16 to 20 h at −20°C. After being washed twice with PBS, cells were gently resuspended in 0.2% Triton X-100 with 1% (wt/vol) bovine serum albumin in PBS for 30 min. Following low-speed centrifugation, cells were incubated with antiagnoprotein antibody in 1% bovine serum albumin in PBS overnight at 4°C. Cells were then washed twice and resuspended in 100 μl of goat anti-rabbit IgG-PE-conjugated secondary antibody (Imgenex, San Diego, CA) for 30 min at room temperature in the dark. As a negative control, samples were left without antiagnoprotein antibody but PE-conjugated secondary antibody alone was applied. After washing cells with PBS, cellular fluorescence was measured using FACScan flow cytometry for detection of GFP (for HIV-1-positive cells) and/or PE (for JCV-positive cells). Flow cytometry was performed with a Coulter EPICS FACScan flow cytometer.
Electrophoretic mobility shift assay.
The TAR RNA sequence of the LTR (5′-UGGGUCUCUCUGGUUAGACCAGAUCUGAGCCUGGGAGCUCUCUGGCUAACUAGGAACCCACUGCUUAAGCCUCA-3′) was end labeled with [γ-32P]ATP using T4 polynucleotide kinase (Boehringer Mannheim, Indianapolis, IN). A 0.3 μM concentration of bacterially produced and eluted GST, GST-agnoprotein, or in vitro-synthesized Tat was incubated with 60,000 cpm of labeled probe in a final volume of 20 μl binding buffer containing 12 mM HEPES (pH 7.9), 4 mM Tris-HCl (pH 7.5), 60 mM KCl, 5 mM MgCl2, 0.8 mM dithiothreitol, 0.5 μg of poly[dI-dC] as nonspecific competitor, 10% glycerol, and 10 μg/ml DNase-free RNase for 1 h at 4°C. The binding mixture was resolved by electrophoresis in a 6% native polyacrylamide-0.5× Tris-borate-EDTA (TBE) gel and analyzed by autoradiography. The integrity and equal loading of proteins used in these assays were verified by SDS-PAGE.
Detection of fluorescent proteins.
U-87MG cells (1 × 105) were transfected with 5 μg of pCFP-Tat or pYFP-agnoprotein, alone or in combination, and then seeded in poly-l-lysine-coated glass chamber slides. Cells were fixed in 4% paraformaldehyde in 1× PBS after 16 h of incubation. Cells were washed in PBS, and proteins were visualized for cyan blue or yellow fluorescence.
Immunocytochemistry.
Primary human fetal astrocytes were infected with HIV-1 and JCV alone or in combination. After 7 days, the cells were seeded on poly-l-lysine-coated glass chamber slides at low density and were fixed in 10% buffered formalin after 24 h. Fixed cells were blocked with 5% bovine serum albumin in PBS for 2 h and incubated with antiagnoprotein rabbit polyclonal primary antibodies for 1 h. Cells were then washed three times with PBS-0.01% Tween 20 at 10-min intervals and incubated with fluorescein isothiocyanate (FITC)-conjugated antirabbit secondary antibody for 45 min. Cells were then blocked with 5% bovine serum albumin in PBS for 2 h and incubated with anti-Tat rabbit polyclonal primary antibody for 1 h, after which the cells were washed three times with PBS-0.01% Tween 20 at 10-min intervals and incubated with an antirabbit rhodamine-conjugated secondary antibody for 45 min. Finally, the slides were washed three times with PBS, mounted, and visualized by fluorescence microscopy.
Immunohistochemistry.
Tissue samples were obtained from the archives of the Manhattan Brain Bank at Mt. Sinai School of Medicine. These had been previously fixed in 10% buffered formalin, embedded in paraffin, and sectioned at a thickness of 4 μm. After deparaffination and antigen retrieval with citrated buffer heated to 97°C, a primary anti-Tat antibody was applied overnight at room temperature (courtesy of Avindra Nath, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD). After being rinsed thoroughly with PBS, samples were incubated with a Texas red-tagged secondary antibody for 1 h. Then rabbit polyclonal antiagnoprotein antibody (24) was incubated overnight, and, finally, after being rinsed thoroughly with PBS, a fluorescein-tagged secondary antibody was incubated and sections were examined. All fluorescent images were captured using an inverted, fluorescent Nikon microscope with deconvolution software (SlideBook 4.0.1.34; Intelligent Imaging, Denver, CO).
RESULTS
Expression and subcellular localization of agnoprotein and Tat.
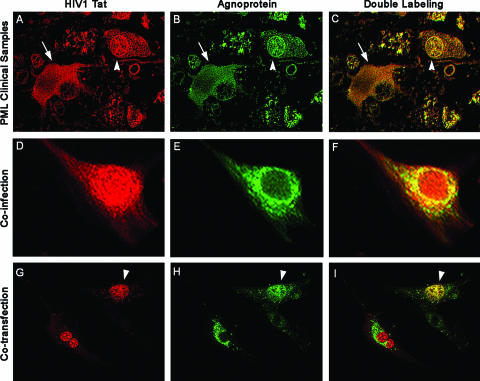
JCV agnoprotein is mostly a perinuclear protein with a limited nuclear appearance both in cell culture and in clinical samples (20, 24, 46, 47). In cell culture, HIV-1 Tat is mainly found in the nuclei and nucleoli with some accumulation in the cytoplasm. Of note, in earlier studies cytoplasmic Tat has been demonstrated in reactive astrocytes of AIDS patients with PML (23, 24). Here, we examined the expression and subcellular localization of agnoprotein and Tat in clinical samples from AIDS patient brain with PML. Samples from a total of 12 autopsy cases of HIV-related PML were obtained from archives of the Manhattan Brain Bank at Mount Sinai School of Medicine in New York, NY. The clinical signs and symptoms of the patients depended on the location of the demyelinated lesions and included headaches, motor deficits, pareses, paresthesias, and cortical blindness in one case with occipital lesions. The brain tissue samples were analyzed by double immunostaining using antiagnoprotein and anti-Tat antibodies. Results from immunohistochemical experiments revealed that in bizarre astrocytes the majority of Tat and agnoprotein is accumulated in the cytoplasm (Fig. 1A and B), although there was a limited number of astrocytes exhibiting both cytoplasmic presence and nuclear presence of these proteins (Fig. 1A). Superimposition of fluorochromes in both cases demonstrates clear colocalization of Tat and agnoprotein in cytoplasm and/or nuclei of bizarre astrocytes (Fig. 1C). Next, we examined the subcellular localization of these two proteins in primary culture of human fetal astrocytes after infection with JCV and HIV-1 by immunofluorescence and were able to detect Tat in both nuclei and cytoplasm and agnoprotein mainly in the cytoplasm of infected cells (Fig. 1D and E). Evaluation of these cells by deconvolution microscopy revealed the colocalization of both proteins in the cytoplasmic compartment (Fig. 1F). In a third approach, we assessed the subcellular colocalization of Tat and agnoprotein in human astrocytic cells after transfection with plasmids expressing these proteins. In this experiment, U-87MG cells were transfected with yellow fluorescent protein (YFP)-agnoprotein and CFP-Tat encoding plasmids either alone or in combination. Once expressed alone, YFP-agnoprotein was mostly accumulated in the cytoplasmic compartment of cells, whereas CFP-Tat was predominantly localized in the nuclei (Fig. 1G and H). Interestingly, in cells transfected with both agnoprotein- and Tat-producing plasmids, some levels of agnoprotein were detected in the nuclei. Conversely, Tat protein was also detected in the cytoplasm, suggesting that coproduction of agnoprotein and Tat in cells may alter their preferred subcellular compartmentalization (Fig. 1I).
FIG. 1.
Colocalization of HIV-1 Tat and JCV agnoprotein in astrocytes. (A to C) Immunofluorescence for the HIV-1 transactivator protein Tat demonstrates robust expression in the cytoplasm and weaker expression in the nuclear compartment of bizarre astrocytes within demyelinated plaques in a case of AIDS-related PML (A; rhodamine). The tissue obtained from the archives of the Manhattan Brain Bank at Mt. Sinai School of Medicine, which had been previously fixed in 10% buffered formalin and embedded in paraffin, was sectioned at a thickness of 4 μm. After deparaffination and antigen retrieval with citrated buffer heated to 97°C, a primary anti-Tat antibody was applied overnight at room temperature (courtesy of Avindra Nath, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD). After being rinsed thoroughly with PBS, samples were incubated with a Texas red-tagged secondary antibody for 1 h. Then rabbit polyclonal antiagnoprotein antibody (24) was incubated overnight, and finally, after a thorough rinsing with PBS, a fluorescein-tagged secondary antibody was incubated and sections were visualized in an inverted, fluorescent Nikon microscope with deconvolution software (SlideBook 4.0.1.34; Intelligent Imaging, Denver, CO). The JCV late regulatory product, agnoprotein, is detected in the cytoplasm of the same astrocytes (B; fluorescein). Superimposition of both fluorochromes shows colocalization of both proteins in the cytoplasm (arrow) of the majority of cells and in the nuclei (arrowhead) of few bizarre astrocytes (C; double labeling). (D to F) Primary astrocytes were coinfected with HIV-1 and JCV, and by immunofluorescence we detected Tat in both nuclei and cytoplasm of infected cells (D; rhodamine), and agnoprotein in the cytoplasm (E; fluorescein). Deconvolution demonstrates the colocalization of both proteins in the cytoplasmic compartment (F). (G to I) U-87MG cells cotransfected, as previously described (20), with plasmids expressing Tat (CFP-Tat) and agnoprotein (YFP-agnoprotein) show Tat localization mainly in the nuclei, with some cells showing nuclear and cytoplasmic labeling (G), and agnoprotein in the cytoplasm (H), where it colocalizes with Tat (I). Original magnification in all panels, ×1,000. YFP-agnoprotein has been previously described (20). CFP-Tat was created by removing the Tat gene from cytomegalovirus (CMV)-Tat with BglII and EcoRI and cloning it into the BamHI and EcoRI sites of pECFP-C1.
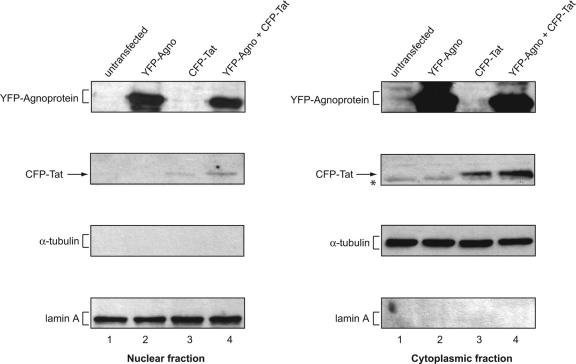
To further verify the subcellular localization of Tat and agnoprotein in astrocytes, U-87MG cells were transfected with YFP-agnoprotein and CFP-Tat expression plasmids and then cytoplasmic and nuclear proteins were prepared and analyzed by Western blotting. As shown in Fig. 2, agnoprotein was detected in both nuclear and cytoplasmic fractions of cells transfected with YFP-agnoprotein expression plasmid either alone or together with CFP-Tat expression plasmid. We observed a noticeably higher level of agnoprotein in the cytoplasmic fraction compared to the nuclear fractions. In parallel, a similar experiment was performed for the detection of Tat protein. Again, Tat was detected in the cytoplasm and nuclear fraction of astrocytes transfected with CFP-Tat. The level of Tat was slightly higher in the cytoplasm in the presence of agnoprotein. The purity of the nuclear fractions was assessed by Western blotting for the presence of α-tubulin. As seen, α-tubulin was detected in the cytoplasm, but not in nuclear fractions (Fig. 2). Furthermore, results from Western blotting showed the presence of nuclear marker (lamin A) only in the nuclear but not in the cytoplasmic fractions.
FIG. 2.
Detection of Tat and agnoprotein in cytoplasmic and nuclear fractions. Nuclear and cytoplasmic proteins were prepared from equal numbers of U-87MG cells cotransfected with YFP-agnoprotein and CFP-Tat-expressing plasmids as indicated above each lane. Protein extracts prepared from an equal number of cells were analyzed by a series of Western blots using antiagnoprotein antibody (24), anti-HIV-1 Tat (R705 from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), antitubulin antibody, and anti-lamin A antibody.
Coinfection of human primary astrocytes with JCV and HIV-1.
To assess the importance and frequency of JCV coinfection with HIV-1 on the expression and replication of HIV-1, human primary culture of astrocytes was transduced with HIV-1(NL4-3) GFP-Vpr (72). After 24 h, cells were infected with Mad-1/SVEdelta JCV at a multiplicity of infection of 1.0 (67). In parallel, control uninfected cells or cells infected with JCV or HIV-1 alone were prepared at day 7. Cells were harvested and, after fixation in formaldehyde and ethanol and treatment with 0.2% Triton X-100, were incubated with antiagnoprotein antibody, followed by incubation with goat anti-rabbit IgG-PE-conjugated secondary antibody. In a negative control, samples were left without antiagnoprotein antibody but PE-conjugated secondary antibody was applied. After washing cells with PBS, cellular fluorescence was measured using FACScan flow cytometry for detection of GFP (for HIV-1-positive cells) and/or PE (for JCV-positive cells). Results from this experiment revealed nearly 81% of the cells with expression of agnoprotein, pointing to the efficient infection of the cells with JCV. In the presence of HIV-1, the number of JCV-positive cells was increased, exceeding 94.7%, which corroborates the previous observation demonstrating stimulation of JCV gene transcription by HIV-1 Tat (11, 37, 59). In vitro infection of the primary culture of astrocytes with HIV-1 was poor, as only 3.15% of the cells were GFP positive, indicative of HIV-1 replication in these cells. This value was decreased to 1.5% in the presence of JCV infection, suggesting that coinfection of the HIV-1-infected cells with JCV can decrease the level of HIV-1 gene expression and replication in these cells.
Effect of JCV agnoprotein on Tat-mediated activation of the HIV-1 LTR.
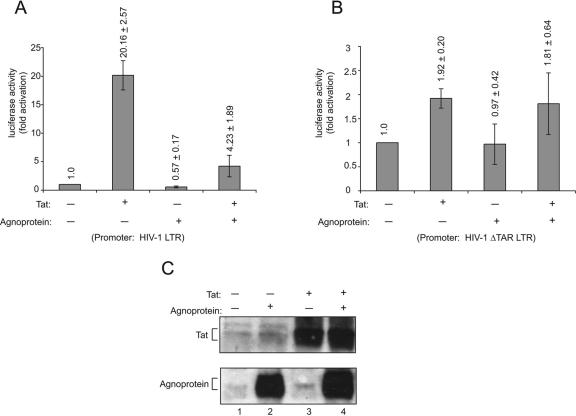
In light of our results showing colocalization of agnoprotein and Tat, in the next series of experiments we examined the effect of agnoprotein on Tat activation of HIV-1 promoter. Toward this end, primary culture of human fetal astrocytes was transfected with an HIV-1 LTR reporter plasmid either alone or together with plasmids expressing agnoprotein and HIV-1 Tat. As anticipated, expression of Tat significantly enhanced the level of full-length LTR promoter activity in the transfected cells (Fig. 3A). In accord with the previous results (8, 17, 31, 62-64, 75), expression of Tat in astrocytes also led to a modest activation of the LTR with no TAR region (Fig. 3B). Interestingly, coexpression of agnoprotein in the cells caused a noticeable decrease in the level of Tat activation of the TAR-containing full-length HIV-1 LTR but showed no effect on the modest activation of ΔTAR LTR by Tat (Fig. 3A and B). The presence of Tat and agnoprotein in the transfected cells was monitored by Western blot analysis (Fig. 3C).
FIG. 3.
Effect of JCV agnoprotein on Tat-mediated activation of the HIV-1 LTR. Primary human fetal astrocytes were prepared according to a modified procedure based on the methods of Cole and de Vellis (16) and Yong and Antel (79). Astrocytes were plated (2 × 105 in 60-mm plate) and maintained in regular growth medium (Dulbecco's modified Eagle's medium-F-12 supplemented with 15% fetal bovine serum). Cells were then transfected using FuGENE 6 transfection reagent with 1 μg of luciferase-based reporter constructs containing full-length (−450 to +80) HIV-LTR (A) or a deletion mutant of the LTR lacking the TAR sequence (+3 to +80) (B). Transfections were carried out in the absence or presence of plasmids (1 μg) expressing either Tat or agnoprotein under control of the cytomegalovirus (CMV) promoter (58). The concentration of DNA in each transfection mixture remained constant by adding pCDNA3. Forty hours after transfection, cells were harvested and luciferase enzymatic activity was measured. The average values of multiple experiments are presented as n-fold effects with a baseline remaining as an arbitrary unit of 1. (C) Western blot analysis of protein extract from the transfected cells (as indicated in panel A) for detection of Tat and agnoprotein.
Identification of the region within Tat which is important for its interaction with agnoprotein.
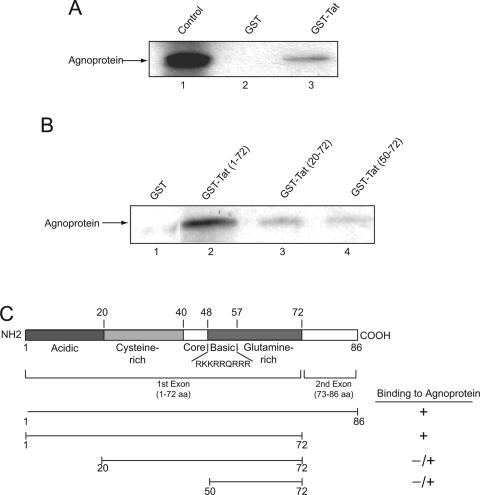
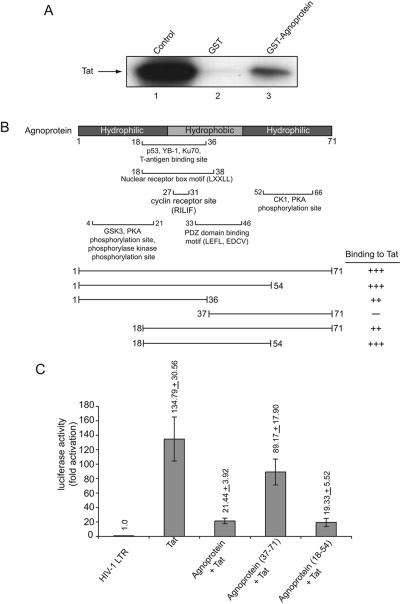
These observations provided a rationale to investigate the physical interaction of JCV agnoprotein with HIV-1 Tat. In vitro-synthesized full-length agnoprotein was prepared and incubated with GST-Tat and GST, and the ability of agnoprotein to interact with Tat was tested by pull-down assay. Figure 4A illustrates a typical gel analysis showing detection of agnoprotein in fractions bound to GST-Tat, but not GST alone. To further verify the specificity of this interaction, by determining the region within Tat that associates with agnoprotein, total protein extracts from NIH 3T3 cells expressing agnoprotein (20) were prepared and mixed with GST, GST-Tat 1-86, and the various deletion mutants of Tat in fusion with GST as indicated in Fig. 4B. From the intensity of the band corresponding to agnoprotein, it was evident that the region within the C terminus of Tat spanning amino acids 72 to 86 can be deleted with no negative impact on Tat interaction with agnoprotein. The N terminus of Tat between residues 1 and 20 is important for its binding to agnoprotein as the removal of this region noticeably decreased Tat-agnoprotein association. Further deletion of Tat that removes the residues between 20 and 50 further decreased Tat association with agnoprotein. Thus, the region of Tat that spans residues 1 to 50 is critical for interaction of Tat and agnoprotein. The integrity of the bacterially produced GST-Tat and its mutants was examined by gel electrophoresis (data not shown). Similar to that in NIH 3T3, the interaction of Tat and agnoprotein was observed in human astrocytic cells (data not shown). Figure 4C schematizes the structural organization of Tat, the mutants that were used in this experiment, and the levels of Tat binding to agnoprotein.
FIG. 4.
Association of agnoprotein with HIV-1 Tat and identification of the agnoprotein binding region within Tat. (A) In vitro-synthesized 35S-labeled full-length agnoprotein was incubated with either GST or GST-Tat 1-86 (58) immobilized on glutathione-Sepharose beads. Beads were washed extensively, and protein complexes were resolved by SDS-PAGE and analyzed by autoradiography. (B) For the mapping assay, total protein extract from NIH 3T3 cells expressing agnoprotein (20) was incubated with either GST, GST-Tat (1-72), or the deletion mutants of Tat fused to GST as indicated in the figure, which were immobilized on glutathione-Sepharose beads. After washing bead-protein complexes, the bound proteins were analyzed by SDS-PAGE followed by Western blot analysis using antibody against JCV agnoprotein (24). (C) Schematic representation of Tat protein depicting the various domains of Tat and the arginine-rich domain spanning residues 48 to 57. The ability of Tat and its deletion mutants to interact with agnoprotein is highlighted on the right.
Identification of the Tat binding region within agnoprotein and functional interaction of agnoprotein and Tat.
In a reciprocal experiment, in vitro-synthesized Tat protein was incubated with GST or GST-agnoprotein and the level of Tat binding was examined by gel electrophoresis. A band corresponding to Tat was detected in the elution fraction obtained from GST-agnoprotein, but not GST alone (Fig. 5A).
FIG. 5.
Physical and functional interaction of agnoprotein and Tat. (A) In vitro-synthesized 35S-labeled full-length Tat was incubated with either GST or GST-agnoprotein (full-length; amino acids 1 to 71) (55) immobilized on glutathione-Sepharose beads. Beads were washed extensively, and the protein complexes associated with GST or GST-agnoprotein were resolved by SDS-PAGE and analyzed by autoradiography. (B) For mapping, in vitro-synthesized 35S-labeled full-length Tat was incubated with either GST, GST-agnoprotein (1-71), or the deletion mutants of agnoprotein (as indicated) fused to GST and immobilized on glutathione-Sepharose beads. The bound proteins were analyzed by SDS-PAGE followed by autoradiography. (B) Structural organization of agnoprotein illustrating the various domains of agnoprotein. The ability of agnoprotein and its deletion mutants to interact with Tat is depicted on the right as follows: +++, strong interaction; ++, reduced interaction; +, weak interaction; and −, no interaction. (C) Effect of agnoprotein mutants on transcriptional activity of Tat. Primary human fetal astrocytes (2 × 105 in 60-mm plate) were transfected using FuGENE 6 transfection reagent with 1 μg of full-length (−450 to +80) HIV-LTR fused to the luciferase gene in the absence or presence of plasmids encoding Tat (1 μg) and full-length agnoprotein (1-71) (1 μg) and deletion mutants which demonstrated strong binding activity (18-54) or no binding ability (37-71) to Tat. The concentration of DNA in each transfection mixture remained constant by adding pCDNA3. Forty hours after transfection, cells were harvested and luciferase enzymatic activity was measured. Average values of multiple experiments are presented as n-fold effects.
As illustrated in Fig. 5B, the 71-amino-acid agnoprotein has a peculiar feature in which its N- and C-terminal domains are hydrophilic while the central portion of the protein is relatively hydrophobic. The predicated secondary structure suggests the presence of several potential helix-turn-helix domains, and the central domain may adopt an α-helical configuration. To evaluate the importance of the various regions of agnoprotein in its interaction with Tat, in vitro-synthesized Tat protein was mixed with deletion mutants of agnoprotein in fusion with GST, and its binding was examined by gel electrophoresis. Results from the binding assay revealed that a region between amino acid residues 18 and 54 is the primary domain for binding to Tat protein (Fig. 5B). From the comparison of the intensity of the bands obtained from GST-agnoproteins 18-71 and 18-54, it seems that the C-terminal domain of agnoprotein spanning amino acids 54 to 71 can have a negative effect on the binding of the region of agnoprotein between residues 18 and 54 with Tat protein.
To correlate the results from binding studies with the functional ability of agnoprotein to suppress Tat activation of the LTR, we selected two mutants of agnoprotein—one which exhibits strong binding ability to Tat and the other with no binding activity to Tat—for use in the transfection assay. As seen in Fig. 5C, agnoprotein mutant 37-71, which had no binding activity to Tat, showed no drastic inhibitory effect upon the LTR, whereas agnoprotein mutant 18-54 with strong binding to Tat severely hampered the level of Tat activation of the LTR in astrocytes. This observation suggests that the physical interaction of Tat and agnoprotein is an important event in the functional interaction of these proteins upon HIV-1 gene transcription.
Effect of agnoprotein on the interaction of Tat with TAR RNA, cyclin T1, and cdk9.
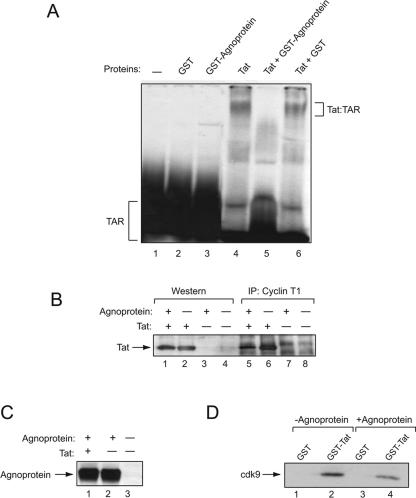
In a TAR-dependent manner, Tat exerts its activity by interacting with the TAR RNA sequence of the LTR, where it can recruit several cellular factors such as cyclin T1 and cdk9 that potentiate RNA polymerase II activity (3, 34, 48, 49). As a first step to assess the impact of the formation of a Tat-agnoprotein complex on the interaction of Tat with TAR RNA, we performed an RNA band-shift assay using synthetic TAR RNA as a probe (22, 36, 41, 74). 32P-labeled TAR RNA was incubated with Tat in the absence and presence of GST and GST-agnoprotein. As shown in Fig. 6A, results from gel analysis ruled out the binding of GST and GST-agnoprotein to TAR RNA, yet demonstrated the ability of GST-agnoprotein to block formation of the Tat-TAR complex which was detected upon the addition of Tat protein to the TAR RNA probe. Thus, these observations indicate that the association of Tat and agnoprotein can have a negative impact on the interaction of Tat and TAR. As stated above, in addition to TAR, Tat interacts with cyclin T1 to stimulate transcription of the LTR via a TAR-dependent pathway. As such, in the next experiment we determined whether the interaction of Tat with cyclin T1 is affected by agnoprotein of JCV. Protein extracts from cells with or without agnoprotein expression were utilized in immunoprecipitation followed by Western blot analysis to assess binding of cyclin T1 with endogenously expressed Tat. Results shown in Fig. 6B illustrate reduced binding of Tat and cyclin T1 in cells that express agnoprotein, suggesting that the interaction of Tat and agnoprotein may have a negative impact on Tat-cyclin T1 complex formation. Expression of agnoprotein in the transfected cells was assessed by direct Western blot assay (Fig. 6C). Similarly, our results from the GST pull-down assay showed that agnoprotein had a moderate effect on the interaction of Tat and cdk9, a partner of cyclin T1 which is responsible for the phosphorylation of the polymerase II carboxyl terminus (Fig. 6D).
FIG. 6.
Effect of agnoprotein on the interaction of Tat with TAR and cyclin T1. (A) Electrophoretic mobility shift assay. Approximately 60,000 cpm of synthetic 32P-labeled TAR RNA (5′UGGGUCUCUCUGGUUAGACCAGAUCUGAGCCUGGGAGCUCUCUGGCUAACUAGGGAACCCACUGCUUAAGCCUCA-3′) was incubated for 1 h on ice with 0.3 μM eluted GST, GST-agnoprotein, or in vitro-synthesized Tat in 20 μl binding buffer containing 12 mM HEPES (pH 7.9), 4 mM Tris-HCl (pH 7.5), 60 mM KCl, 5 mM MgCl2, 0.8 mM dithiothreitol, 0.5 μg of poly(dI-dC) as nonspecific competitor, 10% glycerol, and 10 μg/ml DNase-free RNase. The binding mixture was resolved on a 6% polyacrylamide-0.5× TBE gel and analyzed by autoradiography. Integrity and equal loading of proteins used in the assay were verified by SDS-PAGE. (B) Agnoprotein negatively affects binding of Tat to cyclin T1. HL3T1 cells (HeLa cells with stably integrated HIV-1 LTR in the genome) were transfected with plasmids expressing Tat or agnoprotein alone or in combination. Total protein extract was prepared, and 250 μg was incubated with an anti-cyclin T1 antibody. Immunocomplexes were immunoprecipitated (IP) with the addition of protein A-Sepharose beads, resolved by SDS-PAGE, and analyzed by Western blotting using an anti-Tat antibody. The presence of Tat was verified by Western blotting (lanes 1 to 4). Radiograms were analyzed by the Quantity One program (Molecular Imager FX; Bio-Rad), and binding activity was determined by analyzing the intensity of bands (adjusted volume of counts per mm2). A total of 12.5% of Tat was bound to cyclin T1 in the absence of agnoprotein, and only 3% of Tat was found in the complex with cyclin T1 in the presence of agnoprotein. (C) Expression of agnoprotein in the transfected cells was tested by Western blot analysis. (D) Presence of agnoprotein affects binding of Tat to cdk9. Total protein extracts from U-87MG cells transfected with pCDNA3 or pCMV-agnoprotein were incubated with either GST or GST-Tat 1-86 immobilized on glutathione-Sepharose beads. After washing, protein complexes were resolved by SDS-PAGE and analyzed by Western blotting using anti-cdk9 antibody. GST proteins were used at a 1 μM concentration in pull-down assays.
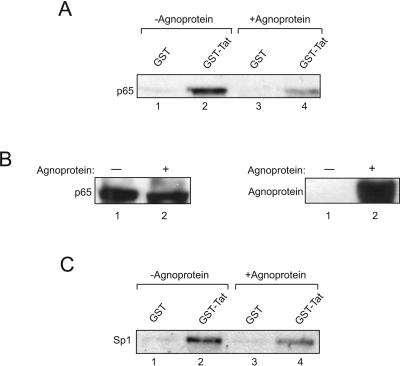
Effect of agnoprotein on the interaction of Tat with the p65 subunit of NF-κB and Sp1.
In the next series of experiments, we focused our attention on the effect of agnoprotein on the interaction of Tat with upstream DNA binding transcription factors whose involvement in Tat activation of the LTR had been previously demonstrated. First, we tested Tat interaction with the p65 subunit of NF-κB as cross-interaction between Tat and upstream transcription activators, particularly the p65 subunit of NF-κB (2, 28, 33, 42, 70, 71, 80), plays an important role in Tat activation of the LTR in susceptible cells, including astrocytic cells (61, 75). In this respect, protein extracts from cells expressing agnoprotein and the control cells with no agnoprotein were incubated with GST or GST-Tat fusion protein. The complexes were analyzed by Western blotting using anti-p65 antibody. As shown in Fig. 7A, under similar conditions, the intensity of the band corresponding to p65 from cells expressing agnoprotein is less than that seen in the control cells (compare lanes 2 and 4), indicating that the interaction of agnoprotein and Tat may also interfere with the ability of Tat to associate with p65. This event may not be attributed to the levels of p65 in the control and agnoprotein-expressing cells as tested by Western blot analysis of p65 in these cells (Fig. 7B).
FIG. 7.
Effect of agnoprotein on the interaction of Tat with p65 and Sp1. (A) Total protein extracts (250 μg) from U-87MG cells transfected with pCDNA3 or pCMV-agnoprotein were incubated with either GST or GST-Tat 1-86 immobilized on glutathione-Sepharose beads. After washing, protein complexes were resolved by SDS-PAGE and analyzed by Western blotting using anti-p65 antibody. GST proteins were used in a 1 μM concentration in pull-down assays. (B) Expression of p65 and agnoprotein was verified by direct Western blotting. (C) Total protein extracts (250 μg) from U-87MG cells transfected with pCDNA3 or pCMV-agnoprotein were incubated with either GST or GST-Tat 1-86 immobilized on glutathione-Sepharose beads. After washing, protein complexes were resolved by SDS-PAGE and analyzed by Western blotting using anti-Sp1 antibody. GST proteins were used in a 1 μM concentration in pull-down assays.
Next, we investigated the interaction of Tat and Sp1 in the presence of agnoprotein. Of note, earlier studies demonstrated that physical interaction of Tat and Sp1 may have an impact on the level of LTR promoter activity (15, 32, 77). Our results, as seen in Fig. 7C, show a modest negative effect of agnoprotein on the cross-communication between Tat and Sp1. Altogether, these observations demonstrate that the physical contact between agnoprotein and Tat can interfere with some of the critical events involved in the interaction of Tat with its viral (TAR) and cellular (p65 and cyclin T1/cdk9) partners and hence decrease the level of LTR transcription in astrocytic cells.
DISCUSSION
Here we provide evidence for the potential cross-interaction of JCV and HIV-1 through the regulatory proteins Tat and agnoprotein and the impact of this interaction on expression of the HIV-1 genome. Results from infection studies showed that human fetal astrocytes can be coinfected with JCV and HIV-1 and the presence of JCV in cells alters HIV-1 gene expression. At the same time, JCV gene expression was slightly increased in HIV-1-replicating cells. We demonstrate that the physical interaction of Tat and agnoprotein partially affects their subcellular localization and has a negative impact on the interaction of Tat with its target RNA, TAR, and the critical upstream transcription factors such as the p65 subunit of NF-κB. Earlier results from several laboratories have ascribed an important role for Tat in recruiting pTEFb, the positive elongation factor b composed of cdk9 and cyclin T complex, in close proximity to the transcription start site via its association with TAR (3, 27, 38, 69). Our results show that agnoprotein can interfere with the interaction of Tat with cyclin T and cdk9. Thus, it is likely that the interaction of agnoprotein with Tat negatively affects its cross-association with TAR through the cyclin T-cdk9 complex. More recently, it has been demonstrated that Tat activity upon the HIV-1 LTR can be inhibited by HEXIM1 (26). The inactive form of pTEFb, which is associated with inhibited cdk9 kinase activity, forms a large complex consisting of HEXIM1 and 7SK small nuclear RNA (40, 45, 76, 78). The interaction of agnoprotein with Tat that interferes with the Tat-cyclin T complex may result in the presence of free cyclin T1, which, in turn, can form a complex with HEXIM1. Thus, it is plausible to envision cooperativity between agnoprotein and HEXIM1-7SK snRNA in disruption of active pTEFb-Tat-TAR complex. On the other hand, the interaction of agnoprotein and Tat may alter the subcellular distribution of Tat, leading to its retention in the cytoplasm. Under these circumstances, Tat may not exert its nuclear function such as transcriptional activation of several cellular genes, including cytokines such as tumor necrosis factor alpha, whose downstream transcription factor, NF-κB, is critical for LTR activity. Altogether, these observations suggest that agnoprotein may utilize both direct and indirect pathways to interfere with transcriptional activation of the LTR by Tat in astrocytes upon its association with Tat. It is known that HIV-1 is poorly replicated in astrocytes in cell culture (9, 39, 43, 44). There have been several speculations and experimental data that may explain, at least in part, the inability of astrocytes to fully support HIV-1 gene expression and replication. While the exact events leading to nonproductive replication of HIV-1 in astrocytes remain to be elucidated, HIV-1 interaction with other pathogens in astrocytes may contribute to the level of HIV-1 gene expression and replication in these cells. In earlier observations, it was demonstrated that the JCV early gene product, T antigen, had the capacity to stimulate transcription of the HIV-1 LTR (29). On the other hand, results from transcription studies revealed the ability of Tat to stimulate transcription of the JCV genome in glial cells (59). Tat activation of the JCV late promoter led to the notion that the molecular dialogue between HIV-1 and JCV may contribute to a higher incidence of PML in people with AIDS than in any other immunosuppressed individuals (59). Activation of the late gene of JCV by Tat can allow, in addition to expression of the viral capsid proteins, expression of JCV agnoprotein, whose function is important for JCV replication. Our results on the effect of agnoprotein on Tat activation of the LTR suggest that a delicate balance in the level of JCV and HIV-1 gene expression can control the level of JCV and HIV-1 gene transcription in astrocytes. On the other hand, our preliminary data show that cross-interaction of Tat with agnoprotein has less negative effect on JCV gene expression and its activation by Tat. Thus, it is likely that while agnoprotein may also interfere with Tat interaction with the TAR-like sequence within JCV (13, 14, 37), the secondary pathway by which Tat can stimulate the JCV genome (4, 7, 10, 18, 25) may remain operative in the presence of agnoprotein. Our results further identified a region within the N terminus of agnoprotein that has a helix-loop-helix structure as a potential Tat binding site. The ability of this small domain of agnoprotein to decrease Tat function may provide a new biological tool for the development of molecules that interfere with the activation of HIV-1 LTR by Tat.
Acknowledgments
We wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for their continued support, insightful discussions, and sharing of reagents and ideas, particularly Martyn White for his insightful comments and critical reading of the manuscript. We would like to thank S. Morgello, Director of the NIH-funded Manhattan Brain Bank at Mt. Sinai School of Medicine, for providing the brain tissue samples from patients with PML. We also thank C. Schriver for editorial assistance.
This work was made possible by grants awarded by NIH to K.K.
REFERENCES
- 1.Anderson, C. E., G. S. Tomlinson, B. Pauly, F. W. Brannan, A. Chiswick, R. Brack-Werner, P. Simmonds, and J. E. Bell. 2003. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol. Appl. Neurobiol. 29:378-388. [DOI] [PubMed] [Google Scholar]
- 2.Barboric, M., R. M. Nissen, S. Kanazawa, N. Jabrane-Ferrat, and B. M. Peterlin. 2001. NF-kappa B binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell 8:327-337. [DOI] [PubMed] [Google Scholar]
- 3.Beiniasz, P. D., T. A. Grdina, H. P. Bogerd, and B. R. Cullen. 1999. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc. Natl. Acad. Sci. USA 96:7791-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger, J. R. 2003. Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions. J. Neurovirol. 9(Suppl. 1):38-41. [DOI] [PubMed] [Google Scholar]
- 5.Berger, J. R., and M. Concha. 1995. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J. Neurovirol. 1:5-18. [DOI] [PubMed] [Google Scholar]
- 6.Berger, J. R., B. Kaszovitz, M. J. Post, and G. Dickinson. 1987. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann. Intern. Med. 107:78-87. [DOI] [PubMed] [Google Scholar]
- 7.Berger, J. R., A. Chauhan, D. Galey, and A. Nath. 2001. Epidemiological evidence and molecular basis of interactions between HIV and JC virus. J. Neurovirol. 7:329-338. [DOI] [PubMed] [Google Scholar]
- 8.Berkhout, B., A. Gatignol, A. B. Rabson, and K. T. Jeang. 1990. TAR-independent activation of the HIV-1 LTR: evidence that tat requires specific regions of the promoter. Cell 62:757-767. [DOI] [PubMed] [Google Scholar]
- 9.Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 10.Chen, N. N., C.-F. Chang, G. L. Gallia, D. A. Kerr, E. M. Johnson, C. P. Krachmarov, S. M. Barr, R. J. Frisque, B. Bollag, and K. Khalili. 1995. Cooperative action of cellular proteins YB-1 and Pur alpha with the tumor antigen of the human JC polyomavirus determines their interaction with the viral lytic control element. Proc. Natl. Acad. Sci. USA 92:1087-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chepenik, L. G., A. P. Tretiakova, C. P. Krachmarov, E. M. Johnson, and K. Khalili. 1998. The single-stranded DNA binding protein, Purα, binds HIV-1 TAR RNA and activates HIV-1 transcription. Gene 210:37-44. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury, M., J. P. Taylor, H. Tada, J. Rappaport, F. Wong-Staal, S. Amini, and K. Khalili. 1990. Regulation of the human neurotropic virus promoter by JCV-T-antigen and HIV-1 tat protein. Oncogene 5:1737-1742. [PubMed] [Google Scholar]
- 13.Chowdhury, M., J. P. Taylor, C.-F. Chang, J. Rappaport, and K. Khalili. 1992. Evidence that a sequence similar to TAR is important for induction of the JC virus late promoter by human immunodeficiency virus type 1 Tat. J. Virol. 66:7355-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury, M., M. Kundu, and K. Khalili. 1993. GA/GC-rich sequence confers Tat responsiveness to human neurotropic virus promoter, JCVL, in cells derived from CNS. Oncogene 8:887-892. [PubMed] [Google Scholar]
- 15.Chun, R. F., O. J. Semmes, C. Neuveut, and K.-T. Jeang. 1998. Modulation of Sp1 phosphorylation by human immunodeficiency virus type 1 Tat. J. Virol. 72:2615-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, R., and J. de Vellis. 1997. Astrocyte and oligodendrocyte cultures, p. 117-130. In S. Federoff and A. Richardson (ed.), Protocols for neural cell culture, 2nd ed. Humana Press, Totowa, NJ.
- 17.Dandekar, D. H., K. N. Ganesh, and D. Mitra. 2004. HIV-1 Tat directly binds to NF kappaB enhancer sequence: role in viral and cellular gene expression. Nucleic Acids Res. 32:1270-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel, D. C., Y. Kinoshita, M. A. Khan, L. Del Valle, K. Khalili, J. Rappaport, and E. M. Johnson. 2004. Internalization of exogenous human immunodeficiency virus-1 protein, Tat, by KG-1 oligodendroglioma cells followed by stimulation of DNA replication initiated at the JC virus origin. DNA Cell Biol. 23:858-867. [DOI] [PubMed] [Google Scholar]
- 19.Darbinian-Sarkissian, N., A. Darbinyan, J. Otte, S. Radhakrishnan, B. E. Sawaya, A. Arzumanyan, G. Chipitsyna, Y. Popov, J. Rappaport, S. Amini, and K. Khalili. 2006. p27SJ, a novel protein in St. John's Wort that suppresses expression of HIV-1 genome. Gene Ther. 13:288-295. [DOI] [PubMed] [Google Scholar]
- 20.Darbinyan, A., N. Darbinian, M. Safak, S. Radhakrishnan, A. Giordano, and K. Khalili. 2002. Evidence for dysregulation of cell cycle by human polyomavirus, JCV, late auxiliary protein. Oncogene 21:5574-5581. [DOI] [PubMed] [Google Scholar]
- 21.Darbinyan, A., K. M. Siddiqui, D. Slonina, N. Darbinian, S. Amini, M. K. White, and K. Khalili. 2004. Role of JC virus agnoprotein in DNA repair. J. Virol. 78:8593-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darfeuille, F., A. Arzumanov, S. Gryaznov, M. J. Gait, C. Di Primo, and J. J. Toulme. 2002. Loop-loop interaction of HIV-1 TAR RNA with N3′-P5′ deoxyphosphoramidate aptamers inhibits in vitro Tat-mediated transcription. Proc. Natl. Acad. Sci. USA 99:9709-9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Valle, L., S. Croul, S. Morgello, S. Amini, J. Rappaport, and K. Khalili. 2000. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J. Neurovirol. 6:221-228. [DOI] [PubMed] [Google Scholar]
- 24.Del Valle, L., J. Gordon, S. Enam, S. Delbue, S. Croul, S. Abraham, S. Radhakrishnan, M. Assimakopoulou, C. D. Katsetos, and K. Khalili. 2002. Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J. Natl. Cancer Inst. 94:267-273. [DOI] [PubMed] [Google Scholar]
- 25.Enam, S., T. M. Sweet, S. Amini, K. Khalili, and L. Del Valle. 2004. Evidence for involvement of transforming growth factor beta 1 signaling pathway in activation of JC virus in human immunodeficiency virus 1-associated progressive multifocal leukoencephalopathy. Arch. Pathol. Lab. Med. 128:282-291. [DOI] [PubMed] [Google Scholar]
- 26.Fraldi, A., F. Varrone, G. Napolitano, A. A. Michels, B. Majello, O. Bensaude, and L. Lania. 2005. Inhibition of Tat activity by the HEXIM1 protein. Retrovirology 2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garber, M. E., T. P. Mayall, E. M. Suess, J. Meisenhelder, N. E. Thompson, and K. A. Jones. 2000. CDK9 autophosphorylation regulates high-affinity binding of the human immunodeficiency virus type 1 Tat-P-TEFb complex to TAR RNA. Mol. Cell. Biol. 20:6958-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Martinez, L. F., G. Mavankal, J. M. Neveu, W. S. Lane, D. Ivanov, and R. B. Gaynor. 1997. Purification of a Tat-associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 16:2836-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendelman, H. E., W. Phelps, L. Feigenbaum, J. M. Ostrove, A. Adachi, P. M. Howley, G. Khoury, H. S. Ginsberg, and M. A. Martin. 1986. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc. Natl. Acad. Sci. USA 83:9759-9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 31.Harrich, D., J. Garcia, R. Mitsuyasu, and R. Gaynor. 1990. TAR-independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 9:4417-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeang, K.-T., R. Chun, N. H. Lin, A. Gatignol, C. G. Glabe, and H. Fan. 1993. In vitro and in vivo binding of human immunodeficiency virus type 1 Tat protein and Sp1 transcription factor. J. Virol. 67:6224-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, K. A., and B. M. Peterlin. 1994. Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem. 63:717-743. [DOI] [PubMed] [Google Scholar]
- 34.Kashanchi, F., G. Piras, M. F. Radonovich, J. F. Duvall, A. Fattaey, C. M. Chiang, R. G. Roeder, and J. N. Brady. 1994. Direct interaction of human TFIID with the HIV-1 transactivator tat. Nature 367:295-299. [DOI] [PubMed] [Google Scholar]
- 35.Khalili, K., M. K. White, H. Sawa, K. Nagashima, and M. Safak. 2004. The agnoprotein of polyomaviruses: a multifunctional auxiliary protein. J. Cell. Physiol. 207:1-7. [DOI] [PubMed] [Google Scholar]
- 36.Kolb, G., S. Reigadas, C. Boiziau, A. van Aerschot, A. Arzumanov, M. J. Gait, P. Herdewijn, and J. J. Toulme. 2005. Hexitol nucleic acid-containing aptamers are efficient ligands of HIV-1 TAR RNA. Biochemistry 44:2926-2933. [DOI] [PubMed] [Google Scholar]
- 37.Krachmarov, C. P., L. G. Chepenik, S. Barr-Vagell, K. Khalili, and E. M. Johnson. 1996. Activation of the JC virus Tat-responsive transcriptional control element by association of the Tat protein of human immunodeficiency virus 1 with cellular protein Pur alpha. Proc. Natl. Acad. Sci. USA 93:14112-14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak, Y. T., D. Ivanov, J. Guo, E. Nee, and R. B. Gaynor. 1999. Role of the human and murine cyclin T proteins in regulating HIV-1 tat activation. J. Mol. Biol. 288:57-69. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy, M., J. He., and C. Wood. 1998. HIV-1 strain-associated variability in infection of primary neuroglia. J. Neurovirol. 4:80-89. [DOI] [PubMed] [Google Scholar]
- 40.Michels, A. A., V. T. Nguyen, A. Fraldi, V. Labas, M. Edwards, F. Bonnet, I. Lania, and O. Bensaude. 2003. MAQ1 and 7SK RNA interaction with CDK9/cyclin T complexes in a transcription-dependent manner. Mol. Cell. Biol. 23:4859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murchie, A. I., B. Davis, C. Isel, M. Afshar, M. J. Drysdale, J. Bower, A. J. Potter, I. D. Starkey, T. M. Swarbrick, S. Mizra, C. D. Prescott, P. Vaglio, F. Aboul-ela, and J. Karn. 2004. Structure-based drug design targeting an inactive RNA conformation: exploiting the flexibility of HIV-1 TAR RNA. J. Mol. Biol. 336:625-638. [DOI] [PubMed] [Google Scholar]
- 42.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711-713. (Erratum, 344:178, 1990.) [DOI] [PubMed] [Google Scholar]
- 43.Neumann, M., B. K. Felber, A. Kleinschmidt, B. Froese, V. Erfle, G. N. Pavlakis, and R. Brack-Werner. 1995. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J. Virol. 69:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann, M., E. Afonina, F. Ceccherini-Silberstein, S. Schlicht, V. Erfle, G. N. Pavlakis, and R. Brack-Werner. 2001. Nucleocytoplasmic transport in human astrocytes: decreased nuclear uptake of the HIV Rev shuttle protein. J. Cell Sci. 114:1717-1729. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen, V. T., T. Kiss, A. A. Michels, and O. Bensaude. 2001. 7SK small nuclear RNA binds to and inhibits the activity of cdk9/cyclin T complexes. Nature 414:322-325. [DOI] [PubMed] [Google Scholar]
- 46.Okada, Y., S. Endo, H. Takahashi, H. Sawa, T. Umemura, and K. Nagashima. 2001. Distribution and function of JCV agnoprotein. J. Neurovirol. 7:302-306. [DOI] [PubMed] [Google Scholar]
- 47.Okada, Y., H. Sawa, S. Endo, Y. Orba, T. Umemura, H. Nishihara, A. C. Stan, S. Tanaka, H. Takahashi, and K. Nagashima. 2002. Expression of JCV Agnoprotein in progressive leukoencephalopathy brain. Acta Neuropathol. (Berlin) 104:130-136. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto, H., C. T. Sheline, J. L. Corden, K. A. Jones, and B. M. Peterlin. 1996. Trans-activation by human immunodeficiency virus Tat protein requires the C-terminal domain of RNA polymerase II. Proc. Natl. Acad. Sci. USA 93:11575-11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parada, C. A., and R. G. Roeder. 1996. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature 384:375-378. [DOI] [PubMed] [Google Scholar]
- 50.Petito, C. K., E. S. Cho, W. Lemann, B. A. Navia, and R. W. Price. 1986. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J. Neuropathol. Exp. Neurol. 45:635-646. [DOI] [PubMed] [Google Scholar]
- 51.Price, R. W., B. J. Brew, and M. Rosenblum. 1990. The AIDS dementia complex and HIV-I brain infection: a pathogenetic model of virus-immune interaction, p. 269-290. In B. H. Waksman (ed.), Immunologic mechanisms in neurologic and psychiatric disease. Raven Press, New York, N.Y. [PubMed]
- 52.Radhakrishnan, S., J. Gordon, L. Del Valle, J. Cui, and K. Khalili. 2004. Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection. J. Virol. 78:7264-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Safak, M., and K. Khalili. 2001. Physical and functional interaction between viral and cellular proteins modulate JCV gene transcription. J. Neurovirol. 7:288-292. [DOI] [PubMed] [Google Scholar]
- 54.Safak, M., and K. Khalili. 2003. An overview: human polyomavirus, JC virus, and its associated disorders. J. Neurovirol. 9(Suppl. 1):3-9. [DOI] [PubMed] [Google Scholar]
- 55.Safak, M., R. Barrucco, A. Darbinyan, Y. Okada, K. Nagashima, and K. Khalili. 2001. Interaction of JC virus Agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J. Virol. 75:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safak, M., B. Sadowska, R. Barrucco, and K. Khalili. 2002. Functional interaction between JC virus late regulatory agnoprotein and cellular Y-box binding transcription factor, YB-1. J. Virol. 76:3828-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito, Y., L. R. Sharer, L. G. Epstein, J. Michaels, M. Mintz, M. Louder, K. Golding, T. A. Cvetkovich, and B. M. Blumberg. 1994. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissue. Neurology 44:474-481. [DOI] [PubMed] [Google Scholar]
- 58.Sawaya, B. E., K. Khalili, J. Gordon, R. Taube, and S. Amini. 2000. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J. Biol. Chem. 275:35209-35214. [DOI] [PubMed] [Google Scholar]
- 59.Tada, H., J. Rappaport, M. Lashgari, S. Amini, F. Wong-Stahl, and K. Khalili. 1990. Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc. Natl. Acad. Sci. USA 87:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi, K., S. L. Wesselingh, D. E. Griffin, J. C. McArthur, R. T. Johnson, and J. D. Glass. 1996. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann. Neurol. 39:705-711. [DOI] [PubMed] [Google Scholar]
- 61.Taylor, J. P., and K. Khalili. 1994. Activation of HIV-1 transcription by Tat in cells derived from the CNS: evidence for the participation of NF-kappaB. Adv. Neuroimmunol. 4:291-303. [DOI] [PubMed] [Google Scholar]
- 62.Taylor, J. P., R. Pomerantz, O. Bagasra, M. Chowdhury, J. Rappaport, K. Khalili, and S. Amini. 1992. TAR-independent transactivation by Tat in cells derived from the CNS: a novel mechanism of HIV-1 gene regulation. EMBO J. 11:3395-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor, J. P., M. Kundu, and K. Khalili. 1993. TAR-independent activation of HIV-1 requires the activation domain but not the RNA-binding domain of Tat. Virology 195:780-785. [DOI] [PubMed] [Google Scholar]
- 64.Taylor, J. P., R. J. Pomerantz, G. Raj, F. Kashanchi, J. N. Brady, S. Amini, and K. Khalili. 1994. Central nervous system-derived cells express a κB-binding activity that enhances human immunodeficiency virus type 1 transcription in vitro and facilitates TAR-independent transactivation by Tat. J. Virol. 68:3971-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tornatore, C., R. Chandra, J. R. Berger, and E. O. Major. 1994. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481-487. [DOI] [PubMed] [Google Scholar]
- 66.Trillo-Pazos, G., A. Diamanturos, L. Rislove, T. Menza, W. Chao, P. Belem, S. Sadiq, S. Morgello, L. Sharer, and D. J. Volsky. 2003. Detection of HIV-1 DNA in microglia/macrophages, astrocytes, and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 13:144-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vacante, D. A., R. Traub, and E. O. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170:353-361. [DOI] [PubMed] [Google Scholar]
- 68.Walker, D. L. 1985. Progressive multifocal leukoencephalopathy, p. 503-524. In P. J. Vinken, G. W. Bruyn, H. L. Klawans, and J. C. Kloetsier (ed.), Handbook of clinical neurology, vol. 47. Elsevier, New York, N.Y. [Google Scholar]
- 69.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 70.West, M. J., A. D. Lowe, and J. Karn. 2001. Activation of human immunodeficiency virus transcription in T cells revisited: NF-κB p65 stimulates transcriptional elongation. J. Virol. 75:8524-8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westendorp, M. O., V. A. Shatrov, K. Schulze-Osthoff, R. Frank, M. Kraft, M. Los, P. H. Krammer, W. Dröge, and V. Lehmann. 1995. HIV-1 Tat potentiates TNF-induced NF-kappaB activation and cytotoxicity by altering the cellular redox state. EMBO J. 14:546-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White, S. M., M. Renda, N.-Y. Nam, E. Klimatcheva, Y. Zhu, J. Fisk, M. Halterman, B. J. Rimel, H. Federoff, S. Pandya, J. D. Rosenblatt, and V. Planelles. 1999. Lentivirus vectors using human and simian immunodeficiency virus elements. J. Virol. 73:2832-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wright, C. M., B. K. Felber, H. Paskalis, and G. N. Pavlakis. 1986. Expression and characterization of the transactivator of HTLV-III/LAV virus. Science 234:988-992. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto, R., M. Katahira, S. Nishikawa, T. Baba, K. Taira, and P. K. Kumar. 2000. A novel RNA motif that binds efficiently and specifically to the Tat protein of HIV and inhibits the transactivation by Tat of transcription in vitro and in vivo. Genes Cells 5:371-388. [DOI] [PubMed] [Google Scholar]
- 75.Yang, L., G. F. Morris, J. M. Lockyer, M. Lu, Z. Wang, and C. B. Morris. 1997. Distinct transcriptional pathways of TAR-dependent and TAR-independent human immunodeficiency virus type-1 transactivation by Tat. Virology 235:48-64. [DOI] [PubMed] [Google Scholar]
- 76.Yang, Z., Q. Zhu, K. Luo, and Q. Zhou. 2001. The 7SK small nuclear RNA inhibits the cdk9/cyclin T1 kinase to control transcription. Nature 414:317-322. [DOI] [PubMed] [Google Scholar]
- 77.Yedavalli, V. S. R. K., M. Benkirane, and K. T. Jeang. 2003. Tat and trans-activation-responsive (TAR) RNA-independent induction of HIV-1 long terminal repeat by human and murine cyclin T1 requires Sp1. J. Biol. Chem. 278:6404-6410. [DOI] [PubMed] [Google Scholar]
- 78.Yik, J. H., R. Chen, R. Nishimura, J. L. Jennings, A. J. Link, and Q. Zhou. 2003. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol. Cell 12:971-982. [DOI] [PubMed] [Google Scholar]
- 79.Yong, V. W., and J. P. Antel. 1997. Culture of neural cells from human brain biopsies, p. 81-96. In S. Federoff and A. Anderson (ed.), Protocols for neural cell culture. Humana Press, Totowa, N.J.
- 80.Zhu, Y., T. Pe'ery, J. Peng, Y. Ramanathan, N. Marshall, T. Marshall, B. Amendt, M. B. Mathews, and D. H. Price. 1997. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 11:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]