Abstract
Human papillomaviruses (HPVs) replicate only in the terminally differentiating epithelium of the skin and mucosa. While infection of basal keratinocytes is considered a requirement for permissive infection, it remains unclear whether virions can specifically target basal cells for adsorption and uptake following epithelial wounding. We present evidence that HPV binds specifically to laminin 5 (LN5), a component of the extracellular matrix (ECM) secreted by migrating and basal keratinocytes. HPV type 11 capsids colocalized with LN5 in the ECM secreted by vaginal keratinocytes. Binding of both virions and virus-like particles to purified LN5 and to the LN5-rich ECM secreted by cultured keratinocytes was effectively blocked by pretreatment with anti-LN5 antibodies. HPV capsid binding to human cervical mucosa sections included the basement membrane which contains LN5. Cultured keratinocytes expressing α6 integrin, a transmembrane protein known to bind LN5, were readily infected by virions preadsorbed to LN5-containing substrates, whereas mutant keratinocytes lacking α6 integrin were relatively resistant to infection via this route. These findings suggest a model of natural HPV infection in which proliferating keratinocytes expressing α6 integrin at the site of epithelial wounding might be targeted by virions adsorbed transiently to LN5 secreted by migrating keratinocytes.
Human papillomavirus (HPV) particles have been shown to adsorb to the plasma membranes of cultured cells via membrane-associated heparan sulfate proteoglycans (HSPGs) (18, 20, 33) or α6 integrin (CD49f) (15, 26). Multiple HSPGs including CD44, syndecans and glypicans are expressed on the membranes of keratinocytes throughout the epidermis and mucosa (22, 29). α6 integrin expression is generally restricted to basal keratinocytes where this transmembrane protein pairs with β4 integrin and contributes to the nucleation of hemidesmosomes connecting the keratin cytoskeleton to the basement membrane (BM) (reviewed in reference 28). Results from experiments utilizing several in vitro infection models suggest that the importance of a particular receptor in HPV adsorption/infection may differ between cell lines and viral genotypes (12, 30, 33).
In addition to binding directly to membrane-associated glycoproteins, we recently found that HPV capsids are also capable of binding a component of the extracellular matrix (ECM) secreted by keratinocytes, but not by nonkeratinocyte cell lines (12). Here we show evidence that this secreted HPV adsorption receptor is laminin 5 (LN5), an epithelial laminin secreted by migrating keratinocytes as they invade wounded epithelium (reviewed in reference 27). In the context of the ECM secreted by cultured keratinocytes, HPV virions can use LN5 as an extracellular “transreceptor” by transiently binding LN5 and subsequently transferring to entry receptors on adjacent cells. In another viral system, human immunodeficiency virus (HIV) is hypothesized to transiently bind DC-SIGN (CD209) on immature dendritic cells within the epithelium and thereby be transported to the CD4/CCR5 receptor complex on T cells within secondary lymphoid tissues (17). Somewhat analogously, our findings suggest that HPV virions and pseudovirions bound to secreted LN5 can be transferred later to the membrane receptors on infectible cells and most efficiently to cells expressing α6 integrin. These findings suggest a model of natural infections in which LN5, secreted by migrating keratinocytes within epithelial wounds, may contribute to the targeting of HPV virions to the proliferating cells of the regenerating basal epithelium.
MATERIALS AND METHODS
Cells and viral particles.
HaCaT cells (5) were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). COS-7 cells were maintained in DMEM with 5% FBS. KH-SV and BOUA-SV cells were cultured with complete KGM (CC-3101; Cambrex) containing KGM SingleQuots (CC-4131; bovine pituitary extract, human epidermal growth factor, insulin, hydrocortisone, and gentamicin/amphotericin B). BOUA-SV-neo and BOUA-SV-α6 cells were grown in complete KGM containing G418 (400 μg/ml). Low-passage (less than five passages) human vaginal keratinocytes (provided by Craig Meyers) were grown in complete KGM. 293TT cells (provided by John Schiller) were cultured in DMEM supplemented with 10% FBS and hygromycin (400 μg/ml). HPV type 11 (HPV-11) virions were produced in xenografts in immunocompromised mice as previously described (21). L1-only virus-like particles (VLPs) were produced in insect cells infected with recombinant baculovirus encoding L1 (9) or alternatively produced in 293TT cells transfected with codon-optimized L1 (6). VLPs were isolated from cell lysates using fractionation following centrifugation in cesium chloride (insect cells) or Optiprep (Accurate Chemical) followed by cesium chloride (293TT cells). Gradient fractions were analyzed for VLP content by an enzyme-linked immunosorbent assay (ELISA) using a panel of monoclonal antibodies (MAbs) binding various epitopes to determine fractions rich in properly folded L1. Infectious pseudovirus particles were produced using 293TT cells according to the method previously described (6). Plasmids expressing L1, L2, and secreted alkaline phosphatase (seAP) were provided by the laboratory of John Schiller.
Binding of HPV-11 to LN5 in solid-phase ELISA.
Microtiter plates (96-well plates) were precoated with LN5 (200 ng/well), purified laminin from human placenta (LNmix) (200 ng/well), bovine serum albumin (BSA) (200 ng/well), or heparin-BSA (35 μg/well) overnight in 50 mM sodium carbonate (pH 9.6). Wells were rinsed with phosphate-buffered saline (PBS) (pH 7.4) and blocked for 2 h with 5% nonfat milk. Select wells were then treated with or without rabbit serum (1:100) in milk for an additional hour, rinsed, and then incubated with HPV-11 virions (1.6 × 109 particles/well) or L1-only VLPs (0.7 μg protein/well) in milk. Bound virus particles were detected using H11.B2 supernatant (1:100) (10), AP-conjugated rabbit anti-mouse antibody (Pierce), and p-nitrophenyl phosphate (Sigma). Final readings shown were obtained by subtracting the mean optical density readings for control wells having the same precoating substance (e.g., LN5) but lacking viral particles from the readings for the corresponding wells incubated with viral particles. Purified laminin 5 (also known as kalinin and epiligrin) was isolated from the conditioned medium of SCC-25 cells by antibody affinity chromatography as previously described (23). Human laminin from human placenta purified by an antibody targeting the β1-subunit of laminin was purchased from Chemicon (AG56P). Successful LN5 and laminin binding to wells was indicated in control wells using reactive antibodies (data not shown). Heparin-BSA was made by the conjugation of heparin (Sigma H-1027) to BSA using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) (Pierce) according to the manufacturer's recommended protocol.
Coimmunoprecipitation of VLPs and LN5.
HPV-11 L1-only VLPs (2.5 μg protein) were combined for 2.5 h on ice with 1 μg BSA or LN5 in 80 μl binding/wash buffer (BupH modified Dulbecco's PBS [Pierce]) supplemented with heat-inactivated BSA (125 μg/ml) to reduce nonspecific binding. ImmunoPure Immobilized Protein A Plus beads (Pierce) preconjugated to rabbit immunoglobulin G (IgG) (I5006; Sigma) or anti-LN5 (ab14509; Abcam) were added to the VLP mixtures and tumbled for 75 min at 4°C in binding/wash buffer supplemented with 0.01% Triton X-100 (Sigma) and heat-inactivated BSA (100 μg/ml). Bead complexes were isolated by centrifugation and washed five times with 1.5 ml binding/wash buffer and then boiled for 8 min in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading dye. Released proteins were separated using SDS-PAGE (7.5%) and transferred to polyvinylidene difluoride. The blot was blocked and subsequently probed in PBS containing 2% nonfat dry milk and 0.1% Span-20. HPV-11 L1 protein was detected by incubation with MAb H16.D9 (11). Bound H16.D9 was detected using a rabbit anti-mouse antibody conjugated to horseradish peroxidase (Pierce). The blot was then incubated with Supersignal West Pico chemiluminescent substrate (Pierce).
Immunocytochemistry.
Cells were seeded on glass coverslips at 8 × 104 cells/well on a 12-well plate and stained as indicated the following day. For Fig. 1a, cells were rinsed with PBS and then fixed with cold methanol. For Fig. 1b and c, rinsed cells were removed with gentle pipetting following a 10-min incubation in 10 mM EDTA followed by additional rinsing with PBS. Residual ECM was either fixed with methanol prior to staining (Fig. 1b) or stained on ice without fixation (Fig. 1c). All coverslips were blocked with 2% BSA (in PBS) which was also used for rinsing and as diluent. Virion binding to keratinocyte ECM was performed using 1010 particles/coverslip. All experiments using VLPs were performed using 12 μg protein/coverslip. Bound capsids were detected using H11.H3, a neutralizing MAb that recognizes a conformationally sensitive surface epitope of L1 (10). Antibodies to human proteins used for these experiments were anti-LN5 rabbit (Rb) serum, purified Rb anti-LN5 (ab14509; Abcam), purified Rb antilaminin (AB1953; Chemicon), anti-collagen type VII (LH7.2; Sigma), anti-CD49f (GoH3; BD PharMingen), anti-heparan sulfate (F58-10E4; Seikagaku), antiperlecan (7B5; Zymed), anti-HSPG (MAB458; Chemicon), anti-syndecan-1 (B-B4; Immunotech), and anti-CD44 (A3D8; Sigma). Purified rat IgG (catalog no. I4131; Sigma), rabbit IgG (catalog no. I5006; Sigma), and naïve rabbit serum were used as control antibodies. Blocking experiments in Fig. 1 were performed using each antibody at 10 μg/ml. Fluorophore-labeled secondary antibodies used were anti-mouse Alexa Fluor 488 and 594, anti-rabbit Alexa Fluor 488 and 594, and anti-rat Alexa Fluor 564 (Molecular Probes). All coverslips were stained with Hoechst 33342 (Molecular Probes) to indicate DNA. Fluorescence microscopy was performed using the Nikon Eclipse E600. Photographs were digitally prepared using Adobe Photoshop. Within each figure, all images were photographed and digitally prepared in an identical manner.
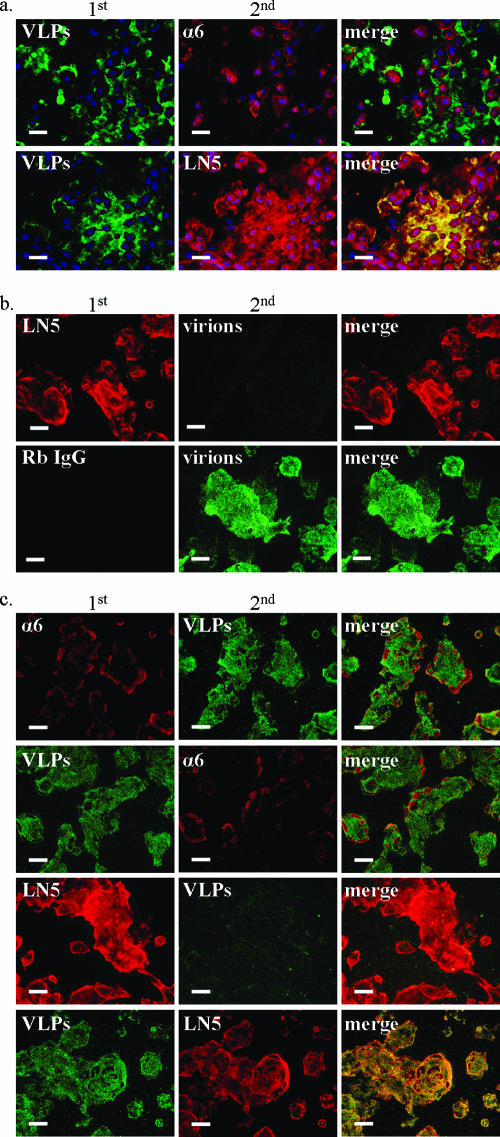
FIG. 1.
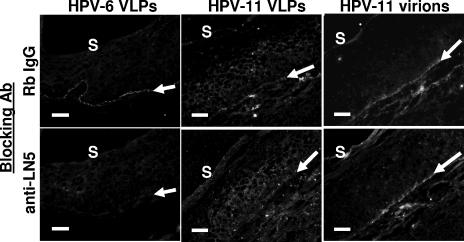
HPV capsids bind LN5 in the ECM of cultured keratinocytes. (a) Low-passage primary human vaginal keratinocytes were grown on glass coverslips for 24 h. After fixation and blocking with BSA, cells were first incubated with HPV-11 VLPs for 20 min. Coverslips were next incubated simultaneously with two antibodies, one to indicate bound VLPs (H11.H3) (green) and the other to indicate either α6 integrin (GoH3) or LN5 (ab14509) (red). This incubation was followed by labeled secondary antibodies and nuclear staining with Hoechst (blue). (b) HaCaT cells were removed from coverslips with EDTA, fixed with methanol, and blocked with BSA. Coverslips were first incubated for 2 h at 4°C with anti-LN5 (ab14509) or Rb IgG (all bound rabbit antibodies are indirectly labeled red), followed by binding with authentic HPV-11 virions (green) for 20 min. Coverslips were subsequently treated with H11.H3 and then with secondary antibodies and Hoechst stain. Similar results were obtained using VLPs and the fixed ECM of vaginal keratinocytes. (c) A blocking experiment was performed on ice using the unfixed ECM of HaCaT cells. As indicated in the panels in the first column, BSA-blocked coverslips were incubated initially either with GoH3 (first row), anti-LN5 (ab14509) (third row), rat IgG or rabbit IgG (not shown) or with HPV-11 L1-VLPs (second and fourth rows). After rinsing, coverslips were next incubated with the alternate treatment indicated in the panels of the second column (antibodies or VLPs). All coverslips were then incubated with H11.H3 to indicate bound VLPs followed by appropriate secondary antibodies and Hoechst stain (blue [absent, indicating successful cell removal]). α6 integrin and LN5 are shown as red, while bound VLPs are green. Bars, 50 μm.
Immunohistochemistry.
Frozen cervical sections from anonymous donors were fixed with cold acetone prior to incubation with viral particles and antibodies using 3% BSA (in PBS, pH 7.4) as the diluent. A pilot experiment showed that acetone-fixed sections stained identically to unfixed sections that were stained prior to fixation. HPV-11 virion binding experiments were performed using 1.3 × 109 particles/cervical section in a total volume of 40 μl. VLPs were used at 1.4 μg protein/section. Antibodies used to indicate human markers and particle binding were the same as those described above under “Immunocytochemistry.” For colocalization studies, confocal microscopy was utilized using the Leica TCS SP2. For competition/blocking experiments, we utilized fluorescence microscopy (on the Nikon Eclipse E600) to increase the stringency of these assays.
Heparinase treatment of sections of cervical mucosa.
Based upon the protocol previously described (8), the digestion buffer consisted of 50 mM HEPES, 50 mM sodium acetate, 150 mM NaCl, 9 mM CaCl2, and 0.1% BSA (pH 7.0). Heparinase II (catalog no. H6512; Sigma) and heparinase III (catalog no. H8891; Sigma) were reconstituted with digestion buffer immediately before use. Frozen sections were fixed on slides with cold acetone, dried briefly, and then covered with 100 μl digestion buffer with or without heparinase II and III (1.4 mIU each). All slides were incubated at 37°C within an immunochamber for 5 h with enzyme exchange occurring every 90 min. Following digestion, all slides were rinsed with PBS, kept in PBS overnight at 4°C, and stained as described the following day. Heparinase II and III were previously utilized for the removal of detectable HS from sections of human skin (8). Likewise, we found that this treatment eliminated all specific staining by the anti-HS MAb (F58-104E) from the sections of cervical mucosa (see Fig. S1b in the supplemental material).
Infection assays using authentic HPV-11 virions.
To create the ECM substrates, culture plates were seeded with HaCaT or COS-7 cells at 8 × 104 cells/cm2. Confluent monolayers were removed the next day using 10 mM EDTA as previously described (12) with cell removal confirmed by visual inspection. Residual ECM was rinsed repeatedly with PBS and visually inspected to ensure complete cell removal. Wells were then incubated with HPV-11 virions (109 particles in 0.5 ml culture medium/well on a 12-well plate) for 5 h at 37°C followed by repeated rinsing with PBS to remove unbound virions prior to seeding with the indicated cell lines (1.1 × 105 cells/well). On the basis of the findings of a previous study (14), cells were harvested at 2 days (COS-7), 4 days (human vaginal keratinocytes), or 5 days postinfection. All infections were carried out in culture medium lacking G418. For the antibody blocking experiments shown in Fig. 7, each control antibody contained the same azide concentration as did the experimental antibody. Successful transient HPV infection was measured by quantitative reverse transcription-PCR (QRT-PCR) using the Quantitect Probe RT-PCR kit (QIAGEN) and primers and probes (IDT) as previously described (13). Relative levels of viral E1^E4 transcripts in 1 μg total RNA (extracted using TRIzol [Invitrogen]) were determined relative to an endogenous cellular transcript (TATA-binding protein) using multiplex reactions with TaqMan probes.
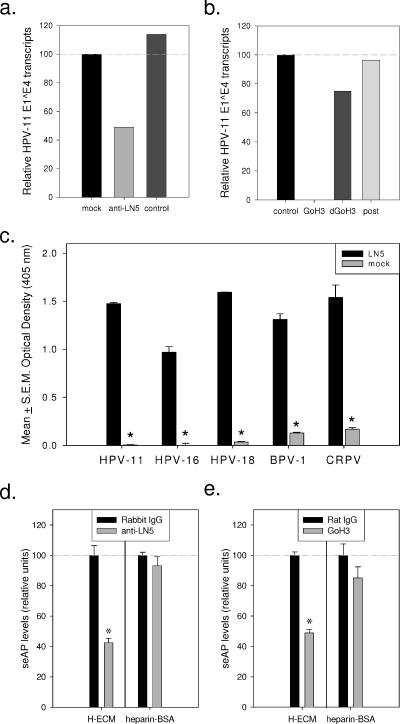
FIG. 7.
Effects of anti-LN5 and anti-α6 integrin antibodies on HPV infection. (a) Wells precoated with HaCaT ECM were incubated with medium only (mock), LN5 antiserum (1:25), or control rabbit serum (1:25) (control) for 3.5 h at 4°C. Unbound antibodies were removed by rinsing, and wells were treated with HPV-11 virions for 30 min at 37°C. Unbound particles were removed by rinsing prior to seeding each well with uninfected HaCaT cells. Viral E1^E4 transcripts were assayed by QRT-PCR. (b) 75% confluent HaCaT cells were inoculated with HPV-11 virions (150 particle/cell) in 1 ml growth medium and immediately treated with 5 μg of a control MAb (control), GoH3, or heat-denatured GoH3 (dGoH3), with a companion well left untreated (post). Twelve hours postinfection, all monolayers were rinsed and given fresh medium containing neutralizing MAb (H11.H3). Six hours later, the previously untreated well (post) was treated with 5 μg GoH3 to examine nonspecific effects of this antibody on viral infection. Infection was assayed by QRT-PCR. (c) Wells of a 96-well culture plate were precoated with LN5-rich ECM or left untreated prior to blocking with BSA (see Materials and Methods). Blocked wells were incubated with pseudovirions for 1 h at 37°C, rinsed, and seeded with uninfected 293TT cells. Three days later, the spent medium was analyzed for levels of seAP. BPV-1, bovine papillomavirus type 1; CRPV, cottontail rabbit papillomavirus. (d) Wells of a 96-well plate were precoated with LN5-rich ECM (ECM of HaCaT cells [H-ECM]) or heparin-BSA and then blocked with BSA. Select wells were then treated with 2 μg anti-LN5 or rabbit IgG (in BSA) for 2 additional hours at 4°C. All wells were then rinsed and incubated with HPV-11 pseudovirions for 1 h at 37°C. Wells were rinsed again and seeded with 293TT cells as described above. (e) HPV-11 pseudovirions were incubated for 1 h in BSA-blocked wells which were precoated with either LN5-rich ECM or heparin-BSA. After the wells were rinsed, they were seeded with 293TT cells, and select wells were immediately treated with 0.5 μg GoH3 or rat IgG. Values that were significantly different from the values for wells treated with the control antibody (P values of <0.05 by Student's t test) are indicated by asterisks.
Pseudovirion infection assays.
For precoating with LN5-rich ECM, wells of a 96-well plate were seeded with 3 × 104 HaCaT cells/well and incubated overnight at 37°C. HaCaT cells were removed with 10 mM EDTA. For precoating with heparin-BSA, wells were incubated overnight at 37°C with 20 μg/well heparin-BSA in PBS (pH 7.4). Prior to pseudovirion binding, all wells were blocked with heat-inactivated BSA (10 mg/ml) containing 0.02% sodium azide at 4°C for 2.5 h. Wells were rinsed three times with PBS and then incubated with infectious pseudovirions in culture medium lacking phenol red and hygromycin at 37°C for 1 h. Unbound pseudovirus particles were removed by rinsing four times with PBS. Wells were then seeded with 3 × 104 293TT cells/well. For the antibody blocking experiments in Fig. 7, each control antibody contained the same azide concentration as did the experimental antibody. Three days postseeding, 30 μl of spent medium from each well was transferred to a microtiter plate and combined with 100 μl p-nitrophenyl phosphate (1 mg/ml) (pH 9.5) followed by colorimetric analysis (at 405 nm).
RESULTS
HPV particles bind LN5 in the ECM of keratinocytes.
Previously we showed that HPV virions, pseudovirions, and L1-only VLPs bound similarly to an ECM component secreted by normal human epidermal keratinocytes and various keratinocyte-derived cell lines (12). To investigate whether this HPV adsorption receptor was also secreted by a more relevant cell type, primary vaginal keratinocytes were utilized (Fig. 1a). As seen previously with primary skin keratinocytes, the staining of vaginal keratinocytes showed high levels of LN5 (using ab14509) bound to large areas adjacent to the cells. As expected, staining of α6 integrin (using GoH3) was restricted to plasma membranes, presumably at the basal surface. Although the anti-LN5 and GoH3 antibodies showed the ability to bind epitopes underneath the fixed cells, the VLPs are evidently too large to access this area. As previously seen with the normal human epidermal keratinocytes (12), large numbers of VLPs bound to areas adjacent to the fixed vaginal keratinocytes where they colocalized with LN5.
To determine whether the anti-LN5 antibody could block authentic virion binding to the adsorption receptor in the ECM, HaCaT cells were removed from coverslips using EDTA followed by gentle pipetting, a treatment we have used previously to expose the ECM of cultured keratinocytes (12). The methanol-fixed ECM was then incubated with anti-LN5 prior to exposure to HPV-11 virions (Fig. 1b). Virion binding to the adsorption receptor present in the ECM was effectively blocked by anti-LN5 antibody compared with large amounts of virions bound to ECM on coverslips receiving the control antibody. Similar results were obtained using VLPs and human vaginal keratinocytes (data not shown).
We next addressed the possibility that interactions between HPV capsids and LN5 might be altered by methanol fixation. Coverslips coated with unfixed HaCaT cell ECM were sequentially treated with anti-α6 integrin (GoH3), anti-LN5, or anti-HS (F58-10E4) followed by incubation with VLPs or alternatively incubated first with VLPs and subsequently with one of the antibodies (Fig. 1c). In independent experiments with unfixed ECM, the anti-LN5 antibody effectively blocked capsid binding when applied to coverslips before the VLPs and colocalized with bound VLPs when applied after VLPs. These results were similar to what we have previously observed using VLPs and methanol-fixed ECM (data not shown) and demonstrate that capsid binding to LN5 is not an artifact of fixation. Although the staining of α6 integrin by GoH3 was improved with the use of unfixed ECM, the pretreatment of ECM with GoH3 did not affect VLP binding patterns compared with coverslips pretreated with the control antibody (data not shown), and the pattern and intensity of both VLP binding and α6 integrin staining were unaffected by the order of treatment. As we had previously seen using fixed ECM (12), HS staining was very weak in the unfixed ECM, and like GoH3, the anti-HS MAb (F58-10E4) did not block or colocalize well with bound VLPs (data not shown).
The ECM secreted by cultured keratinocytes is known to be composed predominately of LN5 (7). Multiple experiments utilizing indirect immunofluorescence to stain the EDTA-resistant ECM beneath cultured keratinocytes consistently revealed that this complex lacks detectable amounts of the selected HSPGs, collagen VII, and laminins containing the β-subunit (Table 1). This agrees with a previous report that the ECM underneath cultured keratinocytes is almost entirely LN5 which is not glycosylated by sulfated moieties (7). Low levels of HS and α6 integrin could be detected in some experiments, and staining was improved when unfixed ECM were stained on ice; however, neither marker colocalized with bound HPV particles. These observations support the hypothesis that HPV capsids bind the ECM of keratinocytes predominately through interactions with LN5, the primary component of the ECM secreted by these cells.
TABLE 1.
Summary of immunofluorescence studies of markers present in the ECM of cultured keratinocytes and cervical mucosa
| Marker | Antibody | Presence of marker ina:
|
||||
|---|---|---|---|---|---|---|
| ECM (in vitro)
|
Human cervical mucosa
|
|||||
| Unfixed | Fixed (meOH)b | Basement membrane | Basal layer epithelium | Suprabasal layers | ||
| LN5 | ab14509 | ++++ | ++++ | ++++ | − | − |
| Laminins (β1-subunit) | AB1953 | ND | − | ++++ | − | − |
| Collagen VII | LH7.2 | − | − | ++++ | − | − |
| α6 integrin (CD49f) | GoH3 | ++ | +/− | ++++ | ++ | + |
| Heparan sulfate | F58-10E4 | + | +/− | ++++ | − | +/− |
| Perlecan | 7B5 | − | − | +++ | − | − |
| BM-HSPG | MAB458 | − | − | + | − | − |
| CD44 | A3D8 | − | − | − | ++ | ++ |
| Syndecan-1 | B-B4 | − | − | − | + | ++++ |
For data within the ECM columns, the EDTA-resistant ECM produced by HaCaT cells was analyzed using each antibody listed. Additional, more-limited studies with these antibodies were also performed using several other keratinocyte-derived cell lines as well as primary keratinocytes from vagina and skin. Data for the staining of cervical sections was collected from experiments using four different patient samples. Symbols: ++++, very strong staining; +++, strong staining; ++, moderate staining; +, weak staining; −, absent; +/−, weak staining visible only in a fraction of experiments. ND, staining not done under this condition.
Fixed with methanol (MeOH).
HPV capsid binding to purified laminin 5.
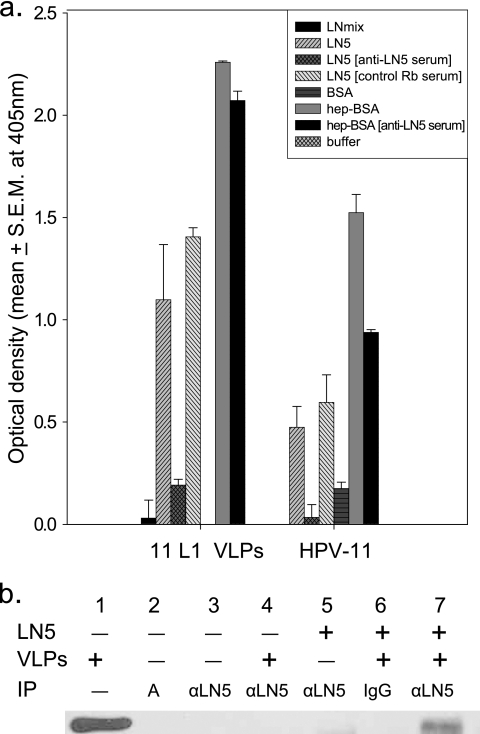
We next evaluated the ability of purified LN5, outside the context of the keratinocyte ECM, to bind HPV particles. LN5, isolated by antibody affinity chromatography (23), was applied to microtiter plates and analyzed for its ability to bind HPV-11 virions or L1-only VLPs (Fig. 2a). In multiple independent experiments, wells precoated with purified LN5 effectively bound viral particles, and capsid binding was blocked by anti-LN5. Wells coated with BSA or a commercially available mixture of laminins 1, 2, 3, 6, 8, and 10, purified from human placenta, failed to bind viral particles. The successful immobilization of both LN5 and the control laminin mixture from human placenta (LNmix) was verified in control wells by the use of anti-LN5 and antilaminin antibodies (data not shown). As previously reported, wells precoated with heparin-coupled BSA also bound HPV capsids effectively (18). Consistent with the ECM binding experiments, the binding of authentic HPV-11 virions to purified LN5 mirrors that of L1-only VLPs, indicating that the interaction between LN5 and the HPV capsids is largely mediated by the major capsid protein. Additional biochemical evidence for L1 binding to LN5 is shown in the coimmunoprecipitation of VLPs with purified LN5 using anti-LN5 complexed with protein A-agarose (Fig. 2b).
FIG. 2.
Binding of HPV particles to purified LN5. (a) For ELISA, triplicate wells of a 96-well microtiter plate were precoated with purified LN5, laminin from human placenta (LNmix), BSA, or heparin-BSA (hep-BSA) as described in Materials and Methods. After the wells were blocked, selected wells were incubated for 1 h with anti-LN5 rabbit serum or control Rb serum (shown in brackets). The entire plate was then rinsed and incubated with HPV-11 virions or L1-only VLPs followed by H11.B2 MAb to detect bound capsids. In parallel experiments, the commercially available anti-LN5 (ab14509) effectively blocked virion and VLP adsorption, while the control antibody (anti-LN [AB1953]) had no effect (data not shown). Values are means ± standard errors of the means (S.E.M.) (error bars). (b) For coimmunoprecipitation experiments, HPV-11 L1 VLPs or BSA were preincubated with (+) or without (−) purified LN5 and then mixed with protein A agarose prebound to anti-LN5 or rabbit IgG. Agarose complexes were washed to remove unbound proteins and analyzed by SDS-PAGE and immunoblotting for L1. Lane 1 contains input VLPs (2.5 μg). Lane 2 contains protein A agarose only (A). Lanes 3, 4, 5, and 7 contain protein A agarose conjugated to ab14509 (αLN5). Lane 6 contains protein A agarose conjugated to rabbit IgG (IgG).
Binding of HPV-11 virions to human cervical mucosa.
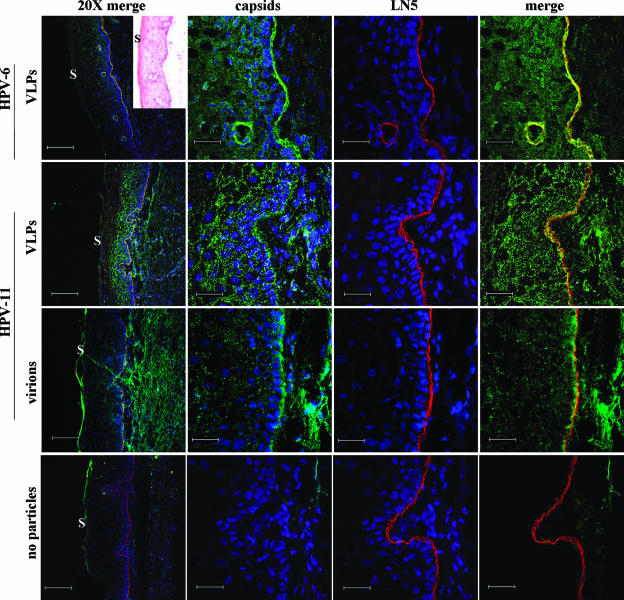
HPV VLPs and pseudovirions can bind directly to the BM of sections of frozen human cervical mucosa (12). To extend these observations using authentic virions, sections of cervical mucosa were exposed to HPV-11 virions prior to incubation with antibodies targeting various components of the BM. In multiple experiments, the L1-only VLPs of HPV-6 and HPV-11 bound directly to the BM as demonstrated by colocalization with anti-LN5 using confocal microscopy (Fig. 3). Differently from the VLPs, however, HPV-11 virions primarily bound the mucosal surface of the BM, possibly indicating interactions with the lamina lucida.
FIG. 3.
Confocal images of human cervical mucosa bound with HPV particles. HPV-6 VLPs, HPV-11 VLPs, or HPV-11 virions were incubated with sections of cervical mucosa for 45 min followed by H6.B10.5 or H11.B2 MAb (green), and anti-LN5 (ab14509) (red). No-particle control sections were treated with all three primary antibodies. All sections were subsequently incubated with labeled secondary antibodies and Hoechst stain (blue [not shown only in the rightmost panels]). Insert to aid in tissue orientation shows an hematoxylin and eosin stain of a companion cervical section. The location of the outer surface of the mucosa where present (S) is indicated. The leftmost panels show triple staining of mucosa at a magnification of ×45 (bars, 150 μm). The remaining three sets of panels show ×227 image including BM (bars, 30 μm). Hoechst staining is not shown in the fourth column merge for greater clarity.
Unlike the ECM beneath cultured keratinocytes which is almost entirely LN5, the BM of epithelial tissue contains a complex milieu of molecules (reviewed in reference 24). Data from multiple immunohistochemistry experiments using cervical mucosa from four patients is summarized in Table 1. Nevertheless, to determine whether anti-LN5 antibodies can block capsid binding to the BM of cervical sections, tissue sections were incubated with anti-LN5 prior to binding with virus particles (Fig. 4). The binding of HPV-6 VLPs to the BM was reproducibly but incompletely blocked by pretreatment with anti-LN5. Repeated experiments showed that the binding of HPV-11 VLPs was affected to a lesser extent than was the binding of HPV-6 VLPs. In contrast to in vitro binding experiments using keratinocyte ECM and purified LN5 (Fig. 1b and 2a), we saw no clear-cut evidence that anti-LN5 blocked the binding of HPV-11 virions to the cervical mucosa.
FIG. 4.
Blocking of basement membrane with anti-LN5 prior to capsid binding. Sequential sections of cervical mucosa were incubated for 40 min with anti-LN5 or rabbit IgG antibody (Ab) at 25 μg/ml followed by rinsing and incubation with VLPs or virions for 45 min. Sections were then exposed to H6.B10.5 or H11.B2 together with a low concentration of anti-LN5 (to indicate the position of the BM) followed by fluorescently labeled secondary antibodies and Hoechst stain (not shown). Gray-scale images show green channel only (bound particles). The location and angle of LN5 staining are shown by the white arrows for orientation. The location of the outer surface of the mucosa (S) is indicated. Bars, 50 μm.
Because HPV capsids have been shown to bind directly to HS and α6 integrin (15, 20), which are both present in large amounts at the BM of cervical mucosa, we performed additional experiments in which cervical sections were pretreated with heparinase II and III in the presence or absence of anti-LN5 or with anti-α6 integrin in the presence or absence of anti-LN5 prior to binding HPV-11 virions (see Fig. S1 in the supplemental material) (data not shown). Each of these pretreatments proved ineffective in blocking virion binding to the mucosal surface of the BM, emphasizing the complexity of in vivo binding.
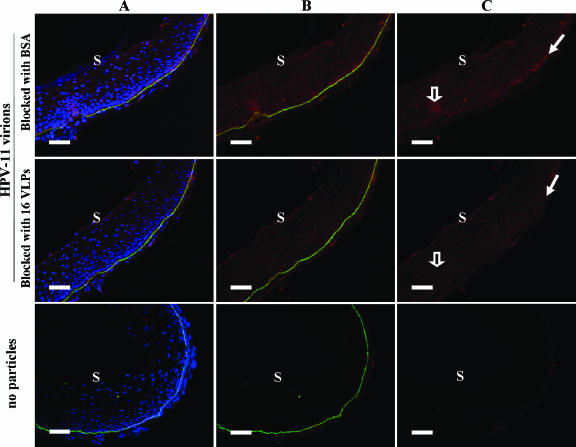
Additional experiments were performed to determine whether the binding of HPV-11 virions to cervical sections can be blocked by pretreatment with HPV-16 capsids. Sections of cervical mucosa were prebound with HPV-16 L1-VLPs prior to treatment with HPV-11 virions (Fig. 5). We consistently found reduced virion binding along the BM of cervical sections prebound with the VLPs. These experiments do not identify the specific receptor(s) that is bound by these particles but do demonstrate at least an overlap in receptor use by the capsids of diverse HPV genotypes (31). Additionally, these results support the hypothesis that particle binding at and near the BM is largely specific and mediated by L1.
FIG. 5.
Blocking the binding of HPV-11 virions to cervical sections using HPV-16 VLPs. Sequential sections of cervical mucosa were treated with HPV-16 L1-VLPs or with BSA only prior to incubation with authentic HPV-11 virions. Sections were then incubated with H11.A3.2 (red) and anti-LN5 (green) followed by appropriate secondary antibodies and Hoechst stain (blue). Triple staining is shown in column A. LN5 and HPV-11 virions are shown in column B, and virions alone are shown in column C. The wide hollow arrows in the BSA-blocked control section (top right) show virions bound to the suprabasal mucosa, while the narrow solid arrows indicate virions bound to the BM. Binding to both areas was blocked in the sequential cervical section that was first treated with HPV-16 VLPs. The location of the outer surface of the mucosa (S) is indicated. Bars, 50 μm.
Infection of cell lines by HPV virions bound to LN5-rich ECM.
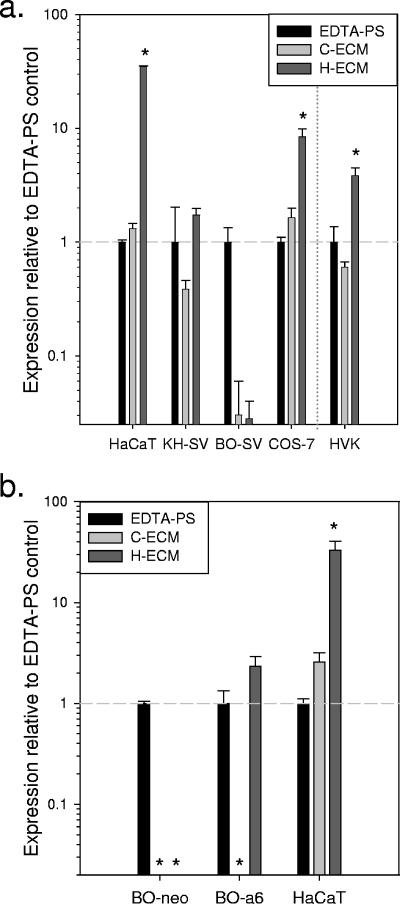
Authentic HPV virions adsorb well to the LN5-rich ECM of HaCaT cells and can be transferred to adjacent cells, resulting in transient infection in vitro (12). Because LN5 is the major ligand of the α6β4 integrin receptor at the hemidesmosomes, we hypothesized that α6 integrin, a proposed HPV receptor (15), might be important for the infection of attached cells by virions preadsorbed to LN5. To test this hypothesis, we first utilized BOUA-SV cells, a keratinocyte-derived cell line which is homozygous null for α6 integrin (16). In independent experiments, all cell lines expressing α6 integrin were highly infectible by HPV-11 adsorbed to the LN5-rich ECM of HaCaT cells, whereas the BOUA-SV cells were only weakly infected by virions adsorbed to this LN5-rich substrate (Fig. 6a). By visual inspection and quantitation of RNA harvested, all cell lines, including the BOUA-SV cells, adhered quickest and grew best on the LN5-rich ECM of HaCaT cells. These observations indicated that the poor ability of BOUA-SV cells to be infected via the HaCaT ECM was not simply because this cell line could not adhere to and proliferate on this substrate. BOUA-SV cells express the α3 integrin (our own unpublished data), which can interact directly with LN5 (7).
FIG. 6.
Infection of cultured cells by HPV-11 virions preadsorbed to various substrates. (a) Wells of a cell culture plate were coated with the ECM of COS-7 cells (C-ECM) or HaCaT cells (H-ECM) using EDTA removal of cells as described in Materials and Methods. Empty control wells were also treated with EDTA (EDTA-PS). All substrates were rinsed two times with PBS following EDTA treatment and prior to immediate incubation with HPV-11 virions suspended in culture medium for 5 h at 37°C. Following this incubation for viral attachment, all wells were rinsed repeatedly with PBS to remove unattached virions. Wells were then seeded with uninfected HaCaT, KH-SV, or BOUA-SV (BO-SV) cells or COS-7 cells or in a separate experiment, with human vaginal keratinocytes (HVK). (b) As described above for panel a, EDTA-resistant ECM substrates produced by COS-7 (C-ECM) or HaCaT cells (H-ECM) or EDTA-treated control wells (EDTA-PS) were incubated with HPV-11 virions in culture medium at 37°C, rinsed, and seeded with uninfected BOUA-SV-neo, BOUA-SV-α6, or HaCaT cells. In panels a and b, viral E1^E4 transcripts were assayed by QRT-PCR. The levels of viral transcripts (means ± standard errors of the means [error bars] of replicates) for each cell line are shown relative to the E1^E4 transcript levels measured in control wells (EDTA-PS) for that same cell line (horizontal dashed line). Values that were significantly different from the values for EDTA-PS wells for that cell line (P values of <0.05 by Student's t test) are indicated by asterisks.
To determine whether the ability to be infected by HPV-11 attached to a LN5-rich substrate could be restored, BOUA-SV cells transduced with α6 integrin (BOUA-SV-α6) or the empty neomycin-resistant expression vector (BOUA-SV-neo) (33) were seeded onto various substrates preadsorbed with HPV-11 virions (Fig. 6b). In each of three independent experiments, BOUA-SV-α6 cells demonstrated a significant gain of function in their ability to take up virions adsorbed to the LN5-rich ECM. Like the parental cell line, BOUA-SV-neo cells remained less susceptible to infection by HPV-11 virions adsorbed to the LN5-rich ECM, although infection could be detected by QRT-PCR. Both BOUA-SV-α6 and BOUA-SV-neo cells grew best on the LN5-rich ECM, demonstrating that the different infection profiles of these two cell lines were not due to differential growth on this substrate.
On the basis of our results utilizing the BOUA-SV cell lines, we next evaluated the ability of anti-LN5 and anti-α6 integrin (GoH3) to inhibit infection by HPV virions. ECM-coated wells were treated with anti-LN5 antibody prior to HPV-11 virion adsorption and seeding with uninfected HaCaT cells (Fig. 7a). Alternatively, adherent uninfected HaCaT cells were inoculated with HPV-11 virions in the presence of GoH3 (Fig. 7b). Both the anti-LN5 treatment prior to virion adsorption to ECM and the treatment with GoH3 at the time of infection resulted in reduced viral E1^E4 transcripts present in the treated cells consistent with reduced HPV-11 particle uptake. It should be noted that while pilot experiments demonstrated that HaCaT cell adherence and growth on the ECM-treated wells was not impaired by either antibody (data not shown), previous studies have demonstrated that under certain conditions, GoH3 can alter HaCaT cell signaling, proliferation, and migration (3, 19).
To further explore the possible role of LN5 in papillomavirus infection, we utilized pseudovirions delivering a reporter gene for secreted alkaline phosphatase (6) (Fig. 7c). HPV-11, HPV-16, HPV-18, cottontail rabbit papillomavirus, and bovine papillomavirus type 1 pseudovirions were each incubated in replicate wells of a 96-well plate precoated with LN5-rich ECM or BSA only. Following a 1-hour adsorption period at 37°C, wells were rinsed to removed unattached particles and then seeded with 293TT cells, a cell line which expresses α6 integrin (data not shown). Relative levels of seAP present in the spent medium collected 3 days later indicated much higher levels of infection in wells precoated with the LN5-rich ECM. This suggests that multiple papillomavirus types can adsorb to human LN5 and be transferred to adjacent cells.
We next explored infection by HPV-11 pseudovirions using anti-LN5 and GoH3. Wells of a 96-well cell culture plate were precoated either with LN5-rich ECM or with a heparin-BSA conjugate. After the wells were blocked with BSA and prior to pseudovirion adsorption, select wells were treated with anti-LN5 or control antibody (Fig. 7d). Following pseudovirion adsorption and extensive rinsing to remove unattached pseudovirions, wells were seeded with uninfected 293TT cells. Immediately after seeding, additional select wells were treated with GoH3 or the control antibody (Fig. 7e). Measuring seAP present in the spent medium 3 days later revealed that ECM-coated wells treated with either the anti-LN5 antibody or the anti-α6 integrin antibody contained significantly lower seAP levels than companion wells treated with the control antibodies. No significant difference was present in the wells precoated with heparin-BSA, consistent with the hypothesis that α6 integrin is important in HPV capsid transfer from LN5 to the plasma membrane. These data should be interpreted with caution, however, as cells treated with GoH3 were less confluent than cells treated with the control antibody (on either substrate at 20 h postseeding), indicating a secondary effect of this antibody treatment (data not shown). Similarly to what we saw with the HaCaT cells, the anti-LN5 antibody did not inhibit 293TT cell adhesion or growth on either the ECM or heparin-BSA-coated substrates.
DISCUSSION
Natural HPV infections are thought to be permissive for viral replication only when virions are endocytosed by mitotically active keratinocytes. Because HSPGs, such as syndecans and CD44, are expressed on the membranes of keratinocytes throughout much of the mucosa and epidermis (1, 22, 29), it is unclear how HS might participate in actively targeting virions to basal keratinocytes. Additionally, while HS appears to play at least a limited role in the infection of keratinocyte-derived cell lines by HPV-11 virions (12, 33), this glycosaminoglycan showed no involvement in the infection of primary human foreskin keratinocytes by HPV-31b virions (30). Therefore, the importance of HS in natural HPV infections remains a question for future studies.
In contrast to the ubiquitous expression of HSPGs throughout the epithelium, α6 integrin, another candidate HPV receptor, is characteristically expressed by basal keratinocytes where it contributes to the structure of hemidesmosomes anchoring these cells to the BM. Because of highly polarized expression by cultured keratinocytes, experiments demonstrating a role for α6 integrin in HPV adsorption have utilized suspended cells (15, 26, 36). While it is known that adherent BOUA-SV cells, which do not express α6 integrin, are capable of transient infection by HPV-11 (14, 33) and BPV-4 (34), these experiments do not rule out a role for this integrin in natural infections.
Our findings show that secreted LN5 can function in vitro as an extracellular transreceptor for infection by HPV virions and pseudovirions. LN5 is a uniquely epithelial laminin secreted as a heterotrimeric complex by migrating and basal keratinocytes. Evidence of specific binding of HPV capsids to LN5 is found in the observations that HPV capsids bind selectively to LN5 both in solid-phase ELISA and coimmunoprecipitation experiments (Fig. 2). Additionally, both virion and VLP binding to purified LN5 and to the LN5-rich ECM secreted by cultured keratinocytes can be effectively blocked by anti-LN5 antibodies (Fig. 1 and 2). Successful transfer of virus particles from LN5 to membrane-associated receptors on adjacent cells appears to be facilitated by expression of α6 integrin, which binds LN5.
The basement membrane of skin and mucosa contains multiple components, including three laminins (LN5, LN6, and LN10) (reviewed in reference 2). The commercially available human laminin mixture used in Fig. 2a was purified by a MAb targeting the β1-subunit of laminin and is described as containing LN6 and LN10. A polyclonal anti-LN antibody reactive against the laminin mixture stained the BM well but did not react with the ECM secreted by cultured keratinocytes. Together with the colocalization experiments and blocking experiments (Fig. 3 and 4), these data suggest that HPV L1 capsid binding to the BM is mediated at least in part by LN5, although participation by other epithelial laminins remains a possibility.
Given both the high levels of LN5, α6 integrin, and HS present at the BM and the high avidities of the HPV capsid-receptor interactions, it is perhaps more surprising that the anti-LN5 antibody effectively blocked HPV-6 VLP binding to the BM than it is that this same antibody failed to block HPV-11 binding. Disappointing to us was that even in combination with GoH3 (anti-α6 integrin) or the heparinase treatment, the anti-LN5 antibody showed no consistent ability to reduce virion binding. Even in the relatively simple context of suspended HaCaT cells, however, the GoH3 MAb could reduce HPV-6 VLP binding by only 60% (15). The effects of HS removal by heparinase on transient HPV infection in vitro are controversial (12, 30, 33). Even if LN5 were the sole receptor used by HPV-11 virions in our cervical mucosa binding experiments, the relative complexity of the BM may itself hinder the ability of antibodies targeting LN5 to block viral binding sites as effectively as we saw in the ECM binding experiments (Fig. 1).
Evidence suggests that the orientation of LN5 within the epidermal BM is nonrandom, with the long axis of LN5 oriented obliquely across the lamina lucida/densa border and the COOH terminus projecting toward the plasma membrane of the basal keratinocytes (25, 32). A nonrandom organization of LN5 and other BM components may influence HPV-11 virion binding in favor of the lamina lucida as suggested in Fig. 3. The ability of HPV-16 L1-VLPs to block HPV-11 virion binding (Fig. 5) supports the assertion that these particles are binding specifically to these sections of cervical mucosa and demonstrates that the interaction is mediated primarily by L1 and is genotype independent (Fig. 7c).
The cervical sections utilized for these experiments showed staining by the anti-HS MAb (clone F58-104E) only at the BM (see Fig. S1 in the supplemental material). It seems likely that undetected HS is expressed throughout most of the mucosa as suggested by syndecan-1 and CD44 staining. Presumably the heparinase treatment, which completely eliminated staining by anti-HS at the BM (see Fig. S1b in the supplemental material), also reduced HS present throughout the mucosa. Therefore, it is surprising that the binding of HPV-11 virions to basal and suprabasal keratinocytes was not obviously affected by the heparinase treatment (see Fig. S1 in the supplemental material). These findings suggest that an HS-independent adsorption mechanism may also exist on the suprabasal keratinocytes.
Our data indicate that α6 integrin, the natural binding partner of LN5 at hemidesmosomes (specifically as α6β4 integrin), may directly or indirectly facilitate the successful transfer of HPV virions from the LN5-rich ECM to the adjacent cells. Multiple cell lines including BOUA-SV cells ectopically expressing α6 integrin were readily infectible by HPV-11 virions preadsorbed to a LN5-rich ECM, while the parental BOUA-SV and vector-only BOUA-SV-neo cells were relatively resistant to infection by this route (Fig. 6). This pattern was repeatedly observed even though the LN5-rich ECM substrate secreted by HaCaT cells is capable of adsorbing large numbers of virus particles. The fact that COS-7 cells, which express α6β1 but not α6β4 integrin (26), are readily infected by LN5-adsorbed virions (Fig. 6a) indicates that β4 integrin is unlikely to be required for efficient transfer of HPV virions.
Efforts to inhibit HPV-11 infection using anti-LN5 and anti-α6 integrin antibodies indicated a partial inhibition by each antibody (Fig. 7). Contrary to binding assays which generally utilize fixed or metabolically inactive cells, infection is a complex biologic process requiring the postinfection culture of live cells with their associated intracellular signaling and metabolic pathways. Because targeting cell surface adhesion molecules (e.g., α6 integrin) and their ligands (e.g., LN5) with antibodies can affect cell adhesion, proliferation, and migration, experiments measuring the effects of such antibodies on viral infection must be interpreted with caution.
In vitro binding studies showed that anti-LN5 could effectively block large numbers of HPV-11 virions and VLPs from adsorbing to the LN5-rich ECM (Fig. 1). However, the same antibody only partially reduced in vitro infection with HPV-11 virions and pseudovirions (Fig. 7a and d). Our in vitro immunocytochemistry experiments showed that while the great majority of possible HPV binding sites on the LN5-rich ECM were effectively blocked by the specific antibody, a small fraction of input capsids were able to adsorb to the treated substrate (Fig. 1b). While viral particles bound to these unblocked binding sites were relatively undetectable by immunofluorescence, successful particle adsorption is clearly indicated by the more-sensitive in vitro infection assays, which utilize far fewer input particles.
LN5 is deposited at the edge of epithelial wounds within 8 h following injury (reviewed in reference 27). By adsorbing to the LN5 secreted by migrating keratinocytes at the site of wounded epithelium, HPV virions within the wound may be positioned to interact with the keratinocytes reconstituting the basal layer. As α6β4 integrin of hemidesmosomes interacts directly with LN5, we postulate that the LN5-adsorbed virions may be transferred directly to α6 integrin, a transmembrane protein expressed characteristically by migrating and basal keratinocytes and previously shown to bind HPV particles (15).
This model of natural infection includes a possible role for HS in the initial attachment of HPV virions within the epithelial wound. Virions bound nonspecifically throughout the epithelium could then be targeted to the proliferating keratinocytes. In the HIV system it has been demonstrated that HIV virions can bind transiently to HS moieties on the surfaces of nonpermissive cells for several days prior to successful transfer to target cells expressing CD4/CCR5 (4). Within the epithelial wound, the transfer of HPV virions from nonspecific HS receptors on suprabasal keratinocytes to other receptors secreted by (e.g., LN5) or expressed on (e.g., α6 integrin) proliferating keratinocytes may be facilitated by the rapid downregulation of HS and HSPG within the wound (1, 29). Additionally, HS on proliferating keratinocytes might participate in the transfer of HPV virions from LN5 to the surfaces of these cells. Heparin, and at least two syndecans expressed by keratinocytes (syndecan-2 and syndecan-4) bind the preprocessed form of LN5 secreted by migrating keratinocytes (35).
The ability of HPV to bind both secreted and membrane-associated adsorption receptors could help virions both to be immobilized within the epithelium following wounding and to be targeted to the appropriate cells. The slow entry kinetics of HPV virions adsorbed to membrane-associated receptors in vitro (14) might reveal a viral strategy to prevent the rapid uptake of virions by nonproliferating keratinocytes within the wounded epithelium. Secretion of LN5 by migrating keratinocytes invading the wound to reestablish the basal layer may function to help target HPV virions, bound nonspecifically within the wound, to the proliferating cells of the reconstituted basal layer.
Supplementary Material
Acknowledgments
We thank C. Meyers, N. Fusenig, R. Kirnbauer, and J. Schiller for their help in providing cells and plasmids used in these studies.
This work was supported by Public Health Service grant RO1 CA47622 from the National Cancer Institute, National Institutes of Health, and the Jake Gittlen Golf Tournament. Immunofluorescence studies were supported by NIH grant CA40145 (G. Clawson) and the Penn State College of Medicine Core Facilities.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Andriessen, M. P., J. van den Born, M. A. Latijnhouwers, M. Bergers, P. C. van de Kerkhof, and J. Schalkwijk. 1997. Basal membrane heparan sulphate proteoglycan expression during wound healing in human skin. J. Pathol. 183:264-271. [DOI] [PubMed] [Google Scholar]
- 2.Aumailley, M., A. El Khal, N. Knoss, and L. Tunggal. 2003. Laminin 5 processing and its integration into the ECM. Matrix Biol. 22:49-54. [DOI] [PubMed] [Google Scholar]
- 3.Becker, C., P. Buttler, and H. G. Graber. 2002. Influence of anti-CD49f and anti-CD29 monoclonal antibodies on mitotic activity of epithelial cells (HaCaT) and gingival fibroblasts in vitro. Eur. J. Oral Sci. 110:137-143. [DOI] [PubMed] [Google Scholar]
- 4.Bobardt, M. D., A. C. Saphire, H. C. Hung, X. Yu, B. Van der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 5.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, W. G., M. C. Ryan, and P. J. Gahr. 1991. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell 65:599-610. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Z., K. Meyer, A. C. Rapraeger, and A. Friedl. 2000. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB J. 14:137-144. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, N. D., R. Kirnbauer, J. T. Schiller, S.-J. Ghim, R. Schlegel, and J. W. Kreider. 1994. Human papillomavirus types 6 and 11 have antigenically distinct strongly-immunogenic conformationally-dependent neutralizing epitopes. Virology 205:329-335. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, N. D., J. W. Kreider, N. M. Cladel, S. D. Patrick, and P. A. Welsh. 1990. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J. Virol. 64:5678-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, N. D., C. A. Reed, N. M. Cladel, K. Hall, and G. S. Leiserowitz. 1996. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology 224:477-486. [DOI] [PubMed] [Google Scholar]
- 12.Culp, T. D., L. R. Budgeon, and N. D. Christensen. 2006. Human papillomaviruses bind a basal extracellular matrix component secreted by keratinocytes which is distinct from a membrane-associated receptor. Virology 347:147-159. [DOI] [PubMed] [Google Scholar]
- 13.Culp, T. D., and N. D. Christensen. 2003. Quantitative RT-PCR assay for HPV infection in cultured cells. J. Virol. Methods 111:135-144. [DOI] [PubMed] [Google Scholar]
- 14.Culp, T. D., and N. D. Christensen. 2004. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology 319:152-161. [DOI] [PubMed] [Google Scholar]
- 15.Evander, M., I. H. Frazer, E. Payne, Y. M. Qi, K. Hengst, and N. A. J. McMillan. 1997. Identification of the α6 integrin as a candidate receptor for papillomaviruses. J. Virol. 71:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gache, Y., C. Romero-Graillet, A. Spadafora, C. Lepinard, P. Descamps, C. B. Bardon, J. P. Ortonne, and G. Meneguzzi. 1998. A novel homozygous mutation affecting integrin alpha6 in a case of junctional epidermolysis bullosa with pyloric atresia detected in utero by ultrasound examination. J. Investig. Dermatol. 111:914-916. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hintermann, E., M. Bilban, A. Sharabi, and V. Quaranta. 2001. Inhibitory role of alpha 6 beta 4-associated erbB-2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on alpha 3 beta 1 integrin. J. Cell Biol. 153:465-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce, J. G., J. S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 21.Kreider, J. W., M. K. Howett, A. E. Leure-Dupree, R. J. Zaino, and J. A. Weber. 1987. Laboratory production in vivo of infectious human papillomavirus type 11. J. Virol. 61:590-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundqvist, K., and A. Schmidtchen. 2001. Immunohistochemical studies on proteoglycan expression in normal skin and chronic ulcers. Br. J. Dermatol. 144:254-259. [DOI] [PubMed] [Google Scholar]
- 23.Marinkovich, M. P., G. P. Lunstrum, and R. E. Burgeson. 1992. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J. Biol. Chem. 267:17900-17906. [PubMed] [Google Scholar]
- 24.McMillan, J. R., M. Akiyama, and H. Shimizu. 2003. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J. Dermatol. Sci. 31:169-177. [DOI] [PubMed] [Google Scholar]
- 25.McMillan, J. R., M. Akiyama, and H. Shimizu. 2003. Ultrastructural orientation of laminin 5 in the epidermal basement membrane: an updated model for basement membrane organization. J. Histochem. Cytochem. 51:1299-1306. [DOI] [PubMed] [Google Scholar]
- 26.McMillan, N. A., E. Payne, I. H. Frazer, and M. Evander. 1999. Expression of the alpha6 integrin confers papillomavirus binding upon receptor-negative B-cells. Virology 261:271-279. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen, B. P., M. C. Ryan, S. G. Gil, and W. G. Carter. 2000. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr. Opin. Cell Biol. 12:554-562. [DOI] [PubMed] [Google Scholar]
- 28.Nievers, M. G., R. Q. Schaapveld, and A. Sonnenberg. 1999. Biology and function of hemidesmosomes. Matrix Biol. 18:5-17. [DOI] [PubMed] [Google Scholar]
- 29.Oksala, O., T. Salo, R. Tammi, L. Hakkinen, M. Jalkanen, P. Inki, and H. Larjava. 1995. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 43:125-135. [DOI] [PubMed] [Google Scholar]
- 30.Patterson, N. A., J. L. Smith, and M. A. Ozbun. 2005. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. J. Virol. 79:6838-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roden, R. B., R. Kirnbauer, A. B. Jenson, D. R. Lowy, and J. T. Schiller. 1994. Interaction of papillomaviruses with the cell surface. J. Virol. 68:7260-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousselle, P., D. R. Keene, F. Ruggiero, M. F. Champliaud, M. Rest, and R. E. Burgeson. 1997. Laminin 5 binds the NC-1 domain of type VII collagen. J. Cell Biol. 138:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafti-Keramat, S., A. Handisurya, E. Kriehuber, G. Meneguzzi, K. Slupetzky, and R. Kirnbauer. 2003. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J. Virol. 77:13125-13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibbet, G., C. Romero-Graillet, G. Meneguzzi, and M. S. Campo. 2000. Alpha6 integrin is not the obligatory cell receptor for bovine papillomavirus type 4. J. Gen. Virol. 81:327-334. [DOI] [PubMed] [Google Scholar]
- 35.Utani, A., M. Nomizu, H. Matsuura, K. Kato, T. Kobayashi, U. Takeda, S. Aota, P. K. Nielsen, and H. Shinkai. 2001. A unique sequence of the laminin alpha 3 G domain binds to heparin and promotes cell adhesion through syndecan-2 and -4. J. Biol. Chem. 276:28779-28788. [DOI] [PubMed] [Google Scholar]
- 36.Yoon, C. S., K. D. Kim, S. N. Park, and S. W. Cheong. 2001. α6 integrin is the main receptor of human papillomavirus type 16 VLP. Biochem. Biophys. Res. Commun. 283:668-673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.