Abstract
Perturbation of the function of endoplasmic reticulum (ER) causes stress leading to the activation of cell signaling pathways known as the unfolded protein response (UPR). Severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) uses ER as a site for synthesis and processing of viral proteins. In this report, we demonstrate that infection with SARS-CoV induces the UPR in cultured cells. A comparison with M, E, and NSP6 proteins indicates that SARS-CoV spike (S) protein sufficiently induces transcriptional activation of several UPR effectors, including glucose-regulated protein 78 (GRP78), GRP94, and C/EBP homologous protein. A substantial amount of S protein accumulates in the ER. The expression of S protein exerts different effects on the three major signaling pathways of the UPR. Particularly, it induces GRP78/94 through PKR-like ER kinase but has no influence on activating transcription factor 6 or X box-binding protein 1. Taken together, our findings suggest that SARS-CoV S protein specifically modulates the UPR to facilitate viral replication.
Severe acute respiratory syndrome (SARS) is a highly lethal infectious disease in human caused by a newly recognized coronavirus termed SARS coronavirus (SARS-CoV) (42), close relatives of which have recently been found in various species of bats (24, 29). Like other coronaviruses, SARS-CoV is an enveloped and positive-stranded RNA virus that has a large genome of ∼30 kb. It replicates in the cytoplasm, and its life cycle is closely associated with the endoplasmic reticulum (ER). The viral activities have a profound impact on ER function (30, 37). Particularly, SARS-CoV hijacks the ER to process its structural and nonstructural proteins (22, 55).
In eukaryotes, the ER is the processing factory for proteins destined for secretion or membrane insertion (43). During the replication of SARS-CoV, substantial amounts of viral proteins are produced. Some of them, such as the spike (S) and matrix (M) proteins, are heavily modified transmembrane proteins (22, 55). This raises the possibility that the accumulation of nascent and unfolded SARS-CoV proteins in the lumen of ER might rapidly exceed its folding capacity, thereby perturbing the normal cellular function of ER.
Perturbation of ER function causes stress. ER stress activates multiple cell-signaling pathways to regulate gene expression at both transcriptional and translational levels. These pathways, collectively termed the unfolded protein response (UPR), adjust the biosynthetic burden and capacity of the ER to maintain homeostasis (43). When the damage to the ER is severe or persistent, the UPR triggers apoptosis (5, 58). To date, three key proximal sensors of the UPR, namely, activating transcription factor 6 (ATF6), inositol-requiring enzyme 1 (IRE1), and PKR-like ER kinase (PERK), have been identified. All three are ER-resident transmembrane proteins, and they govern three branches of the UPR signaling that activate different subsets of genes encoding ER chaperones, folding enzymes, and other proteins required for protein folding, maturation, and degradation (43).
The effects of the UPR could either be beneficial or detrimental to viral infection. For instance, protein chaperones produced in response to ER stress enhance the folding of viral proteins, but elevated expression of protein degradation factors could lead to inactivation of these proteins. To survive ER stress, viruses have developed different strategies to modulate the UPR (7, 12, 45, 46, 54, 57, 59). For one example, infection with hepatitis C virus (HCV) has dual effects on the UPR. On one hand, the expression of E2, core, and NS5A proteins activates the promoter of glucose-regulated protein 78 (GRP78) and GRP94 through PERK and ATF6 (3, 6, 31, 41, 47). Thus, the folding of viral proteins could be stimulated. On the other hand, the IRE1-X box-binding protein 1 (XBP1) pathway of the UPR, which governs protein refolding and degradation, is suppressed by HCV (48). Hence, HCV proteins would not be inactivated through this pathway (49). Likewise, human cytomegalovirus (CMV) activates PERK, IRE1, and XBP1 but suppresses ATF6 and a protein degradation factor known as ER degradation-enhancing α-mannosidase I-like protein (17, 50). In another example, a cytopathic strain of bovine viral diarrhea virus activates PERK and induces apoptosis through the UPR (21). Therefore, different viruses differentially regulate the UPR for their own benefits (15). However, whether and how coronaviruses impact the UPR in infected cells are not understood.
To shed light on the molecular and cellular basis of SARS-CoV pathogenesis, we set out to study the influence of SARS-CoV on ER stress and the UPR. In the present study, we demonstrated the general effect of SARS-CoV infection on ER stress and the modulation of the UPR by SARS-CoV S protein. We first examined the impact of infection with SARS-CoV on the expression of ER chaperones GRP78 and GRP94. Next we compared the regulatory roles of several SARS-CoV proteins in the transcriptional activation of the UPR effectors and identified S protein to be a critical modulator of the UPR. Lastly, we characterized the differential modulatory activity of S for three branches of the UPR signaling. Our findings have implications in the pathogenesis of SARS and the development of antivirals against SARS-CoV.
MATERIALS AND METHODS
Virus and cells.
The GZ50 strain of SARS-CoV was propagated in FRhK-4 or Vero cells in a biosafety level 3 laboratory as described previously (42). FRhK-4 cells infected with SARS-CoV were harvested to 1× sodium dodecyl sulfate (SDS) gel loading buffer (50 mM Tris-Cl [pH 6.8], 2% SDS, 10% glycerol, 1% β-mercaptoethanol, and 0.02% bromophenol blue) at 24 h postinfection (hpi). 293FT cells stably expressing simian virus 40 large T antigen and all other cell lines were grown in Dulbecco's modified Eagle medium containing 10% fetal bovine serum, 100 U/ml penicillin G, and 100 mg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere supplied with 5% CO2. For reporter assays of infected cells, Vero cells were transfected with GRP94-Luc or GRP78-Luc 24 h before infection with SARS-CoV. For inactivation of SARS-CoV, 2-ml aliquots of virus were exposed to UVC irradiation (254 nm) for 15 min as described elsewhere (11).
Plasmids and primers.
Expression plasmids for eIF2α dominant active (DA) and dominant negative (DN) mutants S51D and S51A (39) were kindly provided by David Ron. Human PERK and its DN mutant K621M (34) were a generous gift from Ronald Wek. pCHOP-Luc reporter plasmid containing −644 to +91 of human C/EBP homologous protein (CHOP) promoter was from Nai Sum Wong (23). pGRP78-Luc and pGRP94-Luc reporter constructs (60) were supplied by Kazutoshi Mori. pGRP78-Luc contains −304 to +34 of human GRP78 promoter, and pGRP94-Luc carries a promoter fragment corresponding to −363 to +34 of human GRP94 gene. Both promoters have multiple copies of the ER stress response element (60). In addition, the GRP78 promoter harbors a functional ATF/CRE site (33). pUPRE-Luc and pGal-Luc reporter plasmids and expression vector for Gal-ATF6 have been described elsewhere (8, 27).
cDNAs encoding SARS-CoV S, E, M, and NSP6 proteins were PCR amplified from molecular clones of the SARS-CoV subgenome (37). The primers used for amplification of the S gene were 5′-CAACAGAGTTGTGGTTTCAAGT-3′ and 5′-CGCCAATAACAAGCCATCCGAAAG-3′. The resulting PCR fragment was subcloned into pGEM-T Easy vector (Promega). To express V5-tagged S protein, another set of primers (5′-CACCATGTTTATTTTCTTATTATTTCTTAC-3′ and 5′-TGTGTAATGTAATTTGACACCCTT-3′) was used and the fragment was subcloned into pLenti-Topo vector (Invitrogen). To subclone E/M/NSP6 genes into pLenti vector, the following sets of primers were employed: for amplification of E, 5′-GCGGATCCACCATGTACTCATTCGTTTCGGAAGAAAC-3′ and 5′-CCGCTCGAGCCGACCAGAAGATCAGGAACTCC-3′; for amplification of M, 5′-GCGGATCCACCATGGCAGACAACGGTACTATTA-3′ and 5′-CCGCTCGAGCCCTGTACTAGCAAAGCAATATTGTC-3′; and for amplification of NSP6, 5′-GCGGATCCACCATGGGTAAGTTCAAGAAAATTGTTAAGG-3′ and 5′-CCGCTCGAGCCCTGTACAGTAGCAACCTTGATA-3′. The V5 tag is at the C terminus of the S, E, M, and NSP6 proteins.
Primers 5′-GTTGAGAACCAGGAGTTAAGACAG-3′ and 5′-CAGAGGGTATCTCTAAGACTAGGG-3′ were used to amplify both unspliced and spliced XBP1 transcripts. Primers 5′-GCAGGGGGGAGCCAAAAGGG-3′ and 5′-TGCCAGCCCCAGCGTCAAAG-3′ were used for amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Antibodies.
Rabbit polyclonal anti-V5, mouse monoclonal anti-α-tubulin, and mouse monoclonal anti-β-actin were from Sigma. Rat monoclonal anti-GRP94 and mouse monoclonal anticalnexin were from Affinity Bioreagents. Rabbit polyclonal antiserum against SARS-CoV S protein was from Imgenex. Rabbit polyclonal antiserum against total eIF2α was from Santa-Cruz Biotechnology. Rabbit polyclonal antiserum against eIF2α phosphorylated on serine 51 was from Cell Signaling Technology.
Luciferase reporter assays.
Dual luciferase reporter assays were performed using a reagent kit from Promega as described previously (8, 44). Luminescence was measured with an LB9570 luminometer (EG&G). Relative luciferase activity (RLA) was calculated by normalizing readouts of firefly luciferase to those of Renilla luciferase expressed from a control plasmid (pRLSV40 from Promega) cotransfected into the cells.
Western blotting.
Western blot analysis was carried out as described previously (10, 20). Briefly, cells were lysed in radioimmunoprecipitation buffer (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) supplemented with 2 mM phenylmethylsulfonyl fluoride and other protease inhibitors (Complete protease inhibitor cocktail from Roche). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis. Proteins were transferred to Immobilon-P membranes (Millipore). Blots were blocked with 5% nonfat milk diluted in Tris-buffered saline (pH 7.6) containing 0.5% Tween 20 (TBS-T), followed by incubation with primary antibodies. After TBS-T washes, blots were further incubated with the appropriate secondary antibodies conjugated with horseradish peroxidase (Amersham). Proteins were visualized using chemiluminescence detection kits from Amersham.
Confocal immunofluorescence microscopy.
Confocal microscopy was performed as previously described (9, 38, 62). Briefly, transfected cells were fixed in ice-cold acetone-methanol (1:1) for 10 min. Cells were washed with phosphate-buffered saline (PBS) (pH 7.4) and blocked with 3% bovine serum albumin in PBS. The cells were then incubated with the appropriate antibodies. The coverslips containing cells were mounted on a glass slide using Mowiol prepared in glycerol-PBS. Confocal immunofluorescence microscopy was then performed on a Bio-Rad MRC1024 system, and the images were captured with the help of the LaserSharp software.
RESULTS
Infection with SARS-CoV induces ER stress.
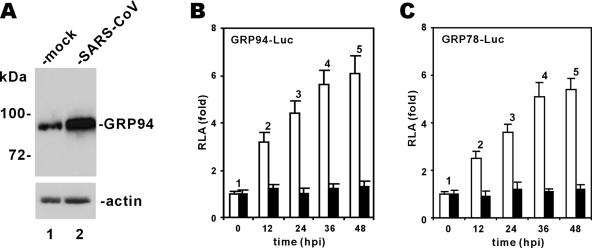
HCV and several other viruses induce ER stress and the UPR in infected cells (15, 49). To investigate whether infection with SARS-CoV might also have an impact on ER stress, we used commercial antibodies for GRP94 and GRP78 to determine whether their expression is induced in SARS-CoV-infected FRhK4 cells. GRP94 and GRP78 are molecular chaperones and sensitive markers of ER stress (43). Among several commercial antibodies we used, only one could react specifically and reproducibly with a discrete protein band of GRP94 in the infected cells. Using this antibody, we detected a 4.8-fold increase in the steady-state level of GRP94 in SARS-CoV-infected cells (Fig. 1A; lane 2 compared to lane 1). This finding is generally consistent with results from global proteomic analysis of SARS-CoV-infected cells, in which GRP94 and GRP78 have been found to be overexpressed (19).
FIG. 1.
Infection with SARS-CoV induces GRP94 and GRP78. (A) Induction of GRP94 expression. FRhK4 cells were either mock infected or infected with SARS-CoV. Cells harvested at 24 hpi were lysed and immunoblotted with anti-GRP94 and anti-β-actin. (B and C) Transcriptional activation of GRP94 and GRP78 promoters. Vero cells were transfected with pGRP94/78-Luc plasmid at 24 h before infection with SARS-CoV (□) or with UVC-inactivated SARS-CoV (▪). Cells were harvested for dual luciferase assay at the indicated time points. RLA values shown represent the means ± standard deviations of the results from three separate infections. Control plasmid pRLSV40 expressing Renilla luciferase was cotransfected into cells and used for normalization of transfection efficiency.
To further analyze the influence of SARS-CoV infection on transcriptional activation of GRP94 and GRP78 genes, we transfected luciferase reporter constructs driven by GRP94/78 promoters (60) into Vero cells before infection with SARS-CoV. We then harvested cells at 12 to 48 hpi for measurement of RLA. Progressively increased RLA values in SARS-CoV-infected cells indicated the activation of GRP94/78 promoters (Fig. 1B and C, white columns 2 to 5 compared to white column 1). In contrast, the RLA values remained constant in cells incubated with SARS-CoV inactivated by UV irradiation (UVC for 15 min; Fig. 1B and C, black columns 1 to 5). Thus, infection with SARS-CoV induces ER stress through transcriptional activation of GRP78/94.
ER stress is induced by SARS-CoV S protein.
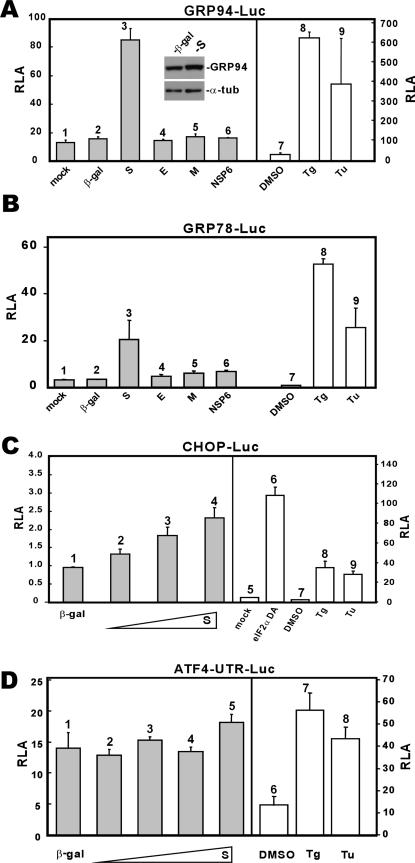
SARS-CoV encodes several transmembrane proteins that are synthesized and likely accumulate in the ER (22, 55). To investigate whether these proteins might perturb the function of ER leading to the UPR, we expressed SARS-CoV S, E, M, and NSP6 proteins in 293FT cells. All four proteins have at least one transmembrane domain and are abundantly expressed in infected cells (37). We observed that among the four, only S activated transcription from GRP94/78 promoters to approximately fivefold (Fig. 2A and B, column 3 compared to columns 1 and 2). In the same experiment, treatment with thapsigargin (Tg) and tunicamycin (Tu), two well-known stimuli of ER stress, led to approximately 10- to 30-fold activation of luciferase expression (Fig. 2A and B, columns 8 and 9 compared to column 7). In contrast, none of the other three proteins significantly stimulated GRP94/78 promoters (Fig. 2A and B, columns 4 to 6 compared to columns 1 and 2). The activation of GRP94 expression was also confirmed by Western blotting, which shows a 2.4-fold increase of GRP94 protein level in S-expressing cells as normalized to the level of α-tubulin (Fig. 2A, inset). In addition, S could modestly stimulate transcription from the promoter of CHOP (Fig. 2C, columns 2 to 5 compared to columns 1 and 6 to 9), a major component of the ER stress-induced apoptosis pathway (23, 35, 40), whereas it did not significantly affect translation of ATF4 (Fig. 2D). The activation of ATF4 translation involving upstream open reading frames in the 5′ untranslated region represents one major mechanism by which cells respond to ER stress (32, 52). In our experiment, we observed that ATF4 translation was slightly increased only when S was most abundantly expressed in cells (Fig. 2D, column 5).
FIG. 2.
Influence of SARS-CoV proteins on the UPR. (A and B) SARS-CoV S protein activates GRP94 and GRP78 promoters. 293FT cells were transiently cotransfected with pGRP78/94-Luc plus a pLenti-based expression vector for the indicated protein. Control cells transfected with pGRP78/94-Luc alone were treated with dimethyl sulfoxide (DMSO), Tg (300 nM), or Tu (5 μg/ml) for 16 h. Cells were harvested 48 hpi for dual luciferase assay. Expression levels of GRP94 and α-tubulin (α-tub) in β-galactosidase (β-gal)- and S-expressing cells were verified by Western blotting (inset). (C) SARS-CoV S protein modestly activates CHOP promoter. 293FT cells were cotransfected with pCHOP-Luc and escalating amounts of pLenti-S. Cells were harvested for dual luciferase assay as described for panel A. A DA form of eIF2α was used as a positive control in this analysis, as were Tg (300 nM) and Tu (5 μg/ml). Transcription from the CHOP promoter is significantly induced by eIF2α DA, Tg, and Tu. (D) SARS-CoV S protein does not significantly affect ATF4 translation. 293FT cells were cotransfected with pATF4-UTR-Luc and escalating amounts of pLenti-S. Cells were harvested for dual luciferase assay as described for panel A. Tg (300 nM) and Tu (5 μg/ml) were used as positive controls in this analysis. pATF4-UTR-Luc contains the 5′ UTR of human ATF4 fused to the coding region of firefly luciferase. This construct is similar to those described for mouse ATF4 (32, 52).
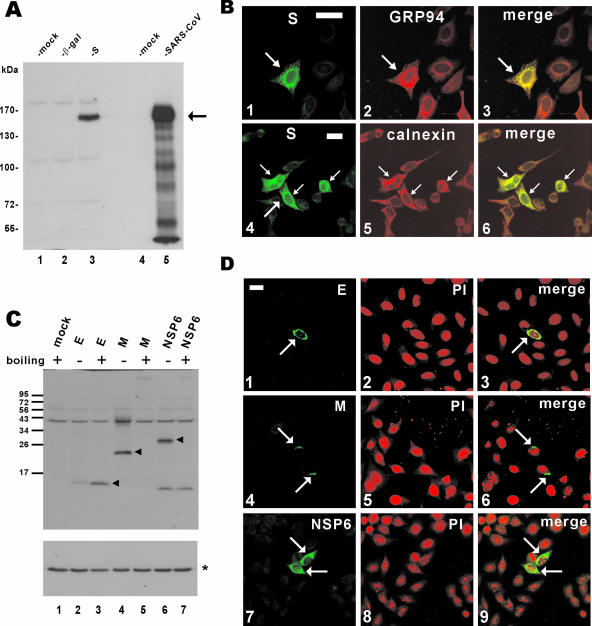
The expression of S and other SARS-CoV proteins (E, M, and NSP6) was verified by Western blotting and confocal immunofluorescence microscopy (Fig. 3). In particular, S protein was effectively expressed, although the relative abundance of S in the transiently transfected cells was lower than in SARS-CoV-infected cells (Fig. 3A, column 3 compared to column 5). While a fluorescent signal above the background level was detected in the whole cell expressing S, a significant amount of S protein was found to accumulate in the ER, as demonstrated by substantial colocalization with ER markers GRP94 and calnexin (Fig. 3B, panels 3 and 6). In addition, E, M, and NSP6 proteins were also efficiently expressed in cultured cells (Fig. 3C and D). Consistent with a previous report on M protein (28), we found that both M and NSP6 proteins were aggregated and could not be detected in the protein blot after boiling of the samples (Fig. 3C; compare lane 5 to lane 4 and lane 7 to lane 6).
FIG. 3.
Expression of SARS-CoV proteins in cultured cells. (A) Western blot analysis of S protein expression. pLenti-β-gal-transfected and pLenti-S-transfected 293FT cells (lanes 2 and 3) and SARS-CoV-infected FRhk4 cells (lane 5) were lysed and immunoblotted with rabbit polyclonal anti-S antibody. The arrow highlights S protein. (B) Subcellular localization of SARS-CoV S protein. HeLa cells were transfected with pLenti-S and then costained with rabbit anti-V5 and either rat anti-GRP94 (panel 2) or mouse anti-calnexin (panel 5). The S and GRP94-calnexin fluorescent signals are shown in merged images, and colocalization is shown in yellow (panels 3 and 6). Transfected cells are highlighted with arrows. Bar, 30 μm. (C and D) Expression of other SARS-CoV proteins. 293FT cells were transfected with pLenti-E/M/NSP6, and protein expression was analyzed by immunoblotting (C) or confocal immunostaining (D) with rabbit polyclonal anti-V5 antibody. To prevent thermal aggregation of M and NSP6 (28), protein samples were not heated before being loaded onto the SDS-polyacrylamide gel electrophoresis gel. Nuclear morphology of cells was visualized by propidium iodide (PI) staining. In panel C, the arrowheads highlight E/M/NSP6 proteins and the asterisk indicates the control protein α-tubulin.
Taken together, our results suggest that differential activation of the UPR by SARS-CoV is mediated at least in part through S protein.
Differential regulation of the UPR pathways by SARS-CoV S protein.
ER stress induces three major pathways of the UPR signaling that are mediated through PERK kinase, IRE1, and ATF6, respectively (43). The phosphorylation of translation initiation factor eIF2α by PERK in response to ER stress shuts off general translation of cellular proteins but stimulates the expression of molecular chaperones (33) and viral proteins (15). For example, Sindbis virus subgenomic 26S mRNA is translated efficiently in the presence of phosphorylated eIF2α (53) and Semliki Forest virus has a translation enhancer element that counteracts the translational repression induced by eIF2α phosphorylation (36).
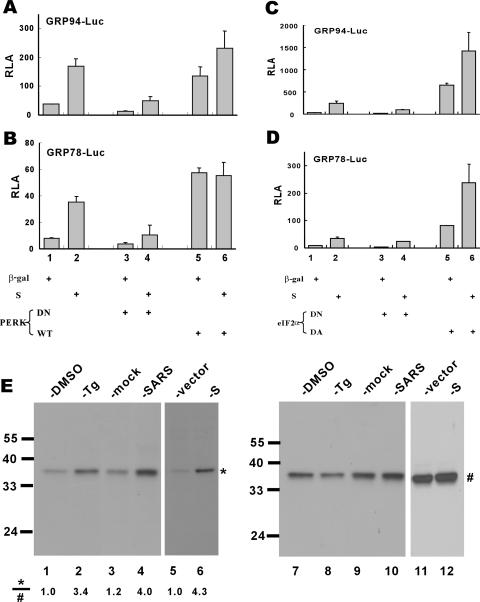
GRP94/78 promoters have been shown to be upregulated in response to PERK activation and eIF2α phosphorylation (2, 33). To investigate whether the activation of GRP94/78 promoters by SARS-CoV S protein might be mediated through PERK and eIF2α phosphorylation, we employed expression plasmids for PERK, eIF2α, and their DN or DA mutants. Because DN forms of PERK and eIF2α constitutively inhibit phosphorylation of eIF2α (34, 39), the activation of GRP94/78 by S would be compromised if PERK and eIF2α were crucially involved. Indeed, PERK DN and eIF2α DN effectively blocked basal and S protein-induced activation of GRP94/78 promoters (Fig. 4A to D, columns 3 and 4 compared to columns 1 and 2), whereas PERK wild-type and eIF2α DA stimulated these promoters (Fig. 4A to D, columns 5 and 6 compared to columns 1 and 2). Hence, PERK activity and eIF2α phosphorylation are required for the activation of ER stress by S protein.
FIG. 4.
Activation of GRP94/GRP78 by SARS-CoV S protein requires PERK and eIF2α phosphorylation. 293FT cells were cotransfected with pGRP78/94-Luc and expression vectors for the indicated combinations of proteins. Cells were harvested for dual luciferase assay as described for Fig. 2 (A to D). The steady-state levels of phosphorylated (*) and total (#) eIF2α in cells treated with DMSO (lanes 1 and 7) or Tg (300 nM; lanes 2 and 8), mock infected (lanes 3 and 9) or infected with SARS-CoV (lanes 4 and 10), and transfected with pLenti-Topo empty vector alone (lanes 5 and 11) or with pLenti-S (lanes 6 and 12) were verified by Western blotting (E). Treatment with Tg was used as a positive control in this experiment. WT, wild type.
To verify directly the phosphorylation of eIF2α in S-expressing and SARS-CoV-infected cells, we determined the steady-state amounts of phosphorylated eIF2α and total eIF2α by Western blotting with specific antibodies (Fig. 4E). Infection with SARS-CoV led to approximately fourfold elevation of the relative amount of phosphorylated eIF2α (Fig. 4E; compare lane 4 to lane 3). In line with this, the expression of S alone sufficiently induced a 4.3-fold increase in the level of phosphorylated eIF2α (Fig. 4E; compare lane 6 to lane 5).
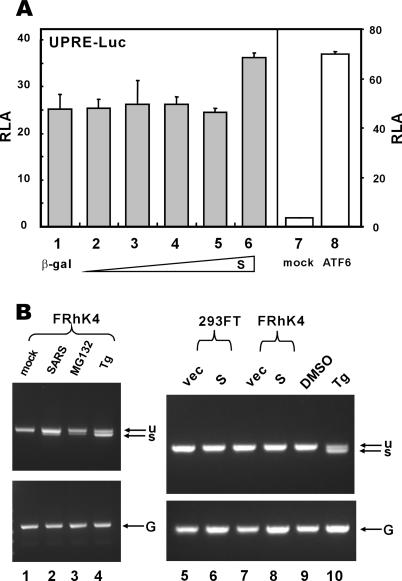
The other two major regulators of the UPR signaling are IRE1 and ATF6, the activation of which leads to reprogramming of the cell and induction of ER-associated degradation (5, 43, 58). To assess the influence of S protein on the IRE1 pathway, we utilized a luciferase reporter construct driven by the UPR element (UPRE), which was thought to be recognized by IRE1 effector XBP1 and other bZIP transcription factors likely involved in ER stress (8, 27, 61). The expression of S did not stimulate the UPRE-dependent transcriptional activity over a wide range of doses but had weak stimulatory effect at the highest concentration (Fig. 5A, columns 1 to 6). To determine whether S protein activates IRE1, leading to unconventional splicing of XBP1, we also performed reverse transcription-PCR to check for spliced XBP1 (Fig. 5B). In this experiment we used as positive controls Tg and proteosome inhibitor MG132, both of which are known stimuli of XBP1 splicing (26). While treatment with Tg or MG132 resulted in the production of a fast-migrating band corresponding to spliced XBP1 (Fig. 5B, lanes 3, 4, and 10), the expression of S in 293FT and FRhK4 cells had no influence on the activation of IRE1 and XBP1 (Fig. 5B; lane 6 compared to lane 5 and lane 8 compared to lane 7). Notably, in these same cells and under the same conditions, S was able to activate transcription from GRP78/94 promoters (Fig. 2 and data not shown). To our surprise, a weak band of spliced XBP1 was also detectable in cells infected with SARS-CoV (Fig. 5B; lane 2 compared to lane 1). Thus, infection with SARS-CoV probably induces IRE1 and XBP1 activation at a low level through an unknown but S-independent mechanism.
FIG. 5.
SARS-CoV S protein does not stimulate UPRE or XBP1 splicing. (A) 293FT cells were cotransfected with pUPRE-Luc and escalating amounts of pLenti-S. In the control group, an expression plasmid for a constitutively active version of ATF6 containing 1 to 373 amino acids was cotransfected. Cells were harvested for dual luciferase assay as described for Fig. 2. ATF6 (1-373) is known to stimulate UPRE, as reported previously (8). (B) 293FT and FRhK4 cells were mock infected (lane 1), infected with SARS-CoV (lane 2), transfected with pLenti-Topo empty vector (vec; lanes 5 and 7), transfected with pLenti-S plasmid (lanes 6 and 8), or treated with MG132 (lane 3), Tg (lanes 4 and 10), or DMSO (lane 9) for 8 h. Unspliced (u) and spliced (s) forms of XBP1 transcript were examined by reverse transcription-PCR as described previously (26). GAPDH mRNA (G) was also detected as a positive control.
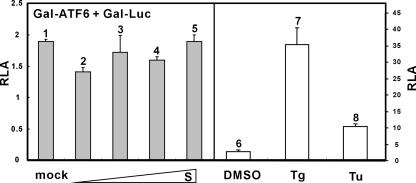
Next we asked whether S might activate ATF6 by use of a Gal-ATF6 fusion protein. This protein is ambiently tethered to the ER through the transmembrane domain of ATF6. Activation and subsequent nuclear translocation of this fusion protein lead to stimulation of reporter expression driven by Gal4-binding sites (27). As such, we observed that Tg and Tu induced activation of Gal-ATF6 (Fig. 6). In contrast, escalating amounts of S had no influence on Gal-ATF6 activity (Fig. 6, columns 2 to 5 compared to columns 1, 7, and 8). Thus, SARS-CoV S protein specifically activated PERK but did not significantly affect IRE1/XBP1 or ATF6.
FIG. 6.
SARS-CoV S protein does not stimulate ATF6-dependent transcriptional activity. 293FT cells were cotransfected with pGal-Luc, pGal-ATF6, and escalating amounts of pLenti-S. Cells were harvested for dual luciferase assay as described for Fig. 2. Tg (300 nM) and Tu (5 μg/ml) were used as positive controls in this analysis.
DISCUSSION
Here we demonstrated the induction of ER stress and the UPR by SARS-CoV infection through S protein (Fig. 1 and Fig. 2). Particularly, we showed that S protein modulated ER stress differentially by stimulating PERK but sparing the other two branches of the UPR signaling mediated through IRE1 and ATF6 (Fig. 4 to 6).
SARS-CoV is highly pathogenic, and its S protein is thought to play a pivotal role in viral pathogenesis (55). In particular, S protein mediates receptor binding, induces membrane fusion, and elicits neutralizing immune response (14). Our demonstration of the modulation of ER stress and the UPR by SARS-CoV S protein suggests a new role for this multifunctional protein after viral entry. This modulation of the UPR likely represents a viral strategy to combat cellular response and to facilitate viral replication. The effect of S on the UPR was shown mainly in the transcriptional activation of intraluminal ER chaperones GRP94/78 through PERK and eIF2α phosphorylation (Fig. 4). Increased expression of these chaperones would enhance the folding and processing of SARS-CoV proteins that are abundantly expressed during viral replication. The induction of CHOP by S is milder (Fig. 2C). In addition, S had little or no influence on ATF4 translation (Fig. 2D), XBP1 splicing (Fig. 5), or ATF6 nuclear translocation (Fig. 6). Because CHOP is a major regulator of ER stress-associated apoptosis (5, 40, 58), activation of CHOP at an early stage of viral infection is undesirable. Hence, SARS-CoV benefits from selective modulation of these events by S protein.
We also found that infection with SARS-CoV led to a slight increase in the level of spliced XBP1 through an as-yet-unknown mechanism independent of S (Fig. 5B). Because XBP1 activation is a double-edged sword that promotes protein folding and apoptosis (43), this low level of XBP1 activation might be required during the course of SARS-CoV infection to enhance protein folding while avoiding the deleterious effects of ER stress-induced apoptosis. Further experiments are required to shed light on the significance of XBP1 activation in SARS-CoV biology as well as the viral protein(s) responsible for this activation.
Viral modulation of ER stress and the UPR signaling has just begun to be understood. Other viruses such as HCV and CMV modulate ER stress and the UPR by activating PERK and inhibiting XBP1 or ER degradation-enhancing α-mannosidase I-like protein (18, 48). It is natural that different viruses adopt different strategies in their modulation of the UPR (15). We showed that SARS-CoV S protein used a unique strategy to deal with ER stress by stimulating PERK but exerting no effect on IRE1 or ATF6 (Fig. 4 to 6). Consistent with this, infection with SARS-CoV does not induce apoptosis in at least some cells or at an early stage of infection (25). It remains to be elucidated whether S protein from other coronaviruses might also exhibit similar modulatory activity on ER stress. Since the S protein of coronavirus NL63 (13, 51) uses the same angiotensin-converting enzyme 2 receptor as SARS-CoV (16), it will be of particularly great interest to see whether it also modulates ER stress and the UPR. Currently we are in the process of comparing in detail the ER stress-modulatory activities of S proteins from SARS-CoV, coronavirus NL63 (13, 51), coronavirus HKU1 (56), and coronavirus 229E.
Induction of ER stress by S protein has a significant impact on cell homeostasis and may contribute to viral pathogenesis. For example, the UPR is activated in response to the release of ER calcium as induced by drugs such as Tg (43). On the other hand, the disruption of calcium homeostasis is a feature of viral enterotoxin exemplified by rotavirus NSP4 (1). Because SARS-CoV also replicates commonly in small and large intestines, leading to diarrhea (55), it will be of particularly great interest to see whether SARS-CoV proteins might sufficiently induce calcium release from ER and cause diarrhea by acting as an NSP4-like viral enterotoxin. In another perspective, a recent study has revealed a link between ER stress and systemic inflammatory response (61). This induction of acute phase response genes by ER stress is mediated by regulated cleavage of CREB-H transcription factor (8) and probably other transmembrane bZIP factors such as LZIP/CREB3 in the same protein subfamily (20). These consequences of ER stress and the UPR could be relevant to the pathogenesis of SARS.
Modulation of ER stress and the UPR by SARS-CoV reveals a novel opportunity for pharmaceutical intervention of SARS. Due to the importance of ER stress in various human diseases, including viral infection, small molecules that specifically counteract ER stress have been under intense investigation (5). In this regard, one selective inhibitor of eIF2α dephosphorylation has recently been found to be effective for the inhibition of herpes simplex virus replication (4). Additionally, drugs that modulate ER stress have also been shown to inhibit the production of infectious CMV virions (18). Because antivirals highly effective for the treatment of SARS have not been identified (55), further investigations on the use of various ER stress-modulating pharmaceutical agents for anti-SARS-CoV therapy are warranted.
Acknowledgments
We thank K. Mori, D. Ron, R. Web, and N. S. Wong for gifts of plasmids; R. Altmeyer for helpful discussion; and A. C. S. Chun, W. L. Lai, and N. S. Wong for critical reading of the manuscript.
This work was supported by the Research Fund for the Control of Infectious Disease (grant 01030222 to D.-Y.J.) from the Research Council of Hong Kong Health, Welfare and Food Bureau. D.-Y.J. is a Leukemia and Lymphoma Society Scholar.
B.Z. was the co-principal investigator of this study.
REFERENCES
- 1.Ball, J. M., T. Peng, C. Q. Y. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister, P., S. Luo, W. C. Skarnes, G. Sui, E. Seto, Y. Shi, and A. S. Lee. 2005. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol. Cell. Biol. 25:4529-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benali-Furet, N. L., M. Chami, L. Houel, F. De Giorgi, F. Vernejoul, D. Lagorce, L. Buscail, R. Bartenschlager, F. Ichas, R. Rizzuto, and P. Paterlini-Bréchot. 2005. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene 24:4921-4933. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, M., K. F. Bryant, C. Jousse, K. Long, H. P. Harding, D. Scheuner, R. J. Kaufman, D. Ma, D. M. Coen, D. Ron, and J. Yuan. 2005. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307:935-939. [DOI] [PubMed] [Google Scholar]
- 5.Boyce, M., and J. Yuan. 2006. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 13:363-373. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. W., and P. A. Egan. 2005. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J. 19:1510-1512. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, G., Z. Feng, and B. He. 2005. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2α dephosphorylation by the γ134.5 protein. J. Virol. 79:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, K. T., H. J. Zhou, C. M. Wong, J. M. F. Lee, C. P. Chan, B. Q. Qiang, J. G. Yuan, I. O. L. Ng, and D. Y. Jin. 2005. The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nucleic Acids Res. 33:1859-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ching, Y. P., S. F. Chan, K. T. Jeang, and D. Y. Jin. 2006. Retroviral oncoprotein Tax targets coiled-coil centrosomal protein TAX1BP2 to induce centrosome overduplication. Nat. Cell Biol. 8:717-724. [DOI] [PubMed] [Google Scholar]
- 10.Chun, A. C. S., and D. Y. Jin. 2003. Transcriptional regulation of mitotic checkpoint gene MAD1 by p53. J. Biol. Chem. 278:37439-37450. [DOI] [PubMed] [Google Scholar]
- 11.Darnell, M. E., K. Subbarao, S. M. Feinstone, and D. R. Taylor. 2004. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 121:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimcheff, D. E., S. Askovic, A. H. Baker, C. Johnson-Fowler, and J. L. Portis. 2003. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 77:12617-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouchier, R. A., N. G. Hartwig, T. M. Bestebroer, B. Niemever, J. C. de Jong, J. H. Simon, and A. D. Osterhaus. 2004. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. USA 101:6212-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher, T. M., and M. J. Buchmeier. 2001. Coronavirus spike proteins in viral entry and pathogenesis. Virology 279:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, B. 2006. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13:393-403. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann, H., K. Pyrc, L. van der Hoek, M. Geier, B. Berkhout, and S. Pohlmann. 2005. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 102:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isler, J. A., A. H. Skalet, and J. C. Alwine. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79:6890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isler, J. A., T. G. Maguire, and J. C. Alwine. 2005. Production of infectious human cytomegalovirus virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. J. Virol. 79:15388-15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, X. S., L. Y. Tang, J. Dai, H. Zhou, S. J. Li, Q. C. Xia, J. R. Wu, and R. Zeng. 2005. Quantitative analysis of severe acute respiratory syndrome (SARS)-associated coronavirus-infected cells using proteomic approaches: implications for cellular responses to virus infection. Mol. Cell. Proteomics 4:902-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, D. Y., H. L. Wang, Y. Zhou, A. C. S. Chun, K. V. Kibler, Y. D. Hou, H. Kung, and K. T. Jeang. 2000. Hepatitis C virus core protein-induced loss of LZIP function correlates with cellular transformation. EMBO J. 19:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan, R., L. Wang, T. M. Graczyk, T. M. Block, and P. R. Romano. 2002. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 76:9588-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai, M. M. C., and K. V. Holmes. 2001. Coronaviruses, p. 1163-1185. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 23.Lai, W. L., and N. S. Wong. 2005. ROS mediates 4HPR-induced posttranscriptional expression of the Gadd153 gene. Free Rad. Biol. Med. 38:1585-1593. [DOI] [PubMed] [Google Scholar]
- 24.Lau, S. K. P., P. C. Y. Woo, K. S. M. Li, Y. Huang, H. W. Tsoi, B. H. L. Wong, S. S. Y. Wong, S. Y. Leung, K. H. Chan, and K. Y. Yuen. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 102:14040-14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law, H. K., C. Y. Cheung, H. Y. Ng, S. F. Sia, Y. O. Chan, W. Luk, J. M. Nicholls, J. S. M. Peiris, and Y. L. Lau. 2005. Chemokine upregulation in SARS coronavirus infected human monocyte derived dendritic cells. Blood 106:2366-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, A. H., N. N. Iwakoshi, K. C. Anderson, and L. H. Glimcher. 2003. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. USA 100:9946-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, K., W. Tirasophon, X. Shen, M. Michalak, R. Prywes, T. Okada, H. Yoshida, K. Mori, and R. J. Kaufman. 2002. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16:452-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, Y. N., L. K. Chen, H. C. Ma, H. H. Yang, H. P. Li, and S. Y. Lo. 2005. Thermal aggregation of SARS-CoV membrane protein. J. Virol. Methods 129:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, W., Z. Shi, M. Yu, W. Ren, C. Smith, J. H. Epstein, H. Wang, G. Crameri, Z. Hu, H. Zhang, J. Zhang, J. McEachern, H. Field, P. Daszak, B. T. Eaton, S. Zhang, and L. F. Wang. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676-679. [DOI] [PubMed] [Google Scholar]
- 30.Liao, Y., Q. Yuan, J. Torres, J. P. Tam, and D. X. Liu. 2006. Biochemical and functional characterization of the membrane association and membrane permeabilizing activity of the severe acute respiratory syndrome coronavirus envelope protein. Virology 349:264-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberman, E., Y.-L. Fong, M. J. Selby, Q.-L. Choo, L. Cousens, M. Houghton, and T. S. B. Yen. 1999. Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J. Virol. 73:3718-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, P. D., H. P. Harding, and D. Ron. 2004. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo, S., P. Baumeister, S. Yang, S. F. Abcouwer, and A. S. Lee. 2003. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through an upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 278:37375-37385. [DOI] [PubMed] [Google Scholar]
- 34.Ma, K., K. M. Vattem, and R. C. Wek. 2002. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 277:18728-18735. [DOI] [PubMed] [Google Scholar]
- 35.Marciniak, S. J., C. Y. Yun, S. Oyadomari, I. Novoa, Y. Zhang, R. Jungreis, K. Nagata, H. P. Harding, and D. Ron. 2004. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInerney, G. M., N. L. Kedersha, R. J. Kaufman, P. Anderson, and P. Liljeström. 2005. Importance of eIF2α phosphorylation and stress granule assembly in alphavirus translation regulation. Mol. Biol. Cell 16:3753-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nal, B., C. Chan, F. Kien, L. Siu, J. Tse, K. Chu, J. Kam, I. Staropoli, B. Crescenzo-Chaigne, N. Escriou, S. van der Werf, K. Y. Yuen, and R. Altmeyer. 2005. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J. Gen. Virol. 86:1423-1434. [DOI] [PubMed] [Google Scholar]
- 38.Ng, D. C. H., S. F. Chan, K. H. Kok, J. W. P. Yam, Y. P. Ching, I. O. L. Ng, and D. Y. Jin. 2006. Mitochondrial targeting of growth suppressor protein DLC2 through the START domain. FEBS Lett. 580:191-198. [DOI] [PubMed] [Google Scholar]
- 39.Novoa, I., H. Zeng, H. Harding, and D. Ron. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153:1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyadomari, S., and M. Mori. 2004. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11:381-389. [DOI] [PubMed] [Google Scholar]
- 41.Pavio, N., P. R. Romano, T. M. Graczyk, S. M. Feinstone, and D. R. Taylor. 2003. Protein synthesis and endoplasmic reticulum stress can be modulated by the hepatitis C virus envelope protein E2 through the eukaryotic initiation factor 2α kinase PERK. J. Virol. 77:3578-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peiris, J. S. M., S. T. Lai, L. L. M. Poon, Y. Guan, L. Y. C. Yam, W. Lim, J. Nicholls, W. K. S. Yee, W. W. Yan, M. T. Cheung, V. C. C. Cheng, K. H. Chan, D. N. C. Tsang, R. W. H Yung, T. K. Ng, and K. Y. Yuen. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schröder, M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74:739-789. [DOI] [PubMed] [Google Scholar]
- 44.Siu, Y. T., K. T. Chin, K. L. Siu, E. Y. W. Choy, K. T. Jeang, and D. Y. Jin. 2006. TORC1 and TORC2 proteins are required for Tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J. Virol. 80:7052-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, J. A., S. C. Schmechel, A. Raghavan, M. Abelson, C. Reilly, M. G. Katze, R. J. Kaufman, P. R. Bohjanen, and L. A. Schiff. 2006. Reovirus induces and benefits from an integrated cellular stress response. J. Virol. 80:2019-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su, H. L., C. L. Liao, and Y. L. Lin. 2002. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J. Virol. 76:4162-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tardif, K. D., K. Mori, and A. Siddiqui. 2002. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J. Virol. 76:7453-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tardif, K. D., K. I. Mori, R. J. Kaufman, and A. Siddiqui. 2004. Hepatitis C virus suppresses the IRE1-Xbp1 pathway of the unfolded protein response. J. Biol. Chem. 279:17158-17164. [DOI] [PubMed] [Google Scholar]
- 49.Tardif, K. D., G. Waris, and A. Siddiqui. 2005. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 13:159-163. [DOI] [PubMed] [Google Scholar]
- 50.Tirosh, B., N. N. Iwakoshi, B. N. Lilley, A. Lee, L. H. Glimcher, and H. L. Ploegh. 2005. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class 1 major histocompatibility complex products. J. Virol. 79:2768-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Hoek, L., K. Pyrc, M. F. Jebbink, W. Vermeulen-Oost, R. J. Berkhout, K. C. Wolthers, P. M. Wertheim-Van Dillen, J. Kaandorp, J. Spaargaren, and B. Berkhout. 2004. Identification of a new human coronavirus. Nat. Med. 10:368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vattem, K. M., and R. C. Wek. 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 101:11269-11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ventoso, I., M. A. San, S. Molina, J. J. Berlanga, L. Carrasco, and M. Esteban. 2006. Translational resistance of late alphavirus mRNA to eIF2α phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 20:87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watowich, S. S., R. I. Morimoto, and R. A. Lamb. 1991. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J. Virol. 65:3590-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss, S. R., and S. Navas-Martin. 2005. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 69:635-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo, P. C. Y., S. K. P. Lau, C.-M. Chu, K.-H. Chan, H.-W. Tsoi, Y. Huang, B. H. L. Wong, R. W. S. Poon, J. J. Cai, W.-K. Luk, L. L. M. Poon, S. S. Y. Wong, Y. Guan, J. S. M. Peiris, and K.-Y. Yuen. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu, A., A. R. Bellamy, and J. A. Taylor. 1998. BiP (GRP78) and endoplasmin (GRP94) are induced following rotavirus infection and bind transiently to an endoplasmic reticulum-localized virion component. J. Virol. 72:9865-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu, C., B. Bailly-Maitre, and J. C. Reed. 2005. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Investig. 115:2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu, Z., G. Jensen, and T. S. B. Yen. 1997. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J. Virol. 71:7387-7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida, H., K. Haze, H. Yanagi, T. Yura, and K. Mori. 1998. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins: involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273:33741-33749. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, K., X. Shen, J. Wu, K. Sakaki, T. Saunders, D. T. Rutkowski, S. H. Back, and R. J. Kaufman. 2006. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124:587-599. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, Y., Y. P. Ching, A. C. S. Chun, and D. Y. Jin. 2003. Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J. Biol. Chem. 278:12530-12536. [DOI] [PubMed] [Google Scholar]