Abstract
We have previously shown that cytomegalovirus (CMV) can reactivate in lungs of nonimmunosuppressed patients during critical illness. Our recent work has shown that polymicrobial bacterial sepsis can trigger reactivation of latent murine CMV (MCMV). We hypothesize that MCMV reactivation following bacterial sepsis may be caused by inflammatory mediators. To test this hypothesis, BALB/c mice latently infected with Smith strain MCMV received sublethal intraperitoneal doses of lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), or saline. Lung tissue homogenates were evaluated for viral reactivation 3 weeks after mediator injection. Because LPS is known to signal via Toll-like receptor 4 (TLR-4) in mice, further studies blocking this signaling mechanism were performed using monoclonal MTS510. Finally, mice were tested with intravenous TNF-α to determine whether this would cause reactivation. All mice receiving sublethal intraperitoneal doses of LPS, TNF-α, or IL-1β had pulmonary reactivation of latent MCMV 3 weeks following injection, and LPS caused MCMV reactivation with kinetics similar to those for sepsis. When TLR-4 signaling was blocked, exogenous LPS did not reactivate latent MCMV. Intravenous TNF-α administration at near-lethal doses did not reactivate MCMV. Exogenous intraperitoneal LPS, TNF-α, and IL-1β are all capable of reactivating CMV from latency in lungs of previously healthy mice. LPS reactivation of MCMV appears dependent on TLR-4 signaling. Interestingly, intravenous TNF-α did not trigger reactivation, suggesting possible mechanistic differences that are discussed. We conclude that inflammatory disease states besides sepsis may be capable of reactivating CMV from latency.
The pathobiology of cytomegalovirus (CMV) in the immunocompetent host is currently being defined. CMV is a ubiquitous herpes family virus that commonly infects humans, causing a mild self-limited primary infection in immunocompetent hosts. Like other betaherpesviruses, CMV then establishes latency in which infectious virus is undetectable in host tissues until some stimulus causes reactivation. Episodes of reactivation are known to be pathogenic in immunosuppressed populations, such as AIDS patients or transplant recipients (53, 56), and recent studies with previously immunocompetent critically ill patients have also suggested pathogenicity (11, 12, 14, 15, 25, 26, 31, 44). As our understanding evolves, it appears that CMV may become a potential pathogen for anyone with a latent infection, and not for just the immunosuppressed.
Because of its pathogenic implications, the mechanism by which CMV reactivation occurs has received considerable attention. There are numerous stimuli suggested or known to reactivate CMV from latency, including immunosuppression (6, 39, 42, 45), allogeneic stimulation (23, 55), catecholamines (49), and bacterial infections (10, 18) (more thoroughly reviewed in reference 29). Both human and murine major immediate early (MIE) enhancer/promoter regions of CMV contain numerous nuclear factor kappa B (NF-κB) consensus sequences (19, 30). Activation of this enhancer/promoter region is thought to be the critical first step in reactivation from latency, and therefore a mechanism of NF-κB-stimulated reactivation of latent CMV has been proposed.
This hypothesis suggests that any mediator or signal capable of activating NF-κB might be capable of reactivating CMV from latency. In vitro work has supported this hypothesis, showing that NF-κB is stimulatory to immediate early gene expression (5, 8, 16, 17), which is thought to be the critical first step in reactivation from latency. Perhaps not surprisingly, inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and lipopolysaccharide (LPS), which are known to stimulate NF-κB activation, are also stimulatory to the CMV MIE promoter in vitro (40, 48, 57). There are also numerous in vivo data, for animals as well as for human patients, which support this proposed mechanism (18, 21, 30, 34, 41, 54). Although the preponderance of evidence supports the hypothesis that inflammation is linked to CMV reactivation, to date this causality has not been demonstrated in an in vivo system.
Reactivation studies are problematic in human hosts because of obvious ethical limitations. Therefore, in vivo studies of CMV reactivation have required the development and use of animal models. Although Kondo et al. have described a model to study reactivation in vitro (36), these experiments occur outside the context of the immune system, which is critical in the reactivation process. Fortunately, murine CMV (MCMV) infection has been well characterized and is similar to human CMV (9, 27). In susceptible mouse strains, intraperitoneal inoculation of MCMV causes acute infection, with subsequent development of latency in host tissues (9, 10). Like its human CMV counterpart, MCMV has been shown to develop latency in many organs, including lung tissue (4, 9, 37, 38), and MCMV can be reactivated from latency in vivo by a variety of stimuli (6, 22). This model affords a unique opportunity to study the reactivation of CMV, and its pathological consequences, in immunocompetent hosts.
Using this murine model, we have recently demonstrated that sepsis induced by cecal ligation and puncture (CLP) can trigger in vivo reactivation of latent MCMV in lungs of previously immunocompetent mice (10). Sepsis is known to stimulate release of numerous inflammatory molecules, including LPS, TNF-α, and interleukin-1β (IL-1β) among others. All three of these mediators stimulate activation of NF-κB (reviewed in reference 3), and in keeping with the NF-κB reactivation hypothesis, any of the three could be considered as candidates to stimulate MCMV reactivation.
We therefore hypothesized that bacterial sepsis causes reactivation of latent CMV via elaboration of inflammatory mediators, such that introduction of these inflammatory mediators into the latently infected host would recapitulate the reactivation of MCMV demonstrated in our sepsis model. We report herein results of these experiments, which demonstrate that LPS can indeed reactivate latent MCMV in a manner similar to CLP and that LPS-induced reactivation occurs via Toll-like receptor 4 (TLR-4) signaling. Further, data are presented showing that the intraperitoneal inflammatory mediators TNF-α and IL-1β are capable of stimulating reactivation. Finally, we confirm previously published results demonstrating that intravenous TNF-α is incapable of reactivating latent MCMV.
MATERIALS AND METHODS
Animals.
Female BALB/c mice (Harlan, Indianapolis IN) of 6 to 8 weeks of age were used in this study. All animals were housed in a large animal facility and isolated from other mice, in adherence with the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (43) following protocol approval by our Institutional Review Board. Mice were euthanized by cervical dislocation under inhalation anesthesia. Mouse lungs and salivary gland were dissected aseptically, frozen immediately in liquid nitrogen, and then stored at −80°C.
Viral infection and development of latency.
Purified Smith strain (VR-194/1981) murine CMV was obtained from ATCC (Rockville, MD). Primary CMV infection was achieved by intraperitoneal (i.p.) injection of 5 × 104 PFU Smith strain MCMV. Following MCMV infection, mice were maintained for 16 weeks, at which point a cohort of animals underwent evaluation by reverse transcription PCR (RT-PCR), in vitro plaque assay (IVPA), and focused expansion assay (FEA) to confirm latency (see below).
Inflammatory mediator monoclonal antibodies.
LPS from Escherichia coli 055:B5, recombinant murine TNF-α, and recombinant murine IL-1β were obtained from Sigma. Monoclonal antibody (MAb) MTS510 was obtained from eBiosciences (San Diego, CA). For intraperitoneal studies, the following doses were used unless otherwise specified; for LPS, 15 μg/kg of body weight i.p.; for recombinant TNF-α, 2 μg/0.2 ml/mouse i.p.; and for IL-1β, 0.2 μg/0.2 ml/mouse i.p. For MAb experiments, each animal received 100 μg i.p. of either MTS510 or an isotypic rat immunoglobulin G (IgG) (14-9924 and 14-4321; eBiosciences).
PCR primers.
The following primers were used for all CMV PCRs and RT-PCRs. Primers were designed using DNASTAR software (Madison, WI). β-Actin sequences are 5′ ATT GTG ATG GAC TCC GGT GA and 3′ AGC TCA TAG CTC TTC TCC AG. Sequences for murine CMV glycoprotein B (GB) genes were obtained from GenBank (NCBI). Primers were as follows: GB 5′ TCA TCA ACT CGA CGA AGC TC and GB 3′ ATC TCG TCC AGG CTG AAC AC (gives 298-bp product). CMV nested primers were as follows: GB 5′ CTG GGC GAG AAC AAC GAG AT and GB 3′ CGC AGC TCT CCC TTC GAG TA (gives 235-bp product).
PCR.
DNA was extracted from tissues using a QIAamp tissue kit (QIAGEN GmbH, Hilden, Germany). DNA extracted from tissue homogenates was eluted in 100 μl of distilled water and stored at −20°C until analysis. DNA was amplified in a total volume of 25 μl with 200 nM of each primer and 1.0 U of Taq DNA polymerase (GIBCO BRL) added in 2.5 μl of a PCR buffer (50 mM KCl, 20 mM Tris-HCl [pH 8.4], and 1.5 mM MgCl2). PCRs were carried out using a Perkin Elmer 9700 thermocycler (PE Applied Biosystems, Foster City, CA) by use of the following program: initial denaturation for 4 min at 94°C, 35 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 53°C, and elongation for 30 s at 72°C, followed by a final elongation for 7 min at 72°C and then holding at 4°C. Primers used for transcription of GB and β-actin genes are listed above. β-Actin transcripts were used as a cellular transcript control. Amplification products were separated by electrophoresis in 1% agarose gels, and gels were stained with ethidium bromide.
RT-PCR and nested PCR.
RNA was extracted from tissues using TRIzol reagent (GIBCO BRL, Carlsbad, CA), dissolved in 100 μl diethyl pyrocarbonate water, and stored at −80°C. Reverse transcription (RT) reactions were carried out using 1 to 5 μg RNA digested with 1 U DNase I (GIBCO BRL) at 37°C for 20 min. The RT reaction was performed in a total volume of 20 μl containing 60 mM KCl, 15 mM Tris-HCl (pH 8.4), 3 mM MgCl2, 10 mM dithiothreitol, 20% (vol/vol) glycerol, 1 mM each deoxynucleoside triphosphate, 12.5 pM random primer (GIBCO BRL), and 2.5 U Super transcriptase (GIBCO BRL). Primer annealing was done for 10 min at 25°C and reverse transcription was carried out for 30 min at 42°C, followed by digestion with 1 U RNase H and then denaturation for 5 min at 95°C. To control for DNA contamination, every sample had a concomitant parallel experiment involving a no-RT reaction.
Following the RT reaction, 2 μl of the resulting cDNA was amplified using the same conditions as outlined above for PCRs. If this first reaction yielded no visible product, a second (nested) PCR was performed using 1 μl of this first PCR product. Using serial dilutions of a plasmid containing CMV GB, we have previously estimated the sensitivity of our nested reaction to detect between 3 and 30 copies/100 ng of RNA, which is 2 or 3 orders of magnitude more sensitive than our first-round reactions (unpublished data).
In vitro plaque assay.
For in vitro plaque assays, mouse embryo fibroblasts (MEFs) were grown to confluence in six-well plates in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL). Following centrifugation (1,000 × g, 10 min) of 5 ml of homogenized tissue, 1 ml of supernatant was placed in each well. These plates were then centrifuged at 1,000 × g and incubated at 37°C in 5% CO2 for 3 to 4 h. Plates were washed three times with phosphate-buffered saline (PBS) and then covered with 3 ml of 1% agar in DMEM. Following 6 to 7 days of incubation (37°C in 5% CO2), plates were fixed in 10% formalin, stained with 1% crystal violet, and analyzed for plaque formation by low-power phase-contrast microscopy.
Assay of infectivity in tissue by FEA in culture.
FEA was performed using the techniques previously described by Kurz et al. (38). Briefly, tissues were subjected to Dounce homogenization at 4°C, and homogenates were used in an appropriate dilution of DMEM for infection of MEFs. Monolayers of MEFs (5 × 106 cells in a 75-cm2 flask) were inoculated with 2 ml of freeze-thawed and homogenized tissue and centrifuged (1,000 × g for 30 min at 20°C). After centrifugation, supernatants were discarded, and cells were washed twice with PBS. Viral replication was allowed to proceed for 72 h at 37°C in a 5%-CO2 humidified atmosphere. After incubation, indicator MEFs were detached by trypsinization (PBS [pH 7.2] containing 0.05% wt/vol trypsin and 0.02% wt/vol EDTA), a process terminated by the addition of DMEM containing 10% (vol/vol) fetal calf serum. Cells were sedimented at 500 × g for 10 min, taken up in PBS, and dissolved in the extraction buffer of TRIzol reagent (GIBCO BRL). RNA was isolated, and analysis for viral RNA by RT-PCR was performed.
RESULTS
LPS causes pulmonary MCMV reactivation.
Cecal ligation and puncture is known to introduce LPS into the peritoneal cavity via gram-negative organisms. Because a 50% lethal dose model of CLP has been shown in our previous work to cause pulmonary reactivation of latent MCMV in 100% of mice (10), we hypothesized that LPS might be the first step in a cascade of events that triggers MCMV reactivation from latency. To test this hypothesis, mice latently infected with MCMV received either a near-lethal dose of LPS (15 μg/kg i.p.; n = 2), a sequential dose of LPS (15 μg/kg i.p. repeated after 1 week; n = 2), or saline (controls; n = 2). Three weeks after injection, pulmonary tissues from surviving mice were evaluated by RT-PCR and IVPA for MCMV reactivation.
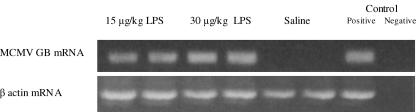
All mice receiving saline or LPS survived, and all mice that received LPS (regardless of dosing regimen) reactivated MCMV, demonstrated by MCMV GB mRNA transcription (Fig. 1). Molecular reactivation was confirmed for all mice with IVPA analysis (180 mean ± 58 standard error of the mean [SEM] PFU). Control animals latently infected with MCMV and receiving saline injection alone showed no evidence of reactivation (0/2) by RT-PCR (Fig. 1) or by IVPA or FEA (data not shown). We therefore conclude that a nonlethal dose of LPS will reactivate latent MCMV and further that intraperitoneal LPS may be the first critical step in the pulmonary reactivation of latent MCMV after intra-abdominal sepsis.
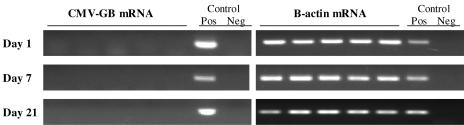
FIG. 1.
LPS stimulates reactivation of MCMV from latency. RT-PCR of lung homogenates from mice latently infected with MCMV shows that LPS administration stimulates reactivation of viral transcription. In vitro plaque assay confirmed the presence of infectious virus in all LPS-treated mice (4/4), and the more sensitive focused expansion assay showed no reactivation (0/2) in mice receiving saline alone (data not shown). Two dose regimens in comparison to saline (0.2 ml i.p.) are shown: 15 μg/kg/0.2 ml i.p. and 30 μg/kg/0.2 ml i.p. Positive and negative controls refer to technique controls. No-RT reactions were also performed in parallel for MCMV GB and β-actin and were negative (data not shown).
Kinetics of LPS-induced pulmonary MCMV reactivation.
The kinetics of reactivation of MCMV from latency have been previously defined for a 50% lethal dose model of CLP, with molecular reactivation becoming detectable between 1 and 2 weeks after CLP and becoming maximal at 3 weeks after CLP (10). To test the hypothesis that LPS-induced MCMV reactivation occurs with kinetics similar to those seen after CLP, mice latently infected with MCMV received a sublethal dose of LPS (15 μg/kg i.p.). In previous experiments, we have been unable to demonstrate CMV GB gene expression before 7 days after reactivation stimulus (unpublished data). Therefore, cohorts of five mice were sacrificed at 1, 2, or 3 weeks after LPS injection, and pulmonary tissues were evaluated by RT-PCR and IVPA or FEA for MCMV reactivation.
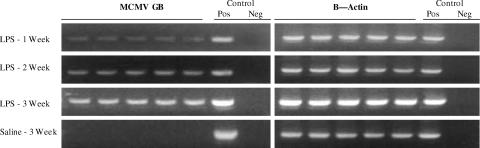
All animals receiving LPS reactivated MCMV in lung tissue (Fig. 2). The kinetics of reactivation following LPS administration were similar to those seen after CLP (10), with MCMV reactivation becoming detectable by nested RT-PCR at week 1 after LPS (five of five animals), persisting to be detectable with nested RT-PCR at week 2 (five of five), and finally becoming more easily detectable with first round RT-PCR by week 3 (five of five). Lung tissue molecular reactivation was confirmed for five of five week 3 animals with IVPA (198 mean ± 27 SEM PFU). Sham surgery has been previously shown to be insufficient to stimulate reactivation (10), and therefore it was not surprising that control animals latently infected with MCMV that received saline injection alone showed no evidence of reactivation (0/5) by RT-PCR (Fig. 2) or by IVPA or FEA (data not shown). GB gene expression is not detectable in our sepsis model before day 7, and this was confirmed after LPS stimulation at 3 days postinjection, with 0/4 mice showing detectable GB gene expression at this early time point (data not shown). We therefore conclude that the kinetics of reactivation after intraperitoneal LPS injection are similar to those seen following CLP.
FIG. 2.
Kinetics of LPS-induced MCMV reactivation. Mice latently infected with MCMV received a sublethal dose of LPS (15 μg/kg/0.2 ml i.p.), and cohorts of five were analyzed at 1-, 2-, and 3-week intervals for MCMV GB and β-actin transcription. RT-PCR of lung homogenates from mice latently infected with MCMV shows LPS-induced reactivation of viral transcription is similar to that seen following bacterial peritonitis, becoming detectable by 1 week and persisting for at least 3 weeks after LPS injection. Detection of reactivation required nested RT-PCR at weeks 1 and 2; reactivation became detectable by less sensitive first-round RT-PCR by week 3. Molecular reactivation was confirmed by IVPA with recovery of live virus from five of five week 3 mice receiving LPS (mean ± SEM, 198 ± 27 PFU). Mice receiving saline alone (0.2 ml i.p.) were evaluated at week 3 only and showed no evidence of reactivation by RT-PCR (see above) or by IVPA or focused expansion assay (data not shown). Each lane represents results from one mouse. Positive (Pos) and negative (Neg) controls refer to technique controls. No-RT reactions were also performed in parallel for MCMV GB and β-actin and were all negative (not shown).
LPS reactivates MCMV via a TLR-4-mediated mechanism.
LPS is known to signal via TLR-4 in mice (1, 28, 47, 52). We therefore hypothesized that LPS-triggered reactivation of latent MCMV might result from signaling through this TLR-4 receptor pathway. To test this hypothesis, mice latently infected with MCMV (n = 3) received MAb MTS510, which blocks TLR-4/MD-2 complexes (1), or an isotypic rat IgG (n = 2). Two hours after injection with MAbs, mice received a sublethal dose of LPS (15 μg/kg i.p.), and 3 weeks after this LPS challenge, pulmonary tissues were evaluated by RT-PCR and IVPA or FEA for MCMV reactivation.
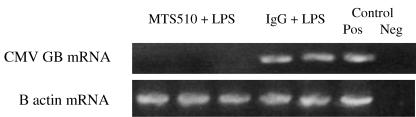
All mice survived, and none of the mice that received MTS510 MAb (0/3) showed evidence of CMV reactivation following LPS administration either by RT-PCR (Fig. 3) or by IVPA or FEA (data not shown). In contrast, mice receiving LPS after isotypic IgG showed transcriptional reactivation (two of two) of latent MCMV, similar to LPS-treated animals (Fig. 3). We therefore conclude that LPS-induced reactivation of latent MCMV is likely a consequence of TLR-4 signaling.
FIG. 3.
LPS reactivates MCMV via a TLR-4-mediated mechanism. Mice latently infected with MCMV received MTS510, a monoclonal antibody that blocks TLR-4 signaling (n = 3), or an isotypic rat IgG (n = 2) prior to receiving a sublethal dose of LPS (15 μg/kg/0.2 ml i.p.). Lung homogenates for each mouse were analyzed 3 weeks after LPS injections for reactivation of MCMV. RT-PCR shows that MTS510 prevents reactivation of viral transcription, while isotypic IgG did not prevent reactivation. Each lane represents results from one mouse. Positive (Pos) and negative (Neg) controls refer to technique controls. No-RT reactions were also performed in parallel for MCMV GB and β-actin and were all negative (not shown).
Exogenous TNF-α or IL-1β reactivates latent MCMV.
In addition to the release of LPS, CLP-induced polymicrobial sepsis causes local, systemic, and distant end organ production of numerous inflammatory mediators (2, 20, 24, 50, 51). To test the hypothesis that LPS/TLR-4 signaling is dispensable to sepsis-induced MCMV reactivation, mice latently infected with MCMV received a sublethal intraperitoneal dose of recombinant TNF-α (2 μg; n = 5), IL-1β (0.2 μg; n = 5), or saline (n = 5). Three weeks after TNF-α, IL-1β, or saline challenge, pulmonary tissues were evaluated by RT-PCR and IVPA for MCMV reactivation. These experiments were performed in duplicate to give 10 mice in each group.
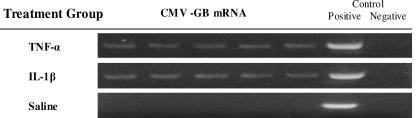
Intraperitoneal inoculation of TNF-α caused pulmonary reactivation of MCMV in 10/10 mice. MCMV GB mRNA transcription was detected in all animals, with representative results (n = 5) shown (Fig. 4). These animals also had recovery of live virus in five of five tested by IVPA (170 mean ± 34 SEM PFU). Similarly, intraperitoneal IL-1β inoculation caused pulmonary reactivation in 10/10 mice. MCMV GB mRNA transcription was detected in all 10 animals (representative results for 5 animals are shown) (Fig. 4), and these animals also showed recovery of live virus in 5/5 tested by IVPA (156 mean ± 29 SEM PFU). Consistent with our previous experiments, saline-challenged mice showed no evidence of reactivation by either MCMV mRNA transcription, IVPA, or FEA (data not shown). We therefore conclude that intraperitoneal inflammatory mediators, specifically TNF-α and IL-1β, are capable of causing reactivation of MCMV from latency. These results also support the hypothesis that LPS/TLR-4 signaling is indeed dispensable in polymicrobial sepsis-induced MCMV reactivation.
FIG. 4.
Intraperitoneal TNF-α or IL-1β stimulates pulmonary MCMV reactivation. Mice latently infected with MCMV received sublethal doses of either TNF-α (2 μg i.p.) or IL-1β (0.2 μg i.p.). Lung homogenates from each mouse were analyzed by RT-PCR for molecular reactivation of MCMV 3 weeks after inflammatory mediator injections. TNF-α stimulated viral mRNA transcription in 10/10 mice. Similarly, IL-1β caused viral mRNA transcription in 10/10 mice. Molecular reactivation was confirmed by IVPA, with recovery of live virus from five of five week 3 mice receiving TNF-α (mean ± SEM, 170 ± 34 PFU) and from five of five mice receiving IL-1β (mean ± SEM, 156 ± 29 PFU). As in previous experiments, controls receiving saline alone showed no evidence of MCMV reactivation, either by RT-PCR (above) or by IVPA and focused expansion analyses (data not shown). Each experiment was performed in duplicate to give 10 in each group, and representative results from one experiment (5 per group) are shown. Each lane represents results from one mouse. Positive and negative controls refer to technique controls. No-RT reactions were also performed in parallel for MCMV GB and β-actin and were all negative (not shown).
Intravenous TNF-α does not reactivate latent MCMV.
Recent studies have suggested that TNF-α administered intravenously at near-lethal doses is insufficient to reactivate MCMV from latency (30, 54), which is in conflict with our new findings. Because significant differences exist between previously published models and ours, it seemed prudent to determine whether TNF-α administered intravenously could trigger reactivation of latent MCMV in our model system. Mice latently infected with MCMV therefore received a near-lethal intravenous dose of recombinant TNF-α (1 μg; n = 10). Pulmonary tissues from surviving mice were evaluated by RT-PCR and IVPA for MCMV reactivation 1, 7, or 21 days after TNF-α challenge.
Intravenous administration of TNF-α did not cause pulmonary reactivation of MCMV in surviving mice at any time point. MCMV GB mRNA transcription was not detectable in lungs (Fig. 5) or salivary glands (not shown) of any animals, nor was live virus recovered by either IVPA or FEA (data not shown). We therefore conclude that there is a mechanistic difference between stimulation by intraperitoneal TNF-α and that by intravenous TNF-α, wherein intraperitoneal TNF-α causes MCMV reactivation and intravenous TNF-α does not.
FIG. 5.
Intravenous TNF-α does not stimulate pulmonary MCMV reactivation. Mice latently infected with MCMV received near-lethal doses of TNF-α (1 μg/mouse) intravenously. Lung homogenates from surviving mice were analyzed by RT-PCR for molecular reactivation of MCMV 1, 7, or 21 days after TNF-α injection. Intravenous TNF-α did not stimulate CMV GB mRNA transcription in any mouse. Similarly, in vitro plaque assay and focused expansion analyses showed no productive virus (data not shown). Each lane represents results from one mouse, and positive (Pos) and negative (Neg) controls refer to technique controls. β-Actin results confirm that RNA were recovered for each sample, and no-RT reactions performed in parallel demonstrated no DNA contamination (not shown).
DISCUSSION
Results of this study demonstrate that intraperitoneal inflammatory mediators can stimulate reactivation of latent MCMV in the lungs of previously immunocompetent mice. As a continuation of our previous work, which has demonstrated that polymicrobial sepsis induced by cecal ligation and puncture can trigger pulmonary reactivation of latent MCMV (10), we sought to determine whether individual mediators released during polymicrobial sepsis were capable of triggering MCMV reactivation. Indeed, one of the main effectors of gram-negative sepsis, LPS, is capable of triggering reactivation of latent MCMV. This reactivation occurs in a manner similar to that seen after CLP and apparently occurs via a TLR-4-mediated mechanism. Other by-products of CLP-induced polymicrobial sepsis, including intraperitoneal TNF-α and IL-1β, also appear capable of reactivating MCMV. This effect may be limited to intraperitoneal mediators, as intravenous TNF-α administration appears insufficient to trigger pulmonary MCMV reactivation.
We began our dissection of the polymicrobial sepsis-induced CMV reactivation pathway by focusing on the proinflammatory molecule LPS. LPS has been shown previously to be stimulatory to the CMV MIE promoter in vivo (41). LPS is known to stimulate peritoneal macrophages via TLR-4, activating numerous signaling pathways, including NF-κB, and causing elaboration of TNF-α and IL-1β (1, 33, 60). Because the MCMV major immediate early promoter is rich in NF-κB binding sequences (19, 30) and NF-κB activation has been correlated with CMV immediate early 1 (IE1) gene transcription in vivo (30), it was expected that activation of TLR-4 by LPS might reactivate MCMV, which was indeed the case (Fig. 1 and 2). Conversely, blocking TLR-4 signaling with MAb specific to TLR-4 receptors should prevent reactivation, which was also demonstrated (Fig. 3). We can therefore conclude that intraperitoneal LPS causes MCMV reactivation, and we suggest that this occurs by a TLR-4-mediated pathway.
Polymicrobial sepsis triggers other innate immune responses besides TLR-4 signaling, and we felt that TLR-4 signaling might be one of several signaling pathways capable of triggering MCMV reactivation. The MCMV major immediate early promoter not only is rich in NF-κB consensus sequences but also contains putative transcription factor binding sites for AP-1, SP1, and CREB/ATF (30). Both TNF-α and IL-1β stimulate NF-κB activation (reviewed in reference 35), as well as the activation of AP-1 and other molecules, and we therefore felt that either of these mediators alone might be capable of triggering MCMV reactivation just as LPS does. Indeed, intraperitoneal inoculation of either of these mediators caused reactivation of latent MCMV (Fig. 4). We thus conclude that the inflammatory mediators TNF-α and IL-1β are capable of reactivating MCMV from latency. Furthermore, while TLR-4 stimulation with LPS can reactivate MCMV, it is not absolutely necessary for MCMV reactivation.
Although intraperitoneal TNF-α was sufficient to reactivate MCMV, there was an interesting discrepancy in the ability of intravenous TNF-α administration to trigger reactivation of MCMV. Previous investigations have shown that TNF-α is stimulatory to the major immediate early promoter (48, 57) and that immediate early gene transcription occurs following intravenous TNF-α administration (30, 54). Despite these data, previous investigations have been unable to trigger full reactivation from latency by utilizing intravenous TNF-α as a trigger (30, 54). These previous investigations utilized slightly different models of latency/reactivation and focused primarily on early time points (1 to 14 days) following TNF-α administration. We were concerned that reactivation was perhaps occurring later and felt it important to repeat these experiments using our model. Despite slight differences in the latency models and a longer wait to detect reactivation (21 days), we were unable to demonstrate MCMV reactivation, either in lungs (Fig. 5) or in salivary glands (not shown), after intravenous TNF-α administration. As surprising as this discrepancy is to us, our findings concur with previously published data, which, taken collectively, indicate that a single intravenous bolus of TNF-α is not a sufficient stimulus to reactivate latent MCMV.
There are several possible explanations for the discrepancy seen between intraperitoneal and intravenous TNF-α administration in causing pulmonary MCMV. There are significant differences in the physiology elicited by intravenous versus intraperitoneal administration of inflammatory mediators. One example is intravenous administration of LPS, which causes significantly elevated circulating serum levels of TNF-α that return to baseline within 4 h after administration (59). In contrast, intraperitoneal LPS causes much lower levels of circulating serum TNF-α, but these low levels persist for at least 24 h (59). Intravenous TNF-α causes an abrupt change in physiology, but the half-life of TNF-α in the bloodstream is very short. While this quick burst of TNF-α is adequate to stimulate the CMV IE promoter (30, 54), it would appear that this brief stimulus is insufficient to cause full blown reactivation. Intraperitoneal LPS, TNF-α, or IL-1β, therefore, may cause more of a smoldering persistent low-level systemic inflammatory process or induction of secondary mediators, which may be necessary for pulmonary reactivation. This difference in physiology might explain the apparent discrepancy between intravenous TNF-α and intraperitoneal TNF-α as a stimulus for MCMV reactivation. It is possible, then, that peritoneal inflammation acts to elicit additional intrinsic or extrinsic signals required for the progression of the reactivation program to the production of progeny virus in the lungs, as suggested by both the two-step model proposed by Hummel et al. (30) and the multistep model proposed by others (39, 54).
An alternative hypothesis that we find equally appealing is that the reactivation of latent MCMV seen after intraperitoneal inflammatory mediator administration is mediated by intraperitoneal macrophages. Peritoneal macrophages are known to harbor latent MCMV (46). Peritoneal macrophages are stimulated by polymicrobial sepsis, LPS, TNF-α, or IL-1β and in response activate NF-κB (7, 28, 32, 58). Given that NF-κB is stimulatory to the MIE promoter, stimulation of latently infected peritoneal macrophages could reactivate MCMV. If in fact MCMV reactivation is occurring locally in the peritoneal macrophage, then this local reactivation could then spread viremically, in a manner similar to that seen following primary infection. Active MCMV demonstrated in distant organs (i.e., lungs) might thus be a consequence of the redistribution of reactivated virus. This is a significant departure from current thinking and is not considered in other proposed models of MCMV reactivation. Nevertheless, the hypothesis that restricted local reactivation occurs (in this case peritoneal) and is followed by virus spread is consistent with all of the data presented herein, and studies to validate it further are ongoing.
Given the findings of these studies, we propose the possible mechanism for MCMV reactivation illustrated in Fig. 6. LPS is a component of polymicrobial sepsis and appears capable of reactivating latent MCMV by signaling through the TLR-4 receptor. The kinetics of this LPS reactivation are similar to those seen after induction of polymicrobial sepsis, and we therefore suspect that LPS plays a major role in polymicrobial sepsis-induced MCMV reactivation. Despite the central role of LPS, other mediators can also reactivate MCMV from latency in the lungs, and we therefore propose that LPS is sufficient but dispensable in polymicrobial sepsis-induced MCMV reactivation. Because all of the factors tested, LPS, TNF-α, and IL-1β, are known to stimulate NF-κB activation, we also suspect that NF-κB activation plays a critical role in MCMV reactivation, and this is also the subject of ongoing studies.
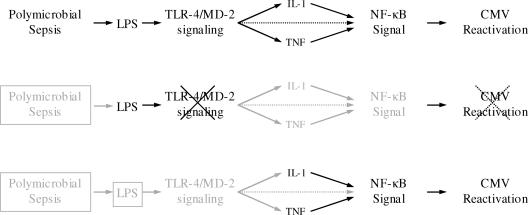
FIG. 6.
Proposed paradigm for MCMV reactivation by intraperitoneal inflammatory mediators. Polymicrobial sepsis, through a variety of pathways, is capable of reactivating latent MCMV. One component released during polymicrobial sepsis, LPS, is capable of reactivating MCMV when introduced exogenously. LPS appears to trigger MCMV reactivation through TLR-4 signaling. TLR-4 signaling is not required for sepsis-triggered reactivation, because other mediators, such as TNF-α and IL-1β, are capable of triggering CMV reactivation.
There remain several unresolved issues that warrant future investigation. First, early molecular events preceding GB expression were not studied. We have never seen GB gene expression without preceding IE1 or DNA polymerase expression, which we have studied extensively in our sepsis model (reference 10 and unpublished observations). Reddehase and coworkers have shown very elegantly that there is a checkpoint between IE1/IE3 and later gene expression (39, 54). Because our inflammatory mediator experiments seemed to overcome this checkpoint, allowing full reactivation, we chose not to evaluate these early events in our studies. Why and how this checkpoint is overridden following intraperitoneal and not intravenous inflammatory mediator administration is an interesting question that will require more extensive examination. Second, results of this study seem to be “all or none,” and we suspect that this is a consequence of using near-lethal doses of LPS, TNF-α, or IL-1β in order to maximize chances for reactivation. The minimum levels of these inflammatory mediators required to cause reactivation, as well as the relative potencies of the mediators used in these studies, have not yet been defined. Finally, the characteristics of later events following reactivation, such as the duration of the reactivation episodes as well as the effects of repeated reactivations on the host, have yet to be studied.
Despite these unresolved questions, the clinical implications of these findings are significant, suggesting that any patient latently infected with CMV is at risk for reactivation. Clinical immunosuppression is clearly not required for CMV reactivation, which broadens the definition of who is at risk for reactivation. We have already established that CMV reactivation can be triggered by bacterial infection (10), which occurs commonly in critically ill patients. In addition, these new results suggest that patients with any inflammatory disease state, and more specifically, those that involve overexpression of TNF-α or IL-1β, might be at risk for CMV reactivation. At first blush, this might not seem important in the case of the immunocompetent host, where CMV reactivation has not been previously considered to be pathogenic. Recently published work from our group, however, has shown that CMV reactivation is injurious in this setting (13), and this pathology might explain the increased morbidity seen in critically ill patients who suffer CMV reactivation.
In conclusion, results of these experiments serve to further elucidate the mechanism by which polymicrobial sepsis stimulates reactivation of CMV from latency. The preponderance of published evidence supports a linkage between inflammation and reactivation of latent CMV, and we therefore felt that it was logical to hypothesize that MCMV reactivation could be triggered by inflammatory mediators in vivo. It is clear from these data that inflammatory mediators are capable of stimulating reactivation, and this has significant implications in the clinical setting. The differences in the intraperitoneal reactivation versus intravenous reactivation by TNF-α are intriguing and will require study and likely further modification of our currently proposed paradigms of CMV reactivation.
Acknowledgments
This work was supported by NIH grant R01GM066115.
REFERENCES
- 1.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. [DOI] [PubMed] [Google Scholar]
- 2.Ayala, A., and I. H. Chaudry. 1996. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock 1(Suppl. 6):S27-S38. [PubMed] [Google Scholar]
- 3.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 4.Balthesen, M., M. Messerle, and M. J. Reddehase. 1993. Lungs are a major organ site of cytomegalovirus latency and recurrence. J. Virol. 67:5360-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedict, C. A., A. Angulo, G. Patterson, S. Ha, H. Huang, M. Messerle, C. F. Ware, and P. Ghazal. 2004. Neutrality of the canonical NF-κB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro. J. Virol. 78:741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevan, I. S., C. C. Sammons, and C. Sweet. 1996. Investigation of murine cytomegalovirus latency and reactivation in mice using viral mutants and the polymerase chain reaction. J. Med. Virol. 48:308-320. [DOI] [PubMed] [Google Scholar]
- 7.Cao, W. G., M. Morin, C. Metz, R. Maheux, and A. Akoum. 2005. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biol. Reprod. 73:565-570. [DOI] [PubMed] [Google Scholar]
- 8.Caposio, P., M. Dreano, G. Garotta, G. Gribaudo, and S. Landolfo. 2004. Human cytomegalovirus stimulates cellular IKK2 activity and requires the enzyme for productive replication. J. Virol. 78:3190-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, T., C. Pomeroy, and M. C. Jordan. 1993. Detection of latent cytomegalovirus DNA in diverse organs of mice. J. Infect. Dis. 168:725-729. [DOI] [PubMed] [Google Scholar]
- 10.Cook, C., X. Zhang, B. McGuinness, M. Lahm, D. Sedmak, and R. Ferguson. 2002. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J. Infect. Dis. 185:1395-1400. [DOI] [PubMed] [Google Scholar]
- 11.Cook, C. H., L. C. Martin, J. K. Yenchar, M. C. Lahm, B. McGuinness, E. A. Davies, and R. M. Ferguson. 2003. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit. Care Med. 31:1923-1929. [DOI] [PubMed] [Google Scholar]
- 12.Cook, C. H., J. K. Yenchar, T. O. Kraner, E. A. Davies, and R. M. Ferguson. 1998. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am. J. Surg. 176:357-360. [DOI] [PubMed] [Google Scholar]
- 13.Cook, C. H., Y. Zhang, D. D. Sedmak, L. C. Martin, S. Jewell, and R. M. Ferguson. 2006. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit. Care Med. 34:842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtsinger, L. J., W. G. Cheadle, M. J. Hershman, K. Cost, and H. C. Polk, Jr. 1989. Association of cytomegalovirus infection with increased morbidity is independent of transfusion. Am. J. Surg. 158:606-611. [DOI] [PubMed] [Google Scholar]
- 15.Cushing, D., S. Elliot, E. Caplan, S. Batlas, R. Sridhara, and J. Wade. 1993. Herpes simplex virus and cytomegalovirus excretion associated with increased ventilator days in trauma patients. J. Trauma 25:161. [Google Scholar]
- 16.DeMeritt, I. B., L. E. Milford, and A. D. Yurochko. 2004. Activation of the NF-κB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J. Virol. 78:4498-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMeritt, I. B., J. P. Podduturi, A. M. Tilley, M. T. Nogalski, and A. D. Yurochko. 2006. Prolonged activation of NF-κB by human cytomegalovirus promotes efficient viral replication and late gene expression. Virology 346:15-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docke, W. D., S. Prosch, E. Fietze, V. Kimel, H. Zuckermann, C. Klug, U. Syrbe, D. H. Kruger, R. von Baehr, and H. D. Volk. 1994. Cytomegalovirus reactivation and tumour necrosis factor. Lancet 343:268-269. [DOI] [PubMed] [Google Scholar]
- 19.Dorsch-Hasler, K., G. M. Keil, F. Weber, M. Jasin, W. Schaffner, and U. H. Koszinowski. 1985. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc. Natl. Acad. Sci. USA 82:8325-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebong, S. J., D. R. Call, G. Bolgos, D. E. Newcomb, J. I. Granger, M. O'Reilly, and D. G. Remick. 1999. Immunopathologic responses to non-lethal sepsis. Shock 12:118-126. [DOI] [PubMed] [Google Scholar]
- 21.Fietze, E., S. Prosch, P. Reinke, J. Stein, W. D. Docke, G. Staffa, S. Loning, S. Devaux, F. Emmrich, and R. von Baehr. 1994. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation 58:675-680. [PubMed] [Google Scholar]
- 22.Furrarah, A. M., and C. Sweet. 1994. Studies of the pathogenesis of wild-type virus and six temperature-sensitive mutants of mouse cytomegalovirus. J. Med. Virol. 43:317-330. [DOI] [PubMed] [Google Scholar]
- 23.Guedes, M. I. M. C., J. M. Risdahl, B. Wiseman, and T. W. Molitor. 2004. Reactivation of porcine cytomegalovirus through allogeneic stimulation. J. Clin. Microbiol. 42:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjiminas, D. J., K. M. McMasters, J. C. Peyton, and W. G. Cheadle. 1994. Tissue tumor necrosis factor mRNA expression following cecal ligation and puncture or intraperitoneal injection of endotoxin. J. Surg. Res. 56:549-555. [DOI] [PubMed] [Google Scholar]
- 25.Heininger, A., G. Jahn, C. Engel, T. Notheisen, K. Unertl, and K. Hamprecht. 2001. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit. Care Med. 29:541-547. [DOI] [PubMed] [Google Scholar]
- 26.Heininger, A., U. Vogel, C. Aepinus, and K. Hamprecht. 2000. Disseminated fatal human cytomegalovirus disease after severe trauma. Crit. Care Med. 28:563-566. [DOI] [PubMed] [Google Scholar]
- 27.Henson, D., R. D. Smith, and J. Gehrke. 1966. Non-fatal mouse cytomegalovirus hepatitis. Combined morphologic, virologic and immunologic observations. Am. J. Pathol. 49:871-888. [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 29.Hummel, M., and M. M. Abecassis. 2002. A model for reactivation of CMV from latency. J. Clin. Virol. 25(Suppl. 2):S123-S136. [DOI] [PubMed] [Google Scholar]
- 30.Hummel, M., Z. Zhang, S. Yan, I. DePlaen, P. Golia, T. Varghese, G. Thomas, and M. I. Abecassis. 2001. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J. Virol. 75:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaber, S., G. Chanques, J. Borry, B. Souche, R. Verdier, P.-F. Perrigault, and J.-J. Eledjam. 2005. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest 127:233-241. [Online.] doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 32.Joshi, A. R., C. S. Chung, G. Y. Song, J. Lomas, R. A. Priester, and A. Ayala. 2002. NF-kappaB activation has tissue-specific effects on immune cell apoptosis during polymicrobial sepsis. Shock 18:380-386. [DOI] [PubMed] [Google Scholar]
- 33.Kato, S., Y. Yuzawa, N. Tsuboi, S. Maruyama, Y. Morita, T. Matsuguchi, and S. Matsuo. 2004. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of toll-like receptor 4. J. Am. Soc. Nephrol. 15:1289-1299. [PubMed] [Google Scholar]
- 34.Kim, S. J., T. K. Varghese, Z. Zhang, L. C. Zhao, G. Thomas, M. Hummel, and M. Abecassis. 2005. Renal ischemia/reperfusion injury activates the enhancer domain of the human cytomegalovirus major immediate early promoter. Am. J. Transplant. 5:1606-1613. [DOI] [PubMed] [Google Scholar]
- 35.Kishimoto, T., T. Taga, and S. Akira. 1994. Cytokine signal transduction. Cell 76:253-262. [DOI] [PubMed] [Google Scholar]
- 36.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurz, S., H. P. Steffens, A. Mayer, J. R. Harris, and M. J. Reddehase. 1997. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J. Virol. 71:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurz, S. K., M. Rapp, H. P. Steffens, N. K. Grzimek, S. Schmalz, and M. J. Reddehase. 1999. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J. Virol. 73:482-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurz, S. K., and M. J. Reddehase. 1999. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J. Virol. 73:8612-8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, Y., W.-J. Sohn, D.-S. Kim, and H.-J. Kwon. 2004. NF-kappaB- and c-Jun-dependent regulation of human cytomegalovirus immediate-early gene enhancer/promoter in response to lipopolysaccharide and bacterial CpG-oligodeoxynucleotides in macrophage cell line RAW 264.7. Eur. J. Biochem. 271:1094-1105. [DOI] [PubMed] [Google Scholar]
- 41.Loser, P., G. S. Jennings, M. Strauss, and V. Sandig. 1998. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFκB. J. Virol. 72:180-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayo, D. R., J. A. Armstrong, and M. Ho. 1977. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature 267:721-723. [DOI] [PubMed] [Google Scholar]
- 43.National Research Council. 1985. Guide for the care and use of laboratory animals, publication 86-23. National Academy Press, Washington, D.C.
- 44.Papazian, L., A. Fraisse, L. Garbe, C. Zandotti, P. Thomas, P. Saux, G. Pierrin, and F. Gouin. 1996. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology 84:280-287. [DOI] [PubMed] [Google Scholar]
- 45.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollock, J. L., R. M. Presti, S. Paetzold, and H. W. Virgin IV. 1997. Latent murine cytomegalovirus infection in macrophages. Virology 227:168-179. [DOI] [PubMed] [Google Scholar]
- 47.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 48.Prosch, S., K. Staak, J. Stein, C. Liebenthal, T. Stamminger, H. D. Volk, and D. H. Kruger. 1995. Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNFalpha is mediated via induction of NF-kappaB. Virology 208:197-206. [DOI] [PubMed] [Google Scholar]
- 49.Prosch, S., C. E. Wendt, P. Reinke, C. Priemer, M. Oppert, D. H. Kruger, H. D. Volk, and W. D. Docke. 2000. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 272:357-365. [DOI] [PubMed] [Google Scholar]
- 50.Remick, D. G., D. E. Newcomb, G. L. Bolgos, and D. R. Call. 2000. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13:110-116. [DOI] [PubMed] [Google Scholar]
- 51.Salkowski, C. A., G. Detore, A. Franks, M. C. Falk, and S. N. Vogel. 1998. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect. Immun. 66:3569-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmons, R. L., A. J. Matas, L. C. Rattazzi, H. H. Balfour, Jr., J. R. Howard, and J. S. Najarian. 1977. Clinical characteristics of the lethal cytomegalovirus infection following renal transplantation. Surgery 82:537-546. [PubMed] [Google Scholar]
- 54.Simon, C. O., C. K. Seckert, D. Dreis, M. J. Reddehase, and N. K. A. Grzimek. 2005. Role for tumor necrosis factor alpha in murine cytomegalovirus transcriptional reactivation in latently infected lungs. J. Virol. 79:326-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 56.Spector, S. A., R. Wong, K. Hsia, M. Pilcher, and M. J. Stempien. 1998. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J. Clin. Investig. 101:497-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein, J., H. D. Volk, C. Liebenthal, D. H. Kruger, and S. Prosch. 1993. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. J. Gen. Virol. 74:2333-2338. [DOI] [PubMed] [Google Scholar]
- 58.Yoo, H. G., B. A. Shin, J. S. Park, K. H. Lee, K. O. Chay, S. Y. Yang, B. W. Ahn, and Y. D. Jung. 2002. IL-1beta induces MMP-9 via reactive oxygen species and NF-kappaB in murine macrophage RAW 264.7 cells. Biochem. Biophys. Res. Commun. 298:251-256. [DOI] [PubMed] [Google Scholar]
- 59.Zanetti, G., D. Heumann, J. Gerain, J. Kohler, P. Abbet, C. Barras, R. Lucas, M. P. Glauser, and J. D. Baumgartner. 1992. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor-alpha and to lipopolysaccharide. J. Immunol. 148:1890-1897. [PubMed] [Google Scholar]
- 60.Zhang, F. X., C. J. Kirschning, R. Mancinelli, X.-P. Xu, Y. Jin, E. Faure, A. Mantovani, M. Rothe, M. Muzio, and M. Arditi. 1999. Bacterial lipopolysaccharide activates nuclear factor-kappa B through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 274:7611-7614. [DOI] [PubMed] [Google Scholar]