Abstract
Jaagsiekte sheep retrovirus (JSRV) induces bronchioalveolar tumors in sheep and goats. Expression of the JSRV envelope (Env) protein in mouse airway epithelial cells induces similar tumors, indicating that Env expression is sufficient for tissue-specific tumor formation. Enzootic nasal tumor virus (ENTV) is related to JSRV but induces tumors in the nasal epithelium of sheep and goats. Here we found that ENTV Env can also induce tumors in mice but, unexpectedly, with a phenotype identical to that of tumors induced by the JSRV Env, indicating that factors other than Env mediate the tissue specificity of tumor induction by ENTV.
Jaagsiekte sheep retrovirus (JSRV) is associated with a contagious lung cancer in sheep called ovine pulmonary adenocarcinoma. Viral genomic DNA has been cloned and used to produce virus from cultured cells, and this virus has been shown to induce disease in sheep that is similar to the naturally occurring disease (3, 8). Tumors induced by JSRV arise in the lung periphery and express markers of bronchiolar Clara cells and/or alveolar type II pneumocytes, and they are herein referred to as bronchioalveolar tumors. Recently, we have used adeno-associated virus (AAV) vectors made with AAV type 6 capsid proteins (AAV6 vectors) to show that expression of JSRV Env in lungs of immunodeficient mice is sufficient to induce bronchioalveolar tumors like those seen in sheep (9).
Enzootic nasal tumor virus (ENTV) is associated with nasal tumors in sheep and goats, and two related viruses have been cloned from sheep (ENTV1) (1) and goats (ENTV2) (7). Neither virus has been produced in culture, nor has disease induction by either virus been demonstrated to date. However, ENTV is consistently associated with nasal tumors of sheep and goats, indicating that it is the causative agent (2). Here we have tested whether ENTV Env can also induce tumors and whether this Env specifically induces nasal tumors.
Tumor induction by JSRV and ENTV Env.
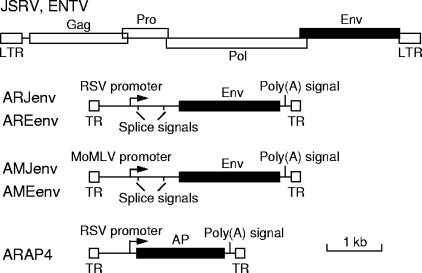
We made a set of four AAV vectors that contained the JSRV and ENTV1 Env coding sequences driven by either the Rous sarcoma virus (RSV) or the Moloney murine leukemia virus promoters (Fig. 1). The JSRV Env coding sequences were obtained from the JSRVJS7 proviral clone (GenBank accession no. AF357971) (3) and the ENTV1 (sheep ENTV) Env coding sequences were obtained from an Env PCR product from affected sheep (gift from Christina Cousens and Mike Sharp) (GenBank accession no. AF401741) (4). Splicing signals were derived from the plasmid pSX2 (6). The AAV vector plasmids were used to make replication-defective AAV6 vectors that were administered by nasal aspiration to Rag-2 knockout immunodeficient mice as described previously (9). We used Rag-2 knockout mice to avoid immune responses against Env that can limit tumor formation (9). All animal experimentation was approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center.
FIG. 1.
Scale drawings of the JSRV/ENTV genome and the AAV vectors. The JSRV coding regions are staggered vertically to indicate the three different reading frames that encode the viral proteins. Abbreviations: LTR, retroviral long terminal repeat; Gag, virion core polyprotein; Pro, protease and dUTPase; Pol, polymerase; Poly(A) signal, polyadenylation signal; MoMLV, Moloney murine leukemia virus; AP, human placental alkaline phosphatase; TR, AAV terminal repeat.
Two or four months later, the mice were killed and tumors were counted (Table 1). All Env expression vectors induced tumors with a bronchioalveolar localization, and no tumors were observed in large airways or in the nose, even though AAV6 vectors can transduce these tissues (5; data not shown). Similar numbers of tumors were induced by the JSRV and ENTV Env vectors with the Moloney murine leukemia virus promoter, and these numbers were substantially higher than the numbers of tumors induced by the vectors with the RSV promoter. The numbers of tumors induced by the JSRV Env vector with the RSV promoter were lower than those induced by the ENTV Env vector with the RSV promoter. However, these numbers were also much lower than the numbers of tumors we previously observed under the same conditions (9) or in the dose-response experiments described below, indicating that the tumor numbers for this preparation of the ARJenv vector were artificially low. Overall, it appears the numbers of tumors induced by the JSRV and ENTV Env proteins are similar.
TABLE 1.
Tumor numbers in Rag-2 knockout mice exposed to Env expression vectorsa
| Env-expressing vector | Mouse no. | Time after vector exposure (mo) | No. of tumors | Mean no. of tumors ± SD for two mice |
|---|---|---|---|---|
| None | 2-1 | 4 | 0 | 0 |
| 2-2 | 4 | 0 | ||
| ARJenv | 3-1 | 2 | 7 | 8 ± 1 |
| 3-2 | 2 | 9 | ||
| 3-3 | 4 | 0b | 5 | |
| 3-4 | 4 | 5 | ||
| AREenv | 5-1 | 2 | 38 | 30 ± 11 |
| 5-2 | 2 | 22 | ||
| 5-3 | 4 | 181 | 131 ± 71 | |
| 5-4 | 4 | 81 | ||
| AMJenv | 4-1 | 2 | 238 | 206 ± 45 |
| 4-2 | 2 | 174 | ||
| 4-3 | 4 | 413 | 448 ± 49 | |
| 4-4 | 4 | 483 | ||
| AMEenv | 6-1 | 2 | 171 | 158 ± 19 |
| 6-2 | 2 | 144 | ||
| 6-3 | 4 | 400 | 323 ± 110 | |
| 6-4 | 4 | 245 |
Each mouse was exposed to 5 × 1010 vg of the indicated Env-expressing AAV6 vector mixed with 5 × 109 vg of the ARAP4 AAV6 vector (to monitor transduction efficiency). Tumors were counted in histologic sections spanning the major dimensions of the four major lung lobes of each mouse.
No alkaline phosphatase staining was detected, indicating that the vector in this case did not reach the lung.
Tumors induced by ENTV Env exhibited both a well-differentiated epithelial appearance typical of adenomas and a less-well-differentiated appearance typical of adenocarcinoma (Fig. 2, top left panel). Tumors were stained brightly with a monoclonal antibody against Env, while there was little Env expression detected outside tumors (Fig. 2, top right panel). Tumors induced by ENTV Env expressed surfactant protein C, a marker for alveolar type II cells, as did normal type II alveolar cells but not airway epithelial cells in the same section (Fig. 2, bottom left panel). Tumor cells expressed little if any of the Clara cell marker CC-10, while as expected, normal small-airway epithelial cells did express CC-10 and normal alveolar cells did not (Fig. 2, bottom right panel). All of these features of tumors induced by ENTV Env are identical to those we previously observed for tumors induced by JSRV Env (9).
FIG. 2.
Lung tumor characteristics 4 months after administration of AAV6 vectors encoding ENTV Env. Top left, hematoxylin and eosin stain showing adenocarcinoma (left) and adenoma (right) (×100 magnification). Top right, recognition of ENTV Env expression in tumors (brown stain) by a monoclonal antibody made against the SU domain of JSRV Env (×40). Bottom left, surfactant protein C antibodies stain tumor and alveolar type II cells (brown) but not airway cells (×100). Bottom right, CC-10 antibodies stain Clara cells (brown) in the airway but not tumor cells (×100). Images shown are from mouse 5-3 (Table 1). Antibody-stained sections were counterstained with methyl green.
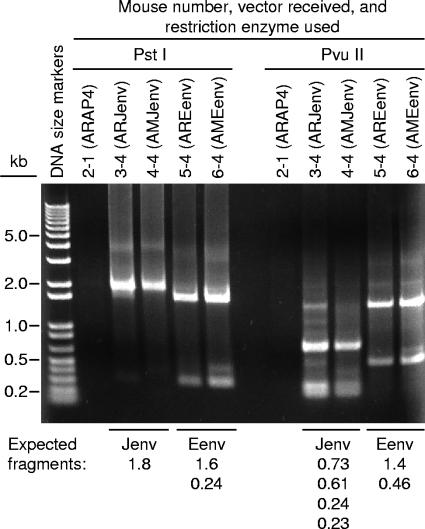
We were surprised that the ENTV and JSRV Env proteins induced apparently identical tumors. To be sure that some mix-up had not occurred, we tested DNA from lungs of tumor-bearing mice for the presence of the correct Env cDNAs. Nested primers were designed against common sequences flanking the Env cDNAs, and these were used to amplify Env sequences present in mice receiving the different vectors. The PCR products were then cut with restriction enzymes that give different patterns of products for each Env cDNA. Analysis of the restriction fragment sizes by gel electrophoresis confirmed that the mice tested received the correct Env cDNAs (Fig. 3). Note that a small amount of ENTV Env cDNA appears to be present in mouse 3-4, which received the JSRV Env vector, and we attribute this result to cross-contamination during DNA preparation. DNA was made by homogenization of lung samples, and it was difficult to entirely clean the homogenizer. We conclude that the expected vector DNAs were present in the mice, in particular, that mice listed as receiving ENTV Env did not mistakenly receive JSRV Env. These results confirm that ENTV Env can induce tumors similar to those induced by JSRV Env.
FIG. 3.
Confirmation of the correct Env DNAs in tumor-bearing mice. DNA samples were extracted from lungs of the indicated mice and were subjected to PCR amplification using nested primers flanking the Env cDNA. The expected 1.8-kb products were obtained for all mice that received Env expression vectors (not shown). These DNA fragments were then cut with PstI and PvuII restriction enzymes and were subjected to gel electrophoresis to determine the origin of the DNAs. The expected Env and the expected sizes of the restriction fragments are indicated below the gel picture.
The AAV6 vector itself appears to play no role in tumorigenesis.
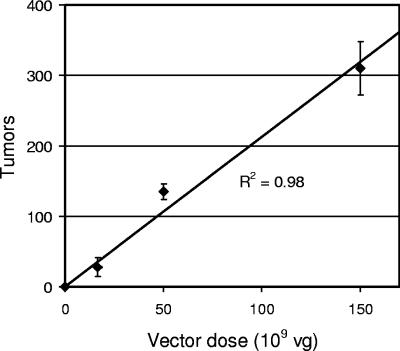
The molecular status of AAV6 vectors following administration to the lung is not known, but multiple forms are expected to be present based on results obtained for other organs and from cultured cells, including single- and double-stranded linear genomes, double-stranded circular genomes, large concatemers, and integrated genomes. While we have never observed direct induction of tumors by ARAP4 in hundreds of animals examined to date, vector effects on DNA metabolism or vector-mediated insertional mutagenesis or gene activation could contribute to tumorigenesis. If so, one might expect an exponential increase in the number of tumors in response to an increased vector dose. We tested this by exposing Rag-2 knockout mice to various doses of the ARJenv vector mixed with a constant low dose of the ARAP4 vector as a control to show that the vector was effectively administered. Tumor number was linearly related to vector dose over a 10-fold range (R2 = 0.98) (Fig. 4), indicating single-hit kinetics of tumor formation and arguing against a stimulatory effect of the AAV6 vector itself on tumor induction.
FIG. 4.
Tumor number increases linearly as a function of vector dose. The indicated amounts of ARJenv vector plus 5 × 109 vg of the ARAP4 vector were administered to mice by nasal aspiration. Tumors in the four major lobes of the lungs of each mouse were counted 5 months later. Error bars indicate standard deviations for groups of three mice exposed to each vector dose.
We also attempted to stimulate tumor induction by including a large excess of the oncogenically inert ARAP4 vector along with the Env expression vector. Administration of a mixture of 5 × 1010 vector genomes (vg) of ARJenv plus 5 × 109 vg of ARAP4 to each of three mice produced 135 ± 11 (mean ± standard deviation) tumors per mouse, while a mixture of 5 × 1010 vg of ARJenv plus 5 × 1011 vg of ARAP4 administered to each of three mice produced 6.7 ± 1.2 tumors per mouse. Thus, there was actually a reduction in tumor number due to inclusion of a large excess of the ARAP4 vector with the Env expression vector, again arguing against a direct stimulatory effect of AAV6 vectors on oncogenesis. The pronounced reduction in tumor number is likely because of competition between vector virions at one or more steps in the process of transduction, for example, at the step of virus binding to a limited number of entry receptors. In conclusion, we find no evidence of a direct effect of the AAV6 vector itself on tumor induction, and it appears that Env expression alone is responsible for tumorigenesis.
In summary, we have shown that the ENTV1 Env can induce lung tumors in mice and therefore is the main, if not the only, oncogenic protein made by ENTV1. However, the location and phenotype of these tumors are similar to those of tumors induced by JSRV Env in mice, and they differ from those of tumors induced in the nasal epithelium of sheep by wild-type ENTV1. The most significant sequence differences between JSRV and ENTV1 occur in the membrane-spanning and intracellular domains of Env (1, 4) and in the promoter/enhancer region of the viral long terminal repeats (1). We plan to test whether the differences in the long terminal repeats can account for the difference in the locations of tumors induced by JSRV and ENTV by using our mouse model for tumorigenesis. It is also possible that differences in sheep and mouse biology account for the difference in disease location, and it will be possible to address this issue by using the AAV6 vectors described here to express the Env proteins in sheep. Lastly, the single-hit kinetics we observed for tumor induction by JSRV Env argue against a role of the AAV6 vector in stimulating tumorigenesis by insertional activation of oncogenes in the host cell genome or by insertional inactivation of tumor suppressor genes, indicating that the Env proteins of JSRV and ENTV1 are each entirely sufficient to induce lethal tumors in animals.
Acknowledgments
We thank James DeMartini for providing the JSRV proviral DNA, Christina Cousens and Mike Sharp for providing the ENTV Env DNA, and Kelly Hudkins-Loya and Charles Alpers for help with the histological and antibody staining.
This work was supported by the Fred Hutchinson Cancer Research Center and by a postdoctoral fellowship to S.K.W. from the National Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Cousens, C., E. Minguijon, R. G. Dalziel, A. Ortin, M. Garcia, J. Park, L. Gonzalez, J. M. Sharp, and M. De las Heras. 1999. Complete sequence of enzootic nasal tumor virus, a retrovirus associated with transmissible intranasal tumors of sheep. J. Virol. 73:3986-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cousens, C., E. Minguijon, M. Garcia, L. M. Ferrer, R. G. Dalziel, M. Palmarini, M. De las Heras, and J. M. Sharp. 1996. PCR-based detection and partial characterization of a retrovirus associated with contagious intranasal tumors of sheep and goats. J. Virol. 70:7580-7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMartini, J. C., J. V. Bishop, T. E. Allen, F. A. Jassim, J. M. Sharp, M. De las Heras, D. R. Voelker, and J. O. Carlson. 2001. Jaagsiekte sheep retrovirus proviral clone JSRV(JS7), derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J. Virol. 75:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirks, C., F.-M. Duh, S. K. Rai, M. I. Lerman, and A. D. Miller. 2002. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76:2141-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbert, C. L., J. M. Allen, and A. D. Miller. 2001. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 75:6615-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller, A. D., and F. Chen. 1996. Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for cell entry. J. Virol. 70:5564-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortin, A., C. Cousens, E. Minguijon, Z. Pascual, M. P. Villarreal, J. M. Sharp, and M. De las Heras. 2003. Characterization of enzootic nasal tumour virus of goats: complete sequence and tissue distribution. J. Gen. Virol. 84:2245-2252. [DOI] [PubMed] [Google Scholar]
- 8.Palmarini, M., J. M. Sharp, M. De las Heras, and H. Fan. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wootton, S. K., C. L. Halbert, and A. D. Miller. 2005. Sheep retrovirus structural protein induces lung tumours. Nature 434:904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]