Abstract
Human immunodeficiency virus type 1 (HIV-1) infects CD4+ T lymphocytes and monocytes/macrophages, incorporating host proteins in the process of assembly and budding. Analysis of the host cell proteins incorporated into virions can provide insights into viral biology. We characterized proteins in highly purified HIV-1 virions produced from human monocyte-derived macrophages (MDM), within which virus buds predominantly into intracytoplasmic vesicles, in contrast to the plasmalemmal budding of HIV-1 typically seen with infected T cells. Liquid chromatography-linked tandem mass spectrometry of highly purified virions identified many cellular proteins, including 33 previously described proteins in HIV-1 preparations from other cell types. Proteins involved in many different cellular structures and functions were present, including those from the cytoskeleton, adhesion, signaling, intracellular trafficking, chaperone, metabolic, ubiquitin/proteasomal, and immune response systems. We also identified annexins, annexin-binding proteins, Rab proteins, and other proteins involved in membrane organization, vesicular trafficking, and late endosomal function, as well as apolipoprotein E, which participates in cholesterol transport, immunoregulation, and modulation of cell growth and differentiation. Several tetraspanins, markers of the late endosomal compartment, were also identified. MDM-derived HIV contained 26 of 37 proteins previously found in exosomes, consistent with the idea that HIV uses the late endosome/multivesicular body pathway during virion budding from macrophages.
As an RNA virus with limited coding capacity, human immunodeficiency virus type 1 (HIV-1) subverts cellular pathways and processes to facilitate many aspects of its replication cycle. It is known that a variety of cellular factors are involved in HIV-1 assembly and budding (13, 20, 42, 44). Typically, HIV-1 is observed by electron microscopy to assemble at and bud from the plasma membrane in T cells and the epithelial cell lines that serve as models for HIV-1 assembly studies (23). In contrast, in macrophages, one of the primary target cell populations in vivo, HIV-1 appears to assemble mostly at internal late endosomal and multivesicular body (MVB) membranes and then bud into these vesicular structures, observable in electron micrographs as internal virion-filled compartments (48, 54, 55, 59). After budding into MVB, these virion-laden vesicles are presumably transported to the cell surface and virus is released from the cell by a normal exocytotic fusion of these structures with the plasma membrane, thereby releasing the contents of the MVB (54, 55).
To date, the differences in viral and cellular protein interactions involved in assembly and budding at the plasma membrane versus the late endosomal assembly pathway remain unclear. Clues to the location and the mechanism by which HIV-1 buds can be provided by the cellular proteins that are incorporated into virions. In the case of macrophage-derived virus, the presence of HLA class II and other late endosomal proteins supports the assembly and budding of HIV-1 in the late-endosome/MVB compartment (45). Also, cellular proteins have been used to identify the cell type that produced the HIV-1 found in patient plasma (37).
In addition to being potential fingerprints for the assembly pathway, cellular proteins found in virions may also play roles in viral pathobiology. The incorporation of cellular proteins such as HLA class II, ICAM-1, and tetraspanins can affect the ability of HIV-1 to infect host cells (7, 43). The presence of HLA class II on virions may contribute to immunopathogenesis, as noninfectious virions carrying HLA class II can induce apoptosis in primary T cells in vitro (18).
Several groups have studied the cellular proteins incorporated into HIV-1 virions produced from lymphocytes and in epithelial cell model systems (reviewed in references 7, 49, and 71); however, the cellular protein content of virions produced from macrophages remains largely unstudied (37, 45). This cell type not only appears to use alternate assembly and budding pathways but also is an important cell lineage for HIV-1 transmission, establishment and maintenance of infection, and pathogenesis (36, 37, 57).
The analysis of cellular proteins in virions is complicated by the presence of non-virion protein-containing particles that can contaminate even virion preparations extensively purified by density gradient centrifugation (1, 25). These particles, mostly microvesicles and exosomes, are produced as part of normal cellular physiology and have been implicated in cell-to-cell signaling and immune activation/surveillance (15, 31, 32, 55, 69). A key difference between the two types of particles is that microvesicles are defined as those particles that bud from the plasma membrane, while exosomes form by budding into late endosomes/MVB vesicles that ultimately fuse with the plasma membrane, releasing these particles from the cell (69). Despite this difference, the two particle types share similarities in morphology and other properties. While these vesicles can be quite heterogeneous, a certain population of these particles has the same density as and approximate size of virion particles, making them quite difficult to remove from virion preparations (1, 25). We have developed a technique by which these contaminating particles can be removed from virion samples by using CD45 affinity depletion with antibody-linked paramagnetic microbeads (72). This method exploits the fact that CD45 is present on many of these vesicles (19, 76) yet is excluded from HIV-1 particles (19, 45). Therefore, CD45 immunoaffinity depletion, combined with density centrifugation, produces highly purified virus preparations.
To better understand the assembly process in macrophages and to identify cellular proteins that might play a role in HIV-1 biology, we examined HIV-1 preparations produced from monocyte-derived macrophages (MDM) that were highly purified by density centrifugation and CD45 immunoaffinity depletion by using liquid chromatography (LC) coupled with electrospray ionization tandem mass spectrometry (MS/MS). Our analysis revealed a large number of cellular proteins not previously described for HIV-1, in addition to proteins previously identified from non-macrophage-derived virus. These results provide important leads for the continued study of HIV-1 assembly, infection, and pathogenesis.
MATERIALS AND METHODS
Virus preparations.
Infectious CCR5-tropic HIV-1 stocks were produced by transfecting 293T cells with the full-length infectious molecular clone NLAD8 (21) by using TransIt 293 reagent (Mirus Corp., Madison, WI). Elutriated monocytes were obtained from HIV-negative donors through the NIH Department of Transfusion Medicine under an NIH institutional research board-approved protocol. To generate macrophages, monocytes (approximately 108 cells) were cultured for seven days on hydrogel-treated plates (ultralow-attachment six-well clusters [catalog no. 3471; Corning, Acton, MA) in RPMI 1640, supplemented with 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, 100 U per ml penicillin, and 100 μg per ml streptomycin. All cell culture products were obtained from Invitrogen (Carlsbad, CA). One half of the MDM from each donor were infected with virus, while the other half served as an uninfected control. Infections were carried out at a multiplicity of infection of >4, and virus production was monitored by a reverse transcriptase assay (28). Input virus was removed from infected cultures by two washings with medium before MDM-derived virus was collected. Culture supernatants were collected over a series of weeks and frozen. Supernatants from the cultures were then thawed, pooled, and clarified by centrifugation (10 min at 2,000 × g) prior to CD45 depletion (see below). All other virus preparations were produced from chronically infected cell lines and purified by sucrose density gradient centrifugation, as previously described (2). Viruses are designated according to the viral strain and cell line in which they were propagated, for example, SIVmne/HuT-78 cl.E11S and HIV-1MN/H9 clone 4.
CD45 immunodepletion.
Virion and vesicle preparations were depleted with anti-CD45 paramagnetic microbeads (catalog no. 130-045-801; Miltenyi Biotec Inc., Southbridge, MA) as previously described (72) with the following modifications. Before use, the microbeads were captured from the stock suspension by applying a magnetic field using one of two Dynal (Brown Deer, WI) magnetic separators, MPC-S or MPC-L. Beads were then washed twice in phosphate-buffered saline (Invitrogen) and resuspended in their original volume. For depletion, clarified cell culture supernatants were treated with anti-CD45 microbeads at a concentration of 2 μl of beads per ml of supernatant, followed by a brief mixing. After 1 h at room temperature, the supernatants were placed in magnetic separators and incubated at 4°C for at least 20 h. CD45 immunoaffinity-depleted supernatants were then removed from the beads by pipetting and centrifuged at 120,000 × g at 4°C for 1 h to pellet virions. Beads were recovered and retained for immunoblot analysis. Fifty milliliters of combined cell culture supernatants from both uninfected and NLAD8-infected MDM cultures was CD45 depleted and density centrifuged to produce the uninfected control and virion preparations analyzed by mass spectrometry.
Immunoblot analysis.
Immunoblot analyses were carried out as previously described (50). The sera used were rabbit anti-Env (reacting with both gp120 and gp41) (Fitzgerald, Concord, MA) and goat anti-p24CA (goat no. 81; AIDS Vaccine Program, NCI—Frederick). Monoclonal antibodies specific for p24 (AIDS Vaccine Program), CD45 (BD-Transduction Laboratories, Inc., San Diego, CA), CD63 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and Tsg101 (Genetex, San Antonio, TX) were also used.
Fractionation and trypsin digestion of protein samples.
Fifteen microliters of sample was prepared in Tris-glycine-sodium dodecyl sulfate (SDS) sample buffer containing 80 mM dithiothreitol (CalBiochem, La Jolla, CA) and incubated for 20 min at 56°C. After cooling at room temperature, the samples were alkylated at a final concentration of 400 mM of iodoacetamide and incubated for 30 min at room temperature in the dark. After alkylation, samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE) on a 1.5-mm-thick 4 to 20% Tris-glycine gel (Invitrogen, Carlsbad, CA), followed by staining with 0.1% (wt/vol) Coomassie blue R-250 in a 40% (vol/vol) methanol, 5% (vol/vol) acetic acid, and water solution. The HIV-1-containing lane was sectioned into 17 contiguous pieces, which were subjected to proteolysis with bovine trypsin (Roche Diagnostics, Mannheim, Germany), according to a previously described protocol (8). The peptides extracted from the gel after digestion were purified by a method adopted from a previously described procedure (60), using 10-μl pipette tips fitted with C18 Empore Discs (3M, Minneapolis, MN) as a microcolumn.
MALDI-TOF mass spectrometry of tryptic digests.
Aliquots containing one third of the extracted peptides were dissolved in 10 μl of 0.1% trifluoroacetic acid (TFA) and purified as described above. The purified material was eluted into a 0.65-ml Eppendorf tube with 2 μl of acetonitrile-0.1% TFA (50/50 [vol/vol]). A 0.5-μl aliquot of the sample was spotted onto a matrix-assisted laser desorption ionization (MALDI) target, followed by the addition of 0.5 μl of matrix solution (2 mg/ml alpha-cyano-4-hydroxycinnamic acid in acetonitrile-0.1% TFA [50/50 {vol/vol}]), containing 10 mM ammonium dihydrogen phosphate, as suggested in reference 79. The mixture was allowed to air dry before analysis. A Voyager-DE Pro time of flight (TOF) mass spectrometer (Perseptive, Houston, TX) was used for analysis. The accelerating voltage was 20 kV, guide wire 0.05%, and grid voltage 95%. The instrument was operated in linear mode under positive ion conditions. A nitrogen laser was used at 337 nm with 150 laser shots averaged per spectrum. Calibration was performed using instrument default settings, and data analysis was carried out using the Data Explorer software included with the Voyager mass spectrometer.
RPLC-MS/MS of tryptic digests and database search.
The remaining two thirds of the peptide samples was dried using vacuum centrifugation and resuspended in 6 μl of 0.1% (vol/vol) TFA. The sample was then analyzed by microcapillary reversed-phase liquid chromatography (μRPLC) using an Agilent 1100 capillary LC system (Agilent Technologies, Inc., Palo Alto, CA), coupled online to an ion trap mass spectrometer (LTQ; Thermo Electron, San Jose, CA). Reversed-phase separations were performed using 75-μm-inside-diameter, 360-μm-outside-diameter, 10-cm-long capillary columns (Polymicro Technologies Inc., Phoenix, AZ) that were packed with 5 μm Jupiter C18 stationary phase slurry (Phenomenex Inc., Torrence, CA). After sample injection, a 20-min wash with 95% mobile phase A (0.1% [vol/vol] formic acid in water) was applied and peptides were eluted using a linear gradient of 5% mobile phase B (0.1% [vol/vol] formic acid in acetonitrile) to 45% mobile phase B over 40 min with a constant flow rate of 0.5 μl/min. The mass spectrometer was operated in a data-dependent MS/MS mode using a normalized collision-induced dissociation (CID) energy of 38%. Dynamic exclusion was applied to minimize redundant selection of peptides previously selected for CID. The temperature of the heated capillary was 180°C, and the electrospray voltage was 1.5 kV. Using SEQUEST (ThermoElectron, San Jose, CA), the CID spectra were compared against those of the EMBL nonredundant protein database. Only peptides having cross-correlation (Xcorr) cutoffs of 1.9 for [M + H]1+, 2.2 for [M + 2H]2+, and 3.5 for [M + 3H]3+ and with delta cross-correlation scores (ΔCn) of at least 0.09 (65) were considered legitimate identifications. These SEQUEST criteria thresholds have been determined previously to result in a 95% confidence level in peptide identification (77).
Transmission electron microscopy.
Micrographs of positive-stained virions were obtained as previously described (27).
RP-HPLC separation and analysis of viral proteins.
Virus sample was disrupted in 8 M guanidine-HCl (Pierce, Rockford, IL) and fractionated by high-performance liquid chromatography (HPLC) to isolate viral proteins under nonreducing conditions. HPLC was performed at a flow rate of 300 μl/min on 2.1- by 100-mm Poros R2/H narrow bore column (Boehringer Mannheim GmbH, Germany), using aqueous acetonitrile-trifluoroacetic acid solvents and a Shimadzu HPLC system equipped with LC-10AD pumps, a SCL-10A system controller, a CTO-10AC oven, a FRC-10A fraction collector, and a SPD-M10AV diode array detector. The gradient of buffer B (0.1% trifluoracetic acid in acetonitrile) was as follows: 10% to 36.5%, 12 min; 36.5% to 37%, 4 min; 37% to 41%, 7 min; 41% to 70%, 12 min; and 70%, 5 min. A temperature of 55°C was maintained during HPLC separation. Peaks were detected by UV absorption at 206 and 280 nm and analyzed by SDS-polyacrylamide gel electrophoresis. Quantitation of purified proteins was performed by dual-color fluorescent protein gel analysis.
Dual-color fluorescent protein gel analysis.
Proteins from lysed virus preparations and HPLC fractions were resolved by SDS-PAGE on 4 to 20% Tris-glycine gels (Invitrogen) under reducing conditions. The p24CA and gp120SU content of the samples were determined by a two-color fluorescence staining assay. Gels with virus samples and a dilution series of purified protein standards were stained with two fluorescent dyes (Molecular Probes, Eugene, OR), SYPRO Pro-Q Emerald (green fluorescence) to detect glycoproteins, such as Env (envelope glycoprotein complex), and SYPRO Ruby (red fluorescence) to detect all proteins, including p24CA. Stained gels were analyzed for fluorescence at 520 nm with UV excitation by using a VersaDoc 3000 imaging system (Bio-Rad Laboratories, Hercules, CA). The gp120SU and p24CA contents of each virion sample were calculated by using the Quantity One software package (Bio-Rad Laboratories) by interpolating the integrated pixel density signals from the unknown samples onto a standard curve derived from a linear regression of density values for serial dilutions of highly purified, quantitative amino acid analysis quantified standards, either recombinant vaccinia-produced HIV-1MN gp120SU (generously provided by B. Puffer and R. Doms, University of Pennsylvania, Philadelphia, PA) or HIV-1MN virion-derived p24CA (AIDS Vaccine Program, Basic Science Program, NCI—Frederick, Frederick, MD). Well characterized reference preparations of SIVmne/HuT-78 cl.E11S and HIV-1MN/H9 clone 4 (AIDS Vaccine Program, Basic Science Program, NCI—Frederick, Frederick, MD) were used to validate this procedure (10).
RESULTS
To examine the proteins found in virions produced by monocyte-derived macrophages, we produced two human MDM cultures from the same donor. One was infected with a CCR5-tropic HIVNLAD8 stock, while the other served as an uninfected control. Thin-section transmission electron microscopy of infected cells revealed that most of the HIV-1 observed was found inside the cell in internal vesicular structures (data not shown), consistent with previous findings that HIV-1 assembles and buds into internal structures in macrophages (48) that have been identified as late endosomes and MVBs (54).
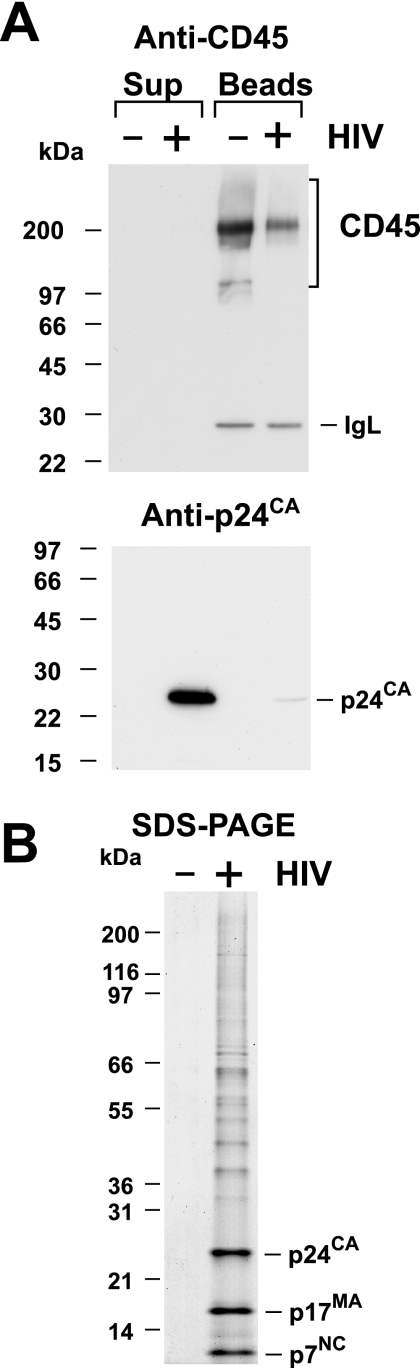
To remove cellular vesicles from virions in cell culture supernatants, we CD45 immunoaffinity depleted clarified cell culture supernatants from these parallel cultures of uninfected and HIVNLAD8-infected MDM by using CD45-antibody-coupled paramagnetic microbeads. This depletion method can remove more than 98% of the CD45 protein from T-cell-derived virus preparations (72). After depletion, the treated supernatants were subjected to our standard density centrifugation procedure to pellet virions away from soluble proteins and other cellular debris. The CD45-depleted preparations as well as the CD45-selected bead-bound fractions (all samples were 5% of the total material by volume) from the uninfected and HIV-infected MDM cultures were analyzed by immunoblot and SDS-PAGE analyses (Fig. 1). CD45 and p24CA immunoblot analyses (Fig. 1A) showed that while there was no detectable CD45 signal in the supernatant samples (the CD45-depleted fraction), there was an evident CD45 signal in the bead sample (the CD45-containing fraction), indicating that most of the CD45 was removed from both infected and uninfected materials. In addition to the CD45 signal in the bead sample, there is a 28-kDa band corresponding to the immunoglobulin light chain (Fig. 1A). This is likely mouse immunoglobulin light chain released from the heavy chain-microbead complex in reduced SDS-PAGE samples and detected by the anti-mouse secondary antibody employed in the analysis. Reacting the same blot with p24CA antiserum showed that virus was present in the CD45-depleted virus sample and not in the CD45-selected bead-bound sample (Fig. 1A).
FIG. 1.
Analysis of CD45-depleted HIV. (A) Immunoblots of CD45-depleted virion preparations (equal amounts by volume) isolated from parallel uninfected (−) and infected (+) cell cultures are presented. Samples from the immunoaffinity-depleted supernatant (Sup) and anti-CD45 bead fractions are identified above their respective lanes. Antibody or antiserum used is indicated above each blot. IgL, immunoglobulin light chain. (B) An analytical Coomassie blue-stained SDS-PAGE gel of CD45-depleted uninfected (−) and HIV-infected (+) preparations (equal amounts by volume) produced from MDM is shown.
The removal of contaminating vesicles from a virus sample should also be reflected in the removal of cellular proteins. An analytical-scale SDS-gel electrophoresis analysis of the CD45-immunodepleted and density-purified samples (3% of the total material by volume) prepared from infected and noninfected cells revealed that all of the detectable protein was removed from the noninfected MDM samples, while viral proteins, as well as cellular proteins, still remained in the infected MDM preparations (Fig. 1B). Thus, the CD45 depletion and density centrifugation removed all of the detectable protein from the uninfected preparation.
Electron microscopy of virion preparations.
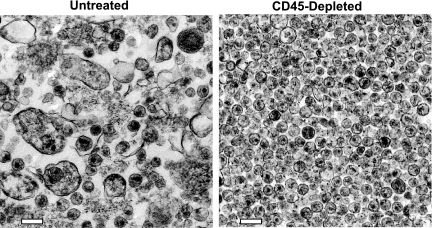
To directly examine the removal of nonviral particles, we analyzed samples of the HIV-1 produced from MDM, processed with and without CD45 depletion, by transmission electron microscopy (Fig. 2). The untreated HIV-1 preparation contained a relatively small number of virions and an abundance of nonviral particles. In contrast, the CD45-depleted virion preparation contained mostly virions and few if any nonviral particles. These data are consistent with our previous observations (72), as well as with the biochemical data obtained for the same specimens, and directly demonstrate that CD45 depletion effectively removed vesicular contamination from the MDM-derived virion preparations.
FIG. 2.
Electron microscopy of HIV-1. Thin-section transmission electron micrographs of untreated and CD45-depleted virus preparations are shown. Samples are identified above their respective micrographs. A white 200-nm bar has been added to each micrograph for reference.
Proteomics of CD45-depleted MDM-derived HIV.
To identify proteins in HIV-1 produced from macrophages, the CD45-depleted HIV-1 preparation (corresponding to 2 μg of HIV-1 p24CA, 30% of the total volume) was fractionated using a preparative-scale SDS-PAGE gel separation. Before SDS-PAGE, the cysteinyl residues within the samples were reduced and alkylated to avoid spontaneous intermolecular disulfide bond formation during electrophoresis. The CD45-depleted virion sample contained a large number of bands with a wide range of sizes (Fig. 3). In contrast, an equal volume (also 30%) of the uninfected control sample, i.e., the CD45-depleted preparation from the uninfected macrophages produced and processed in parallel, contained no detectable proteins in the preparative-scale Coomassie blue-stained gel (data not shown; results were identical to those in Fig. 1B). To analyze the proteins in the HIV-1 containing gel, the lane was sectioned into 17 contiguous slices (Fig. 3); each of these fractions was digested individually with trypsin, and the peptides generated were eluted from the gel by extraction. The LC-MS/MS technique requires a complete tryptic digest of the peptides with low levels of nonspecific cleavage that can occur with overdigestion of the sample with protease. To assess the quality of the eluted peptides, one-third of the peptide material extracted from each gel slice was analyzed by MALDI-TOF MS prior to LC-MS/MS analysis. The MALDI-TOF MS data confirmed that the digested samples contained high-quality peptides and provided a good estimate of the relative protein concentrations in these samples. Therefore, a portion of the peptide samples that were extracted from the gel bands was analyzed by μRPLC-MS/MS. The results showed that all fractions of the gel contained statistically significant peptide identifications (those proteins identified with more than one peptide at a confidence level greater than 97%). A μRPLC-MS/MS analysis of fractions 7 and 8 from the parallel preparative-scale SDS-PAGE analysis of the CD45-depleted uninfected control preparation produced no statistically significant peptide identifications (data not shown). In contrast, the same fractions produced many peptides in the virus sample analysis (Table 1); thus, the proteins identified in the CD45-depleted virus samples appear to be from the virus and not from contaminating vesicle-associated proteins.
FIG. 3.
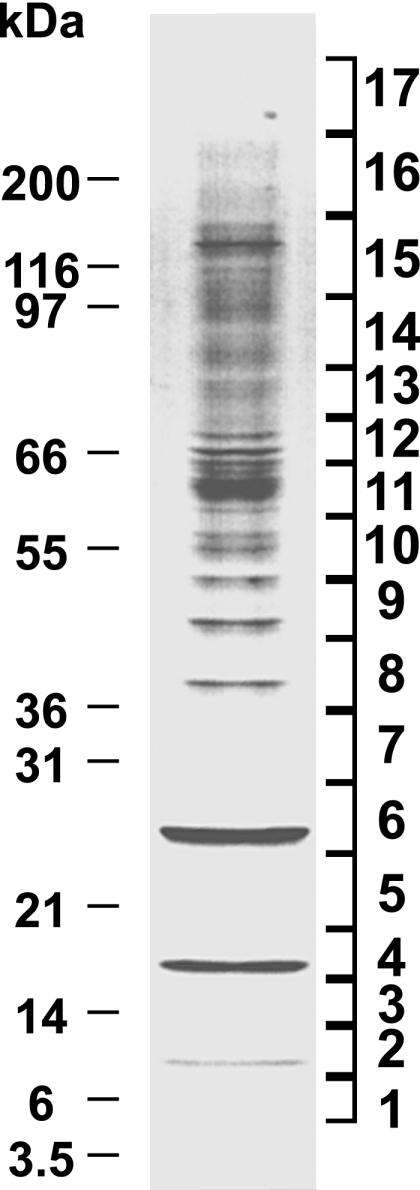
Fractionated preparative SDS-PAGE gel. The Coomassie blue-stained preparative SDS-PAGE (4 to 20%) gel of the CD45-depleted virus sample is shown. Gel fractions taken are indicated at the right and the positions of molecular mass markers are denoted at the left.
TABLE 1.
Cellular proteins in MDM-derived HIV-1aa
| Fraction (size range in kDa) | Protein nameb | Mol mass (Da)c | Particled | Locatione | Function/structure in which protein is involvedf | Accession no.g | No. of peptides |
|---|---|---|---|---|---|---|---|
| 1 (5-7) | 3′,5′-Cyclic phosphodiesterase B | 124,376 | M | Metabolism | Q13370 | 2 | |
| Alpha-actinin 4 (F-actin cross linking protein) | 104,854 | C | Actin cytoskeleton | O43707 | 2 | ||
| 2 (8-11) | S100A10 (p11) | 11,072 | C, M | LE trafficking | P60903 | 5 | |
| S100A11 (S100C) | 11,740 | C, M | Signaling | P31949 | 4 | ||
| S100A6 | 10,180 | C, M | Signaling | P06703 | 2 | ||
| S100A8 | 10,835 | C, M | Signaling | P05109 | 2 | ||
| Histone H4 | 11,236 | Exo | N | Chromatin | P62805 | 3 | |
| Beta-2-microglobulin | 13,714 | Vir | M | Antigen presentation | P61769 | 2 | |
| Syndecan-2 (C-terminal fraction) | 22,174 (P) | M | Adhesion | P34741 | 2 | ||
| Hypothetical protein (fractions 3 [2] and 15 [4]) | 15,258 | ? | ? | Q3MIF5 | 2 | ||
| 3 (11-16) | Epidermal fatty acid-binding protein (E-FABP) | 15,033 | C | Lipid organization | Q01469 | 4 | |
| Histone H2B | 13,775 | Exo | N | Chromatin | P62807 | 4 | |
| Histone H3.1 | 15,273 | Exo | N | Chromatin | P68431 | 2 | |
| Profilin-1 | 14,923 | Exo | C | Actin cytoskeleton | P07737 | 3 | |
| Vesicle-associated membrane protein 8 (VAMP-8) | 11,438 | C | LE trafficking | Q9BV40 | 3 | ||
| Vacuolar ATP synthase subunit G1 | 13,626 | C | Vesicular acidification | O75348 | 3 | ||
| Lysozyme C | 16,537 | S | Bacteriolysis | P61626 | 3 | ||
| Ubiquitin (fractions 4 [3], 6 [2], 8 [2], 10 [2], 12 [2], 15 [2]) | 8,565 | Vir | C, N | UPS/protein trafficking | Q9UEF2 | 3 | |
| Retinoic acid-binding protein II (CRABP-II) | 15,562 | C | Signaling | P29373 | 2 | ||
| Galectin-1 | 14,584 | C, M, S | Signaling | P09382 | 2 | ||
| Coactosin-like protein | 15,814 | C | Actin cytoskeleton | Q14019 | 2 | ||
| Signal recognition particle 14-kDa protein (SRP14) | 14,544 | C | Secretion | P37108 | 2 | ||
| Syndecan-2 (mature protein) | 22,174 (P) | M | Adhesion | P34741 | 2 | ||
| 4 (16-20) | Cyclophilin A (fraction 5 [2]) | 17,881 | Vir | C | Protein folding | P62937 | 8 |
| GAPR-1 | 17,087 | M | Membrane (Golgi, ER) | Q9H4G4 | 5 | ||
| Cofilin (fraction 5 [2]) | 18,502 | Exo, Vir | C | Actin cytoskeleton | P23528 | 4 | |
| Methylmalonyl-coenzyme A epimerase | 18,749 | ? | ? | Q96PE7 | 2 | ||
| RAD21-like 1 | 22,840 | ? | ? | Q5W0X5 | 2 | ||
| Rho-related GTP-binding protein RhoG | 21,309 | M | Signaling | P84095 | 2 | ||
| 40S ribosomal protein S16 | 16,314 | C | Translation | P62249 | 2 | ||
| Ankyrin repeat domain 22 | 21,849 | ? | ? | Q5VYY1 | 2 | ||
| Beta-glucan receptor isoform E | 19,218 | M | Innate immunity | Q96PA7 | 2 | ||
| Clathrin light chain A | 27,077 | M | Endocytosis | P09496 | 3 | ||
| Clathrin light chain B (fraction 3 [3]) | 25,190 | M | Endocytosis | P09497 | 2 | ||
| COP9 signalosome complex subunit 7b | 29,622 | C | Signaling/neddylation | Q9H9Q2 | 2 | ||
| Forkhead box protein G1B | 51,340 | N | Transcription | P55315 | 2 | ||
| Galectin-9 | 39,518 | S | Signaling | O00182 | 2 | ||
| HSPC069 isoform b | 135,937 | ? | ? | Q5QGN2 | 2 | ||
| Prolyl endopeptidase | 80,749 | C | Cellular protease | P48147 | 2 | ||
| 5 (20-24 kDa) | Actin-related protein 2/3 complex subunit 4 | 19,536 | C | Actin cytoskeleton | P59998 | 5 | |
| Vacuolar protein sorting (Vps) 29 human homolog | 20,506 | C, M | Vesicular trafficking | Q9UBQ0 | 2 | ||
| ARF-1 (ADP-ribosylation factor 1) (fraction 4 [3]) | 20,566 | M | Vesicular trafficking | P84077 | 3 | ||
| Rap-1A (Ras-related protein) | 20,987 | M | Signaling | P62834 | 2 | ||
| CDC42 | 21,311 | M | Actin cytoskeleton | P60953 | 2 | ||
| Cyclophilin B | 22,742 | ER | ER protein folding | P23284 | 2 | ||
| Tmp21 | 24,976 | G | Golgi vesicle sorting | P49755 | 2 | ||
| Synaptogyrin-1 | 25,570 | ? | ? | O43759 | 2 | ||
| CD81 (fraction 2 [2]) | 25,809 | Exo, Vir | M | Signaling | P60033 | 1 | |
| Desmoyokin (AHNAK) (fraction 2 [2]) | 312,493 | C, N | Actin cytoskeleton | Q09666 | 2 | ||
| Desmoplakin (DP) (fraction 3 [3]) | 8,331,776 | C | Actin cytoskeleton | P15924 | 3 | ||
| Junction plakoglobin (desmoplakin-3) | 81,498 | C, M | Actin cytoskeleton | P14923 | 3 | ||
| Tsg101 | 43,944 | Exo, Vir | C | Vesicle sorting | Q99816 | 2 | |
| 6 (24-29) | CD9 (fractions 5 [3], 7 [4], 8 [5], 9 [2], 10 [2], 12 [2], 13 [2], and 14 [3]) | 25,285 | Exo | M | Signaling | P21926 | 7 |
| Syntenin 1 | 32,444 | Exo | M | Actin cytoskeleton | O00560 | 6 | |
| Peroxiredoxin 6 | 24,904 | C | Redox control | P30041 | 6 | ||
| Triosephosphate isomerase (TPI) | 26,538 | C | Metabolism | P60174 | 6 | ||
| Rab7 (fraction 5 [4]) | 23,490 | Exo | C | LE trafficking | P51149 | 5 | |
| Phosphoglycerate mutase 1 | 28,673 | C | Catabolism | P18669 | 4 | ||
| Rab8A | 23,668 | M | Vesicular trafficking | P61006 | 4 | ||
| Rab11 | 24,393 | Exo | M | Vesicle trafficking | P62491 | 3 | |
| Rab5C | 23,483 | M | Vesicular transport | P51148 | 3 | ||
| Rab35 | 23,025 | M | Vesicle trafficking | Q15286 | 2 | ||
| Protein kinase C inhibitor protein 1 zeta/delta | 27,745 | C | Signaling | P63104 | 3 | ||
| B-cell receptor-associated protein 31 | 27,860 | ER, G | ER-to-Golgi trafficking | P51572 | 3 | ||
| GlutathioneS-transferase P | 23,225 | C | Redox control | P09211 | 3 | ||
| GTP-binding nuclear protein Ran | 24,423 | N, C | Nuclear shuttling | P62826 | 3 | ||
| LGALS3 protein variant (fraction 4 [3]) | 27,118 | ? | Lectin binding | Q59FR8 | 3 | ||
| Metalloproteinase inhibitor 2 (TIMP-2) | 24,399 | S | Protease inhibitor | P16035 | 2 | ||
| Protein kinase C inhibitor protein 1 beta/alpha | 27,951 | C | Signaling | P31946 | 2 | ||
| Hypothetical protein FLJ43237 | 24,474 | ? | ? | Q6ZUX7 | 2 | ||
| Tetraspanin-14 | 30,691 | ? | ? | Q8NG11 | 2 | ||
| CD258 | 26,351 | C, M, S | Signaling | O43557 | 2 | ||
| Vps28 (human homolog) | 26,558 | C | ESCRT I component | Q9UK41 | 2 | ||
| Rab1A | 22,547 | G | Golgi vesicular sorting | P62820 | 5 | ||
| Transforming protein RhoA | 21,768 | M | Signaling | P61586 | 4 | ||
| Peroxiredoxin 1 | 22,110 | C | Redox control | Q06830 | 3 | ||
| Rac3 | 21,379 | C, M | Signaling | P60763 | 2 | ||
| Renin receptor | 39,008 | M | Signaling | O75787 | 3 | ||
| SNARE protein Ykt6 | 22,418 | ER, G | ER-to-Golgi transport | O15498 | 3 | ||
| Inositol oxygenase (fraction 4 [4]) | 33,010 | C | Catabolism | Q9UGB7 | 2 | ||
| Rac1 | 21,450 | M | Signaling | P63000 | 3 | ||
| Rac2 | 21,429 | C | Signaling | P15153 | 2 | ||
| Rab10 | 22,541 | M | Vesicular transport | P61026 | 2 | ||
| Rab13 | 22,774 | C | Vesicular transport | P51153 | 2 | ||
| Rab5A | 23,659 | M | Endocytosis | P20339 | 2 | ||
| Rab8B | 23,584 | M | Vesicular transport | Q92930 | 2 | ||
| Ral-B | 23,409 | M | Signaling | P11234 | 2 | ||
| Rap-1b | 20,825 | M, C | Signaling | P61224 | 2 | ||
| 7 (29-34) | Annexin A5 | 35,805 | Exo | M | Blood clotting | P08758 | 4 |
| PA28a proteasome subunit | 28,723 | C, N | UPS | Q06323 | 2 | ||
| eEF1A (HIV-1 protease fragmenth) | ∼30,000 | Vir | C | Translation | Q05639 | 3 | |
| HLA class II-DR | 29,966 | Exo | M | Antigen presentation | P01903 | 3 | |
| Chloride intracellular channel protein 1 (NCC27) | 26,792 | N, M, C | Ion transport | O00299 | 2 | ||
| 8 (34-43) | Annexin A2 (calpactin I heavy chain) (fractions 7 [3] and 9 [9]) | 38,472 | Exo | M | Exocytosis | P07355 | 27 |
| ApoE (fractions 2 [3], 3 [2], 4 [6], 5 [5], 6 [11], 7 [7], 9 [8], 10 [2], 12 [5], 13 [2], 14 [4], 15 [3], and 16 [2]) | 36,154 | S | Cholesterol transport | P02649 | 25 | ||
| Annexin A1 | 38,583 | Exo | Exocytosis | P04083 | 7 | ||
| CD53 (fractions 4 [1], 5 [1], 6 [1], and 10 [1]) | 24,341 (G) | Exo | M | Signaling | P19397 | 2 | |
| Glyceraldehyde-3-phosphate dehydrogenase (fraction 5 [2]) | 35,922 | Vir | C | Metabolism | P00354 | 7 | |
| Junctional adhesion molecule A (fraction 3 [2]) | 32,518 (G) | M | Adhesion | Q9Y624 | 5 | ||
| Macrophage capping protein | 38,518 | C, N | Actin cytoskeleton | P40121 | 5 | ||
| Vacuolar ATP synthase subunit d | 40,329 | C, M | Vesicular acidification | P61421 | 5 | ||
| Fructose-1,6-bisphosphatase 1 | 36,683 | C | Catabolism | P09467 | 3 | ||
| Biliverdin reductase A | 33,428 | C | Metabolism | P53004 | 5 | ||
| Malate dehydrogenase | 36,295 | C | Metabolism | P40925 | 3 | ||
| Poly(rC)-binding protein 1 | 37,498 | N | Nuclear export | Q15365 | 3 | ||
| 26S proteasome non-ATPase regulatory subunit 14 | 34,577 | C, N | UPS | O00487 | 2 | ||
| Actin-related protein 2/3 complex subunit 1B (p41-ARC) | 40,819 | C | Actin cytoskeleton | O15143 | 2 | ||
| F-actin capping protein alpha-1 subunit (CapZ alpha-1) | 32,923 | C | Actin cytoskeleton | P52907 | 2 | ||
| Gi2 alpha | 41,548 | Exo | C | Signaling | Q6B6N3 | 2 | |
| N-Acetylglucosamine kinase | 37,244 | C | Carbohydrate metabolism | Q9UJ70 | 2 | ||
| Protein XRP2 | 39,641 | ? | ? | O75695 | 2 | ||
| Transcriptional activator protein PUR-alpha | 34,911 | N | Transcription | Q00577 | 2 | ||
| 1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase-like 4 | 129,725 | ? | Lipid signaling | O75038 | 2 | ||
| Erythroid membrane-associated protein | 52,589 | M, C | Adhesion | Q7Z3X0 | 2 | ||
| Polypyrimidine tract-binding protein 1 (PTB1) | 57,221 | C | Splicing, translation | P26599 | 2 | ||
| 9 (43-48) | Beta actin (fractions 8 [1], 10 [1], 11 [1], and 14 [1]) | 41,736 | Exo, Vir | C | Actin cytoskeleton | P60709 | 5 |
| Alpha-actin-2 (fractions 1 [2], 3 [2], 4 [3], 6 [4],7 [2], 8 [5], 13 [2], 14 [3], 15 [4], and 16 [2]) | 42,009 | Exo, Vir | C | Actin cytoskeleton | P62736 | 5 | |
| 2′,3′-Cyclic nucleotide 3′-phosphodiesterase (fraction 7 [2]) | 47,578 | M | Signaling | P09543 | 4 | ||
| HLA class A (fractions 8 [5], 10 [2], 12 [2]) | 40,846 (G) | Vir | M | Antigen presentation | P30443 | 3 | |
| HLA class B (fraction 8 [2]) | 40,331 (G) | Exo, Vir | M | Antigen presentation | P30460 | 4 | |
| HLA class C | 40,710 (G) | Vir | M | Antigen presentation | P10321 | 2 | |
| CD82 (fractions 10 [2] and 11 [3]) | 29,625 (G) | Exo | M | Signaling | P27701 | 2 | |
| Titin (cellular fragment)i | 3816,262 | C | Contraction | Q8WZ42 | 2 | ||
| Phosphoglycerate kinase 1 (PRP2) | 44,483 | C | Metabolism | P00558 | 2 | ||
| Complement C4b | 75,437 | S | Cell lysis | P01028 | 2 | ||
| 10 (48-56) | 6-Phosphogluconate dehydrogenase | 53,008 | C | Metabolism | P52209 | 10 | |
| LAP3 protein | 56,136 | ? | ? | Q6IAM6 | 6 | ||
| GDP dissociation inhibitor beta (Rab GDIb) | 50,663 | Exo | C, M | Regulation of Rab targeting | P50395 | 6 | |
| eEF1A (elongation factor-1 alpha) (fractions 3 [2], 5 [2], 6 [4], 7 [2], 8 [5], 12 [3], 13 [3], 15 [3], 16 [2]) | 50,141 | Exo, Vir | C | Translation | Q05639 | 5 | |
| Alpha-2-HS-glycoprotein (fetuin-A) (fractions 11 [2], 12 [3], and 16 [2]) | 39,324 (G) | S | Endocytosis | P02765 | 3 | ||
| Serine protease HTRA1 | 51,287 | S | Protease | Q92743 | 5 | ||
| Vimentin (fractions 5 [2] and 9 [2]) | 53,520 | C | IF cytoskeleton | P08670 | 5 | ||
| Annexin A11 | 54,390 | C, N | ? | P50995 | 4 | ||
| Disulfide isomerase ER-60 | 56,782 | ER | Protein folding | P30101 | 4 | ||
| Thymidine phosphorylase (TdRPase) | 49,955 | C | Metabolism | P19971 | 4 | ||
| Adenylyl cyclase-associated protein 1 (CAP1) | 51,542 | M | Actin-RNA-binding regulation | Q01518 | 3 | ||
| Alpha-enolase (fraction 8 [3]) | 47,038 | C, M | Multiple function | P06733 | 3 | ||
| Coronin-1A | 51,026 | C | Actin cytoskeleton | P31146 | 3 | ||
| CD14 | 40,076 (G) | M | LPS receptor | P08571 | 3 | ||
| CD147 | 42,200 (G) | M | Adhesion | P35613 | 2 | ||
| CD48 | 27,683 (G) | M | Signaling | P09326 | 2 | ||
| HU-K4 (fractions 11 [2], 12 [2], and 13 [2]) | 48,771 | ? | ? | Q92853 | 2 | ||
| Rho-GTPase-activating protein 1 | 50,436 | C | Signaling | Q07960 | 2 | ||
| Alpha-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase | 50,862 | G | Glycosylation | P26572 | 2 | ||
| ATP synthase beta chain | 56,560 | Mi | Metabolism | P06576 | 2 | ||
| TCP-1-beta | 57,357 | C | Protein folding | P78371 | 2 | ||
| Tubulin alpha-1 | 49,924 | Exo | C | Microtubules | P68366 | 2 | |
| Vacuolar ATP synthase subunit B | 56,501 | C | Endocytosis | P21281 | 2 | ||
| CD2/LFA-2 (fractions 2, 5, 7, 14 [1], and 16 [1]) | 39,439 (G) | Vir | M | Signaling | P06729 | 1 | |
| Heat shock 40-kDa protein 4 | 44,868 | M | Protein folding | P31689 | 5 | ||
| Hsc70-interacting protein (HIP) | 41,332 | C | Protein folding | P50502 | 3 | ||
| Actin-like protein 3 (ARP3) | 47,371 | C | Actin cytoskeleton | P61158 | 2 | ||
| K-glypican | 62,412 | M | Adhesion | O75487 | 2 | ||
| Pigment epithelium-derived factor (PEDF) | 46,342 | S | Differentiation | P36955 | 2 | ||
| Radixin | 68,564 | M | Cytoskeleton | P35241 | 3 | ||
| Brain acid soluble protein 1 (BASP1, NAP22) | 22,562 | M | Actin cytoskeleton | P80723 | 2 | ||
| Ifapsoriasin | 248,073 | C | IF cytoskeleton | Q5D862 | 2 | ||
| Solute carrier family 12 member 7 | 119,150 | M | Ion transporter | Q9Y666 | 2 | ||
| 11 (56-64) | Pyruvate kinase (fractions 8 [3], 10 [4], 12 [15], 14 [2], and 15 [3]) | 57,805 | C | Metabolism | P14618 | 9 | |
| L-Plastin (lymphocyte cytosolic protein 1) | 70,289 | C | Actin cytoskeleton | P13796 | 9 | ||
| EHD4 (fractions 10 [3] and 12 [5]) | 61,175 | C | Vesicle trafficking | Q9H223 | 4 | ||
| Copine III (Ca2+ kinase) | 60,130 | M | Membrane organization | O75131 | 5 | ||
| Carboxypeptidase M (fraction 12 [3]) | 50,514 (G) | M | Extracellular protease | P14384 | 2 | ||
| Protein kinase C (fraction 12 [4]) | 55,739 | C | Vesicular transport | Q9UNF0 | 2 | ||
| Gamma-glutamyltransferase 1 (fraction 13 [2]) | 61,382 | M | Redox control | P19440 | 2 | ||
| LIR-D1 | 55,903 | M | Adhesion | Q8NG09 | 2 | ||
| Lipid transfer protein II (fraction 13 [2]) | 54,739 | S | Phospholipid transport | P55058 | 3 | ||
| Moesin (fractions 8 [3] and 10 [6]) | 67,689 | C | Membrane-organizing | P26038 | 6 | ||
| 75-kDa glucose-regulated protein (GRP75) | 73,680 | Mt | Protein folding | P38646 | 5 | ||
| 78-kDa glucose-regulated protein (GRP78) | 72,333 | ER | Protein folding | P11021 | 4 | ||
| Annexin A6 | 75,742 | C, M | Signaling | P08133 | 3 | ||
| 12 (64-70) | Membrane matrix metalloproteinase-14 | 65,883 | M | Membrane organization | P50281 | 6 | |
| Hsc70 (fractions 9 [2] and 11 [14]) | 70,898 | Exo, Vir | C | Protein folding | P11142 | 6 | |
| Hsp70-2 (fractions 9 [2] and 11 [6]) | 70,021 | Vir | C | Protein folding | P54652 | 4 | |
| Hsp70-1 (fractions 6 [2] and 11 [12]) | 70,052 | Vir | C | Protein folding | P08107 | 3 | |
| Hsp70-1L (fractions 6 [2] and 11 [5]) | 70,375 | C | Protein folding | P34931 | 2 | ||
| CD58 (fractions 12 [2] and 13 [2]) | 28,147 (G) | M | Adhesion, signaling | P19256 | 2 | ||
| CD84 | 36,871 (G) | M | ? | O15430 | 2 | ||
| Actin interacting protein 1 | 66,062 | C | Actin cytoskeleton | O75083 | 2 | ||
| Hsp60 | 61,055 | C | Protein folding | P10809 | 3 | ||
| Kindlin-3 (fractions 2 [2], 5 [2], 6 [5], 8 [10], 15 [3], 16 [2]) | 75,952 | C | Cytoskeleton | Q86UX7 | 8 | ||
| Thyroxine-binding globulin | 46,325 | S | Thyroxine transport | P05543 | 3 | ||
| Tyrosine-protein phosphatase (SHP-1) | 67,561 | C | Signaling | P29350 | 3 | ||
| Catalase | 59,625 | C | Redox control | P04040 | 2 | ||
| LAMP-1 (CD107a) (fractions 14 [2] and 15 [2]) | 44,773 (G) | M | Endocytic trafficking | P11279 | 2 | ||
| 13 (70-80) | Hsp90 (fractions 15 [2] and 16 [3]) | 83,133 | Exo | C | Protein folding | P08238 | 10 |
| CD98 | 57,945 (G) | M | Amino acid transport | P08195 | 3 | ||
| CD54 (ICAM-1) (fraction 14 [4]) | 57,825 (G) | M | Adhesion | P05362 | 3 | ||
| Quiescin Q6, isoform a (QSCN6) | 82,578 | ? | ? | O00391 | 4 | ||
| Endopeptidase-2 | 84,368 | M | Protease | Q16819 | 3 | ||
| Rho GTPase activating protein 18 | 74,947 | ? | ? | Q8N392 | 2 | ||
| ALB protein (fractions 10 [2], 11 [5], 12 [3], 14 [2], and 15 [2]) | 71,705 | ? | ? | Q5D0D7 | 2 | ||
| Gelsolin | 85,697 | C | Actin cytoskeleton | P06396 | 9 | ||
| Periostin | 93,332 | S | Adhesion | Q15063 | 5 | ||
| Hsp89-alpha-delta-N | 63,252 | ? | Protein folding | O75322 | 4 | ||
| Signal transducer and activator of transcription 1 (STAT1) | 87,335 | C | Signaling | P42224 | 4 | ||
| Matriptase (MT-SP1) | 94,770 | M | Protease | Q9Y5Y6 | 4 | ||
| gp91-phox | 65,205 | M | pH regulation | P04839 | 2 | ||
| Tumor necrosis factor alpha-induced protein 3 | 89,614 | C, N | Signaling | P21580 | 3 | ||
| 26S proteasome regulatory subunit RPN1 | 100,200 | C, N | UPS | Q13200 | 2 | ||
| CDC48/p97 | 89,191 | C, N | Vesicle trafficking | P55072 | 2 | ||
| Vps35 (human homolog) | 91,707 | C, M | Vesicle sorting | Q96QK1 | 2 | ||
| 14 (80-100) | CD18 (integrin beta-2) (fraction 13 [4]) | 84,790 (G) | Exo, Vir | M | Adhesion | P05107 | 19 |
| CD36 (fraction 13 [2]) | 52,922 (G) | M | Adhesion | P16671 | 7 | ||
| CD43 (leukosialin) (fractions 5 [2] and 13 [2]) | 40,322 (G) | Vir | M | Signaling | P16150 | 1 | |
| CD44 (fractions 4 [3], 5 [3], 6 [2], 11 [7], and 13 [3]) | 81,214 (G) | Vir | M | Adhesion | P16070 | 3 | |
| CD61 (integrin beta-3) | 87,214 | M | Adhesion | P05106 | 3 | ||
| CD49c (integrin alpha-3) | 118,698 | M | Adhesion | P26006 | 2 | ||
| CD86 | 37,696 (G) | M | T-cell regulation | P42081 | 2 | ||
| CD276 | 57,235 (G) | M | T-cell regulation | Q5ZPR3 | 2 | ||
| CD315 (fraction 15 [4]) | 98,556 | M | Signaling | Q9P2B2 | 4 | ||
| Na+/K+ ATPase 1 (fraction 15 [2]) | 112,896 | M | Ion transport | P05023 | 10 | ||
| AIP-1/Alix (fractions 2 [2] and 12 [3]) | 96,023 | Exo, Vir | C | LE trafficking | Q8WUM4 | 9 | |
| Hexokinase-3 | 98,919 | C | Microtubule motor | P52790 | 6 | ||
| 94-kDa glucose-regulated protein (GRP94) | 92,469 | ER | Protein folding | P14625 | 5 | ||
| Elongation factor 2 (EF-2) (fraction 13 [6]) | 95,207 | C | Translation | P13639 | 2 | ||
| Importin beta-1 (importin 90) (fraction 13 [2]) | 97,170 | N, C | Nuclear shuttling | Q14974 | 2 | ||
| Vacuolar proton pump subunit 1 | 96,413 | M | Endosome acidification | Q93050 | 2 | ||
| KIFC3 kinesin (fractions 3, 4, 5, 6, 10, and 16 [2]) | 77,866 | C | Vesicle trafficking | Q9BVG8 | 1 | ||
| Putative helicase MOV-10 (fraction 12 [2]) | 113,671 | ? | ? | Q9HCE1 | 5 | ||
| HEM-1 | 128,215 | M | ? | P55160 | 4 | ||
| Vinculin | 123,668 | M | Actin cytoskeleton | P18206 | 3 | ||
| Fibulin-1 | 77,261 | S | Adhesion | P23142 | 2 | ||
| Inter-alpha-inhibitor heavy chain 2 (fractions 12 [2], 13 [2], 14 [2], and 16 [2]) | 106,436 | S | Hyaluronan binding | P19823 | 2 | ||
| 15 (100-130) | CD11b (Mac-1, integrin alpha-M) (fraction 11 [2]) | 27,178 (G) | Exo, Vir | M | Adhesion | P11215 | 11 |
| CD13 (fraction 14 [2]) | 109,380 | M | Signaling | P15144 | 10 | ||
| CD11c (fractions 14 [2] and 16 [2]) | 127,887 | M | Adhesion | P20702 | 8 | ||
| CD29 (integrin beta-1) | 884,654 (G) | M | Adhesion | P05556 | 5 | ||
| CD18 (integrin beta-1 chain) | 88,465 (G) | Exo, Vir | M | Adhesion | P05556 | 2 | |
| CD49e (fibronectin receptor alpha subunit) | 114,536 | M | Adhesion | P08648 | 2 | ||
| CD51 (vitronectin receptor alpha subunit) | 116,052 | M | Adhesion | P06756 | 2 | ||
| CD304 (neurophilin-1) (fractions 14 [3] and 16 [2]) | 103,120 | M | Signaling receptor | O14786 | 2 | ||
| EMILIN-2 | 115,616 | S | Adhesion | Q9BXX0 | 6 | ||
| Thrombospondin-1 | 129,413 | ? | Adhesion | P07996 | 2 | ||
| Myoferlin (fraction 16 [3]) | 234,709 | M | Membrane organization | Q9NZM1 | 6 | ||
| Protein tyrosine phosphatase receptor type C (PTPRC) | 131,130 | M | Signaling | Q5T5R0 | 4 | ||
| Cytoplasmic FMR1 interacting protein isoform 1 | 145,182 | C | Translation/RNA location | Q7L576 | 3 | ||
| Cytoplasmic FMR1 interacting protein isoform 4 | 94,523 | C | ? | Q6ZSX1 | 2 | ||
| Type 3 inositol 1,4,5-trisphosphate receptor | 304,038 | ER | Signaling | Q14573 | 2 | ||
| Nicastrin | 78,411 | M | Protease | Q92542 | 2 | ||
| Ras GTPase-activating-like protein (IQGAP1) (fraction 14 [2]) | 189,252 | M | Actin cytoskeleton | P46940 | 2 | ||
| 16 (130-280) | Fibronectin | 262,607 | S, M | Adhesion | P02751 | 17 | |
| Clathrin heavy chain 1 (fractions 3 [3], 8 [6], 10 [6], 11 [7], 12 [8], 13 [11], 14 [15], and 15 [31]) | 191,614 | C | Endocytosis | Q00610 | 12 | ||
| CD148 | 145,927 (G) | M | Signaling | Q12913 | 2 | ||
| CD169 (sialoadhesin) | 182,624 | M | Adhesion | Q9BZZ2 | 6 | ||
| CD205 | 198,271 | M | Endocytosis | O60449 | 2 | ||
| Tenascin-C | 240,866 | S | Adhesion | P24821 | 3 | ||
| Talin-1 (fractions 14 [2] and 15 [3]) | 269,767 | M | Cytoskeketon membrane | Q9Y490 | 19 | ||
| Complement C3 (fraction 14 [2]) | 187,164 | S | Cell lysis | P01024 | 2 | ||
| NPC-1 (fraction 15 [1]) | 142,167 | C | LE/cholesterol trafficking | O15118 | 1 | ||
| Dermcidin | 11,284 | S | Antimicrobial reaction | P81605 | 2 | ||
| 17 (>280) | Filamin A (FLNa) | 280,759 | S | Actin cytoskeleton | P21333 | 17 | |
| Plectin 1 (PLTN) | 531,732 | C | IF cytoskeleton | Q15149 | 4 |
The CID spectra were compared against those of the EMBL nonredundant protein database by using SEQUEST (ThermoElectron, San Jose, CA). Only those peptides having cross-correlation (Xcorr) cutoffs of 1.9 for [M + H]1+, 2.2 for [M + 2H]2+, and 3.5 for [M + 3H]3+ and with delta cross-correlation scores (ΔCn) of at least 0.09 (65) were considered. Proteins were identified at least by two peptides, either unique peptides in the same fraction or independent observations in different fractions. These SEQUEST criteria thresholds have been determined previously to result in a 95% confidence level in peptide identification (77).
Alternative names are provided in parentheses. Proteins whose observed mass falls within the expected molecular mass range of their fraction are shown in bold. Peptides from a given protein found in more than one fraction are indicated in parentheses with the other fraction numbers in which it was observed. The number of peptides found in the secondary fractions is indicated in brackets.
Theoretical molecular mass for the primary translation product calculated from DNA sequences protein. Glycosylated proteins are indicated by a G. Proteins with a processed cellular form are indicated by a P.
Proteins previously found in virions (Vir) or exosomes (Exo) are indicated.
The locations of the protein are indicated as follows: C, cytoplasmic (can include intracellular vesicles); M, plasma membrane; N, nuclear; S, secreted; ER, endoplasmic reticulum; G, Golgi; P, peroxisomal; and ?, unknown.
Abbreviations: UPS, ubiquitin/proteasome system; IF, intermediate filament; LE, late endosome; ER, endoplasmic reticulum; Reg., regulation; Red/Ox, reduction oxidation; and ?, unknown.
Accession numbers for UniProt (accessible at http://www.pir.uniprot.org/search/textSearch.shtml).
eEF1A (formerly named elongation factor-1 alpha) (12) fragments produced by HIV-1 protease (11, 51).
A 47-kDa truncated version of titin of unknown function was detected in immunoblots of monocyte-derived macrophage cultures (data not shown).
The tandem mass spectra were compared against spectra of the EMBL nonredundant human protein database by using a SEQUEST search program. After filtering the results based on Xcorr (cutoffs of 1.9 for [M + H]1+, 2.2 for [M + 2H]2+, and 3.5 for [M + 3H]3+) and ΔCn (at least 0.09) values, the peptide identifications were segregated into cellular and viral proteins: the 253 unique cellular proteins are shown in Table 1 and the expected set of viral proteins is shown in Table 2. The implications and caveats on these findings are presented in Discussion below.
TABLE 2.
Viral proteins
| Fraction (size in kDa) | Protein namea | No. of peptides |
|---|---|---|
| 1 (5-7) | Pol TF (p6pol, p6*, preprotease) | 1 |
| gp41TM | 1 | |
| gp120SU | 2 | |
| 2 (8-11) | Pol TF (p6pol, p6*, preprotease) | 2 |
| p7NC | 2 | |
| p6Gag | 2 | |
| RNase H | 1 | |
| p17MA | 4 | |
| 3 (11-16) | p24CA | 3 |
| IN | 2 | |
| Tat | 1 | |
| RNase H | 2 | |
| 4 (16-20) | p17MA | 18 |
| Nef | 1 | |
| 5 (20-24) | p17MA | 7 |
| 6 (24-29) | p24CA | 8 |
| Nef | 1 | |
| 7 (29-34) | IN | 1 |
| P17MA | 6 | |
| P24CA | 3 | |
| 9 (43-48) | gp41TM | 2 |
| gp120SU | 3 | |
| 10 (48-56) | Pr55Gag (p17MA) | 2 |
| Pr55Gag (p24CA) | 2 | |
| RT | 1 | |
| 11 (56-64) | Pr55Gag (p17CA) | 1 |
| Pr55Gag (p24CA) | 1 | |
| gp41TM | 5 | |
| 12 (64-70) | RT | 1 |
| Vif | 2 | |
| 13 (70-80) | Nef | 1 |
| 14 (80-100) | Nef | 2 |
| 15 (100-130) | gp120SU | 3 |
Proteins whose observed mass falls within the expected molecular mass range of their fractions are shown in bold. The Pr55Gag peptides detected in fractions 10 and 11 are indicated in parentheses. Pol TF, polymerase transframe; RT, reverse transcriptase; IN, integrase.
Immunoblot analysis of virions.
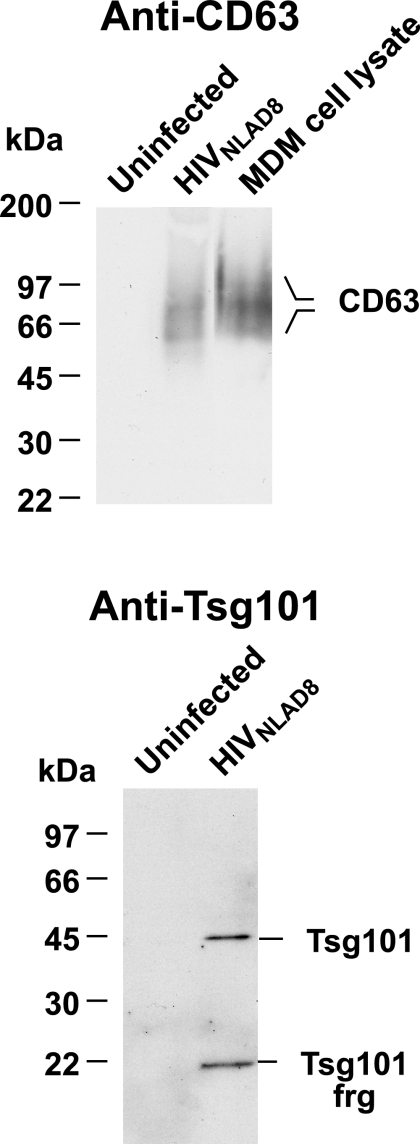
While our μRPLC-MS/MS analysis found a large number of proteins, it failed to detect the CD63 protein, a marker for the late endosomal/MVB system that was previously found in macrophage-produced HIV-1 (45, 54). Failure to identify a protein by μRPLC-MS/MS peptide analysis does not conclusively demonstrate that the protein is not present in the analyzed sample, since the absence of identifiable peptides can be due to several technical factors, especially for highly glycosylated proteins such as CD63. To determine if CD63 was present in our MDM-derived HIV-1 preparations, CD45-depleted preparations from uninfected and infected cultures (equal amounts by volume approximately 200 ng p24CA in the virion sample) were examined by immunoblot analysis. The results revealed that the CD45-depleted virion preparations did indeed contain CD63, confirming that CD63 is present in HIV-1 virions produced from MDM, while there was no signal from the matched CD45-depleted uninfected cell sample (Fig. 4).
FIG. 4.
CD63 and Tsg101 immunoblots of MDM-derived HIV-1. Immunoblots of virion preparations (equal amounts by volume with approximately 200 ng p24CA in the virion sample) isolated from parallel uninfected and HIV-1NLAD8-infected MDM cultures are shown. The samples are identified above their respective lanes. The antibody or antiserum used is indicated above each blot. For the CD63 immunoblot, a lane containing a cell lysate control from uninfected MDM (lysate from 5 × 105 cells) is included. frg, fragment.
Tsg101, a late endosomal sorting protein that is essential for viral budding, has been found in HIV-1 virions produced from HeLa cell and T-cell lines (14, 22, 75). However, we did not find any Tsg101 peptides by our μRPLC-MS/MS analysis in fraction 9, where the full-length protein should migrate in the gel (44 kDa). However, we did find two peptides in fraction 5 that spans 20 to 24 kDa. To confirm the presence of this important protein, we carried out an immunoblot analysis of CD45-depleted preparations from infected and uninfected MDM (equal amounts by volume with approximately 200 ng p24CA in the virion sample). The results revealed a band of 43 kDa that corresponds to full-length Tsg101, as well as a 22-kDa band that confirms the presence of a fragment of Tsg101 as indicated by the peptide sequences in fraction 5. Since this fragment was not detected in MDM cellular lysates (data not shown), it might be a protease cleavage HIV-1 product of some of the Tsg101 incorporated into virions. Other cellular proteins in the virion are known to be cleaved by HIV-1 protease (11, 51, 52, 70). In contrast to the virion sample, the CD45-depleted sample from a parallel, uninfected cell culture contained no detectable Tsg101 signal (Fig. 4). These data confirm that both full-length and truncated forms of Tg101 are present in MDM-derived HIV-1 virions.
Env content of MDM-derived HIV-1.
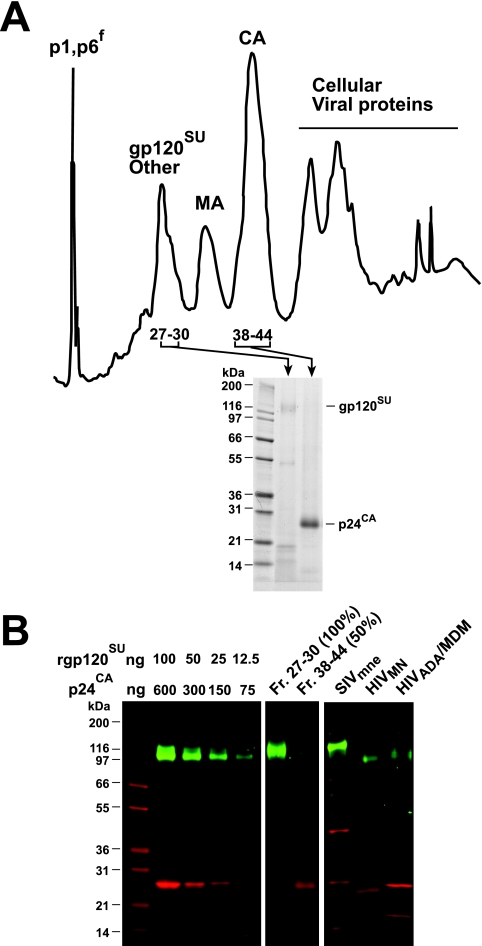
Previously, we found that HIV-1 and simian immunodeficiency virus virions produced from various T-cell lines in which the virus assembles on the cell surface contain between 7 and 14 Env trimers per virion (10, 78). To evaluate whether the alternative MVB-based assembly and budding mechanism used by HIV-1 in macrophages alters the incorporation of Env into virions, the proteins in an MDM-derived HIV-1 preparation that was density gradient-purified twice were separated and collected into fractions by HPLC (Fig. 5). SDS-PAGE gel analysis of the fractions containing p24CA (fractions 38 to 44) showed an intense p24CA band, while those containing gp120SU (fractions 27 to 30) showed only a small amount of signal for gp120SU relative to the large amounts of cellular proteins. Quantitative amino acid analysis to measure gp120SU content was therefore not feasible. To determine the relative contents of Gag and Env in virions, we quantitated the amounts of both Gag (p24CA) and Env (gp120SU) in MDM-produced virion preparations by fluorescence-based quantitation (39). SDS-PAGE gels of virus samples and serial dilutions of purified protein standards were stained first with SYPRO Pro-Q Emerald, a fluorescent dye that detects glycoproteins, and the gp120SU signal was measured. The proteins in the gel were then stained with the fluorescent dye SYPRO Ruby, which reacts with all proteins, and the amount of p24CA present was determined. A merged image of a representative gel is presented in Fig. 5B. The masses of p24CA and gp120SU in the virion samples were then calculated by interpolation of integrated pixel densities for the test samples onto standard curves obtained from a dilution series of calibrated protein standards (Fig. 5B; Table 3). Based on these data, the molar Gag-to-Env ratio was 39 to 1. This corresponds to an average of 12 Env trimers per MDM-derived HIV particle based on an estimated 1,400 Gag molecules per particle. This is the value that we previously have determined by direct enumeration of trimers on individual virions by electron tomography combined with biochemical quantitation of Gag/Env ratios (78). However, by comparing in vitro-produced Gag particles to immature virions, another group has estimated that there are 5,000 Gag molecules per virion (5). While there currently is no clear resolution between the two methods, we favor our direct approach because the virions are produced under native conditions. The number of Env trimers quantified using this method also correlates well with the Gag/Env stoichiometry of other retroviruses (53, 74). For comparison, the two-dye fluorescent analysis of the well-characterized control virus preparations HIV-1MN/H9 clone 4 and SIVmne/HuT-78 cl.E11S yielded results (Table 3) that showed excellent agreement with previous results obtained by alternative methods (6:1 and 60:1 trimers per 1,400 Gag molecules, respectively [10, 78]).
FIG. 5.
Env content analysis of MDM-derived HIV-1. (A) An HPLC chromatogram (A206) of HIV-1ADA produced from MDM is shown. Reversed-phase HPLC of denatured virions was performed as described in Materials and Methods. Viral protein peaks (identified by SDS-PAGE gels) are labeled above the chromatograph, and Coomassie blue-stained SDS-PAGE gel analysis of pertinent corresponding fractions is shown below, with positions of the molecular mass markers denoted at the left. (B) A two-dye SYPRO-stained SDS-PAGE gel is shown. Samples are identified above their respective lanes. For the recombinant gp120SU (rgp120SU) and purified p24CA standards, the amount of material loaded in each lane (in ng) is indicated above each lane. The relative loads, by volume, for the HPLC fractions are indicated in parentheses on the lane labels. The images of these standards have been digitally overlaid and merged for presentation purposes. The relative intensities of these images have not been altered. The positions of molecular mass markers are denoted at the left. p6f, an HIV protease cleavage fragment of p6; Fr., fraction.
TABLE 3.
Quantitation of Env and Gag in virion preparations
| Virus preparation | Amt (pmol) of:
|
Amt of CA/amt of gp120 | Env molecules/ virion in:
|
||
|---|---|---|---|---|---|
| CAa | gp120b | Two-color assayc | Previous reportd | ||
| HIV-1/MDMe | 24 | 0.62 | 39 | 12 | ND |
| HIV-1MN/clone 4 | 5.5 | 0.09 | 63 | 7 | 6 |
| SIVmne/E11S | 3.8 | 0.53 | 7.2 | 67 | 60 |
Calculated from the ng of protein detected by SYPRO orange assay.
Calculated from the ng of protein detected by SYPRO green assay.
Calculations using 1,400 Gag molecules per virion as estimated by Zhu et al. (78).
Previously determined number of Env molecules per virion from amino acid analysis (1,400 Gag molecules per virion) (10). ND, not determined.
Produced from pooled harvests of infected MDM on six-well hydrogel-treated plates (∼107 cells per well).
DISCUSSION
The proteomic analysis of HIV-1 virions produced from monocyte-derived macrophages presented here reveals many new proteins not previously described in HIV-1 virions. These proteins are components of a wide variety of cellular systems. Since viruses rely heavily on cellular systems for their replication, these proteins provide potential clues for viral biology. Of the 253 unique proteins identified, 33 were proteins previously found in virions produced from nonmacrophage cells (Table 1) (49, 71). However, most of the proteins (i.e., 220) had not been previously identified in HIV-1 particles. Many of the observed proteins were found in gel fractions corresponding to their expected size, as deduced from their amino acid sequence. In addition, other proteins, especially the glycosylated proteins, were found in fractions with higher molecular mass than the protein sequence. Other posttranslational modifications, such as phosphorylation and ubiquitination, might also cause proteins to migrate more slowly than expected and be found in higher-molecular-mass bands on the SDS-PAGE gels. The presence of ubiquitin in several higher-molecular-mass fractions (fractions 4, 6, 8, 10, 12, and 15) is consistent with the potential presence of ubiquitin-conjugated proteins in these regions. In contrast, some proteins migrated at a faster rate, reflecting a lower molecular mass than expected for the full-length protein. In some cases, this might be due to HIV-1 protease cleaving cellular proteins (70). For example, eEF1A (formerly elongation factor-1 alpha), which has been previously found in HIV-1 virions in a full-length (53-kDa) form and as a 30-kDa C-terminal-cleaved fragment, was detected in both the 29- to 34-kDa and 34- to 43-kDa fractions (11, 51). Also, immunoblot analysis demonstrated the presence of a truncated form of Tsg101 in virions. A molecular mass lower than expected might also be due to cellular proteins being expressed in truncated forms through alternative splicing of message or posttranslational cleavage by cellular proteases inside the cell. One surprise on our list was a titin peptide in fraction 9 (43 to 48 kDa). However, immunoblot analysis of MDM cell lysate demonstrated the presence of a 47-kDa species of what is normally a 3-MDa protein (L. V. Coren and D. E. Ott, unpublished results). The significance or function of this truncated titin in MDM cells is not known.
Cellular proteins may be incorporated into virions as bystanders, simply by being fortuitously present at the site of budding. Alternatively, such proteins as Tsg101 (fraction 5) and AIP1 (Alix; fractions 2, 12, and 14), which interact with Gag to help form the virion and assist in the budding process (13, 44, 64, 75) might be incorporated because they are active participants in different stages of the viral replication cycle,. Lastly, cellular proteins preferentially incorporated into virions may influence viral biology and pathogenesis, as has been shown for integrins, which can increase infectivity (4, 66), and HLA class II proteins, the presence of which on virions has been associated with an induction of increased levels of apoptosis in vitro (19). Based on these precedents, identification of additional cellular proteins incorporated into virions may provide useful insights into viral biology.
The virion preparations described herein contained proteins from several important cytoplasmic systems, including the actin cytoskeleton, microtubules, ubiquitin/proteasomes, translation, protein chaperones, signal transduction, endosome/exosome, and metabolism. In addition, surface proteins associated with cell signaling, adhesion, and antigen presentation were identified. However, at present, the relevance of these proteins to viral biology is not clear. While all of these proteins provide important possibilities for influencing viral function, the present observations represent a starting point. Further studies will be needed to determine the relevance of these host proteins to viral biology.
One of the current models for HIV-1 budding suggests that the viral components, especially Gag, assemble at either late endosome/MVB vesicles directly or at late endosomal/MVB-derived patches on the plasma membrane before budding (3, 7, 26, 29, 42, 46, 54, 55, 63, 75). In macrophages, immunoelectron microscopy for HIV-1 Gag and cellular markers indicates that the internal vesicles harboring HIV-1 are late endosomal/MVB structures (54). Our data support these observations; we found many common endosomal/MVB proteins in HIV-1 derived from macrophages, including HLA class II, actin, and actin-binding proteins, as well as tetraspanins, e.g., CD9 (fraction 6 and others), tetraspanin-14 (fraction 6), CD53 (fraction 8 and others), CD81 (fractions 2 and 5), CD82 (fractions 10 and 11), and the CD63 late endosome marker. A recent report by Nydegger et al. has found that HIV-1 assembles and buds at membrane regions that contains tetraspanins (CD9, CD63, CD81, and CD82), Vps28, and Tsg101 (47), proteins identified by our analyses.
One of the endosomal/MVB-associated proteins that we found in virions, annexin 2 (fractions 7, 8, and 9), is a cytoplasmic actin-binding protein, which translocates to the plasma membrane in stimulated cells (24). Annexin 2 tightly binds to a member of the S100 family of calcium-binding proteins, S100A10 (p11). Upon binding, annexin 2 and S100A10 form a heterotetramer which is capable of binding two membrane surfaces simultaneously, potentially promoting fusion events (38), and also plays a role in exocytosis (24). The S100A10 (fraction 2) protein was also detected by our analysis, suggesting that HIV-1 derived from MDM incorporates this complex. Other S100 family members were also detected in viral sample (fraction 2) and could play various roles in fusion and membrane organization (16, 38). Recently, annexin 2 was shown to participate in lipid raft organization, as well as docking and fusion of secretory granules with the plasma membrane (exocytosis) in neuroendocrine cells (9). The presence of annexin 2 in HIV-1 viral particles supports the idea that HIV-1 is exploiting endosomal/exosomal pathways. Recently, Ryzhova et al. have shown that annexin 2A binds Gag and that small interfering RNA-mediated depletion of this annexin prevents viral budding from cells (61), suggesting a role for this protein in HIV-1 assembly. Other less studied annexin family members (annexins A5 [fraction 7], A11 [fraction 10], and A6 [fraction 11]) were also identified by our analysis, but their function in the cell is still not clear (24).
In addition to annexins, proteins from other important vesicle trafficking systems were also present. Rab protein family members, which function mostly in vesicle transport (6), are present in MDM-derived HIV-1 (fraction 6). The NPC1 protein, a protein that transports cholesterol through the late endosomal system (33), was also observed (fraction 15 and 16), further supporting the idea that HIV-1 assembly occurs on MVB membranes in macrophages. The sorting of proteins within the cell is regulated by members of the Vps family (13, 14, 20, 22, 42, 44, 75). Our analysis found Tsg101 (the human ortholog of yeast Vps23; fraction 5 and immunoblot), Vps28 (fraction 6), Vps29 (fraction 5), and Vps35 (fraction 13). These proteins are involved in vesicle sorting, and Tsg101 and Vsp28 are both members of the ESCRT I complex which has been shown to be involved in virus budding from the cell (13, 44).
Besides markers of the endosomal compartment, several components of exosomes were also identified by our analysis. Our macrophage-produced viruses contained 26 out of 37 proteins previously observed by a proteomic analysis of exosomes from dendritic cells (68), further supporting the proposal that HIV-1 and exosomes use similar budding mechanisms in macrophages, with the virus exploiting the exosome budding pathway to release virions (28).
Apolipoprotein E (ApoE; fraction 8) is best known for its role in plasma cholesterol transport (for a review, see reference 41) but has also been implicated in immunoregulation and modulation of cell growth and differentiation (35, 40, 41) and was identified in our analysis. Macrophages express ApoE (40), although the levels of expression can vary widely (67). ApoE binds lipid membrane surfaces (62), so the presence of this secreted protein could be the result of binding to the virion surface. Macrophages also endocytose ApoE and recycle it back to the surface (30), raising the possibility that HIV-1 could acquire this protein in an exocytotic compartment, such as the late endosomal system in macrophages. The presence of ApoE on a HIV-1 could potentially facilitate virus entry, by targeting HIV-1 virions to low density lipoprotein receptor-expressing cells, such as monocytes (58).
The list of cellular proteins that we found by our μRPLC-MS/MS analysis is quite large, and several important caveats need to be considered when interpreting these data. First, these proteins were found in a population of virions, and it is unlikely that a single virion contains all of these proteins. In addition, HIV-1 is structurally very heterogeneous (5), so the amount and variety of cellular proteins in any particular virion could be quite different from those of another. Another consideration is that while the μRPLC-MS/MS analysis used here identified many proteins, others proteins (e.g., CD63) were not detected. This reflects the intrinsic difficulty in identifying proteins present in relatively small amounts within complex mixtures, even after gel fractionation. The scoring thresholds used in this study have been shown to provide a confidence level of greater than 97% in the identification of a protein by peptides (17, 56, 73, 77). While at this level of stringency, identification of even a single peptide constitutes solid evidence for the presence of the parent protein in the sample (73), we have presented only those proteins that yielded at least two peptides. Consequently, some proteins may have been excluded from by our μRPLC-MS/MS analysis due to the high-stringency thresholds employed.
Our μRPLC-MS/MS analysis of highly purified HIV-1 produced from infected MDM has identified numerous cellular proteins potentially involved in various aspects of virus replication, especially assembly. However, we also identified proteins whose presence in virions is difficult to interpret mechanistically based on current knowledge. Some proteins simply might be incorporated due to their relatively large amounts at the site of budding. Another explanation is that cellular proteins can have multiple and very different functions, including functions other than those for which they are best known (34). However, it is unlikely that all these unexpected proteins have multiple pertinent functions. While we have applied what we consider to be the best technology available to produce highly purified virions, removing contaminating proteins present (both soluble protein and those in vesicular particles), some of the proteins identified here could be simply adhered to the virions and not functionally incorporated into the particles. Even with this caveat, this proteomic analysis represents important data because it provides leads for further studies of the involvement of cellular proteins and pathways in the HIV-1 replication cycle. Such studies should provide additional information about the assembly and budding pathways used by HIV-1 in infected macrophages, raise interesting questions about the possible contributions of some of these virion-incorporated molecules in HIV-1 pathogenesis, and may point to potential targets for novel approaches to therapeutic intervention in HIV-1 infection.
Acknowledgments
We thank B. Bohn, J. Miller, T. Ireland, M. Poore, and R. Imming for purified viruses and M. Jason de la Cruz for skilled electron microscope assistance.
This project has been funded in whole with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1.Bess, J. W., Jr., R. J. Gorelick, W. J. Bosche, L. E. Henderson, and L. O. Arthur. 1997. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 230:134-144. [DOI] [PubMed] [Google Scholar]
- 2.Bess, J. W., Jr., P. J. Powell, H. J. Issaq, L. J. Schumack, M. K. Grimes, L. E. Henderson, and L. O. Arthur. 1992. Tightly bound zinc in human immunodeficiency virus type 1, human T-cell leukemia virus type 1, and other retroviruses. J. Virol. 66:840-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth, A. M., Y. Fang, J. K. Fallon, J. M. Yang, J. E. Hildreth, and S. J. Gould. 2006. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 172:923-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bounou, S., J. F. Giguere, R. Cantin, C. Gilbert, M. Imbeault, G. Martin, and M. J. Tremblay. 2004. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 18:1294-1296. [DOI] [PubMed] [Google Scholar]
- 5.Briggs, J. A., M. N. Simon, I. Gross, H. G. Krausslich, S. D. Fuller, V. M. Vogt, and M. C. Johnson. 2004. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 11:672-675. [DOI] [PubMed] [Google Scholar]
- 6.Bucci, C., and M. Chiariello. 2006. Signal transduction gRABs attention. Cell Signal. 18:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Cantin, R., S. Methot, and M. J. Tremblay. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79:6577-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman, J. R. 2000. Mass spectrometry of protein and peptides. Humana Press, Totowa, N.J.
- 9.Chasserot-Golaz, S., N. Vitale, E. Umbrecht-Jenck, D. Knight, V. Gerke, and M. F. Bader. 2005. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol. Biol. Cell 16:1108-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertova, E., J. W. Bess, Jr., B. J. Crise, I. R. Sowder, T. M. Schaden, J. M. Hilburn, J. A. Hoxie, R. E. Benveniste, J. D. Lifson, L. E. Henderson, and L. O. Arthur. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 76:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cimarelli, A., and J. Luban. 1999. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 73:5388-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, B. F. C., M. Grunberg-Manago, N. K. Gupta, J. W. B. Hershey, A. G. Hinnebusch, R. J. Jackson, U. Maitra, M. B. Matthews, W. C. Merrick, R. E. Rhoads, N. Sonenberg, L. L. Spremulli, H. Trachsel, and H. O. Voorma. 1996. Prokaryotic and eukaryotic translation factors. Biochimie 78:1119-1122. [PubMed] [Google Scholar]
- 13.Demirov, D. G., and E. O. Freed. 2004. Retrovirus budding. Virus Res. 106:87-102. [DOI] [PubMed] [Google Scholar]
- 14.Demirov, D. G., A. Ono, J. M. Orenstein, and E. O. Freed. 2002. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA 99:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denzer, K., M. J. Kleijmeer, H. F. Heijnen, W. Stoorvogel, and H. J. Geuze. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113:3365-3374. [DOI] [PubMed] [Google Scholar]
- 16.Donato, R. 2003. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 60:540-551. [DOI] [PubMed] [Google Scholar]
- 17.Elias, J. E., W. Haas, B. K. Faherty, and S. P. Gygi. 2005. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat. Methods 2:667-675. [DOI] [PubMed] [Google Scholar]
- 18.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4+ and CD8+ T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 75:1152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, Jr., L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freed, E. O., G. Englund, and M. A. Martin. 1995. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J. Virol. 69:3949-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 23.Gelderblom, H. R. 1991. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS 5:617-638. [PubMed] [Google Scholar]
- 24.Gerke, V., C. E. Creutz, and S. E. Moss. 2005. Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 6:449-461. [DOI] [PubMed] [Google Scholar]
- 25.Gluschankof, P., I. Mondor, H. R. Gelderblom, and Q. J. Sattentau. 1997. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology 230:125-133. [DOI] [PubMed] [Google Scholar]
- 26.Goff, A., L. S. Ehrlich, S. N. Cohen, and C. A. Carter. 2003. Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release. J. Virol. 77:9173-9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonda, M. A., S. A. Aaronson, N. Ellmore, V. H. Zeve, and K. Nagashima. 1976. Ultrastructural studies of surface features of human normal and tumor cells in tissue culture by scanning and transmission electron microscopy. J. Natl. Cancer Inst. 56:245-263. [DOI] [PubMed] [Google Scholar]
- 28.Gorelick, R. J., S. M. Nigida, J. W. Bess, Jr., L. E. Henderson, L. O. Arthur, and A. Rein. 1990. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J. Virol. 64:3207-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould, S. J., A. M. Booth, and J. E. Hildreth. 2003. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 100:10592-10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasty, A. H., M. R. Plummer, K. H. Weisgraber, M. F. Linton, S. Fazio, and L. L. Swift. 2005. The recycling of apolipoprotein E in macrophages: influence of HDL and apolipoprotein A-I. J. Lipid Res. 46:1433-1439. [DOI] [PubMed] [Google Scholar]
- 31.Heijnen, H. F., A. E. Schiel, R. Fijnheer, H. J. Geuze, and J. J. Sixma. 1999. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94:3791-3799. [PubMed] [Google Scholar]
- 32.Hwang, I., X. Shen, and J. Sprent. 2003. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc. Natl. Acad. Sci. USA 100:6670-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ioannou, Y. A. 2005. Guilty until proven innocent: the case of NPC1 and cholesterol. Trends Biochem. Sci. 30:498-505. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery, C. J. 2003. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 19:415-417. [DOI] [PubMed] [Google Scholar]
- 35.Lahiri, D. K. 2004. Apolipoprotein E as a target for developing new therapeutics for Alzheimer's disease based on studies from protein, RNA, and regulatory region of the gene. J. Mol. Neurosci. 23:225-233. [DOI] [PubMed] [Google Scholar]
- 36.Lawn, S. D., T. L. Pisell, C. S. Hirsch, M. Wu, S. T. Butera, and Z. Toossi. 2001. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J. Infect. Dis. 184:1127-1133. [DOI] [PubMed] [Google Scholar]
- 37.Lawn, S. D., B. D. Roberts, G. E. Griffin, T. M. Folks, and S. T. Butera. 2000. Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J. Virol. 74:139-145. [PMC free article] [PubMed] [Google Scholar]
- 38.Lewit-Bentley, A., S. Rety, J. Sopkova-de Oliveira Santos, and V. Gerke. 2000. S100-annexin complexes: some insights from structural studies. Cell Biol. Int. 24:799-802. [DOI] [PubMed] [Google Scholar]
- 39.Louder, M. K., A. Sambor, E. Chertova, T. Hunte, S. Barrett, F. Ojong, E. Sanders-Buell, S. Zolla-Pazner, F. E. McCutchan, J. D. Roser, D. Gabuzda, J. D. Lifson, and J. R. Mascola. 2005. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology 339:226-238. [DOI] [PubMed] [Google Scholar]
- 40.Mahley, R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622-630. [DOI] [PubMed] [Google Scholar]
- 41.Mahley, R. W., and S. C. Rall, Jr. 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1:507-537. [DOI] [PubMed] [Google Scholar]
- 42.Marsh, M., and M. Thali. 2003. HIV's great escape. Nat. Med. 9:1262-1263. [DOI] [PubMed] [Google Scholar]
- 43.Martin, F., D. M. Roth, D. A. Jans, C. W. Pouton, L. J. Partridge, P. N. Monk, and G. W. Moseley. 2005. Tetraspanins in viral infections: a fundamental role in viral biology? J. Virol. 79:10839-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morita, E., and W. I. Sundquist. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20:395-425. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen, D. G., A. Booth, S. J. Gould, and J. E. Hildreth. 2003. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J. Biol. Chem. 278:52347-52354. [DOI] [PubMed] [Google Scholar]
- 46.Nydegger, S., M. Foti, A. Derdowski, P. Spearman, and M. Thali. 2003. HIV-1 egress is gated through late endosomal membranes. Traffic 4:902-910. [DOI] [PubMed] [Google Scholar]
- 47.Nydegger, S., S. Khurana, D. N. Krementsov, M. Foti, and M. Thali. 2006. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J. Cell Biol. 173:795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orenstein, J. M., M. S. Meltzer, T. Phipps, and H. E. Gendelman. 1988. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J. Virol. 62:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott, D. E. 2002. Potential roles of cellular proteins in HIV-1. Rev. Med. Virol. 12:359-374. [DOI] [PubMed] [Google Scholar]
- 50.Ott, D. E., L. V. Coren, T. D. Gagliardi, and K. Nagashima. 2005. Heterologous late-domain sequences have various abilities to promote budding of human immunodeficiency virus type 1. J. Virol. 79:9038-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ott, D. E., L. V. Coren, D. G. Johnson, B. P. Kane, I. Sowder, R. C. Sowder II, Y. D. Kim, R. J. Fisher, X. Z. Zhou, K. P. Lu, and L. E. Henderson. 2000. Actin-binding cellular proteins inside human immunodeficiency virus type 1. Virology 266:42-51. [DOI] [PubMed] [Google Scholar]
- 52.Ott, D. E., L. V. Coren, B. P. Kane, L. K. Busch, D. J. Johnson, I. Sowder, R. C., E. N. Chertova, L. O. Arthur, and L. E. Henderson. 1996. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70:7734-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker, S. D., J. S. Wall, and E. Hunter. 2001. Analysis of Mason-Pfizer monkey virus Gag particles by scanning transmission electron microscopy. J. Virol. 75:9543-9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelchen-Matthews, A., B. Kramer, and M. Marsh. 2003. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 162:443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelchen-Matthews, A., G. Raposo, and M. Marsh. 2004. Endosomes, exosomes and Trojan viruses. Trends Microbiol. 12:310-316. [DOI] [PubMed] [Google Scholar]
- 56.Peng, J., J. E. Elias, C. C. Thoreen, L. J. Licklider, and S. P. Gygi. 2003. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2:43-50. [DOI] [PubMed] [Google Scholar]
- 57.Pisell, T. L., I. F. Hoffman, C. S. Jere, S. B. Ballard, M. E. Molyneux, S. T. Butera, and S. D. Lawn. 2002. Immune activation and induction of HIV-1 replication within CD14 macrophages during acute Plasmodium falciparum malaria coinfection. AIDS 16:1503-1509. [DOI] [PubMed] [Google Scholar]
- 58.Powell, E. E., and P. A. Kroon. 1994. Low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in human mononuclear leukocytes is regulated coordinately and parallels gene expression in human liver. J. Clin. Investig. 93:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raposo, G., M. Moore, D. Innes, R. Leijendekker, A. Leigh-Brown, P. Benaroch, and H. Geuze. 2002. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic 3:718-729. [DOI] [PubMed] [Google Scholar]
- 60.Rappsilber, J., Y. Ishihama, and M. Mann. 2003. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75:663-670. [DOI] [PubMed] [Google Scholar]
- 61.Ryzhova, E. V., R. M. Vos, A. V. Albright, A. V. Harrist, T. Harvey, and F. Gonzalez-Scarano. 2006. Annexin 2: a novel human immunodeficiency virus type 1 Gag binding protein involved in replication in monocyte-derived macrophages. J. Virol. 80:2694-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saito, H., P. Dhanasekaran, F. Baldwin, K. H. Weisgraber, S. Lund-Katz, and M. C. Phillips. 2001. Lipid binding-induced conformational change in human apolipoprotein E. Evidence for two lipid-bound states on spherical particles. J. Biol. Chem. 276:40949-40954. [DOI] [PubMed] [Google Scholar]
- 63.Sherer, N. M., M. J. Lehmann, L. F. Jimenez-Soto, A. Ingmundson, S. M. Horner, G. Cicchetti, P. G. Allen, M. Pypaert, J. M. Cunningham, and W. Mothes. 2003. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4:785-801. [DOI] [PubMed] [Google Scholar]
- 64.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 65.Tabb, D. L., L. L. Smith, L. A. Breci, V. H. Wysocki, D. Lin, and J. R. Yates III. 2003. Statistical characterization of ion trap tandem mass spectra from doubly charged tryptic peptides. Anal. Chem. 75:1155-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tedla, N., E. N. Glaros, U. T. Brunk, W. Jessup, and B. Garner. 2004. Heterogeneous expression of apolipoprotein-E by human macrophages. Immunology 113:338-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thery, C., M. Boussac, P. Veron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena. 2001. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166:7309-7318. [DOI] [PubMed] [Google Scholar]
- 69.Thery, C., L. Zitvogel, and S. Amigorena. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2:569-579. [DOI] [PubMed] [Google Scholar]
- 70.Tomasselli, A. G., J. O. Hui, L. Adams, J. Chosay, D. Lowery, B. Greenberg, A. Yem, M. R. Deibel, H. Zurcher-Neely, and R. L. Heinrikson. 1991. Actin, troponin C, Alzheimer amyloid precursor protein and pro-interleukin 1b as substrates of the protease from human immunodeficiency virus. J. Biol. Chem. 266:14548-14553. [PubMed] [Google Scholar]
- 71.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 72.Trubey, C. M., E. Chertova, L. V. Coren, J. M. Hilburn, C. V. Hixson, K. Nagashima, J. D. Lifson, and D. E. Ott. 2003. Quantitation of HLA class II protein incorporated into human immunodeficiency type 1 virions purified by anti-CD45 immunoaffinity depletion of microvesicles. J. Virol. 77:12699-12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Veenstra, T. D., T. P. Conrads, and H. J. Issaq. 2004. What to do with “one-hit wonders”? Electrophoresis 25:1278-1279. [DOI] [PubMed] [Google Scholar]
- 74.Vogt, V. M., and M. N. Simon. 1999. Mass determination of Rous sarcoma virus virions by scanning transmission electron microscopy. J. Virol. 73:7050-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H. G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist. 2003. The protein network of HIV budding. Cell 114:701-713. [DOI] [PubMed] [Google Scholar]
- 76.Wubbolts, R., R. S. Leckie, P. T. Veenhuizen, G. Schwarzmann, W. Mobius, J. Hoernschemeyer, J. W. Slot, H. J. Geuze, and W. Stoorvogel. 2003. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 278:10963-10972. [DOI] [PubMed] [Google Scholar]
- 77.Yu, L. R., T. P. Conrads, T. Uo, Y. Kinoshita, R. S. Morrison, D. A. Lucas, K. C. Chan, J. Blonder, H. J. Issaq, and T. D. Veenstra. 2004. Global analysis of the cortical neuron proteome. Mol. Cell Proteomics 3:896-907. [DOI] [PubMed] [Google Scholar]
- 78.Zhu, P., E. Chertova, J. Bess, Jr., J. D. Lifson, L. O. Arthur, J. Liu, K. A. Taylor, and K. H. Roux. 2003. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl. Acad. Sci. USA 100:15812-15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu, X., and I. A. Papayannopoulos. 2003. Improvement in the detection of low concentration protein digests on a MALDI TOF/TOF workstation by reducing alpha-cyano-4-hydroxycinnamic acid adduct ions. J. Biomol. Tech. 14:298-307. [PMC free article] [PubMed] [Google Scholar]